Abstract
Cancer is currently ineffectively treated using therapeutic drugs, and is also able to resist drug action, resulting in increased side effects following drug treatment. A novel therapeutic strategy against cancer cells is the use of anticancer peptides (ACPs). The physicochemical properties, amino acid composition and the addition of chemical groups on the ACP sequence influences their conformation, net charge and orientation of the secondary structure, leading to an effect on targeting specificity and ACP-cell interaction, as well as peptide penetrating capability, stability and efficacy. ACPs have been developed from both naturally occurring and modified peptides by substituting neutral or anionic amino acid residues with cationic amino acid residues, or by adding a chemical group. The modified peptides lead to an increase in the effectiveness of cancer therapy. Due to this effectiveness, ACPs have recently been improved to form drugs and vaccines, which have sequentially been evaluated in various phases of clinical trials. The development of the ACPs remains focused on generating newly modified ACPs for clinical application in order to decrease the incidence of new cancer cases and decrease the mortality rate. The present review could further facilitate the design of ACPs and increase efficacious ACP therapy in the near future.
Keywords: therapeutic peptide, anticancer peptide, modified peptide, targeting peptide, cancer, clinical application, clinical trial
1. Introduction
Cancer drug therapy was developed from chemotherapy and radiotherapy to molecular targeting therapy combined with a 'guiding missile', for cancer-targeted delivery to avoid healthy tissue damage (1). For example, in genome targeted therapy, DNAs and RNAs can interfere with the normal host genome, and genetic modification is difficult as the modified genes may mutate the original genome or the off-target (2). Furthermore, immunotherapy with antibodies against cancer cell surface antigens can provide specific delivery, but some healthy cells can express the same targeted antigens, resulting in limited effectiveness (3). Small molecules can also exert antitumor effects on cancer cells, such as C188-9, a STAT3 inhibitor, in head and neck squamous cell carcinoma, and GNS561, a lysosomotropic molecule, in intrahepatic cholangiocarcinoma (4,5). Moreover, these small molecules can be used in drug delivery systems (6); however, they are difficult to synthesize. Therefore, peptides against cancer cells are an alternative therapeutic method in anticancer drug development.
Anticancer peptides (ACPs): What and why?
ACPs, as small peptides containing amino acid sequences, are selective and toxic to cancer cells (7). ACPs are a superior choice of therapeutics compared with antibodies and small molecules due to their high selectivity, high penetration and easy modifications (8-10). Ideally, anticancer therapy should destroy a range of cancer types, but not all healthy cells.
A different property between cancerous and healthy cells is the cell membrane. Numerous anticancer peptides destroy cancer cells via apoptosis and necrosis by membrane lysis or pore formation (11-13). The eukaryotic cell membrane contains cholesterol to protect lytic action by modifying membrane fluidity (14). Moreover, a high level of membrane cholesterol can inhibit lytic activity. It has been shown that membrane fluidity of cancer cells is higher compared with healthy cells (15). Cancer cells also contain more abundant microvilli compared with healthy cells, which increases the cell surface area (16). Furthermore, healthy cells have electrical neutrality, whereas cancer cells contain a negatively charge component on their surface (17), leading to membrane destabilization, cytotoxicity and cancer cell lysis when interacting with small molecules, such as ACPs (18,19). In addition, the primary driving force for the interactions between peptides and the healthy cell membrane is the hydrophobic interactions, while that between peptides and the cancer cell membrane is the electrostatic interactions (20).
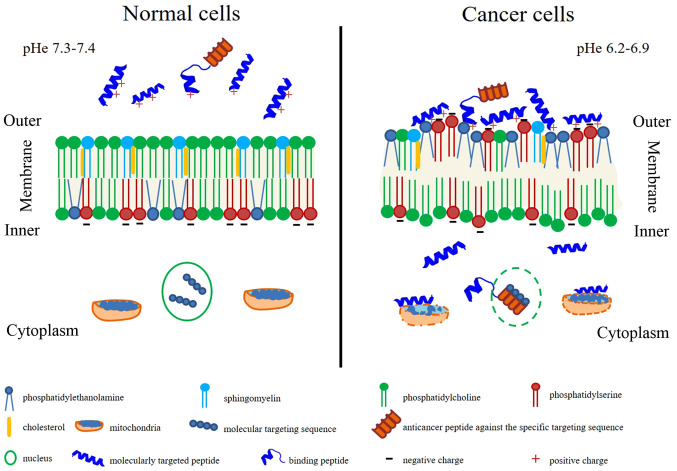
Anticancer medicines contain molecularly targeted drugs with or without 'guiding missiles' to interact with specific molecular targets on cancer cells (21). Besides molecularly targeted drugs, drug-delivery to the cancer cell surface was developed using the most important properties, including high specificity, high selectivity and the binding capability to various targeted drugs, as well as being easy to synthesize and produce (21). Peptide properties can be used both in molecularly targeted drugs and 'guiding missiles' to inhibit cell proliferation or eradicate cancer cells completely, depending on the amino acid residue composition, sequence length, isoelectric point, molecular weight, net charge, hydrophobicity, amphiphilicity, secondary structure and structural orientation (22). These ideal anticancer peptide characteristics are summarized in Fig. 1. Membrane characteristics promote or inhibit drug penetration, drug conformation and/or location within the membrane and sequentially affect therapeutic targets (23). Healthy cell membranes have zwitterion phosphatidylcholine and sphingomyelin in an outer leaflet and anionic phosphatidylserine and the phosphatidylethanonlamine in the inner leaflet with the asymmetric distribution (24). The inner leaflet with the asymmetric distribution is primarily maintained by flippases (phosphatidylserine and phosphatidylethanonlamine from outer to inner membrane), floppases (phosphatidylcholine and cholesterol from inner to outer membrane) and scramblases (facilitated the flip-flop of lipids) (24,25). In contrast, the cancer cell membrane loses this asymmetric distribution and alterations in membrane fluidity, resulting in exposure of negative charge of phosphatidylserine on the surface of the membrane, as well as the locating of phosphatidylethanonlamine on the outer leaflet (26-28). Furthermore, sphingomyelin is decreased in the cancer cell membrane and is associated with tumorigenesis (29). Different lipid composition affects membrane fluidity, influencing drug penetration and biological action (30,31). Extracellular acidity with or without exosome release affects the pH, changing from 7.4 to 6.5 (typical pH of cancer), forming the malignant tumor phenotype (32). The surrounding environment in the acidic extracellular pH (pHe) can promote cancer invasiveness (33). Specific interaction between anticancer peptides and cell membrane components are mostly bound by electrostatic interactions (34).
Figure 1.
Comparisons of membrane characteristics and anticancer peptides action on healthy cells (left) and cancer cells (right). The outer leaflet of the healthy cell membrane presents a neutral net charge leading to non-interaction of anticancer peptides on the healthy cell surface (left), whereas negative net charge on the outer leaflet of the cancer cell membrane could interact with the cationic anticancer peptides (right). In cancer cells, anticancer peptides, particularly in the α-helical form, act as molecularly targeted peptides that can penetrate and directly bind to the specific cancer cell or organelle membranes promoting cancer cell apoptosis. While, binding peptides linking to the anticancer drugs that have no anticancer property, can recognize and penetrate the cancer cell membrane. pHe, extracellular pH.
Anticancer peptides act as either molecularly targeted peptides, which can penetrate and directly bind to the specific cancer cell or organelle membranes, or binding peptides linking to the anticancer drugs (35-37). In cancer cells, anticancer peptides, as molecular targeting peptides, particularly in the α-helical form, penetrate the plasma membrane, the nuclear membrane and/or the mitochondrial membrane exerting pharmacological activity via different mechanisms (such as the inhibition of DNA synthesis or cell division), thus promoting cancer cell apoptosis (38-41). However, binding peptides, also referred to as cancer-targeting peptides or cell-penetrating peptides, that have no anticancer property, can recognize and penetrate the cancer cell membrane (42). Binding peptides can also be used for drug delivery by binding to the anticancer drugs, such as those that are non-penetrable (43).
Amino acid composition and derivatives in peptides also convey anticancer properties
Amino acid residues containing peptides can drive cell permeability (44-46). The amino acid residues that are predominant in peptides with anticancer abilities include glycine, lysine and leucine (47). For example, hydrophobic positively charged lysine- and arginine-rich peptides act as cationic peptides that can interact with membranes via a snorkeling mechanism, including selecting anionic membranes on cancer cells, disrupting cell membrane integrity, penetrating into the membrane and potentially serving a role in cancer cell toxicity (48). Moreover, protonation of histidine under acidic pH conditions means that histidine-containing peptides can induce cancer cytotoxicity via membrane permeability under acidic conditions (49,50). Glutamic and aspartic acid residues present potential anti-proliferative activity on the tumor cells (51). Cysteine residues in ACPs do not serve a role in the selectivity and toxicity for cancer cells, but cysteine-rich domains on a number of cell surface receptors can stabilize and maintain extracellular motif or domain structures (52).
Internal prolines in peptides are crucial for membrane interaction and conformational flexibility, which is the same as glycine residues (53). It has been reported that serine and glycine-free diets can slow tumor growth and enhance antiproliferative effects (54). Methionine, a moderately hydrophobic amino acid, does not serve a major role in ACPs, but its elevated levels can be consumed by cancer cells. Furthermore, a methionine-deficient diet causes a metabolic defect in cancer cells by arresting cancer cell proliferation (55). Phenylalanine, a strongly hydrophobic residue, is highly present in primary tumors and acts as a protective amino acid (56). Phenylalanine-containing peptides can also enhance the affinity for targeting the cancer cell membrane (57). Tyrosine and tryptophan are weakly hydrophobic amino acids; tyrosine does not serve a role in toxicity of ACPs, whereas tryptophan may exert a role in the toxicity of some ACPs against cancer cells such as indolicidin and trans-activator of transportation (TAT)-Ras GTPase-activating protein-326 peptides (19,58,59). However, synthesized peptides containing tryptophan and histidine may decrease cytotoxicity, while those containing tyrosine, phenylalanine or proline may be able to increase cytotoxic activity (60). The tryptophan position on the cell-penetrating peptides serves an important role in entering cancer cells, which subsequently involves an endocytic pathway and binding at the major groove of nuclear DNA (61). The role of amino acid residues on ACPs and on cancer cells is summarized in Table I. Collectively, these findings suggested ACPs should contain cationic and hydrophobic amino acid residues to further form secondary structures that affect cancer cells.
Table I.
Role of amino acid residues on ACP effects in cancer cells, based on previous reports.
| Amino acid residue | Amino acid properties | Action on cancer cells | (Refs.) |
|---|---|---|---|
| Charged residues on ACPs | |||
| Lysine Arginine | Positively charged (basic amino acids), | Disrupt cell membrane integrity and penetrate cell membrane, leading to cancer cell cytotoxicity | (48) |
| Histidine | polar, hydrophilic | Induce cancer cytotoxicity via membrane permeability under acidic condition | (49,50) |
| Glutamic acid Aspartic acid | Negatively charged (acidic amino acids), polar, hydrophilic | Antiproliferative activity on tumor cells | (51) |
| Effect on cancer cell structure | |||
| Cysteine | Polar, non-charged | On numerous cell surface receptors for stabilizing and maintaining extracellular motif/domain structure | (52) |
| Proline | Non-polar, aliphatic residues | Membrane interaction and conformational flexibility, may be able to increase cytotoxic activity | (53,60) |
| Glycine | Membrane interaction and conformational flexibility | (53) | |
| Phenylalanine | Aromatic | Enhance the affinity for target cancer cell membrane, act as protective amino acids of primary tumors and may be able to increase cytotoxic activity | (57,60) |
| Effect on cancer cell metabolism | |||
| Methionine | Polar, non-charged | Reduced methionine will arrest cancer cell proliferation | (55) |
| Tyrosine | Aromatic | May be able to increase cytotoxic activity | (60) |
| Tryptophan | Serve a role in the toxicity of some ACPs to cancer cells, entering cancer cells following an endocytic pathway and then binding at the major groove of nuclear DNA | (19,61) |
ACP, anticancer peptides.
ACPs and the structure-activity relationship (SAR)
The association between ACPs and SAR has been investigated and analyzed using machine learning, and it has been demonstrated that the majority of ACPs contained 21-30 amino acids and were predominately composed of glycine, lysine and leucine (47). In addition, amino acid residues on a peptide influences its anticancer activity depending on the cationic, hydrophobic and amphiphilic properties associated with forming helical structure (62-64). Anticancer activity is primarily determined by the IC50 value associated with cancer cell membrane disruption (62). It has been reported that peptides with a higher hydrophobicity can penetrate into the hydrophobic core of the cancer cell membrane, resulting in cancer cell disruption via necrosis (62). Several studies have aimed to substitute low hydrophobic and neutral or acidic amino acid residues with positively charged amino acid residues, such as lysine and leucine, on the polar and non-polar faces of α-helical peptides (63,65). As a result, high cationic peptides with moderate hydrophobicity can enhance the cytotoxicity of cancer cells (63). Peptides in free-form do not fold in solution, but arrange in an α-helix or β-sheet via electrostatic interaction on the membrane surface of the cells (11).
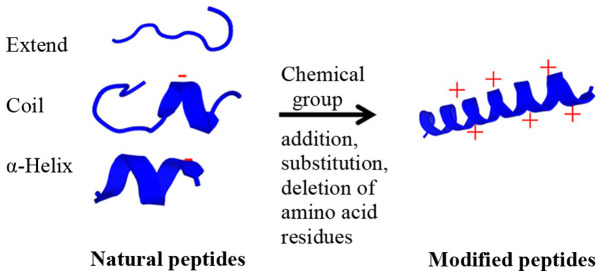
As well as the physicochemical properties, the secondary structure of the peptides is important in cell surface interaction, such as peptide structural orientation (57). The orientation of peptides can enhance the surface-activity for targeted interaction with the cancer cell membrane (66). The angle of the interaction leads to destabilized lipid packing on the cancer cell membrane, thus resulting in membrane penetration (67). Furthermore, modifying peptides by adding chemical groups, including methylation, acetylation or phosphorylation (particularly phosphorylation at tyrosine), can inhibit STAT3 phosphorylation, leading to cancer cell death (68). The potential modification of natural peptides is presented in Fig. 2. Therefore, the results indicated that the secondary structure of ACPs serves a crucial role in peptide-cancer cell membrane interaction, leading to cancer cell disruption and cell death.
Figure 2.
Modification of natural peptides. The natural peptide conformations included the extended, the coiled and/or the α-helical forms with neutral, anionic or cationic properties. These natural peptides are modified by adding the chemical groups (such as acyl and methyl groups) or the positive amino acid residues (such as lysine and arginine) to increase positive net charge and specificity for cancer cell targets. Moreover, the addition, deletion or substitution of the amino acid residues changes the conformation from the extended or coiled peptides to the α-helix form for higher cancer cell penetration. After modification, the cationic α-helix modified peptide exhibits higher efficacy and specificity to the cancer cells.
2. Classification of ACPs
Anticancer peptide creation should consider the peptide structure, mode of action, selectivity and efficacy to specific cancer cells (69,70). In the present review, active peptides were classified into three types depending on their actions, including: i) Molecularly targeted peptides, which directly act on cancer cells via cytotoxic, anti-proliferative and apoptotic activities; ii) 'guiding missile' peptides or binding peptides, which are drug binding peptides used for transporting drugs into the cancer cell targets; and iii) cell-stimulating peptides that indirectly effect other stimulating cells to kill cancer cells, such as via immunomodulatory activities and hormone receptors (71-73).
Molecularly targeted peptides
Molecularly targeted peptides, which are specific to the cancer cell targets, can penetrate, bind and then inhibit or kill cancer cells that are in an important stage of carcinogenesis or proliferation (74). The peptides concerning target cells can be classified into two major groups, including: i) Peptides against only cancer cells, and not against healthy cells (75,76) and, ii) peptides against both cancerous and healthy cells (77). Numerous peptides have selectivity for cancer cells but not healthy cells, such as peptides derived from defensins, lactoferricin B, cecropins, magainin-2 and chrysophsin-1 (22).
The majority of ACPs are collected using the CancerPPD resource for predicting peptide structure and identifying the best ACP for further study (7). In addition, ACPs are identified via computational methods that consider amino acid composition, binary profiles and sequence-based methods (78-80). Membranolytic ACPs are generated de novo using automated designs based on α-helical cationic amphipathic peptide sequences against the cancer cells (81). Anionic molecules in the malignant cells conferring a net negative charge are different from the normal mammalian cell membrane, which have a neutral net charge (17). High cholesterol contents in healthy cells can obstruct the cationic peptide entry via cell fluidity; healthy cells are less fluid compared with cancer cells (15,82). Furthermore, peptides can permeate into the cells, causing mitochondrial swelling with cytochrome c release, followed by apoptosis (83). For example, Mastoparan I, a peptide with a α-helical structure, can act on the negative charge of prostate and liver cancer cell surfaces causing cell injury, cell swelling, cell bursting and then necrosis (84). Moreover, SVS-1 (KVKVKVKVDPLPTKVKVKVK-NH2), as a β-sheet structure, disrupts cell membranes via pore formation in lung-, epidermal- and breast-cancer cells (85,86). Peptides extracted from marine organisms, such as sponges, mollusks, tunicates, bryozoans, algae, fish, soft corals and sea slugs, can act against human cancer cells via, for example, anti-proliferative, cytotoxicity and anti-tubulin activities, as well as suppressing microtubule depolymerization (87).
Amino acid composition of the peptides can act directly against various cancer cell types. For example, highly cationic peptides can enhance cancer cell specificity, while an increase in hydrophobic peptides can decrease the degree of specificity (63). Moreover, polycationic peptides have selectivity against human acute T-cell leukemia via a higher membrane potential compared with healthy cells (88). Lysine and argi-nine-rich peptides with an intact amphipathic helical interface can also enhance cell lysis via membrane lysis mechanisms by penetrating and inducing caspase-3-dependent apoptotic cell death (89). The methods of peptide designing, such as cyclization, hybridization, fragmentation and modification, have potential advantages in increasing drug half-life time in plasma, enhancing stability and activity and decreasing toxicity of ACPS, for improving their therapeutic efficacy (90).
Therapeutic peptides are classified into three classes based on the mechanism of peptide entry into cancer cells, including: i) Pore-forming peptides, which bind to negatively charged molecules on the cancer cell membrane for inducing apoptosis or necrosis; ii) cell-penetrating peptides, which translocate across the plasma membrane and transporting small molecules to oligonucleotides or proteins, known as internalization; and iii) tumor-targeting peptides, which bind to receptors on the cancer cell surface for cell internalization (91). Based on the mechanism of entry, therapeutic peptides are also classified into three groups based on their biological targets, including: i) Signal transduction pathways; ii) cell cycle regulation; and iii) cell death pathways (92,93). For instance, a tumor-penetrating peptide, KLA, exerts pro-apoptotic activity, which disrupts the mitochondrial membrane, leading to programmed cell death in tumors (40). In a tumor suppressor mechanism, kisspeptin-1 metastasis suppressor, a precursor for several shorter peptides, which regularly exhibits decreased expression in metastatic tumors, can suppress colonization of disseminated cancer cells in distant organs and is involved in mechanisms of tumor angiogenesis, autophagy and apoptosis regulation in breast cancer (94). Furthermore, the tubulysin analogue KEMTUB10 can inhibit tubulin polymerization during mammalian cancer cell proliferation, block the G2/M phase of the cell cycle and stimulate apoptosis or cell death via p53, Bcl-2-interacting mediator of cell death and Bcl-2 (95). Although ACPs can induce cancer cell death and specify an expressed molecule to cellular targets, such as a cationic anticancer peptide, temporin-1CEa and melanoma cell surface-expressed phosphatidylserine (96), ACPs have limitations, including drug binding peptide delivery to cancer cell targets (97). Thus, ACPs could be developed for their high penetration into the tumor tissue and tumor cells, as well as high antitumor activity (40). While ACPs can progress from binding to killing cancer cells, in terms of molecular targeting peptides, ACPs cannot be specific or penetrated all cancer cell types, leading to the need for an addition of a binding cancer cell target, such as 'guiding missile' peptides or binding peptides.
'Guiding missile' peptides or binding peptides
Optimizing anticancer drug delivery requires the safety of healthy cells, as well as cancer cell elimination (98). 'Guiding missile' peptides or binding peptides, used as delivery carriers, should hold the poorly stable, non-soluble drugs and control drug-release inside the tumor environments (99). Furthermore, these peptides require specificity, affinity and dose effectiveness (98). Anticancer drug concentration is continually diluted during transport until reaching the target areas. However, drug binding adjuvant and nanoparticles can retain drug concentration during transport to target areas and induce the slow-release of the drug at these target areas (100,101).
Drug concentration and cell and tissue barriers are an obstacle for therapeutic efficacy. Medical application for drug delivery requires biologically active conjugates (cargoes) and/or binding peptides ('guiding missile') to reach specific intracellular targets (102-104). Minimal amino acid sequences of various cell-penetrating peptides, typically comprising 5-30 amino acid residues, especially cationic residues, can pass through tissue and cell membranes using energy-dependent or -independent mechanisms without the interaction of specific receptors (36). Binding peptides can bind to the cargoes with either covalent (mainly disulfide and thioester bonds) or non-covalent bonds (electrostatic and/or hydrophobic interactions between negatively charged cargoes and positively charged peptides) to protect the cargoes from enzymatic degradation (105).
The physical and chemical properties of binding peptides can be categorized into three main classes: Cationic, amphipathic and hydrophobic peptides (42). Firstly, cationic peptides contain highly positive net charges comprising lysines and arginines. Arginine contains a guanidine head group, which is used to form bidentate hydrogen bonds with the negatively charged carboxylic, sulfate and phosphate groups on the cell membrane, resulting in binding peptide internalization into the cells; however, lysine does not contain the guani-dine head group, leading to lower penetration into the cell membrane (106). Secondly, amphipathic peptides, which contain both hydrophilic and hydrophobic amino acids, are classified into primary (covalent binding hydrophobic domain targeting to cell membrane and nuclear localization signal), secondary (α-helical structure with hydrophilic and hydrophobic residues on different sides of the helix or β-sheet for cellular internalization) and proline-rich (pyrolidine ring without hydrogen bonds on α-amino group able to allow cell permeability) peptides (107,108). Hydrophobic peptides contain non-polar amino acids with a low net charge and have a high affinity for the hydrophobic domain of the cell membrane, leading to cellular internalization and translocation across the membrane via energy-independent mechanisms (107,109).
Binding peptides enter target cells via cell penetration (pore formation and membrane destabilization) and endocytosis (macropinocytosis, clathrin or caveolin-mediated endocytosis, and clathrin/caveolin-independent endocytosis with enhanced endosomal escape from a lysosome) depending on physico-chemical properties, size and concentration of the peptides (110). There are various mechanistic studies examining binding peptides depending on their targets. For example, D-form octa-arginines stimulates the intestinal epithelial transport of drugs, such as insulin, via energy-independent unsaturable internalization (111). Furthermore, a specific peptide derived from nuclear localization signal (NLS) and epidermal growth factor receptor pathway substrate 8 (EPS8), called CP-EPS8-NLS, can cross the cellular membrane and interfere with the nuclear translocation of EPS8, leading to inhibited cell viability and proliferation in acute myeloid leukemia (AML) (112). It has also been revealed that cell-penetrating peptide TAT-conjugated gambogic acid promotes tumor apoptosis via reactive oxygen species (ROS)-mediated apoptosis by increasing the ROS level in bladder cancer cells (113).
Development of cell-permeable therapeutic peptides with polar side chains has used advantage of adding methyl groups, asparagine residues and D-amino acids (45). Similarly, another drug delivery system, known as nanoparticles, can carry ACPs to tumor sites without enzymatic degradation and can then enter inaccessible tumor sites (114). However, anticancer drug-carrying nanoparticles should be optimized for synergistic effect, drug release control, circulating stability and drug combination (115). Moreover, binding peptides could be modified to protect enzymatic digestion, penetrate cancer cell or organelle membranes, specifically bind to cancer targets and stimulate biological cells around tumor environments (116).
Cell stimulating peptides
Immune system stimulating peptides
Host defense mechanisms against pathogens or transformed cells, such as the cancer cells, is a novel therapeutic approach that involves recruiting the immune cells into the tumors (117). Antigenic peptide-human leukocyte antigen class I complex respond to cytotoxic CD8+ T-cells against malignant diseases and brain tumors (118). However, ACP-produced vaccines exhibit poor immunogenicity, and thus require adjuvants to increase specific immune responses (119). For example, E75 peptide breast cancer vaccine (Her2 p369-p377) containing polyactin A can increase CD4+ and CD8+ T lymphocytes, enhance proliferation of splenocytes and increase levels of interferon-γ in splenocytes (120). Furthermore, a melittin-RADA32 hybrid peptide hydrogel-linked doxorubicin can recruit activated natural killer cells in the primary melanoma tumor, resulting in growth retardation, as well as activation of dendritic cells of draining lymph nodes and production of cytotoxic T-cells against the remaining tumors (121). Tyrosinase-related protein 2 melanoma antigen peptide nanovaccine combined with CpG adjuvant could slowly result in growth of the melanoma tumor (122). Moreover, the 5-mer peptide, A-P-D-T-R, is a potential target for immunotherapy against breast cancer due to its highly immunogenic property that exists within the variable number of tandem repeats found in all mucins, particularly mucin1, which is increased by 10-fold in adenocarcinomas (123). Some peptide vaccines have been studied in phase I/II clinical trials (124-126). For instance, an adjuvant multi-peptide vaccine (UroRCC) was administered in patients with metastatic renal cell carcinoma following metastasectomy (127). Furthermore, a multipeptide vaccine (IMA950) containing 11 tumor-associated peptides, which targets IMA950 antigens, has been used as a tumor-targeting vaccine involving the T-cell response in grade II and III glioma (128). In metastatic hormone-naïve prostate cancer, the novel human telomerase reverse transcrip-tase (hTERT) peptide vaccine UV1 can induce an immune response, affecting the prostate-specific antigen level (129). A vaccine containing peptides can also be an adjuvant for activating the immune system. For instance, Hp91 peptide has formed the adjuvant for a protein vaccine against human papillomavirus to control cervical cancer (130). Therefore, immune system stimulating peptides are an alternative cancer therapy to control metastasis and eradicate cancer cells by activating host immunity with the specific tumor antigens.
Hormone stimulating peptides
The therapeutic peptides can inhibit cancer cell proliferation by controlling hormone release via their receptors (131). Cancer cells can produce hormones, such as growth hormone-releasing hormone (GHRH), to stimulate the pituitary gland and then the release of growth hormone (132,133). In a previous study, a GHRH antagonist was synthesized to inhibit proliferation in AML cell lines, including K562, THP-1 and KG-1a cells (134). Follicle stimulating hormone (FSH), for which the circulating level is increased by leptin, serves an important role in the initiation and the proliferation of the ovarian cancer cells (135). Moreover, the obese OB3 peptide, a derivative of leptin, may prevent leptin-induced ovarian cancer cells by disrupting leptin-induced ovarian cancer cell proliferation signal via stimulation of STAT3 phosphorylation and estrogen receptor α-activation (135). Furthermore, nanoparticle drug vehicles containing 21-amino acid peptides [YTRDLVYGDPARPGIQGTGTF (D-FP21)] conjugated to polyethylenimine and methoxy polyethylene glycol target the FSH receptor, leading to anti-proliferative effects on ovarian cancer (136). For chemotherapeutic improvement of metastatic hormone-refractory prostate cancer, it was found that the AlkB homolog 2 proliferating cell nuclear antigen (PCNA) interacting motif peptide targeting PCNA, an essential scaffold protein, in combination with docetaxel could decrease prostate volume and inhibit cancer cell regrowth in vivo (137).
3. Development of therapeutic ACPs
The issues with conventional therapeutic agents associated with the majority of cancer drugs, include poor water solubility, lack of target specificity and capability, non-specific distribution, system cytotoxicity and low therapeutic index, can be solved by creating a water-soluble form, targeting the delivery of ACPs, non-systemic side effects and specific treatment efficacy (138). Numerous natural peptides derived from natural products, such as bioactive peptides, are applied in cancer therapy (139). Although naturally bioactive peptides exhibited beneficial biocompatibility and low cytotoxicity, a number of bioactive peptides cannot provide the active targeting, cell uptake, cancer cell cytotoxicity and targeted delivery (140). The natural active peptides can be modified to novel peptides with special properties, including specificity, higher cell penetration, cancer cell cytotoxicity and therapeutic efficacy with no side effect. The present review focused on the therapeutic peptide development from natural peptides to modified peptides and targeting peptides for increasing the specific cancer cell targets.
Natural peptides
Anticancer peptides have been discovered and modified from antimicrobial peptides, and these resources produce natural peptides from various organisms, such as marine, plant, yeast, fungi, bacteria and bovine (141,142). Antimicrobial and anticancer peptides, especially cationic peptides, can kill both bacteria and cancer cells due to the similar negative net charge on their membranes (143). Proteins from nutrients can release bioactive peptides via enzymatic hydrolysis, gastrointestinal digestion or during fermentation (144). Bioactive peptides discovered from natural peptides have an electrostatic interaction between the peptides and cell membrane, leading to cancer cell or mitochondrial membrane disruption and then necrosis or apoptosis (145). For example, bioactive milk-derived peptides released during digestion have a vital role in cancer prevention (146). Moreover, germinated soybean protein-derived peptides from enzymatic hydrolysis exert antiproliferative activity against human colorectal cancer cells (147). It has also been shown that the extracted peptides from Lentinus squarrosulus mushrooms can mediate human lung cancer cells via apoptosis (148). Cyclic peptides isolated from marine cyanobacteria, such as Urumamide, exhibited low proliferative inhibitory activity on human cancer cells (149). Additional examples of natural peptides that have anticancer properties are presented in Table II and Fig. 3A. The majority of natural peptides that exert effects against cancer cell survival are α-helical folding peptides that have cationic properties (150,151). However, a minority of peptides, including other folding with neutral or anionic peptides, are able to disrupt cancer cell survival (152). Recently, a number of anionic antimicrobial peptides that originate from amphibians, including frogs, toads, newts and salamanders across Africa, South America and China, demonstrated anticancer activity (153). Thus, natural ACPs can exhibit both cationic and anionic or neutral properties; also, the majority of cationic peptides are found to have a significant cytotoxic effect against cancer cells compared with anionic or neutral peptides. In the future, these natural ACPs can be modified to further ACP development.
Table II.
Examples of natural peptides against cancer cells.
| Source | Name | Sequence | Net chargea | Structureb | Against cancer cell types | Biological mechanism | (Refs.) |
|---|---|---|---|---|---|---|---|
| Buthus Occitanus tunetanus | RK1 | IDCSKVNLTAECSS | -1 | α-helix | IGR39, U87 cells | Reduce cell proliferation and migration | (154) |
| Cancer stem-like cells | EpCAM peptide-CTLs | VVAGIVVLV GLKAGVIAV VLAFGLLLA RTYWIIIEL SMCWCVNTA SMQGLKAGV ILYENNVIT LLLAAATAT |
0 +1 0 0 0 +1 -1 0 |
Extend Coil α-helix Extend Extend/coil α-helix/coil Coil α-helix |
EpCAM-expressing HepG2 cells | Inhibit tumor growth and induce specific immune response | (155) |
| Cancer stem-like cells | CD44 peptide-specific CTLs | YIFYTFSTV LILAVCIAV SLLALALIL IILASLLAL WLIILASLL VLLQTTTRM GLVEDLDRT TVGDSNSNV |
0 0 0 0 0 +1 -2 -1 |
Extend α-helix α-helix α-helix α-helix Coil/extend α-helix/coil Coil |
CD44 positive MCF-7 tumor cells | Kill tumor cells | (155) |
| Tyrosine-protein kinase Lck | Lck-486 peptide | TFDYLRSVL | 0 | α-helix | Some metastatic tumor cells and T-cells at the tumor site | Inhibit tumor cell growth | (156) |
| Bombina orientalis | Bombinin-BO1 | GIGSAILSAGKSIIKGLAKGLAEHF | +2 | α-helix/coil | Human hepatoma cell lines (Hep G2, SK-HEP-1 and Huh7) | Anti-proliferative effects | (157) |
| Bombina orientalis | Bombinin H-BO1 | IIGPVLGLVGKALGGLL | +1 | α-helix/coil | Human hepatoma cell lines (Hep G2, SK-HEP-1 and Huh7) | Anti-proliferative effects | (157) |
| Ichthyophthirius multifiliis (strain G5) (White spot disease agent) (Ich) | BP100 | KKLFKKILKYL | +5 | α-helix | K562 cells | Promote LDH release | (41) |
| A specific Eps8/EGFR inhibitor | Peptide 327 | EFLDCFQKF | -1 | α-helix | HT-29 cells | Immune response to tumor cell recognition | (158) |
| Anthopleura anjunae (sea anemone) | Anthopleura anjunae anti-tumor peptide (AAP-H) | YVPGP | 0 | Coil | Prostate cancer DU-145 cells | Increase apoptosis via pro-apoptotic proteins | (159) |
Predicted using PepDraw (http://www.tulane.edu/~biochem/WW/PepDraw/index.html).
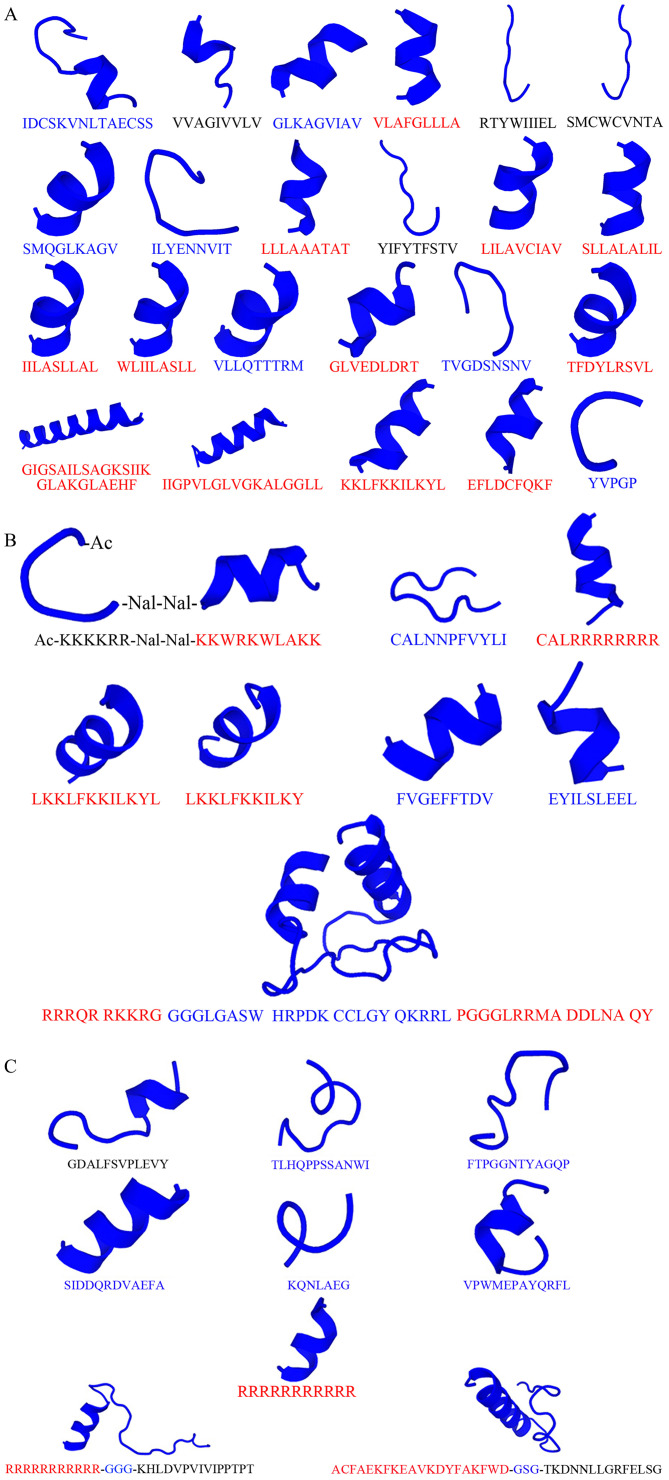
Figure 3.
Conformation of anticancer peptides predicted using PEP-FOLD 3.5 (https://mobyle.rpbs.univ-paris-diderot.fr/cgi-bin/portal.py#forms::PEP-FOLD3). (A) Natural, (B) modified and (C) targeting peptides corresponding to Tables II-IV, respectively, performed in three conformations including extend (black alphabet amino acid sequences), coiled (blue alphabet amino acid sequences) and α-helix (red alphabet amino acid sequences).
Modified peptides
Highly cationic and amphipathic peptide properties can be synthesized and designed via in silico creations. For example, some chemical groups, such as acetylation or amidation, are added into the natural peptides to increase the cationic properties and target cell specificity (162). Replacement of D-amino acids in an amphipathic peptide, KLALKLALKALKAAKLA-NH2, and a hydrophobic interaction can increase the membrane-disrupting effect on high negative surface charge bilayers, which then promotes peptide penetration into the inner membrane regions (163). Moreover, the folding and formation of peptides, such as the α-helix or cyclization, results in an increase in anticancer properties and stability (164,165). It has also been revealed that fewer helical peptides can decrease the bilayer disruption activity (163), and that cyclic peptides can act on cell permeability (45). Furthermore, substitution, deletion or addition of positively charged or polar and non-polar amino acids on natural peptides could modify their properties to improve therapeutic application (164,165). Some modified peptides are displayed in Table III and Fig. 3B.
Table III.
Examples of modified peptides against cancer cells.
| Source | Name | Sequence | Property | Structure | Against cancer cell types | (Refs.) |
|---|---|---|---|---|---|---|
| S1 (Ac-KKWRKWLAKK-NH2) | Nal2-S1 K4R2-Nal2-S1 K6-Nal2-S1 |
Ac-Nal-Nal-KKWRKWLAKK-NH2 Ac-KKKKRR-Nal-Nal-KKWRKWLAKK-NH2 Ac-KKKKKK-Nal-Nal-KKWRKWLAKK-NH2 |
Cationic peptides | Amphipathic α-helical peptides | OECM-1, C9, SAS, A549, PC9, PC9-G | (168) |
| Drosophila Antennapedia homodomain | PFV R8 |
CALNNPFVYLI CALRRRRRRRR |
Neutrala Cationic peptidesa | Coil/extendb α-helical peptidesb | B16, A549 | (169) |
| Ichthyophthirius multifiliis (strain G5) (White spot disease agent) (Ich) | B4 B8 |
LKKLFKKILKYL LKKLFKKILKY |
Cationic peptides | α-helical peptides | K562 | (41) |
| Carcinoembryonic antigen glypican-3 | GPC3(144-152) GPC3(298-306) |
FVGEFFTDV EYILSLEEL |
Anionic peptidesa | Coil/α-helical peptidesb | The HLA-A 02:01 human cancer cell lines, HepG2, Wilm's tumor G-401, SK-N-DZ, HuH-6 | (170) |
| Transactivator of transcript-DV1-Bcl-2 homology 3 | TAT-DV1-BH3 polypeptide | RRRQR RKKRG GGGLGASW HRPDK CCLGY QKRRL PGGGLRRMA DDLNA QY | Cationic peptidea | α-helical-coil- α-helical peptideb | MDAMB-231 and MCF-7 | (171) |
Predicted using PepDraw (http://www.tulane.edu/~biochem/WW/PepDraw/index.html).
Besides the aforementioned modifications, ACPs have been constructed via genetic engineering, including anticancer fusion peptides; for example, the structure of bovine lactoferricin and hexapeptide derived from bovine milk protein for ovarian cancer treatment (166). NT4 peptides bound to GAG chains of heparan sulfate proteoglycans have a modulatory effect on the cancer cell migration and invasion ability (167). A recombinant protein consisting of iRGD (CRGDKGPDC)-conjugated KLA peptide (KLAKLAKKLAKLAK) exerts a pro-apoptotic activity and high penetration to tumor tissue and cells for gastric cancer treatment (40). Collectively, modified peptides can be developed to improve anticancer properties and the effect on the cancer targets directly.
Targeting peptides
The discovery of cancer cell targets can promote cell target specificity to avoid healthy cell damage (172). Targeting peptides on various cancer cell types should bind to cancer cell targets and eliminate cancer cells at the same time (173). Molecular targets in cancer cells are important for clinical therapy, including vascular endothelial growth factor, RAS/mitogen-activated protein kinase pathway inhibitors, aurora kinase inhibitors or endothelin receptor antagonists (174,175). Some molecular targets can induce an immune response, such as cytokines, while others directly bind to specific cancer cell biomarkers (175,176). Upregulation of specific cancer proteins or peptides has been used as cancer targets (139). For instance, high expression levels of MDM2 proto-oncogene (MDM2) and MDM4 regulator of p53 (MDMX), as negative regulators of tumor suppressor protein p53, and upregulated expression of the cell surface receptor CD33 have been targeted for AML therapy (177). Lanthanide oxyfluoride nanoparticle (LONp) bound dual-specific peptide antagonists of MDM2 and MDMX (PMI) and antiCD33-LONp-PMI can activate the p53 pathway, thus inducing AML cell apoptosis (177). Furthermore, upregulated urokinase plasminogen activator receptors (uPAR) on cancer cells are targeted to uptake a specific peptide, 68Ga-labeled AE105 peptide, as uPAR PET-probes, into U87MG tumor cells (178). Examples of the targeting peptides are presented in Table IV and Fig. 3C. Besides disturbing cancer cell survival, targeting peptides for cancer cell labeling was an advantage for cancer cell detection and diagnosis. For example, 99mTc-(tricine)-HYNIC-Lys-FROP peptides were taken up by breast cancer cells for tumor targeting and molecular imaging (179). Therefore, targeting peptides can specifically and directly bind and destroy cancer cells, but not healthy cells. However, their targets are difficult to discover and develop for specific cancer cell therapy.
Table IV.
Examples of targeting peptides bind to specific cancer cells.
| Name | Sequence | Net chargea | Structureb | Targeting cancer cell types | (Refs.) |
|---|---|---|---|---|---|
| CSP-GD CSP-TL CSP-FT |
GDALFSVPLEVY TLHQPPSSANWI FTPGGNTYAGQP |
-2 0 0 |
Extend/coil Coil Coil | Human cervical cancer derived cells (SiH) | (139) |
| CSP-SI CSP-KQ p160 |
SIDDQRDVAEFA KQNLAEG VPWMEPAYQRFL |
-3 0 0 |
Coil/α-helix Coil/α-helix Coil/α-helix | Human cervical cancer derived cells (C-33A) Neuroblastoma and breast (180,181) cancer cell lines | (139) |
| Polyarginine (R11) | RRRRRRRRRRR | +11 | α-helix | Bladder cancer | (182) |
| DN-C16orf74 | RRRRRRRRRRR-GGG-KHLDVPVIVIPPTPT | +11 | α-helix-coli-extend | Pancreatic cancer cells | (183) |
| α-helix HSP70 peptide | ACFAEKFKEAVKDYFAKFWD-GSG-TKDNNLLGRFELSG | 0 | α-helix-coli-extend | Tumor regression on mice B16OVA melanoma models | (184) |
Predicted using PepDraw (http://www.tulane.edu/~biochem/WW/PepDraw/index.html).
4. Anticancer peptides in clinical trials
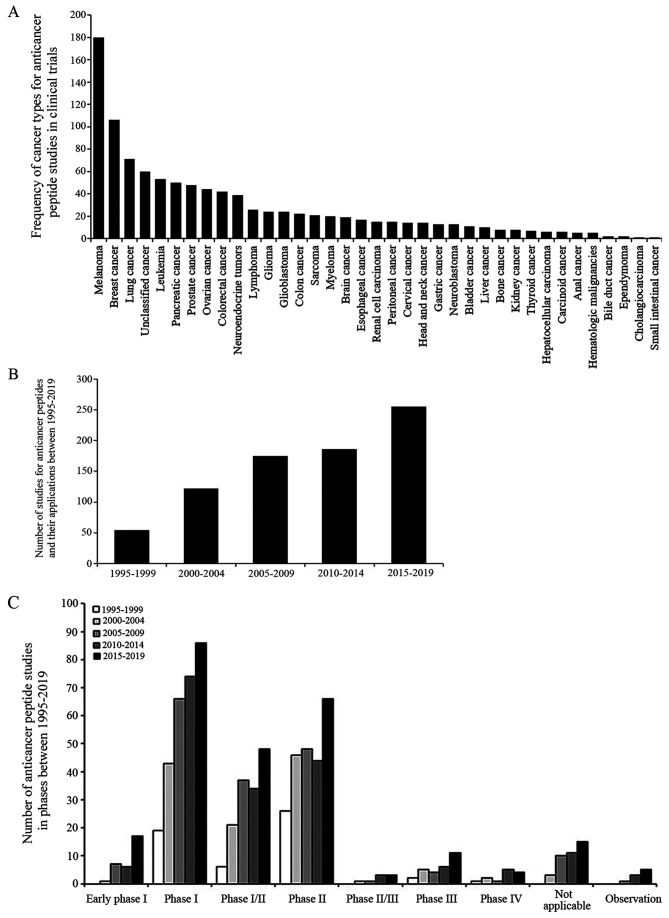
Several synthetic peptide-based drugs and vaccines are currently undergoing clinical trials. The National Library of Medicine (NLM) at the National Institutes of Health (NIH) provides and updates clinical trial information on the ClinicalTrials.gov website. A total of 792 studies between 1995-2019 were identified and searched for 'cancer', 'peptide', 'drug' or 'biological' key words, excluding non-anti-cancer peptide interventions such as behavior and surgery. The search result is presented in Fig. 4.
Figure 4.
Number of ACP studies for the drug and biological intervention. (A) Frequency of cancer types from 792 ACP studies, which were submitted on the ClinicalTrials.gov website, including 36 cancer types and unclassified cancer types. The unclassified cancer types were reported as solid tumors, cancer or neoplasms. (B) Number of ACP studies in every 5-year period between 1995-2019 was continuously increased. (C) Furthermore, from 1995-2019, >98% of these ACP studies were an intervention study type, including clinical trial in early phase I, phase I, I/II, II, II/III, III, IV and not applicable, while <2% of them were an observation study type, which cannot assign a specific intervention or treatment. Source:www.clinicaltrials.gov search on Feb 4, 2020 with drug, biological and peptide key words in cancer. ACP, anticancer peptide.
For example, CIGB-300, an amidated disulfide cyclic undecapeptide fused to the TAT cell-penetrating peptide via a β-alanine spacer, inhibits CK-2-mediated phosphorylation leading to cancer cell apoptosis in patients with cervical and non-small cell lung cancer (185-187). Wilms' tumor 1 (WT1) peptide-based vaccination combined with the adjuvant drug OK-432 administered to pediatric patients with a solid tumor has been demonstrated to be safe for these children (188). Furthermore, WT1-pulsed dendritic cell vaccine has been used to treat patients with surgically resected pancreatic cancer under a phase I study (189). A modified 9-mer WT1 peptide vaccine was also used in patients with gynecological cancer for inducing myeloid dendritic cells, and was demonstrated to be associated with cytotoxic T-cell activation (190). Subsequently, WT1 peptide vaccine therapy was evaluated in patients with gynecological cancer in a phase II clinical trial (191). A target of esophageal squamous cell carcinoma and lung cancer types is lymphocyte antigen 6 complex locus K (LY6K), which is expressed in gastric cancer (192). LY6K-177 peptide vaccine emulsified with Montanide ISA 51 was evaluated in patients with gastric cancer as a phase I clinical trial, and was found to be tolerated by patients with advanced gastric cancer (50% of patients with gastric cancer had stable disease and 16% patients had a tumor contraction effect) (192).
B-cell lymphocytic leukemia and pancreatic cancer have demonstrated a high level of telomerase activity (193). GV1001, a peptide based-cancer vaccine derived from the hTERT (hTERT 616-626; EARPALLTSRLRFIPK), was administrated in patients with non-resectable pancreatic cancer undergoing a dose-escalating phase I/II study (194); GV1001 was capable of inducing CD4+ and CD8+ T-cells, interacting with professional antigen-presenting cells and then engulfing dead tumor tissue or cells (194). Moreover, GV1001 may be a candidate vaccine in patients with B-cell chronic lymphocytic leukemia that exhibit telomerase-specific leukemic cells (195).
A combination of the ACPs and other drugs have also been evaluated in phase I trials, such as cyclodepsipeptide plitidepsin and bevacizumab in refractory solid tumors (196). For the binding peptide strategy, a carrier peptide, as a luteinizing hormone-releasing hormone (LHRH) agonist, is linked to the cytotoxic analogs of LHRH for cancer expressing receptors for LHRH (197). The LHRH agonist under phase II clinical trial exhibits anticancer activity in LHRH receptor-positive cancer types, such as human endometrial, ovarian and prostate cancer (197). Previously, a personalized peptide vaccination (PPV) has been developed as a novel approach for a cancer vaccine to boost the immune response using specific peptides for each patient (198). The peptides for PPV treatment under a randomized phase II trial in patients with bladder cancer were selected from the candidate peptides, according to human leukocyte antigen types and peptide-reactive IgG titers, to observe progression-free survival, overall survival, immune response and toxicity (198). Similarly, 19 mixed peptides were selected from 31 PPVs according to the anti-tumor immunological effect, and the safety profiles for patients with metastatic breast cancer were also assessed in a phase II clinical trial (199). While some peptides, such as gp100:209-217 (210M)/Montanide™ ISA-51/Imiquimod for high risk melanoma and E39 peptide/GM-CSF vaccine plus E39 booster for ovarian cancer, have been approved by the Food and Drug Administration (FDA), these have been improved in clinical therapy, such as peptide boronate bortezomib (200-202). The peptide boronate bortezomib is a reversible 26S proteasome inhibitor, degenerating several intracellular proteins, with antitumor and antiproliferative activities and can be used in multiple myeloma therapy (202). Due to adverse effects, such as hematotoxicity and peripheral neuropathy, poor penetration into solid tumors and low clinical stability and bioavailability, bortezomib was developed for delivery using nanoparticles, and treatment for bortezomib resistant multiple myeloma was improved using target chemical modification during synthetic processes (203,204). Additional ACP examples are presented in Table V. As aforementioned, various cancer vaccines have been produced using ACPs and ACPs combined with adjuvants or drugs, and the effects of carrier peptides on targeting cancer cell directly and/or by activating immune response have been tested in clinical trials for safety, side effects and effectiveness.
Table V.
Examples of ACPs in clinical trials (source: www.ClinicalTrials.gov).
| Phases | Biological peptides | Conditions | Outcomes |
|---|---|---|---|
| Early phase 1 | MUC-1 peptide vaccine, poly ICLC, MUC1 peptide-poly-ICLC adjuvant vaccine | Breast cancer | A positive anti-MUC1 antibody response |
| HER-2/neu peptide vaccine | Breast cancer | Peptide-specific interferon-γ producing T-cell and peptide-specific IL-5 producing T-cell responses | |
| GAA/TT-peptide vaccine and poly-ICLC | Astrocytoma, oligoastrocytoma, glioma | Induction of GAA-specific T-cell response | |
| Peptide vaccine + poly-ICLC | Astrocytoma, oligoastrocytoma, oligodendroglioma | Infiltration of GAA-specific T-cells | |
| Gag:267-274 peptide vaccine | Melanoma | Vaccine peptide-specific CTL response | |
| Phase 1 | HPV16 E7 peptide-pulsed autologous DCs | Cervical cancer | Pulsed autologous DCs immunotherapy |
| NY-ESO-1b peptide plus CpG 7909 and Montanide ISA-5 | Cancer, neoplasm | NY-ESO-1 specific humoral and cellular immunity | |
| Antiangiogenic peptide vaccine | Hepatocellular carcinoma | Peptide specific CTL response | |
| RNF43-721 | Colorectal cancer | Specific CTL induction in vitro | |
| LY6K, VEGFR1, VEGFR2 | Esophageal cancer | Immune responses including LY6K, VEGFR1 and VEGFR2 specific T-cells | |
| HLA-A*0201 or HLA-A*0206- restricted URLC10 peptides | Non-small cell lung cancer | Immunological responses including peptides specific CTL, antigen cascade, regulatory T-cells, cancer antigens and HLA levels. | |
| Phase 1/Phase 2 | MAGE-3.A1 peptide and CpG 7909 | Malignant melanoma | Detectable CTL response |
| VEGFR1-1084, VEGFR2-169 | Pancreatic cancer | Peptide specific CTL response | |
| HER-2/neu peptide vaccine | Breast cancer | HER2-specific T-cell response | |
| HLA-A*2402 or A*0201 restricted peptides | Solid tumors | Various immunological responses including peptides specific CTL, antigen cascade, regulatory T-cells, cancer antigens and HLA levels | |
| Modified CEA peptide | Pancreatic adenocarcinoma | T-cell response with modified CEA peptide | |
| Phase 2 | synthetic human papillomavirus 16 E6 peptide | Cervical cancer | Immunological response to HPV |
| gp100:209-217(210M), HPV 16 | Melanoma | T-cell immunity to gp100 peptide and to | |
| E7:12-20 | E7 12-20 papilloma virus peptide | ||
| WT1 126-134 peptide | Acute myeloid leukemia | Generation of T-cell response | |
| G250 peptide | Metastatic renal cell carcinoma | G250-specific CTL response | |
| Melanoma helper peptide vaccine, multi-epitope melanoma peptide vaccine | Melanoma | CTL response, helper T-cells response to 6MHP | |
| Phase 3 | PR1 leukemia peptide vaccine | Leukemia | Immune response to PR1-HLA-A2 tetramer |
| Phase 4 | Degarelix (LHRH antagonist) | Prostatic neoplasms | Binds to GnRH receptors and blocks interaction with GnRH |
From ClinicalTrials.gov searched on January 31, 2020. CTL, cytotoxic T-cell lymphocytes; GnRH, gonadotropin-releasing hormone; CEA, carcinoembryonic antigen; HPV, human papillomavirus; HER, human epidermal growth factor receptor; VEGFR, vascular endothelial growth factor receptor; MAGE-3, melanoma-associated antigen 3; RNF, ring finger protein; NY-ESO, New York esophageal squamous cell carcinoma; MUC, mucin.
5. Future direction
Although ACPs have a number of disadvantages, such as biological instability, low bioavailability, short half-life, protease sensitivity, poor pharmacokinetics and first-pass metabolism, their most notable advantage is the protein-protein interaction with a target, thus overcoming limitations via designing peptide modifications and conjugation to improve affinity, stability and selectivity (205,206). For example, the peptide BBN7-14 (Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2) composed of natural amino acids has a higher binding affinity with the CFPAC-1 cell line compared with the modified peptide GB-6 (Gln-5-Htp-β-Ala-Nva-Gln-His-NH2) that consists of unnatural amino acids (in vitro). However, in vivo, BBN7-14 has a reduced tumor-targeting ability compared with GB-6, which is stable against protease-mediate degradation and has a slightly lower uptake and slow metabolism (207). Currently, ACPs have been modified to improve specific cancer cell targets and enhance cancer cell elimination. Some anticancer peptides as drugs and vaccinations have been tested in phase I/II clinical trials (175). For example, dTCApFs, a natural hormone peptide for the treatment of advanced or metastatic solid tumors, enters the cells via the Toll/interleukin-1 receptor superfamily, suppresses angiogenic factors and induces anticancer cytokine production and ER stress, leading to cancer cell apoptosis (208). dTCApFs anticancer activity in humans was firstly studied in a phase I clinical trial by investigating the safety and efficacy with regards to both pharmacokinetics and pharmacodynamics, with intravenous dTCApFs (6-96 mg/m2; 3 times/week; in consecutive 28-day cycles) (209). The intravenous dTCApFs is decreased at lower limit of detection in serum after 24-h administration and its concentration in serum is present in dose-dependent manner (209). Furthermore, ACPs have been combined with immunogens for clinical therapeutic improvement (210). Upregulation of molecular cancer targets, such as Ras protein that has been discovered in various cancer cell types (lung, colon and pancreatic), could also be direct targets for ACP development (211). The aim of ACP therapy should promote cancer cell death and intermit tumor regression, without contributing to tumorigenesis and resistance in cancer cell treatment (212). The first ACP approved by the FDA was the peptide boronate bortezomib (Velcade®) for multiple myeloma treatment in 2003 and mantle cell lymphoma in 2006 (213). In the near future, combination therapy with a drug or vaccine containing i) the specific targeting peptides, ii) the ACPs and iii) the cell-penetrating peptides and/or the conjugated delivery materials (such as liposome, nanoparticles or adjuvants) may facilitate the development of cancer therapy with cancer cell specificity, stability, safety and efficacy, without healthy cell eradication (214). ACP construction for specific cancer cell targets, and predictive, preventive and personalized medicine may be beneficial to the cancer research field due to the different complexity of the whole-body system in each individual (215). Besides the aforementioned therapeutic peptides, peptides with specific cancer cell targets are applied to bind the cancer cell targets for cancer detection and therapy (216,217). For example, a sodium pump Na+/K+ ATPase α1-targeted peptide for positron emission tomography imaging of breast cancer, as peptide-based platform on dual-targeted molecular imaging, is able to more obviously visualize the disease state of a patient, leading to improved informed treatment decisions (218,219).
6. Conclusions
ACP therapy affects molecular targets, binds the anticancer drugs and stimulates biological systems involving cancer and healthy cell environments. Notably, natural and synthetic peptides have been developed as novel strategies against cancer types. Natural anticancer peptides can be modified to enable high penetration, specific cancer cell targets, increase efficacy and reduce side effects. A number of ACPs have been demonstrated to be anti-proliferative, apoptotic and proliferation inhibitors in various cancer cell types, both in vitro and in vivo, leading to clinical trials for the evaluation of cancer treatment. The development of drug or vaccine technology could further ACPs in design, synthesis and delivery to eliminate cancer cells directly or by affecting the anticancer immune responses (220). Collectively, it was suggested ACPs may promote cancer drugs or vaccine development to decrease emerging cases and mortality rates in the future.
Acknowledgments
The authors would like to thank Dr Thitinee Vanichapol, Division of Hematology and Oncology, Department of Pediatrics, Faculty of Medicine, Ramathibodi Hospital, Mahidol University for revising the manuscript and providing kind suggestions.
Abbreviations
- ACPs
anticancer peptides
- AML
acute amyloid leukemia
- FSH
follicle stimulating hormone
- GHRH
growth hormone-releasing hormone
- LHRH
luteinizing hormone-releasing hormone
- LONp
lanthanide oxyfluoride nanoparticle
- LY6K
lymphocyte antigen 6 complex locus K
- PCNA
proliferating cell nuclear antigen
- PPV
personalized peptide vaccination
- ROS
reactive oxygen species
- uPAR
urokinase plasminogen activator receptors
- WT1
Wilms' tumor 1
Funding
This study was financially supported by BRAND'S Health Research Award 2017 (Cerebos Award 2017), Children Cancer Fund under the Patronage of HRH Princess Soamsawali and Faculty Staff Development Program of Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand (grant no. BHR2017).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
WC wrote and edited the manuscript and was involved in the creation of the figures and data analysis. All authors were involved in the drafting and revising of the manuscript. All authors read the manuscript and approved the final version.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang SH, Yu J. Structure-based design for binding peptides in anti-cancer therapy. Biomaterials. 2018;156:1–15. doi: 10.1016/j.biomaterials.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Zhang L, Ding N, Yang X, Zhang J, He J, Li Z, Sun LQ. Identification and characterization of DNAzymes targeting DNA methyltransferase I for suppressing bladder cancer proliferation. Biochem Biophys Res Commun. 2015;461:329–333. doi: 10.1016/j.bbrc.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Ingram JR, Blomberg OS, Rashidian M, Ali L, Garforth S, Fedorov E, Fedorov AA, Bonanno JB, Le Gall C, Crowley S, et al. Anti-CTLA-4 therapy requires an Fc domain for efficacy. Proc Natl Acad Sci USA. 2018;115:3912–3917. doi: 10.1073/pnas.1801524115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di JX, Zhang HY. C188-9 a small-molecule STAT3 inhibitor, exerts an antitumor effect on head and neck squamous cell carcinoma. Anticancer Drugs. 2019;30:846–853. doi: 10.1097/CAD.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 5.Brun S, Bassissi F, Serdjebi C, Novello M, Tracz J, Autelitano F, Guillemot M, Fabre P, Courcambeck J, Ansaldi C, et al. GNS561, a new lysosomotropic small molecule, for the treatment of intrahe-patic cholangiocarcinoma. Invest New Drugs. 2019;37:1135–1145. doi: 10.1007/s10637-019-00741-3. [DOI] [PubMed] [Google Scholar]
- 6.Jahangirian H, Kalantari K, Izadiyan Z, Rafiee-Moghaddam R, Shameli K, Webster TJ. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int J Nanomedicine. 2019;14:1633–1657. doi: 10.2147/IJN.S184723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyagi A, Tuknait A, Anand P, Gupta S, Sharma M, Mathur D, Joshi A, Singh S, Gautam A, Raghava GP. CancerPPD: A database of anticancer peptides and proteins. Nucleic Acids Res. 2015;43(Database Issue):D837–D843. doi: 10.1093/nar/gku892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thundimadathil J. Cancer treatment using peptides: Current therapies and future prospects. J Amino Acids. 2012;2012:967347. doi: 10.1155/2012/967347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: Science and market. Drug Discov Today. 2010;15:40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Otvos L., Jr Peptide-based drug design: Here and now. Methods Mol Biol. 2008;494:1–8. doi: 10.1007/978-1-59745-419-3_1. [DOI] [PubMed] [Google Scholar]
- 11.Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta. 2008;1778:357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues EG, Dobroff AS, Taborda CP, Travassos LR. Antifungal and antitumor models of bioactive protective peptides. An Acad Bras Cienc. 2009;81:503–520. doi: 10.1590/S0001-37652009000300015. [DOI] [PubMed] [Google Scholar]
- 13.Droin N, Hendra JB, Ducoroy P, Solary E. Human defensins as cancer biomarkers and antitumour molecules. J Proteomics. 2009;72:918–927. doi: 10.1016/j.jprot.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 15.Sok M, Sentjurc M, Schara M. Membrane fluidity characteristics of human lung cancer. Cancer Lett. 1999;139:215–220. doi: 10.1016/S0304-3835(99)00044-0. [DOI] [PubMed] [Google Scholar]
- 16.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. doi: 10.1182/blood.V89.4.1121. [DOI] [PubMed] [Google Scholar]
- 17.Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009;625:190–194. doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062–3066. [PubMed] [Google Scholar]
- 19.Harris F, Dennison SR, Singh J, Phoenix DA. On the selectivity and efficacy of defense peptides with respect to cancer cells. Med Res Rev. 2013;33:190–234. doi: 10.1002/med.20252. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Huang Y, Feng Q, Chen Y. Tryptophan as a probe to study the anticancer mechanism of action and specificity of alpha-helical anticancer peptides. Molecules. 2014;19:12224–12241. doi: 10.3390/molecules190812224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marqus S, Pirogova E, Piva TJ. Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci. 2017;24:21. doi: 10.1186/s12929-017-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roudi R, Syn NL, Roudbary M. Antimicrobial peptides as biologic and immunotherapeutic agents against Cancer: A comprehensive overview. Front Immunol. 2017;8:1320. doi: 10.3389/fimmu.2017.01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alves AC, Ribeiro D, Nunes C, Reis S. Biophysics in cancer: The relevance of drug-membrane interaction studies. Biochim Biophys Acta. 2016;1858:2231–2244. doi: 10.1016/j.bbamem.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Yamaji-Hasegawa A, Tsujimoto M. Asymmetric distribution of phospholipids in biomembranes. Biol Pharm Bull. 2006;29:1547–1553. doi: 10.1248/bpb.29.1547. [DOI] [PubMed] [Google Scholar]
- 25.Clark MR. Flippin' lipids. Nat Immunol. 2011;12:373–375. doi: 10.1038/ni.2024. [DOI] [PubMed] [Google Scholar]
- 26.Deliconstantinos G. Physiological aspects of membrane lipid fluidity in malignancy. Anticancer Res. 1987;7:1011–1021. [PubMed] [Google Scholar]
- 27.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- 28.Stafford JH, Thorpe PE. Increased exposure of phosphati-dylethanolamine on the surface of tumor vascular endothelium. Neoplasia. 2011;13:299–308. doi: 10.1593/neo.101366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barcelo-Coblijn G, Martin ML, de Almeida RF, Noguera-Salva MA, Marcilla-Etxenike A, Guardiola-Serrano F, Lüth A, Kleuser B, Halver JE, Escribá PV. Sphingomyelin and sphin-gomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc Natl Acad Sci USA. 2011;108:19569–19574. doi: 10.1073/pnas.1115484108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preetha A, Huilgol N, Banerjee R. Comparison of paclitaxel penetration in normal and cancerous cervical model monolayer membranes. Colloids Surf B Biointerfaces. 2006;53:179–186. doi: 10.1016/j.colsurfb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Feng SS, Go ML. Investigation of molecular interactions between paclitaxel and DPPC by Langmuir film balance and differential scanning calorimetry. J Pharm Sci. 2004;93:86–98. doi: 10.1002/jps.10523. [DOI] [PubMed] [Google Scholar]
- 32.Logozzi M, Spugnini E, Mizzoni D, Di Raimo R, Fais S. Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev. 2019;38:93–101. doi: 10.1007/s10555-019-09783-8. [DOI] [PubMed] [Google Scholar]
- 33.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5:786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 34.Jobin ML, Alves ID. On the importance of electrostatic interactions between cell penetrating peptides and membranes: A pathway toward tumor cell selectivity? Biochimie. 2014;107:154–159. doi: 10.1016/j.biochi.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Peyressatre M, Prevel C, Pellerano M, Morris MC. Targeting cyclin-dependent kinases in human cancers: From small molecules to Peptide inhibitors. Cancers (Basel) 2015;7:179–237. doi: 10.3390/cancers7010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raucher D, Ryu JS. Cell-penetrating peptides: Strategies for anticancer treatment. Trends Mol Med. 2015;21:560–570. doi: 10.1016/j.molmed.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Tan S, Chen X, Zhang CY, Zhang Y. Peptide aptamers with biological and therapeutic applications. Curr Med Chem. 2011;18:4215–4222. doi: 10.2174/092986711797189583. [DOI] [PubMed] [Google Scholar]
- 38.Fuertes MA, Castilla J, Alonso C, Perez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 2003;10:257–266. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- 39.Horwitz SB. Taxol (paclitaxel): Mechanisms of action. Ann Oncol. 1994;5(Suppl 6):S3–S6. [PubMed] [Google Scholar]
- 40.Huang Y, Li X, Sha H, Zhang L, Bian X, Han X, Liu B. Tumor-penetrating peptide fused to a pro-apoptotic peptide facilitates effective gastric cancer therapy. Oncol Rep. 2017;37:2063–2070. doi: 10.3892/or.2017.5440. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Shi W, Li J, Liao C, Yang L, Huang W, Qian H. Synthesis and biological evaluation of novel peptides based on antimicrobial peptides as potential agents with antitumor and multidrug resistance-reversing activities. Chem Biol Drug Des. 2017;90:972–980. doi: 10.1111/cbdd.13023. [DOI] [PubMed] [Google Scholar]
- 42.Ramsey JD, Flynn NH. Cell-penetrating peptides transport therapeutics into cells. Pharmacol Ther. 2015;154:78–86. doi: 10.1016/j.pharmthera.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Kapoor P, Singh H, Gautam A, Chaudhary K, Kumar R, Raghava GP. TumorHoPe: A database of tumor homing peptides. PLoS One. 2012;7:e35187. doi: 10.1371/journal.pone.0035187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghasemy S, Garcia-Pindado J, Aboutalebi F, Dormiani K, Teixido M, Malakoutikhah M. Fine-tuning the physicochemical properties of peptide-based blood-brain barrier shuttles. Bioorg Med Chem. 2018;26:2099–2106. doi: 10.1016/j.bmc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Buckton LK, McAlpine SR. Improving the cell permeability of polar cyclic peptides by replacing residues with alkylated amino acids, asparagines, and d-Amino Acids. Org Lett. 2018;20:506–509. doi: 10.1021/acs.orglett.7b03363. [DOI] [PubMed] [Google Scholar]
- 46.Perry SR, Hill TA, de Araujo AD, Hoang HN, Fairlie DP. Contiguous hydrophobic and charged surface patches in short helix-constrained peptides drive cell permeability. Org Biomol Chem. 2018;16:367–371. doi: 10.1039/C7OB02952G. [DOI] [PubMed] [Google Scholar]
- 47.Shoombuatong W, Schaduangrat N, Nantasenamat C. Unraveling the bioactivity of anticancer peptides as deduced from machine learning. EXCLI J. 2018;17:734–752. doi: 10.17179/excli2018-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai YX, Cai XG, Shi W, Bi XZ, Su X, Pan MB, Li HL, Lin HY, Huang WL, Qian H. Pro-apoptotic cationic host defense peptides rich in lysine or arginine to reverse drug resistance by disrupting tumor cell membrane. Amino Acids. 2017;49:1601–1610. doi: 10.1007/s00726-017-2453-y. [DOI] [PubMed] [Google Scholar]
- 49.Navarro S, Aleu J, Jimenez M, Boix E, Cuchillo CM, Nogues MV. The cytotoxicity of eosinophil cationic protein/ribonuclease 3 on eukaryotic cell lines takes place through its aggregation on the cell membrane. Cell Mol Life Sci. 2008;65:324–337. doi: 10.1007/s00018-007-7499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Midoux P, Kichler A, Boutin V, Maurizot JC, Monsigny M. Membrane permeabilization and efficient gene transfer by a peptide containing several histidines. Bioconjug Chem. 1998;9:260–267. doi: 10.1021/bc9701611. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi Y, Yamamoto K, Sato Y, Inoue S, Morinaga T, Hirano E. Combination of aspartic acid and glutamic acid inhibits tumor cell proliferation. Biomed Res. 2016;37:153–159. doi: 10.2220/biomedres.37.153. [DOI] [PubMed] [Google Scholar]
- 52.Oancea E, Teruel MN, Quest AF, Meyer T. Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signaling in living cells. J Cell Biol. 1998;140:485–498. doi: 10.1083/jcb.140.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shamova O, Orlov D, Stegemann C, Czihal P, Hoffmann R, Brogden K, Kolodkin N, Sakuta G, Tossi A, Sahl HG, et al. ChBac3.4: A Novel proline-rich antimicrobial peptide from goat leukocytes. Int J Pept Res Ther. 2009;15:107–119. doi: 10.1007/s10989-009-9170-7. [DOI] [Google Scholar]
- 54.Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, Mackay GM, Labuschagne CF, Gay D, Kruiswijk F, et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544:372–376. doi: 10.1038/nature22056. [DOI] [PubMed] [Google Scholar]
- 55.Kawaguchi K, Han Q, Li S, Tan Y, Igarashi K, Kiyuna T, Miyake K, Miyake M, Chmielowski B, Nelson SD, et al. Targeting methionine with oral recombinant methioninase (o-rMETase) arrests a patient-derived orthotopic xenograft (PDOX) model of BRAF-V600E mutant melanoma: Implications for chronic clinical cancer therapy and prevention. Cell Cycle. 2018;17:356–361. doi: 10.1080/15384101.2017.1405195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gueron G, Anselmino N, Chiarella P, Ortiz EG, Lage Vickers S, Paez AV, Giudice J, Contin MD, Leonardi D, Jaworski F, et al. Game-changing restraint of Ros-damaged phenylalanine, upon tumor metastasis. Cell Death Dis. 2018;9:140. doi: 10.1038/s41419-017-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dennison SR, Whittaker M, Harris F, Phoenix DA. Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr Protein Pept Sci. 2006;7:487–499. doi: 10.2174/138920306779025611. [DOI] [PubMed] [Google Scholar]
- 58.Marchand C, Krajewski K, Lee HF, Antony S, Johnson AA, Amin R, Roller P, Kvaratskhelia M, Pommier Y. Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic Acids Res. 2006;34:5157–5165. doi: 10.1093/nar/gkl667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barras D, Chevalier N, Zoete V, Dempsey R, Lapouge K, Olayioye MA, Michielin O, Widmann C. A WXW motif is required for the anticancer activity of the TAT-RasGAP317-326 peptide. J Biol Chem. 2014;289:23701–23711. doi: 10.1074/jbc.M114.576272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmaditaba MA, Shahosseini S, Daraei B, Zarghi A, Houshdar Tehrani MH. Design, synthesis, and biological evaluation of new peptide analogues as selective cox-2 inhibitors. Arch Pharm (Weinheim) 2017;350:e1700158. doi: 10.1002/ardp.201700158. [DOI] [PubMed] [Google Scholar]
- 61.Bhunia D, Mondal P, Das G, Saha A, Sengupta P, Jana J, Mohapatra S, Chatterjee S, Ghosh S. Spatial position regulates power of tryptophan: Discovery of a major-groove-specific nuclear-localizing, cell-penetrating tetrapeptide. J Am Chem Soc. 2018;140:1697–1714. doi: 10.1021/jacs.7b10254. [DOI] [PubMed] [Google Scholar]
- 62.Huang YB, Wang XF, Wang HY, Liu Y, Chen Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol Cancer Ther. 2011;10:416–426. doi: 10.1158/1535-7163.MCT-10-0811. [DOI] [PubMed] [Google Scholar]
- 63.Yang QZ, Wang C, Lang L, Zhou Y, Wang H, Shang DJ. Design of potent, non-toxic anticancer peptides based on the structure of the antimicrobial peptide, temporin-1CEa. Arch Pharm Res. 2013;36:1302–1310. doi: 10.1007/s12272-013-0112-8. [DOI] [PubMed] [Google Scholar]
- 64.Dennison SR, Harris F, Bhatt T, Singh J, Phoenix DA. A theoretical analysis of secondary structural characteristics of anticancer peptides. Mol Cell Biochem. 2010;333:129–135. doi: 10.1007/s11010-009-0213-3. [DOI] [PubMed] [Google Scholar]
- 65.Wu JM, Jan PS, Yu HC, Haung HY, Fang HJ, Chang YI, Cheng JW, Chen HM. Structure and function of a custom anticancer peptide, CB1a. Peptides. 2009;30:839–848. doi: 10.1016/j.peptides.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Lins L, Brasseur R. Tilted peptides: A structural motif involved in protein membrane insertion? J Pept Sci. 2008;14:416–422. doi: 10.1002/psc.971. [DOI] [PubMed] [Google Scholar]
- 67.Lins L, Decaffmeyer M, Thomas A, Brasseur R. Relationships between the orientation and the structural properties of peptides and their membrane interactions. Biochim Biophys Acta. 2008;1778:1537–1544. doi: 10.1016/j.bbamem.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Mandal PK, Gao F, Lu Z, Ren Z, Ramesh R, Birtwistle JS, Kaluarachchi KK, Chen X, Bast RC, Jr, Liao WS, et al. Potent and selective phosphopeptide mimetic prodrugs targeted to the Src homology 2 (SH2) domain of signal transducer and activator of transcription 3. J Med Chem. 2011;54:3549–3563. doi: 10.1021/jm2000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabernet G, Gautschi D, Muller AT, Neuhaus CS, Armbrecht L, Dittrich PS, Hiss JA, Schneider G. In silico design and optimization of selective membranolytic anticancer peptides. Sci Rep. 2019;9:11282. doi: 10.1038/s41598-019-47568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh M, Kumar V, Sikka K, Thakur R, Harioudh MK, Mishra DP, Ghosh JK, Siddiqi MI. Computational design of biologically active anticancer peptides and their interactions with heterogeneous POPC/POPS Lipid membranes. J Chem Inf Model. 2020;60:332–341. doi: 10.1021/acs.jcim.9b00348. [DOI] [PubMed] [Google Scholar]
- 71.Ray T, Kar D, Pal A, Mukherjee S, Das C, Pal A. Molecular targeting of breast and colon cancer cells by PAR1 mediated apoptosis through a novel pro-apoptotic peptide. Apoptosis. 2018;23:679–694. doi: 10.1007/s10495-018-1485-4. [DOI] [PubMed] [Google Scholar]
- 72.Bohmova E, Machova D, Pechar M, Pola R, Venclikova K, Janouskova O, Etrych T. Cell-penetrating peptides: A useful tool for the delivery of various cargoes into cells. Physiol Res. 2018;67(Suppl 2):S267–S279. doi: 10.33549/physiolres.933975. [DOI] [PubMed] [Google Scholar]
- 73.Levely ME, Mitchell MA, Nicholas JA. Synthetic immunogens constructed from T-cell and B-cell stimulating peptides (T:B chimeras): Preferential stimulation of unique T- and B-cell specificities is influenced by immunogen configuration. Cell Immunol. 1990;125:65–78. doi: 10.1016/0008-8749(90)90063-W. [DOI] [PubMed] [Google Scholar]
- 74.Asao T, Takahashi F, Takahashi K. Resistance to molecu-larly targeted therapy in non-small-cell lung cancer. Respir Investig. 2019;57:20–26. doi: 10.1016/j.resinv.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Han D, Lv T, Liu K, Yang Y, Xu X, Chen Y. Novel peptide myristoly-CM4 induces selective cytotoxicity in leukemia K562/MDR and Jurkat cells by necrosis and/or apop-tosis pathway. Drug Des Devel Ther. 2019;13:2153–2167. doi: 10.2147/DDDT.S207224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen YQ, Min C, Sang M, Han YY, Ma X, Xue XQ, Zhang SQ. A cationic amphiphilic peptide ABP-CM4 exhibits selective cytotoxicity against leukemia cells. Peptides. 2010;31:1504–1510. doi: 10.1016/j.peptides.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 77.Jiang R, Du X, Lonnerdal B. Comparison of bioactivities of talactoferrin and lactoferrins from human and bovine milk. J Pediatr Gastroenterol Nutr. 2014;59:642–652. doi: 10.1097/MPG.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 78.Tyagi A, Kapoor P, Kumar R, Chaudhary K, Gautam A, Raghava GP. In silico models for designing and discovering novel anticancer peptides. Sci Rep. 2013;3:2984. doi: 10.1038/srep02984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hajisharifi Z, Piryaiee M, Mohammad Beigi M, Behbahani M, Mohabatkar H. Predicting anticancer peptides with Chou's pseudo amino acid composition and investigating their muta-genicity via Ames test. J Theor Biol. 2014;341:34–40. doi: 10.1016/j.jtbi.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 80.Chen W, Feng PM, Lin H, Chou KC. iRSpot-PseDNC: Identify recombination spots with pseudo dinucleotide composition. Nucleic Acids Res. 2013;41:e68. doi: 10.1093/nar/gks1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grisoni F, Neuhaus C, Gabernet G, Muller A, Hiss J, Schneider G. Designing anticancer peptides by constructive machine learning. ChemMedChem. 2018;13:1300–1302. doi: 10.1002/cmdc.201800204. [DOI] [PubMed] [Google Scholar]
- 82.Kozlowska K, Nowak J, Kwiatkowski B, Cichorek M. ESR study of plasmatic membrane of the transplantable melanoma cells in relation to their biological properties. Exp Toxicol Pathol. 1999;51:89–92. doi: 10.1016/S0940-2993(99)80074-8. [DOI] [PubMed] [Google Scholar]
- 83.Mai JC, Mi Z, Kim SH, Ng B, Robbins PD. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001;61:7709–7712. [PubMed] [Google Scholar]
- 84.Zhang W, Li J, Liu LW, Wang KR, Song JJ, Yan JX, Li ZY, Zhang BZ, Wang R. A novel analog of antimicrobial peptide Polybia-MPI, with thioamide bond substitution, exhibits increased therapeutic efficacy against cancer and diminished toxicity in mice. Peptides. 2010;31:1832–1838. doi: 10.1016/j.peptides.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 85.Sinthuvanich C, Veiga AS, Gupta K, Gaspar D, Blumenthal R, Schneider JP. Anticancer β-hairpin peptides: Membrane-induced folding triggers activity. J Am Chem Soc. 2012;134:6210–6217. doi: 10.1021/ja210569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaspar D, Veiga AS, Sinthuvanich C, Schneider JP, Castanho MA. Anticancer peptide SVS-1: Efficacy precedes membrane neutralization. Biochemistry. 2012;51:6263–6265. doi: 10.1021/bi300836r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Negi B, Kumar D, Rawat DS. Marine peptides as anticancer agents: A remedy to mankind by nature. Curr Protein Pept Sci. 2017;18:885–904. doi: 10.2174/1389203717666160724200849. [DOI] [PubMed] [Google Scholar]
- 88.Lemeshko VV. Electrical potentiation of the membrane permeabilization by new peptides with anticancer properties. Biochim Biophys Acta. 2013;1828:1047–1056. doi: 10.1016/j.bbamem.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Liu X, Cao R, Wang S, Jia J, Fei H. Amphipathicity determines different cytotoxic mechanisms of lysine- or arginine-rich cationic hydrophobic peptides in cancer cells. J Med Chem. 2016;59:5238–5247. doi: 10.1021/acs.jmedchem.5b02016. [DOI] [PubMed] [Google Scholar]
- 90.Hu C, Chen X, Zhao W, Chen Y, Huang Y. Design and modification of anticancer peptides. Drug Des. 2016;5:1000138–1000147. doi: 10.4172/2169-0138.1000138. [DOI] [Google Scholar]
- 91.Boohaker RJ, Lee MW, Vishnubhotla P, Perez JM, Khaled AR. The use of therapeutic peptides to target and to kill cancer cells. Curr Med Chem. 2012;19:3794–3804. doi: 10.2174/092986712801661004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bidwell GL, III, Raucher D. Therapeutic peptides for cancer therapy. Part I-peptide inhibitors of signal transduction cascades. Expert Opin Drug Deliv. 2009;6:1033–1047. doi: 10.1517/17425240903143745. [DOI] [PubMed] [Google Scholar]
- 93.Raucher D, Moktan S, Massodi I, Bidwell GL., III Therapeutic peptides for cancer therapy. Part II-cell cycle inhibitory peptides and apoptosis-inducing peptides. Expert Opin Drug Deliv. 2009;6:1049–1064. doi: 10.1517/17425240903158909. [DOI] [PubMed] [Google Scholar]
- 94.Ulasov IV, Borovjagin AV, Timashev P, Cristofanili M, Welch DR. KISS1 in breast cancer progression and autophagy. Cancer Metastasis Rev. 2019;38:493–506. doi: 10.1007/s10555-019-09814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lamidi OF, Sani M, Lazzari P, Zanda M, Fleming IN. The tubulysin analogue KEMTUB10 induces apoptosis in breast cancer cells via p53, Bim and Bcl-2. J Cancer Res Clin Oncol. 2015;141:1575–1583. doi: 10.1007/s00432-015-1921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang C, Chen YW, Zhang L, Gong XG, Zhou Y, Shang DJ. Melanoma cell surface-expressed phosphatidylserine as a therapeutic target for cationic anticancer peptide, temporin-1CEa. J Drug Target. 2016;24:548–556. doi: 10.3109/1061186X.2015.1113539. [DOI] [PubMed] [Google Scholar]
- 97.Cao XW, Yang XZ, Du X, Fu LY, Zhang TZ, Shan HW, Zhao J, Wang FJ. Structure optimisation to improve the delivery efficiency and cell selectivity of a tumour-targeting cell-penetrating peptide. J Drug Target. 2018;26:777–792. doi: 10.1080/1061186X.2018.1424858. [DOI] [PubMed] [Google Scholar]
- 98.Shull AY, Hu CA, Teng Y. Zebrafish as a model to evaluate peptide-related cancer therapies. Amino Acids. 2017;49:1907–1913. doi: 10.1007/s00726-017-2388-3. [DOI] [PubMed] [Google Scholar]
- 99.Leite ML, da Cunha NB, Costa FF. Antimicrobial peptides, nanotechnology, and natural metabolites as novel approaches for cancer treatment. Pharmacol Ther. 2018;183:160–176. doi: 10.1016/j.pharmthera.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 101.Dossa F, Acuna SA, Rickles AS, Berho M, Wexner SD, Quereshy FA, Baxter NN, Chadi SA. Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol. 2018;4:930–937. doi: 10.1001/jamaoncol.2017.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu J, Khan AR, Fu M, Wang R, Ji J, Zhai G. Cell-penetrating peptide: A means of breaking through the physiological barriers of different tissues and organs. J Control Release. 2019;309:106–124. doi: 10.1016/j.jconrel.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 103.Duarte D, Fraga AG, Pedrosa J, Martel F, Vale N. Increasing the potential of cell-penetrating peptides for cancer therapy using a new pentagonal scaffold. Eur J Pharmacol. 2019;860:172554. doi: 10.1016/j.ejphar.2019.172554. [DOI] [PubMed] [Google Scholar]
- 104.Park SE, Sajid MI, Parang K, Tiwari RK. Cyclic cell-penetrating peptides as efficient intracellular drug delivery tools. Mol Pharm. 2019;16:3727–3743. doi: 10.1021/acs.molpharmaceut.9b00633. [DOI] [PubMed] [Google Scholar]
- 105.Copolovici DM, Langel K, Eriste E, Langel U. Cell-penetrating peptides:Design, synthesis, and applications. ACS Nano. 2014;8:1972–1994. doi: 10.1021/nn4057269. [DOI] [PubMed] [Google Scholar]
- 106.Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J Am Chem Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- 107.Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol Sci. 2017;38:406–424. doi: 10.1016/j.tips.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 108.Pujals S, Giralt E. Proline-rich, amphipathic cell-penetrating peptides. Adv Drug Deliv Rev. 2008;60:473–484. doi: 10.1016/j.addr.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 109.Marks JR, Placone J, Hristova K, Wimley WC. Spontaneous membrane-translocating peptides by orthogonal high-throughput screening. J Am Chem Soc. 2011;133:8995–9004. doi: 10.1021/ja2017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koren E, Torchilin VP. Cell-penetrating peptides: Breaking through to the other side. Trends Mol Med. 2012;18:385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 111.Kamei N, Onuki Y, Takayama K, Takeda-Morishita M. Mechanistic study of the uptake/permeation of cell-penetrating peptides across a caco-2 monolayer and their stimulatory effect on epithelial insulin transport. J Pharm Sci. 2013;102:3998–4008. doi: 10.1002/jps.23708. [DOI] [PubMed] [Google Scholar]
- 112.Chen Y, Xie X, Wu A, Wang L, Hu Y, Zhang H, Li Y. A synthetic cell-penetrating peptide derived from nuclear localization signal of EPS8 exerts anticancer activity against acute myeloid leukemia. J Exp Clin Cancer Res. 2018;37:12. doi: 10.1186/s13046-018-0682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lyu L, Huang LQ, Huang T, Xiang W, Yuan JD, Zhang CH. Cell-penetrating peptide conjugates of gambogic acid enhance the antitumor effect on human bladder cancer EJ cells through ROS-mediated apoptosis. Drug Des Devel Ther. 2018;12:743–756. doi: 10.2147/DDDT.S161821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benergossi J, Calixto G, Fonseca-Santos B, Aida KL, de Cassia Negrini T, Duque C, Gremiao MP, Chorilli M. Highlights in peptide nanoparticle carriers intended to oral diseases. Curr Top Med Chem. 2015;15:345–355. doi: 10.2174/1568026615666150108125040. [DOI] [PubMed] [Google Scholar]
- 115.Meng F, Han N, Yeo Y. Organic nanoparticle systems for spatiotemporal control of multimodal chemotherapy. Expert Opin Drug Deliv. 2017;14:427–446. doi: 10.1080/17425247.2016.1218464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Conibear AC, Schmid A, Kamalov M, Becker CFW, Bello C. Recent advances in peptide-based approaches for cancer treatment. Curr Med Chem. 2017;27:1174–1205. doi: 10.2174/0929867325666171123204851. [DOI] [PubMed] [Google Scholar]
- 117.Asadzadeh Z, Mohammadi H, Safarzadeh E, Hemmatzadeh M, Mahdian-Shakib A, Jadidi-Niaragh F, Azizi G, Baradaran B. The paradox of Th17 cell functions in tumor immunity. Cell Immunol. 2017;322:15–25. doi: 10.1016/j.cellimm.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 118.Darabi A, Thuring C, Paulsson KM. HLA-I antigen presentation and tapasin influence immune responses against malignant brain tumors-considerations for successful immunotherapy. Anticancer Agents Med Chem. 2014;14:1094–1100. doi: 10.2174/1871520614666140825110001. [DOI] [PubMed] [Google Scholar]
- 119.Li M, Shi HS, Zhang HL, Luo ZC, Wan Y, Lu L, Luo ST, Yang L. bFGF peptide combined with the pVAX-8CpG plasmid as adjuvant is a novel anticancer vaccine inducing effective immune responses against Lewis lung carcinoma. Mol Med Rep. 2012;5:625–630. doi: 10.3892/mmr.2012.968. [DOI] [PubMed] [Google Scholar]
- 120.Wang W, Li Y, Wang Y, Ren S, Li Y, Wang B. Polyactin A is a novel and potent immunological adjuvant for peptide-based cancer vaccine. Int Immunopharmacol. 2018;54:95–102. doi: 10.1016/j.intimp.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 121.Jin H, Wan C, Zou Z, Zhao G, Zhang L, Geng Y, Chen T, Huang A, Jiang F, Feng JP, et al. Tumor ablation and therapeutic immunity induction by an injectable peptide hydrogel. ACS Nano. 2018;12:3295–3310. doi: 10.1021/acsnano.7b08148. [DOI] [PubMed] [Google Scholar]
- 122.Kakwere H, Ingham ES, Allen R, Mahakian LM, Tam SM, Zhang H, Silvestrini MT, Lewis JS, Ferrara KW. Toward personalized peptide-based cancer nanovaccines: A facile and versatile synthetic approach. Bioconjug Chem. 2017;28:2756–2771. doi: 10.1021/acs.bioconjchem.7b00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Apostolopoulos V, McKenzie IF. Cellular mucins: Targets for immunotherapy. Crit Rev Immunol. 1994;14:293–309. doi: 10.1615/CritRevImmunol.v14.i3-4.40. [DOI] [PubMed] [Google Scholar]
- 124.Obara W, Eto M, Mimata H, Kohri K, Mitsuhata N, Miura I, Shuin T, Miki T, Koie T, Fujimoto H, et al. A phase I/II study of cancer peptide vaccine S-288310 in patients with advanced urothelial carcinoma of the bladder. Ann Oncol. 2017;28:798–803. doi: 10.1093/annonc/mdw675. [DOI] [PubMed] [Google Scholar]
- 125.Antonilli M, Rahimi H, Visconti V, Napoletano C, Ruscito I, Zizzari IG, Caponnetto S, Barchiesi G, Iadarola R, Pierelli L, et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial. Int J Oncol. 2016;48:1369–1378. doi: 10.3892/ijo.2016.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Asahara S, Takeda K, Yamao K, Maguchi H, Yamaue H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J Transl Med. 2013;11:291. doi: 10.1186/1479-5876-11-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rausch S, Gouttefangeas C, Hennenlotter J, Laske K, Walter K, Feyerabend S, Chandran PA, Kruck S, Singh-Jasuja H, Frick A, et al. Results of a Phase 1/2 Study in Metastatic Renal Cell Carcinoma Patients Treated with a Patient-specific Adjuvant Multi-peptide Vaccine after Resection of Metastases. Eur Urol Focus. 2019;5:604–607. doi: 10.1016/j.euf.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 128.Dutoit V, Migliorini D, Ranzanici G, Marinari E, Widmer V, Lobrinus JA, Momjian S, Costello J, Walker PR, Okada H, et al. Antigenic expression and spontaneous immune responses support the use of a selected peptide set from the IMA950 glio-blastoma vaccine for immunotherapy of grade II and III glioma. Oncoimmunology. 2018;7:e1391972. doi: 10.1080/2162402X.2017.1391972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lilleby W, Gaudernack G, Brunsvig PF, Vlatkovic L, Schulz M, Mills K, Hole KH, Inderberg EM. Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer. Cancer Immunol Immunother. 2017;66:891–901. doi: 10.1007/s00262-017-1994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Talebi S, Bolhassani A, Azad TM, Arashkia A, Modaresi MH. Immuno-stimulating peptide derived from HMGB1 is more effective than the N-terminal domain of Gp96 as an endogenous adjuvant for improvement of protein vaccines. Protein Pept Lett. 2017;24:190–196. doi: 10.2174/0929866523666161220111833. [DOI] [PubMed] [Google Scholar]
- 131.Glover S, Delaney M, Dematte C, Kornberg L, Frasco M, Tran-Son-Tay R, Benya RV. Phosphorylation of focal adhesion kinase tyrosine 397 critically mediates gastrin-releasing peptide's morphogenic properties. J Cell Physiol. 2004;199:77–88. doi: 10.1002/jcp.10456. [DOI] [PubMed] [Google Scholar]
- 132.Schally AV, Zhang X, Cai R, Hare JM, Granata R, Bartoli M. Actions and potential therapeutic applications of growth hormone-releasing hormone agonists. Endocrinology. 2019;160:1600–1612. doi: 10.1210/en.2019-00111. [DOI] [PubMed] [Google Scholar]
- 133.Munoz-Moreno L, Bajo AM, Prieto JC, Carmena MJ. Growth hormone-releasing hormone (GHRH) promotes metastatic phenotypes through EGFR/HER2 transactivation in prostate cancer cells. Mol Cell Endocrinol. 2017;446:59–69. doi: 10.1016/j.mce.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 134.Jimenez JJ, DelCanto GM, Popovics P, Perez A, Vila Granda A, Vidaurre I, Cai RZ, Rick FG, Swords RT, Schally AV. A new approach to the treatment of acute myeloid leukaemia targeting the receptor for growth hormone-releasing hormone. Br J Haematol. 2018;181:476–485. doi: 10.1111/bjh.15207. [DOI] [PubMed] [Google Scholar]
- 135.Chin YT, Wang LM, Hsieh MT, Shih YJ, Nana AW, Changou CA, Yang YSH, Chiu HC, Fu E, Davis PJ, et al. Leptin OB3 peptide suppresses leptin-induced signaling and progression in ovarian cancer cells. J Biomed Sci. 2017;24:51. doi: 10.1186/s12929-017-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang M, Zhang M, Wang J, Cai Q, Zhao R, Yu Y, Tai H, Zhang X, Xu C. Retro-inverso follicle-stimulating hormone peptide-mediated polyethylenimine complexes for targeted ovarian cancer gene therapy. Drug Deliv. 2018;25:995–1003. doi: 10.1080/10717544.2018.1461956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sogaard CK, Moestue SA, Rye MB, Kim J, Nepal A, Liabakk NB, Bachke S, Bathen TF, Otterlei M, Hill DK. APIM-peptide targeting PCNA improves the efficacy of docetaxel treatment in the TRAMP mouse model of prostate cancer. Oncotarget. 2018;9:11752–11766. doi: 10.18632/oncotarget.24357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 139.Liu X, Peng J, He J, Li Q, Zhou J, Liang X, Tang S. Selection and identification of novel peptides specifically targeting human cervical cancer. Amino Acids. 2018;50:577–592. doi: 10.1007/s00726-018-2539-1. [DOI] [PubMed] [Google Scholar]
- 140.Serrill JD, Wan X, Hau AM, Jang HS, Coleman DJ, Indra AK, Alani AW, McPhail KL, Ishmael JE. Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts. Invest New Drugs. 2016;34:24–40. doi: 10.1007/s10637-015-0303-x. [DOI] [PubMed] [Google Scholar]
- 141.Chakrabarti S, Guha S, Majumder K. Food-derived bioac-tive peptides in human health: Challenges and opportunities. Nutrients. 2018;10:1738. doi: 10.3390/nu10111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sable R, Parajuli P, Jois S. Peptides, Peptidomimetics, and polypeptides from marine sources: A wealth of natural sources for pharmaceutical applications. Mar Drugs. 2017;15:124. doi: 10.3390/md15040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O'Brien-Simpson NM, Hoffmann R, Chia CSB, Wade JD. Editorial: Antimicrobial and Anticancer Peptides. Front Chem. 2018;6:13. doi: 10.3389/fchem.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sultan S, Huma N, Butt MS, Aleem M, Abbas M. Therapeutic potential of dairy bioactive peptides: A contemporary perspective. Crit Rev Food Sci Nutr. 2018;58:105–115. doi: 10.1080/10408398.2015.1136590. [DOI] [PubMed] [Google Scholar]
- 145.Felicio MR, Silva ON, Goncalves S, Santos NC, Franco OL. Peptides with dual antimicrobial and anticancer activities. Front Chem. 2017;5:5. doi: 10.3389/fchem.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mohanty DP, Mohapatra S, Misra S, Sahu PS. Milk derived bioactive peptides and their impact on human health-A review. Saudi J Biol Sci. 2016;23:577–583. doi: 10.1016/j.sjbs.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gonzalez-Montoya M, Hernandez-Ledesma B, Silvan JM, Mora-Escobedo R, Martinez-Villaluenga C. Peptides derived from in vitro gastrointestinal digestion of germinated soybean proteins inhibit human colon cancer cells proliferation and inflammation. Food Chem. 2018;242:75–82. doi: 10.1016/j.foodchem.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 148.Prateep A, Sumkhemthong S, Suksomtip M, Chanvorachote P, Chaotham C. Peptides extracted from edible mushroom: Lentinus squarrosulus induces apoptosis in human lung cancer cells. Pharm Biol. 2017;55:1792–1799. doi: 10.1080/13880209.2017.1325913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 150.Fahs S, Patil-Sen Y, Snape TJ. Foldamers as anticancer therapeutics: Targeting protein-protein interactions and the cell membrane. Chembiochem. 2015;16:1840–1853. doi: 10.1002/cbic.201500188. [DOI] [PubMed] [Google Scholar]
- 151.Dhar A, Mallick S, Ghosh P, Maiti A, Ahmed I, Bhattacharya S, Mandal T, Manna A, Roy K, Singh S, et al. Simultaneous inhibition of key growth pathways in melanoma cells and tumor regression by a designed bidentate constrained helical peptide. Biopolymers. 2014;102:344–358. doi: 10.1002/bip.22505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tanishiki N, Yano Y, Matsuzaki K. Endowment of pH responsivity to anticancer peptides by introducing 2,3-diami-nopropionic acid residues. Chembiochem. 2019;20:2109–2117. doi: 10.1002/cbic.201900226. [DOI] [PubMed] [Google Scholar]
- 153.Dennison SR, Harris F, Mura M, Phoenix DA. An atlas of anionic antimicrobial peptides from amphibians. Curr Protein Pept Sci. 2018;19:823–838. doi: 10.2174/1389203719666180226155035. [DOI] [PubMed] [Google Scholar]
- 154.Khamessi O, Ben Mabrouk H, ElFessi-Magouri R, Kharrat R. RK1, the first very short peptide from Buthus occi-tanus tunetanus inhibits tumor cell migration, proliferation and angiogenesis. Biochem Biophys Res Commun. 2018;499:1–7. doi: 10.1016/j.bbrc.2018.01.133. [DOI] [PubMed] [Google Scholar]
- 155.Choi YJ, Park SJ, Park YS, Park HS, Yang KM, Heo K. EpCAM peptide-primed dendritic cell vaccination confers significant anti-tumor immunity in hepatocellular carcinoma cells. PLoS One. 2018;13:e0190638. doi: 10.1371/journal.pone.0190638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Matsueda S, Itoh K, Shichijo S. Antitumor activity of antibody against cytotoxic T lymphocyte epitope peptide of lymphocyte-specific protein tyrosine kinase. Cancer Sci. 2018;109:611–617. doi: 10.1111/cas.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peng X, Zhou C, Hou X, Liu Y, Wang Z, Peng X, Zhang Z, Wang R, Kong D. Molecular characterization and bioactivity evaluation of two novel bombinin peptides from the skin secretion of Oriental fire-bellied toad, Bombina orientalis. Amino Acids. 2018;50:241–253. doi: 10.1007/s00726-017-2509-z. [DOI] [PubMed] [Google Scholar]
- 158.Xie X, Zhou W, Hu Y, Chen Y, Zhang H, Li Y. A dual-function epidermal growth factor receptor pathway substrate 8 (Eps8)-derived peptide exhibits a potent cytotoxic T lymphocyte-activating effect and a specific inhibitory activity. Cell Death Dis. 2018;9:379. doi: 10.1038/s41419-018-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu ZZ, Ding GF, Huang FF, Yang ZS, Yu FM, Tang YP, Jia YL, Zheng YY, Chen R. Anticancer activity of anthopleura anjunae oligopeptides in prostate cancer DU-145 cells. Mar Drugs. 2018;16 doi: 10.3390/md16040125. pii: E125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shen Y, Maupetit J, Derreumaux P, Tuffery P. Improved PEP-FOLD approach for peptide and miniprotein structure prediction. J Chem Theory Comput. 2014;10:4745–4758. doi: 10.1021/ct500592m. [DOI] [PubMed] [Google Scholar]
- 161.Thevenet P, Shen Y, Maupetit J, Guyon F, Derreumaux P, Tuffery P. PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012;40:W288–W293. doi: 10.1093/nar/gks419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hao Y, Yang N, Teng D, Wang X, Mao R, Wang J. A review of the design and modification of lactoferricins and their derivatives. Biometals. 2018;31:331–341. doi: 10.1007/s10534-018-0086-6. [DOI] [PubMed] [Google Scholar]
- 163.Dathe M, Schumann M, Wieprecht T, Winkler A, Beyermann M, Krause E, Matsuzaki K, Murase O, Bienert M. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry. 1996;35:12612–12622. doi: 10.1021/bi960835f. [DOI] [PubMed] [Google Scholar]
- 164.Sun S, Zhao G, Huang Y, Cai M, Yan Q, Wang H, Chen Y. Enantiomeric effect of d-Amino acid substitution on the mechanism of action of α-helical membrane-active peptides. Int J Mol Sci. 2017;19:67. doi: 10.3390/ijms19010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hicks RP. Antibacterial and anticancer activity of a series of novel peptides incorporating cyclic tetra-substituted C(α) amino acids. Bioorg Med Chem. 2016;24:4056–4065. doi: 10.1016/j.bmc.2016.06.048. [DOI] [PubMed] [Google Scholar]
- 166.Zhou J, Yang X, Zhang W, Wang J, Wei C, Gu F, Lei T, Qin Y. Construction of an Anticancer Fusion Peptide (ACFP) derived from milk proteins and an assay of anti-ovarian cancer cells in vitro. Anticancer Agents Med Chem. 2017;17:635–643. doi: 10.2174/1871520616666160627091131. [DOI] [PubMed] [Google Scholar]
- 167.Bracci L, Mandarini E, Brunetti J, Depau L, Pini A, Terzuoli L, Scali S, Falciani C. The GAG-specific branched peptide NT4 reduces angiogenesis and invasiveness of tumor cells. PLoS One. 2018;13:e0194744. doi: 10.1371/journal.pone.0194744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chu HL, Yip BS, Chen KH, Yu HY, Chih YH, Cheng HT, Chou YT, Cheng JW. Novel antimicrobial peptides with high anticancer activity and selectivity. PLoS One. 2015;10:e0126390. doi: 10.1371/journal.pone.0126390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.He B, Yang D, Qin M, Zhang Y, He B, Dai W, Wang X, Zhang Q, Zhang H, Yin C. Increased cellular uptake of peptide-modified PEGylated gold nanoparticles. Biochem Biophys Res Commun. 2017;494:339–345. doi: 10.1016/j.bbrc.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 170.Tsuchiya N, Hosono A, Yoshikawa T, Shoda K, Nosaka K, Shimomura M, Hara J, Nitani C, Manabe A, Yoshihara H, et al. Phase I study of glypican-3-derived peptide vaccine therapy for patients with refractory pediatric solid tumors. Oncoimmunology. 2017;7:e1377872. doi: 10.1080/2162402X.2017.1377872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liang AL, Qian HL, Zhang TT, Zhou N, Wang HJ, Men XT, Qi W, Zhang PP, Fu M, Liang X, et al. Bifunctional fused polypeptide inhibits the growth and metastasis of breast cancer. Drug Des Devel Ther. 2015;9:5671–5686. doi: 10.2147/DDDT.S90082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Herbert KJ, Ashton TM, Prevo R, Pirovano G, Higgins GS. T-LAK cell-originated protein kinase (TOPK): An emerging target for cancer-specific therapeutics. Cell Death Dis. 2018;9:1089. doi: 10.1038/s41419-018-1131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu D, Gao Y, Qi Y, Chen L, Ma Y, Li Y. Peptide-based cancer therapy: Opportunity and challenge. Cancer Lett. 2014;351:13–22. doi: 10.1016/j.canlet.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 174.Zachos I, Konstantinopoulos PA, Tzortzis V, Gravas S, Karatzas A, Karamouzis MV, Melekos M, Papavassiliou AG. Systemic therapy of metastatic bladder cancer in the molecular era: Current status and future promise. Expert Opin Investig Drugs. 2010;19:875–887. doi: 10.1517/13543784.2010.496450. [DOI] [PubMed] [Google Scholar]
- 175.Amir E, Hughes S, Blackhall F, Thatcher N, Ostoros G, Timar J, Tovari J, Kovacs G, Dome B. Targeting blood vessels for the treatment of non-small cell lung cancer. Curr Cancer Drug Targets. 2008;8:392–403. doi: 10.2174/156800908785133187. [DOI] [PubMed] [Google Scholar]
- 176.Kim-Schulze S, Taback B, Kaufman HL. Cytokine therapy for cancer. Surg Oncol Clin N Am. 2007;16:793–818. viii. doi: 10.1016/j.soc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 177.Niu F, Yan J, Ma B, Li S, Shao Y, He P, Zhang W, He W, Ma PX, Lu W. Lanthanide-doped nanoparticles conjugated with an anti-CD33 antibody and a p53-activating peptide for acute myeloid leukemia therapy. Biomaterials. 2018;167:132–142. doi: 10.1016/j.biomaterials.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vats K, Sharma R, Sarma HD, Satpati D, Dash A. 68Ga-labeled HBED-CC variant of uPAR targeting peptide AE105 compared with 68Ga-NODAGA-AE105. Anticancer Agents Med Chem. 2018;18:1289–1294. doi: 10.2174/1871520618666180316152618. [DOI] [PubMed] [Google Scholar]
- 179.Ahmadpour S, Noaparast Z, Abedi SM, Hosseinimehr SJ. 99mTc-(tricine)-HYNIC-Lys-FROP peptide for breast tumor targeting. Anticancer Agents Med Chem. 2018;18:1295–1302. doi: 10.2174/1871520618666180307142027. [DOI] [PubMed] [Google Scholar]
- 180.Zhang J, Spring H, Schwab M. Neuroblastoma tumor cell-binding peptides identified through random peptide phage display. Cancer Lett. 2001;171:153–164. doi: 10.1016/S0304-3835(01)00575-4. [DOI] [PubMed] [Google Scholar]
- 181.Soudy R, Etayash H, Bahadorani K, Lavasanifar A, Kaur K. Breast cancer targeting peptide binds keratin 1: A new molecular marker for targeted drug delivery to breast cancer. Mol Pharm. 2017;14:593–604. doi: 10.1021/acs.molpharmaceut.6b00652. [DOI] [PubMed] [Google Scholar]
- 182.Du Y, Wang L, Wang W, Guo T, Zhang M, Zhang P, Zhang Y, Wu K, Li A, Wang X, et al. Novel application of cell penetrating R11 peptide for diagnosis of bladder cancer. J Biomed Nanotechnol. 2018;14:161–167. doi: 10.1166/jbn.2018.2499. [DOI] [PubMed] [Google Scholar]
- 183.Sato S, Nakamura T, Katagiri T, Tsuchikawa T, Kushibiki T, Hontani K, Takahashi M, Inoko K, Takano H, Abe H, et al. Molecular targeting of cell-permeable peptide inhibits pancreatic ductal adeno-carcinoma cell proliferation. Oncotarget. 2017;8:113662–113672. doi: 10.18632/oncotarget.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kang T, Huang Y, Zhu Q, Cheng H, Pei Y, Feng J, Xu M, Jiang G, Song Q, Jiang T, et al. Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials. 2018;164:80–97. doi: 10.1016/j.biomaterials.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 185.Garay H, Espinosa LA, Perera Y, Sanchez A, Diago D, Perea SE, Besada V, Reyes O, Gonzalez LJ. Characterization of low-abundance species in the active pharmaceutical ingredient of CIGB-300: A clinical-grade anticancer synthetic peptide. J Pept Sci. 2018;24:e3081. doi: 10.1002/psc.3081. [DOI] [PubMed] [Google Scholar]
- 186.Perea SE, Reyes O, Baladron I, Perera Y, Farina H, Gil J, Rodriguez A, Bacardi D, Marcelo JL, Cosme K, et al. CIGB-300, a novel proapoptotic peptide that impairs the CK2 phosphorylation and exhibits anticancer properties both in vitro and in vivo. Mol Cell Biochem. 2008;316:163–167. doi: 10.1007/s11010-008-9814-5. [DOI] [PubMed] [Google Scholar]
- 187.Rodriguez-Ulloa A, Ramos Y, Gil J, Perera Y, Castellanos-Serra L, Garcia Y, Betancourt L, Besada V, Gonzalez LJ, Fernandez-de-Cossio J, et al. Proteomic profile regulated by the anticancer peptide CIGB-300 in non-small cell lung cancer (NSCLC) cells. J Proteome Res. 2010;9:5473–5483. doi: 10.1021/pr100728v. [DOI] [PubMed] [Google Scholar]
- 188.Hirabayashi K, Yanagisawa R, Saito S, Higuchi Y, Koya T, Sano K, Koido S, Okamoto M, Sugiyama H, Nakazawa Y, et al. Feasibility and immune response of WT1 peptide vaccination in combination with OK-432 for paediatric solid tumors. Anticancer Res. 2018;38:2227–2234. doi: 10.21873/anticanres.12465. [DOI] [PubMed] [Google Scholar]
- 189.Yanagisawa R, Koizumi T, Koya T, Sano K, Koido S, Nagai K, Kobayashi M, Okamoto M, Sugiyama H, Shimodaira S. WT1-pulsed dendritic cell vaccine combined with chemotherapy for resected pancreatic cancer in a phase I study. Anticancer Res. 2018;38:2217–2225. doi: 10.21873/anticanres.12464. [DOI] [PubMed] [Google Scholar]
- 190.Ohno S, Takano F, Ohta Y, Kyo S, Myojo S, Dohi S, Sugiyama H, Ohta T, Inoue M. Frequency of myeloid dendritic cells can predict the efficacy of Wilms' tumor 1 peptide vaccination. Anticancer Res. 2011;31:2447–2452. [PubMed] [Google Scholar]
- 191.Ohno S, Kyo S, Myojo S, Dohi S, Ishizaki J, Miyamoto K, Morita S, Sakamoto J, Enomoto T, Kimura T, et al. Wilms' tumor 1 (WT1) peptide immunotherapy for gynecological malignancy. Anticancer Res. 2009;29:4779–4784. [PubMed] [Google Scholar]
- 192.Ishikawa H, Imano M, Shiraishi O, Yasuda A, Peng YF, Shinkai M, Yasuda T, Imamoto H, Shiozaki H. Phase I clinical trial of vaccination with LY6K-derived peptide in patients with advanced gastric cancer. Gastric Cancer. 2014;17:173–180. doi: 10.1007/s10120-013-0258-6. [DOI] [PubMed] [Google Scholar]
- 193.Vasef MA, Ross JS, Cohen MB. Telomerase activity in human solid tumors. Diagnostic utility and clinical applications. Am J Clin Pathol. 1999;112(1 Suppl):S68–S75. [PubMed] [Google Scholar]
- 194.Bernhardt SL, Gjertsen MK, Trachsel S, Moller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer. 2006;95:1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kokhaei P, Palma M, Hansson L, Osterborg A, Mellstedt H, Choudhury A. Telomerase (hTERT 611-626) serves as a tumor antigen in B-cell chronic lymphocytic leukemia and generates spontaneously antileukemic, cytotoxic T cells. Exp Hematol. 2007;35:297–304. doi: 10.1016/j.exphem.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 196.Aspeslagh S, Awada A, Matos-Pita A, Aftimos P, Bahleda R, Varga A, Soria JC. Phase I dose-escalation study of plitidepsin in combination with bevacizumab in patients with refractory solid tumors. Anticancer Drugs. 2016;27:1021–1027. doi: 10.1097/CAD.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 197.Engel JB, Tinneberg HR, Rick FG, Berkes E, Schally AV. Targeting of peptide cytotoxins to LHRH receptors for treatment of cancer. Curr Drug Targets. 2016;17:488–494. doi: 10.2174/138945011705160303154717. [DOI] [PubMed] [Google Scholar]
- 198.Noguchi M, Matsumoto K, Uemura H, Arai G, Eto M, Naito S, Ohyama C, Nasu Y, Tanaka M, Moriya F, et al. An open-label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin Cancer Res. 2016;22:54–60. doi: 10.1158/1078-0432.CCR-15-1265. [DOI] [PubMed] [Google Scholar]
- 199.Toh U, Saku S, Okabe M, Iwakuma N, Kimitsuki Y, Akashi M, Ogo E, Yamada A, Shichijo S, Itoh K, et al. Development of peptide vaccines for triple-negative breast cancer treatment. Gan To Kagaku Ryoho. 2016;43:1249–1251. In Japanese. [PubMed] [Google Scholar]
- 200.Brown TA, Byrd K, Vreeland TJ, Clifton GT, Jackson DO, Hale DF, Herbert GS, Myers JW, Greene JM, Berry JS, et al. Final analysis of a phase I/IIa trial of the folate-binding protein-derived E39 peptide vaccine to prevent recurrence in ovarian and endometrial cancer patients. Cancer Med. 2019;8:4678–4687. doi: 10.1002/cam4.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mikecin AM, Walker LR, Kuna M, Raucher D. Thermally targeted p21 peptide enhances bortezomib cytotoxicity in androgen-independent prostate cancer cell lines. Anticancer Drugs. 2014;25:189–199. doi: 10.1097/CAD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Korani M, Korani S, Zendehdel E, Nikpoor AR, Jaafari MR, Orafai HM, Johnston TP, Sahebkar A. Enhancing the therapeutic efficacy of bortezomib in cancer therapy using polymeric nanostructures. Curr Pharm Des. 2019;25:4883–4892. doi: 10.2174/1381612825666191106150018. [DOI] [PubMed] [Google Scholar]
- 204.Zhou Y, Liu X, Xue J, Liu L, Liang T, Li W, Yang X, Hou X, Fang H. Discovery of peptide boronate derivatives as histone deacetylase and proteasome dual inhibitors for overcoming bortezomib resistance of multiple myeloma. J Med Chem. 2020;63:4701–4715. doi: 10.1021/acs.jmedchem.9b02161. [DOI] [PubMed] [Google Scholar]
- 205.Garofalo M, Grazioso G, Cavalli A, Sgrignani J. How computational chemistry and drug delivery techniques can support the development of new anticancer drugs. Molecules. 2020;25:1756. doi: 10.3390/molecules25071756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Adlakha S, Sharma A, Vaghasiya K, Ray E, Verma RK. Inhalation delivery of host defence peptides (HDP) using nano-formulation strategies: A pragmatic approach for therapy of pulmonary ailments. Curr Protein Pept Sci. 2020;21:369–378. doi: 10.2174/1389203721666191231110453. [DOI] [PubMed] [Google Scholar]
- 207.Tu Y, Tao J, Wang F, Liu P, Han Z, Li Z, Ma Y, Gu Y. A novel peptide targeting gastrin releasing peptide receptor for pancreatic neoplasm detection. Biomater Sci. 2020;8:2682–2693. doi: 10.1039/D0BM00162G. [DOI] [PubMed] [Google Scholar]
- 208.Ohana J, Sandler U, Kass G, Stemmer SM, Devary Y. dTCApFs, a derivative of a novel human hormone peptide, induces apoptosis in cancer cells through a mechanism involving loss of Golgi function. Mol Clin Oncol. 2017;7:991–999. doi: 10.3892/mco.2017.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stemmer SM, Benjaminov O, Silverman MH, Sandler U, Purim O, Sender N, Meir C, Oren-Apoteker P, Ohana J, Devary Y. A phase I clinical trial of dTCApFs, a derivative of a novel human hormone peptide, for the treatment of advanced/metastatic solid tumors. Mol Clin Oncol. 2018;8:22–29. doi: 10.3892/mco.2017.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Murali R, Kieber-Emmons T. Cancer immunotherapeutics: Evolution of monoclonal antibodies to peptide immunogens. Monoclon Antib Immunodiagn Immunother. 2014;33:179–182. doi: 10.1089/mab.2014.0021. [DOI] [PubMed] [Google Scholar]
- 211.Tan Z, Zhang S. Past, present, and future of targeting ras for cancer therapies. Mini Rev Med Chem. 2016;16:345–357. doi: 10.2174/1389557515666151001154111. [DOI] [PubMed] [Google Scholar]
- 212.Li QX, Yu DH, Liu G, Ke N, McKelvy J, Wong-Staal F. Selective anticancer strategies via intervention of the death pathways relevant to cell transformation. Cell Death Differ. 2008;15:1197–1210. doi: 10.1038/cdd.2008.48. [DOI] [PubMed] [Google Scholar]
- 213.Micale N, Scarbaci K, Troiano V, Ettari R, Grasso S, Zappala M. Peptide-based proteasome inhibitors in anticancer drug design. Med Res Rev. 2014;34:1001–1069. doi: 10.1002/med.21312. [DOI] [PubMed] [Google Scholar]
- 214.Wang X, Chen X, Yang X, Gao W, He B, Dai W, Zhang H, Wang X, Wang J, Zhang X, et al. A nanomedicine based combination therapy based on QLPVM peptide functionalized liposomal tamoxifen and doxorubicin against Luminal A breast cancer. Nanomedicine. 2016;12:387–397. doi: 10.1016/j.nano.2015.12.360. [DOI] [PubMed] [Google Scholar]
- 215.Cheng T, Zhan X. Pattern recognition for predictive, preventive, and personalized medicine in cancer. EPMA J. 2017;8:51–60. doi: 10.1007/s13167-017-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Vadevoo SMP, Gurung S, Khan F, Haque ME, Gunassekaran GR, Chi L, Permpoon U, Lee B. Peptide-based targeted therapeutics and apoptosis imaging probes for cancer therapy. Arch Pharm Res. 2019;42:150–158. doi: 10.1007/s12272-019-01125-0. [DOI] [PubMed] [Google Scholar]
- 217.Sun X, Li Y, Liu T, Li Z, Zhang X, Chen X. Peptide-based imaging agents for cancer detection. Adv Drug Deliv Rev. 2017;110-111:38–51. doi: 10.1016/j.addr.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ehlerding EB, Sun L, Lan X, Zeng D, Cai W. Dual-targeted molecular imaging of cancer. J Nucl Med. 2018;59:390–395. doi: 10.2967/jnumed.117.199877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang Q, Li SB, Zhao YY, Dai DN, Du H, Lin YZ, Ye JC, Zhao J, Xiao W, Mei Y, et al. Identification of a sodium pump Na(+)/K(+) ATPase alpha1-targeted peptide for PET imaging of breast cancer. J Control Release. 2018;281:178–188. doi: 10.1016/j.jconrel.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 220.Perez SA, von Hofe E, Kallinteris NL, Gritzapis AD, Peoples GE, Papamichail M, Baxevanis CN. A new era in anticancer peptide vaccines. Cancer. 2010;116:2071–2080. doi: 10.1002/cncr.24988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.