Abstract
In a relatively short period of time, treatment strategies for metastatic melanoma have radically changed leading to an unprecedented improvement in patient survival. In this period, immunotherapy options have evolved from cytokine-based approaches to antibody-mediated inhibition of immune checkpoints, cancer vaccines and pharmacological modulation of the melanoma microenvironment. Combination of immunotherapy strategies and the association of immune checkpoint inhibitors (ICIs) with BRAF V600 targeted therapy show encouraging results. The future of drug development in this field is promising. The comprehension of primary and acquired resistance mechanisms to ICIs and the dissection of melanoma immunobiology will be instrumental for the development of new treatment strategies and to improve clinical trial design. Moreover, biomarker discovery will help patient stratification and management during immunotherapy treatment. In this review, we summarize landmark clinical trials of immune checkpoint inhibitors in advanced melanoma and discuss the rational for immunotherapy combinations. Immunotherapy approaches at early stage of clinical development and recent advances in melanoma immunotherapy biomarker development are also discussed.
Keywords: melanoma, immunotherapy, PD-1, CTLA-4, tumor microenvironment
1. Introduction
Skin cancers are among the most common cancers diagnosed in the United States. The incidence of melanoma of the skin has risen in the last three decades and although melanoma accounts for approximately 1% of all skin cancers it causes most of the skin cancer deaths (1). When melanoma is diagnosed in its early stages, surgical resection of the lesion is associated with favorable prognosis. However, for locally advanced and metastatic disease surgery is no longer sufficient. The 5-year survival for localized melanoma is 99%, but it is 20% when distant metastases are present (1).
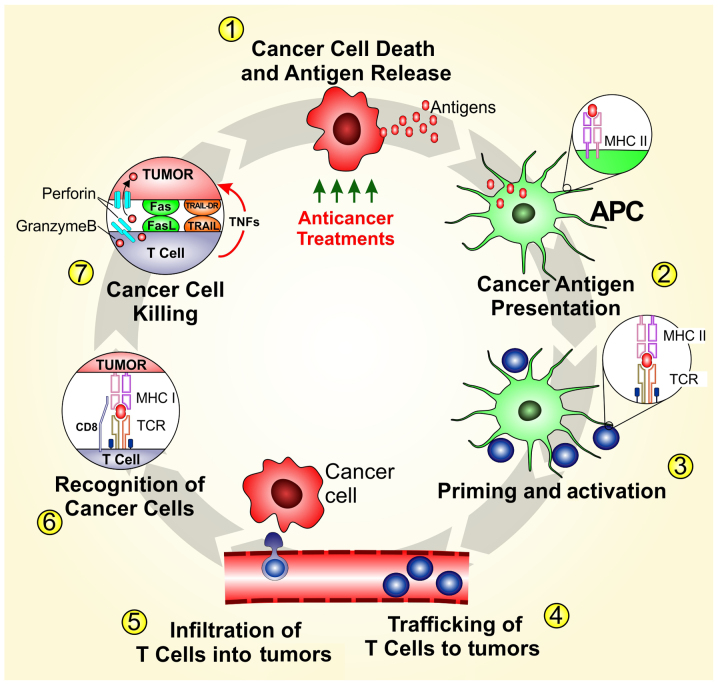
Melanoma is one of the most sensitive tumors to immune modulation. Several factors may explain melanoma cell susceptibility to immune system activation including high tumor mutational load due to ultraviolet light exposure, expression of cancer testis antigens and mimicry of melanocyte lineage proteins with pathogen-associated antigens (3-5). In this context T-cell response seems to play a central role to keep the melanoma at bay. Tumor infiltrated lymphocytes (TILs) are central to the development of an anti-tumor immune response and a subset of TILs demonstrate cytolytic activity against autologous tumors in melanoma patients (Fig. 1) (6). Their presence also correlates with increased survival and reduced risk of metastasis (6). In the past decades several clinical trials aimed at eliciting T-cell response with local or systemic immunomodulatory drugs such as interferon (IFN)-α (7,8), interleukin (IL)-2 (9,10), cancer vaccines (11,12) and adoptive cell transfer (13). Despite some evidence of activity, these trials failed to demonstrate sustained benefit in metastatic melanoma patients. More recently, immune checkpoint inhibitors (ICIs) against cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) have dramatically changed the management of both unresectable and metastatic melanoma as well as those at high risk for recurrence after resection (Table I) (14-16). Unfortunately, primary and secondary resistance and the absence of predictive markers of response are challenging problems with ICIs therapy (17). Combination of immunotherapy strategies aim to improve response and overcome resistance, while biomarker discovery is fundamental for the optimization of patient selection.
Figure 1.
The cancer immunity cycle.
Table I.
Landmark clinical trials of immunotherapy in locally advanced and metastatic melanoma.
| Trial name | Primary outcome | Treatment arms | ORR (%) | Median PFS (months) | Median OS (months) | 1yr-RFS (%) |
|---|---|---|---|---|---|---|
| CA184-002 (20) | OS | gp100 vaccine | 1.5 | 2.8 | 6.4 | - |
| gp100 vaccine+ipilimumab | 5.7 | 2.8 | 10.0 | - | ||
| ipilimumab | 11.0 | 2.9 | 10.1 | - | ||
| CA184-024 | OS | Dacarbazine | 10.3 | 3.0 | 9.1 | - |
| Dacarbazine+ipilimumab | 15.2 | 3.0 | 11.2 | - | ||
| CheckMate 066 (32) | OS | Dacarbazine | 14.4 | 2.2 | 11.2 | - |
| Nivolumab | 42.9 | 5.1 | 37.5 | - | ||
| KEYNOTE-006 (28,30) | PFS, OS | Ipilimumab | 11.9 | 3.4 | 16.0 | - |
| Pembrolizumab q2w | 33.7 | 5.6 | 32.7a - | |||
| Pembrolizumab q3w | 32.9 | 4.1 | ||||
| CheckMate 067 (37) | PFS, OS | Ipilimumab | 19.0 | 2.9 | 19.9 | - |
| Nivolumab | 45.0 | 6.9 | 36.9 | - | ||
| Nivolumab+ipilimumab | 58.0 | 11.5 | NR | - | ||
| OPTiM (46) | Durable response lasting ≥6 months | GM-CSF | Not reported | Not reported | 18.9 | - |
| T-VEC | Not reported | Not reported | 23.3 | - | ||
| CheckMate 238 (34) | RFS | Nivolumab | - | - | - | 70.5 |
| Ipilimumab | - | - | - | 60.8 | ||
| EORTC1325/ KEYNOTE-054 (35) | RFS | Pembrolizumab | - | - | - | 75.4 |
| Placebo | - | - | - | 61.0 |
ORR, overall response rate; OS, overall survival; PFS, progression-free survival; RFS, relapse-free survival; NR, not reached; q2w, every two weeks; q3w, every three weeks.
Pooled data from the pembrolizumab arms.
Herein, we review and discuss the rational of approved immunotherapy treatments, of preclinical data and ongoing clinical trials of combination strategies for advanced stage and metastatic melanoma. An overview is also provided of biomarker discovery and evidence on the role of gut microbioma in melanoma immunotherapy.
2. CTLA-4 blockade
CTLA-4 is an inhibitory checkpoint receptor that blocks T-cell activation and induces immune suppression (Fig. 2) (18). In 1996 Allison and colleagues showed that CTLA-4 blockade could attenuate the growth of several implanted murine tumors (19). In 2011 ipilimumab, a fully human monoclonal antibody (mAb) IgG1 that inhibits the interaction between CTLA-4 and its ligands, was the first ICI approved by the FDA. In previously treated patients with advanced melanoma, ipili-mumab improved median overall survival (OS) compared with gp100 peptide vaccine (10.6 vs. 6.4 months) (20). At 3-years, the survival rate was 22% followed by a plateau of the survival curve for up to 10 years (21). Combination strategies of ipilimumab with IL-2 or Peg-IFN failed to show any improvement over ipilimumab monotherapy (22,23). Tremelimumab, another monoclonal antibody targeting CTLA-4, failed to demonstrate a survival benefit over standard chemotherapy in a phase III clinical trial (24). In the adjuvant setting, a randomized clinical trial in resected stage III patients showed that ipilimumab improves relapse-free survival (RFS) and OS compared to placebo. However, more than 50% or patients experienced grade 3-4 adverse events with ipilimumab and 5 patients (1.1%) died due to immune related adverse events (25).
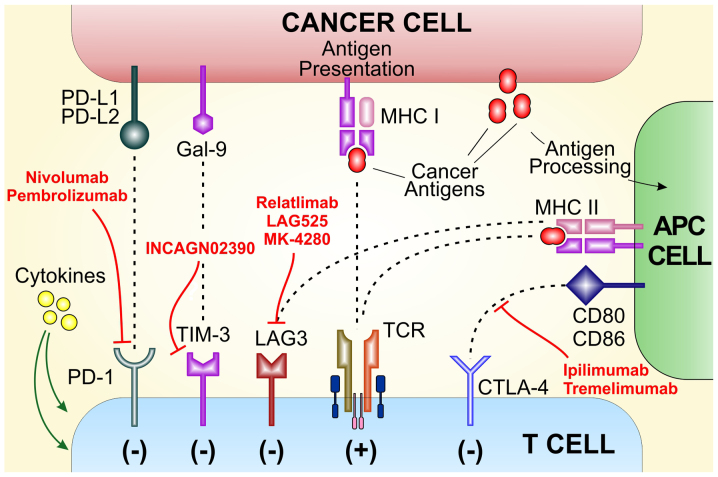
Figure 2.
Immune checkpoints and their inhibitors in advanced melanoma. PD, programmed death; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; LAG3, lymphocyte activation gene 3; TCR, T cell receptor; CTLA-4, cytotoxic T-lymphocyte antigen-4.
3. PD-1 blockade
PD-1 is an immune checkpoint with a central role in immunopathology and tumor immune surveillance through effector T-cell inhibition (Fig. 2) (18). In 2014, two mAbs targeting PD-1 (nivolumab and pembrolizumab) received FDA approval, becoming first line treatment option in metastatic melanoma. Randomized clinical trials have shown that monotherapy with nivolumab or pembrolizumab is superior to ipilimumab alone (26). Pembrolizumab monotherapy in treatment naïve and previously treated patients showed sustained response rates of 30-40% (26-28). In treatment of naïve patients pembrolizumab showed a 3-year OS rate of 51% and a 5-year rate of 41% (29,30). Clinical trials of nivolumab monotherapy provided durable response rate of 32% in untreated patients and 40% in previously treated melanoma (16,31). Three-year survival rate for nivolumab in previously untreated patients is 42% (32) while 5-year survival rate in previously treated patients with nivolumab monotherapy is 35% (15). Cross study comparisons of homogeneous groups of patients treated with pembrolizumab or nivolumab monotherapy have similar results regarding clinical endpoints and adverse event rates (33). At this time, no good predictive biomarkers for anti-PD-1 mAbs are available and clinical benefit is reached regardless of PD-L1 status (32). Since 2017, adjuvant immunotherapy with single agent anti-PD-1 mAb is the first treatment option in patients with resected stage III disease. Nivolumab improved RFS compared to ipilimumab with lower toxicities (34). In a phase 3 double-blind trial pembrolizumab treatment resulted in significantly longer RFS than placebo with no new toxicities compared to other pembrolizumab monotherapy trials (35).
4. Immune checkpoint inhibitor combinations
Monotherapy with ICIs is associated with significant improvement in patient survival, however, response rates are low. With the attempt to increase the number of patients who benefit from ICI therapy, combination of anti-CTLA-4 mAb plus anti-PD-1 mAb have been evaluated in prospective clinical trials. In particular, two randomized trials showed that the combination of anti-CTLA-4 plus anti-PD-1 mAbs results in increased clinical benefit compared to single agent ipilimumab or nivolumab. The Checkmate-067, a phase 3 randomized clinical trial compared ipilimumab plus nivolumab to nivolumab alone and ipilimumab alone in unresectable/metastatic melanoma. Response rates were 57.6, 43.7 and 19%, respectively, and 5-year survival rates were 52% in the combination arm, 44% in the nivolumab group and 26% in the ipilimumab arm (36,37). The combination arm showed increased toxicity compared to each monotherapy treatment arm. Specifically, treatment-related adverse events of any grade occurred in 95% of patients in the combination treatment group compared to 82% in the nivolumab arm and 86% in the ipilimumab group. Grade 3 or 4 treatment related adverse events occurred in 55% of the patients in the nivolumab plus ipilimumab group, 16.3% of the patients in the nivolumab group and 27.3% of those in the ipilimumab group (36). In 2015 the FDA approved the combination ipilimumab plus nivolumab on the basis of overall response rate and progression-free survival (PFS) improvement. In an attempt of reducing the toxicity burden of the combination, different dosing schedule by reducing ipilimumab dose and keep more standard dose anti-PD-1 single agents were investigated. Although the ORR appears to be conserved, the expected difference in terms of efficacy and safety are small and only results from larger trial will be conclusive (38,39). In patients with primary or secondary resistance to single agent PD-1 mAbs, ICIs combination or ipilimumab alone represent potential treatment strategies (40,41). Currently, the respective benefits of combination immunotherapy versus sequential immunotherapy are not yet fully understood and is object of an open debate in the clinical and scientific community. On one hand combination strategies are associated with increased adverse events that can be justified by the intent of gaining long-term disease response. On the other hand, the subset of patients who benefit more from the combination is unknow potentially exposing patients to unnecessary toxicities (42).
Brain metastases are a common cause of disabling neurologic complications and poor prognosis in patients with metastatic melanoma. The phase 2 clinical trial CheckMate-204 enrolled patients with small, untreated and asymptomatic brain metastasis and showed that ipilimumab plus nivolumab have clinically meaningful intracranial efficacy (56% of intracranial response). The safety profile was similar to those reported for the combination in patients without brain metastasis (43). Another phase 2 clinical trial compared the combination of nivolumab plus ipilimumab versus nivolumab alone. Despite the small sample size, ICIs combination was superior to nivolumab monotherapy with a higher proportion of patients achieving intracranial response (44).
No data are yet available on the potential benefit of anti-CTLA-4 plus anti-PD-1 combination in the adjuvant setting. Results from the clinical trial CheckMate-915 (NCT03068455) that compare nivolumab monotherapy to nivolumab plus ipilimumab in resected stage III melanoma are awaited.
5. Talimogene laherparepvec (T-VEC)
T-VEC is a type I herpes simplex virus genetically modified to preferentially replicate in tumor cells, enhance antigen loading of MHC class I and express granulocyte-macrophage colony-stimulating factor (GM-CSF) to increase tumor antigen presentation by dendritic cells (DCs) (45). In 2015 T-VEC received approval by the FDA for advanced melanoma. A phase 3 trial in unresected stage IIIB-IV melanoma showed that intratumoral administration of T-VEC improved response rate compared with GM-CSF (26 vs. 6%). Few responses were obtained in distant non-injected lesions (mainly lung and visceral sites) while the majority of responses were limited to the site of injection and regional non-injected lesions (46). The combination of T-VEC and ICIs has shown interesting results. The phase II study of T-VEC plus ipilimumab versus ipilimumab alone in patients with advanced melanoma showed an improvement in ORR (39 vs. 18%, respectively) (47). In the phase Ib trial evaluating the association of T-VEC plus pembrolizumab, confirmed objective response rate was 62%, with a complete response rate of 33% per immune-related response criteria (48). The results of the phase III trial MASTERKEY-265/KEYNOTE-034 of T-VEC plus pembrolizumab compared with pembrolizumab alone are awaited (NCT02263508).
6. Other immunotherapy strategies
Despite the impressive results obtained with currently approved ICI treatments strategies, primary and secondary resistance represent major clinical challenges. Several promising immunomodulatory targets have been included in ongoing clinical trials mainly in association with approved anti-PD-1 and anti-CTLA-4 mAbs (Fig. 2).
Inhibitors of lymphocyte activation gene-3 (LAG-3) and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) in clinical development
LAG-3 is an immune checkpoint receptor found on the cell surface of effector T cells and regulatory T cells. It is an inhibitor regulator of T cell response, activation and growth (49). Relatlimab, LAG525, and MK-4280 are mAbs that targets LAG-3 and are currently under investigation in combination with pembrolizumab or nivolumab (50). While the inhibition of LAG-3 alone has minor effect on T-cell reactivation, the combination with anti-PD-1 mAb has shown encouraging results (51,52).
TIM-3 is an immune checkpoint expressed on IFN-γ-producing T cells, FoxP3+ Treg cells and macrophages and DCs where it suppresses their responses upon interaction with their ligands. In vivo blockade of TIM-3 with other check-point inhibitors enhances anti-tumor immunity and suppresses tumor growth in several preclinical tumor models (53). INCAGN02390 is a TIM-3 inhibitor that is undergoing trial for the treatment of advanced solid tumor including melanoma (NCT03652077).
IDO inhibitors
Indoleamine 2,3-dioxygenase 1 (IDO1) is an enzyme involved in tryptophan catabolism with a central immunosuppressive function within the tumor microenvironment (54). Several IDO inhibitors (indiximod, epacadostat and BMS-986205) are currently evaluated in clinical trials in association with pembrolizumab, nivolumab or ipilimumab (54). Regrettably, the phase III clinical trial ECHO-301/KEYNOTE-252 in advanced melanoma failed to demonstrate PFS benefit in the arm of pembrolizumab with epcadostat compared to pembrolizumab alone (55).
Cytokines
Cytokines are the first class of immunomodula-tory agents that have found clinical application in melanoma. Indeed, IL-2 and IFN-α are both FDA approved for adjuvant treatment in melanoma (7,56). Other cytokines such as IL-12, IL-15, IL-18, IL-21 and GM-CSF have shown interesting results in preclinical and clinical settings. However, single agent cytokine strategy does not appear feasible due to their pleiotropic activity and the critical toxicity profile especially at high dose (56). With this in mind, NTRK-214 is a prodrug of conjugated IL-2, retaining the same amino acid sequence as human recombinant IL-2. The IL-2 core is conjugated to 6 releasable polyethylene glycol (PEG) chains that in vivo slowly release generating active IL-2 conjugates (57). An ongoing phase I/II clinical trial aims to evaluate the tolerability and efficacy of NTRK-214 with nivolumab and ipilimumab plus nivolumab (NCT02983045).
Modulation of the tumor microenvironment and the innate immune system
Tilsotolimod is a synthetic TLR-9 agonist oligonucleotide that acts on macrophages and DCs and can stimulate antigen presentation and T cell activation and proliferation. Intratumoral tilsotolimod in combination with ipilimumab in PD-1 inhibitor refractory metastatic melanoma is well tolerated and shows significant clinical benefit (ORR 38%) and durable response (58). These favorable results have led to an ongoing phase III study of tilsotolimod plus ipilimumab versus ipilimumab alone (NCT03445533). Another intratumoral TLR-9 agonist, SD-101, is in clinical development. The phase Ib/II clinical trial SYNERGY-001/KEYNOTE-184 evaluates the combination of SD-101 and pembrolizumab in patients with unresectable stage IIIC-IV melanoma and naïve to PD-1 axis inhibitors. Preliminary results show that the combination is well-tolerated, with promising high response rates and PFS (59). CD40 is expressed on macrophages and other antigen-presenting cells and its agonists stimulate maturation and increase macrophage killing activity against tumor cells (60). On the other hand, tumor-associated macrophages can be characterized by tumor-promoting phenotype (61). This phenotype is a consequence, among other factors, of the continuous activation of the colony-stimulating factor-1 (CSF-1) axis (62). An ongoing phase I/Ib trial is evaluating the safety and efficacy of the CSF-1 receptor inhibitor, cabiralizumab, combined with the CD40 agonist, APX005M, with or without nivolumab in patients with advanced melanoma (NCT03502330).
Vaccines
Therapeutic cancer vaccines aim at inducing a specific immune response against tumor antigens. In melanoma patients, peptide vaccines have been tested in association with ipilimumab, but failed to demonstrate an advantage compared to ipilimumab alone (20,63). In a phase I clinical trial, tremelimumab plus MART-1 peptide-pulsed DCs resulted in objective and durable tumor responses compared to each agent alone (64). A phase I trial in patients with pretreated advanced melanoma showed that autologous monocyte-derived DCs electroporated with synthetic mRNA (TriMixDC-MEL) are immunogenic and have antitumor activity (65). TriMixDC-MEL combined with ipilimumab has shown 38% of durable tumor responses in a phase II trial (66). The tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine uses yeast cell wall particles to load tumor lysate into autologous DCs. The phase IIb trial of TLPLDC vs. placebo in resected stage III/IV patients showed an increased 24-month DFS. The trial showed also a potential synergistic effect of TLPLDC plus ipilimumab to be confirmed in a phase III study evaluating adjuvant TLPLDC plus ipilimumab versus ipilimumab alone in resected stage IV patients (67).
Adoptive T cell transfer
The presence of tumor-reactive T cells has been associated with the success of ICIs (68,69). When patients do not have functional tumor-antigen-specific T cells with high-affinity T cell receptors (TCRs), T cell therapies can transfer such T cell populations by either expanding pre-existing anti-tumor T cells or by using gene-therapy to alter T cells to become melanoma-specific with a high-affinity TCR (70). ACT of autologous tumor infiltrating lymphocytes with high-dose IL-2 was the first clinical trial to show that tumor-reactive T cells could mediate melanoma regression (71). However, TIL has not been approved as anti-cancer treatment yet due to lack of results from sufficiently powered prospective randomized clinical trials (13,72). In order to improve clinical benefit and survival, TIL therapy could be combined with other immunotherapies (70). ACT through chimeric antigen receptor (CAR) T-cell therapy has been successful in hematological malignancies. However, less response was seen in the treatment of solid tumors such as melanoma (73). Combination of CAR-T therapy and immune checkpoint blockade, targeted therapy might induce desired clinical responses (73). The adoptive transfer of autologous T cells transduced with a retrovirus encoding a TCR against an HLA-A*0201 restricted NY-ESO-1 epitope is a potentially effective treatment for some refractory metastatic melanoma patients (74).
7. Combination of immune checkpoint inhibitors and targeted therapy
Targeted therapy and ICIs have radically changed the management of different tumor types, including advanced stage melanoma (75). However, both approaches have limitations, including limited duration of response with targeted therapy and low overall response rate without clear predictive biomarkers in patients treated with ICIs. Therefore, great interest has been shown on the possibility of combination strategies that could take advantage from the high response rate of targeted therapy with the long-term disease control of ICIs. Despite some contrasting preclinical results that have been observed on the association of BRAF and MEK inhibitors with immunotherapy, various trials are ongoing investigating the association of MAPK inhibitors with ICIs and other immunotherapy strategies (76). Several trials with CTLA-4 inhibitors and MAPK inhibitors have raised concerns due to the toxicities associated with the combinations that led to early trial termination (77,78). More tolerable and with good disease control rates seem to be the association of PD-1/PD-L1 axis inhibitors with BRAF and MEK inhibitors (79). In this context, study design is fundamental to properly conduct clinical trial of combination strategies without increasing toxicities. The ongoing phase III clinical trial DREAMseq (NCT02224781) studies how well initial treatment works with ipilimumab and nivolumab followed by dabrafenib and trametinib, and compares it to initial treatment with dabrafenib and trametinib followed by ipilimumab and nivolumab in treating patients with stage III-IV BRAF V600 melanoma.
8. Host microbiota and response to immunotherapy
The improvement of clinical efficacy of immunotherapy strategies is a central goal of translational and clinical research in immunooncology. In recent years, several factors have been shown to influence the immune response during ICI treatment (80-83). In this context, the human microbiota seems to be an important modulator of the immune system response in different physiopathological conditions, including cancer development and response to anti-cancer treatment (84). In a melanoma mouse model, different commensal gut microbiota composition was associated with difference in melanoma growth and spontaneous antitumor immunity which were eliminated upon cohousing or after fecal transfer (85). In a RET melanoma mouse model with dysbiosis induced by antibiotic treatment, gut colonization with Akkermansia muciniphila restored responsiveness to PD-1 blockade compared to control and increased the recruitment of CD4+ T lymphocytes into mouse tumor beds (86). In 112 melanoma patients treated with anti-PD-1 mAbs, significant differences were observed in the gut microbiome of responders versus non-responders. Analysis of patient fecal microbiome samples showed significantly higher species diversity and relative abundance of the Ruminococcaceae family in responding patients. Moreover, mice receiving fecal microbiota transplantation (FMT) with stool from responders to PD-1 inhibitors also exhibited improved response to anti-PD-L1 therapy compared to mice transplanted with stool from melanoma patients that did not respond to anti-PD-L1 therapy (87). Several preclinical and clinical works show the relevance of the modulation of the gut microbiota in ICIs efficacy (88-91). Taken together these data sustain potential use of the human microbiome as a predictive biomarker of response to ICIs. Moreover, the modulation of specific component of the human microbiome by FMT or oral supplementation through probiotics may improve response rates and other clinical endpoints of ICIs therapy in cancer patients. Prospective randomized clinical trials are awaited to better understand the impact of such approaches.
9. Immunotherapy biomarkers
Targeted therapy derives its efficacy from the presence of a specific tumor feature, such as the BRAF V600 mutation, that drives tumor growth and that represent a specific biomarker of response to the drug targeting the aberrant pathway. In melanoma, primary and secondary resistance to targeted therapy are challenging problems and several studies have tried to improve BRAF V600 detection of prognostic and predictive markers (92-94). Despite great efforts, melanoma immunotherapy and especially ICIs, which are already approved for clinical use, lack biomarker response. This is especially urgent due to the relatively low response rate of immunotherapy. Regarding inhibitors of PD-1/PD-L1 axis, PD-L1 expression assessed by immunohistochemistry (IHC) staining has been used as biomarkers in several clinical trials. Different PD-L1 IHC antibodies with non-homogeneous cut-off values among studies have generated contrasting results on the role of PD-L1 for patient stratification (26,31,36). Although PD-L1 status is not currently considered a valid stratification marker, it warrants further analysis since it may indicate underlying biological insights (95). More comprehensive models are under investigation to better characterize the tumor microenvironment and define predictive biomarkers for immunotherapy. In this context an important study by Tumeh and collaborators (96) has shown that the presence of CD8+ T cells that cause upregulation of PD-L1 expression on melanoma cells at the invasive tumor margin might more clearly explain primary resistance or responsiveness to anti-PD-1 mAbs. Gene-expression profile has been suggested as a marker of response (97). IFNγ secreted by CD8+ T cells is the principal mediator of intratumoural antitumor inflammation, a gene expression profile termed 'T-cell-inflamed tumor' has been associated with response to diverse immunotherapies including IL-2, vaccines, ICIs and cancer vaccine (98,99). Moreover, primary and secondary resistance to PD-1/PD-L1 pathway inhibitors is associated with a low IFNγ gene expression signature that can be mediated by activation of PTEN and WNT/β-catenin pathway, impairment of JAK2 signaling or alteration of antigen presentation through structural or functional impairment of MHC class I mediated antigen presentation (100-103). A study with the largest whole exome sequencing and transcriptome sequencing analysis of tumor material from patients with metastatic melanoma receiving immune-checkpoint inhibitors has been published (104). The study supports the correlations between baseline immune infiltrate and treatment response, but also show inconsistent associations of tumor mutational burden, and prove that multiple novel genomic and transcriptomic features predict selective response, including features associated with MHC-I and MHC-II antigen presentation (104). Moreover, the authors constructed predictive models integrating clinical, genomic and transcriptomic characteristics to identify patients with melanoma with intrinsic resistance to anti-PD1 mAb (104). Recently, a growing body of evidence is trying to correlate ICI efficacy to the expression levels of PD-L1 detected in circulating tumor DNA (105,106). Finally, studies have proposed the analysis of matrix metalloproteinase (MMPs), known to be strictly involved in melanoma growth (107,108), as good indicator of response to immunotherapy. Moogk and colleagues (109) showed an inverse association between anti-tumor T-cell response and MMP-23 expression in primary melanoma tumors treated with adjuvant immunotherapy. The authors concluded that MMP-23 expression is associated with shorter periods of PFS and therefore may represent a potential therapeutic target in melanoma, as well as a possible biomarker for evaluating response of melanoma patients to immunotherapies.
10. Conclusions
The number of effective treatments for patients with metastatic melanoma have increased rapidly in recent few years. In this scenario, immunotherapy combinations and immunotherapy plus targeted therapy hold great expectation. Moreover, results from the clinical trial DREAMseq are eagerly awaited and will shed light on the best sequential treatment option in metastatic melanoma patients with BRAF V600E. Given the complexity of the antitumor immune response, the identification of biomarkers of response and the mechanisms associated with primary and secondary resistance are of utmost importance. These insights will allow the next generation of immunotherapy approaches that will be tailored on specific melanoma genomic features and its tumor microenvironment characteristics.
Acknowledgments
Not applicable.
Funding
This study was partially supported by the Italian League against Cancer (LILT).
Availability of data and materials
Not applicable
Authors' contributions
GCL conceived the work, performed bibliographic research and wrote the manuscript, SC prepared the figures and provided critical revisions, LF performed bibliographic research and provided critical revisions, DAS, ML contributed to the conception of the work and provided critical revisions. All authors agreed on the final version of the manuscript.
Ethics approval and consent to participate
Not applicable
Patient consent for publication
Not applicable
Competing interest
GCL, SC, LF, ML no competing interest to declare, DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Leonardi GC, Falzone L, Salemi R, Zanghì A, Spandidos DA, McCubrey JA, Candido S, Libra M. Cutaneous melanoma: From pathogenesis to therapy (Review) Int J Oncol. 2018;52:1071–1080. doi: 10.3892/ijo.2018.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Candido S, Rapisarda V, Marconi A, Malaponte G, Bevelacqua V, Gangemi P, Scalisi A, McCubrey JA, Maestro R, Spandidos DA, et al. Analysis of the B-RafV600E mutation in cutaneous melanoma patients with occupational sun exposure. Oncol Rep. 2014;31:1079–1082. doi: 10.3892/or.2014.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawakami Y, Rosenberg SA. T-cell recognition of self peptides as tumor rejection antigens. Immunol Res. 1996;15:179–190. doi: 10.1007/BF02918248. [DOI] [PubMed] [Google Scholar]
- 5.Faramarzi S, Ghafouri-Fard S. Melanoma: A prototype of cancer-testis antigen-expressing malignancies. Immunotherapy. 2017;9:1103–1113. doi: 10.2217/imt-2017-0091. [DOI] [PubMed] [Google Scholar]
- 6.Lee N, Zakka LR, Mihm MC, Jr, Schatton T. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology. 2016;48:177–187. doi: 10.1016/j.pathol.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, Rao U. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: Results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 8.Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29:241–252. doi: 10.1016/S0305-7372(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 9.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: Long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–S14. [PubMed] [Google Scholar]
- 10.Rosenberg SA. IL-2: The first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: Are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohaan MW, van den Berg JH, Kvistborg P, Haanen JBAG. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: A viable treatment option. J Immunother Cancer. 2018;6:102. doi: 10.1186/s40425-018-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, Kluger H, Sznol M, Carvajal R, Lawrence D, Atkins M, Powderly J, Sharfman W, Puzanov I, et al. Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. Cancer Res. 2016;76:CT001. [Google Scholar]
- 16.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 17.Christofi T, Baritaki S, Falzone L, Libra M, Zaravinos A. Current perspectives in cancer immunotherapy. Cancers (Basel) 2019;11:1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 19.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weide B, Martens A, Wistuba-Hamprecht K, Zelba H, Maier L, Lipp HP, Klumpp BD, Soffel D, Eigentler TK, Garbe C. Combined treatment with ipilimumab and intratumoral interleukin-2 in pretreated patients with stage IV melanoma-safety and efficacy in a phase II study. Cancer Immunol Immunother. 2017;66:441–449. doi: 10.1007/s00262-016-1944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brohl AS, Khushalani NI, Eroglu Z, Markowitz J, Thapa R, Chen YA, Kudchadkar R, Weber JS. A phase IB study of ipilimumab with peginterferon alfa-2b in patients with unre-sectable melanoma. J Immunother Cancer. 2016;4:85. doi: 10.1186/s40425-016-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. KEYNOTE-006 investigators Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 27.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 28.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 29.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582–588. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–1251. doi: 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 31.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 32.Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, Di Giacomo AM, Mortier L, Hassel JC, Rutkowski P, McNeil C, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: Three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019;5:187–194. doi: 10.1001/jamaoncol.2018.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss SA, Wolchok JD, Sznol M. Immunotherapy of melanoma: Facts and hopes. Clin Cancer Res. 2019;25:5191–5201. doi: 10.1158/1078-0432.CCR-18-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, et al. CheckMate 238 Collaborators Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 35.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 36.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 38.Kirchberger MC, Hauschild A, Schuler G, Heinzerling L. Combined low-dose ipilimumab and pembrolizumab after sequential ipilimumab and pembrolizumab failure in advanced melanoma. Eur J Cancer. 2016;65:182–184. doi: 10.1016/j.ejca.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Long GV, Atkinson V, Cebon JS, Jameson MB, Fitzharris BM, McNeil CM, Hill AG, Ribas A, Atkins MB, Thompson JA, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): An open-label, phase 1b trial. Lancet Oncol. 2017;18:1202–1210. doi: 10.1016/S1470-2045(17)30428-X. [DOI] [PubMed] [Google Scholar]
- 40.Zimmer L, Apuri S, Eroglu Z, Kottschade LA, Forschner A, Gutzmer R, Schlaak M, Heinzerling L, Krackhardt AM, Loquai C, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47–55. doi: 10.1016/j.ejca.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Long GV, Robert C, Blank C, Ribas A, Mortier L, Schachter J, Middleton MR, et al. Outcomes in patients treated with ipilimumab after pembrolizumab in KEYNOTE-006. Eur J Cancer. 2017;72:S128–S129. doi: 10.1016/S0959-8049(17)30500-2. [DOI] [Google Scholar]
- 42.Ascierto PA, Butterfield LH, Demaria S, Ferris RL, Freeman GJ, Lo RS, Mantovani A, Nathan P, Hamid O, Politi K, et al. The great debate at 'Immunotherapy Bridge 2018', Naples, November 29th, 2018. J Immunother Cancer. 2019;7:221. doi: 10.1186/s40425-019-0683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer RA, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 45.Conry RM, Westbrook B, McKee S, Norwood TG. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum Vaccin Immunother. 2018;14:839–846. doi: 10.1080/21645515.2017.1412896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 47.Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36:1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribas A, Dummer R, Puzanov I, Vander Walde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174:1031–1032. doi: 10.1016/j.cell.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 49.Durham NM, Nirschl CJ, Jackson CM, Elias J, Kochel CM, Anders RA, Drake CG. Lymphocyte activation gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PLoS One. 2014;9:e109080. doi: 10.1371/journal.pone.0109080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ascierto PA, Bono P, Bhatia S, Melero I, Nyakas MS, Svane I, Larkin J, Gomez-Roca C, Schadendorf D, Dummer R, et al. LBA18Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti-PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Ann Oncol. 2017;28(Suppl 5):v605–v649. doi: 10.1093/annonc/mdx440.011. [DOI] [Google Scholar]
- 53.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricciuti B, Leonardi GC, Puccetti P, Fallarino F, Bianconi V, Sahebkar A, Baglivo S, Chiari R, Pirro M. Targeting indoleamine-2,3-dioxygenase in cancer: Scientific rationale and clinical evidence. Pharmacol Ther. 2019;196:105–116. doi: 10.1016/j.pharmthera.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, Arance A, Carlino MS, Grob JJ, Kim TM, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–1097. doi: 10.1016/S1470-2045(19)30274-8. [DOI] [PubMed] [Google Scholar]
- 56.Nicholas C, Lesinski GB. Immunomodulatory cytokines as therapeutic agents for melanoma. Immunotherapy. 2011;3:673–690. doi: 10.2217/imt.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, Sheng D, Liu X, Sims PW, VanderVeen LA, et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin Cancer Res. 2016;22:680–690. doi: 10.1158/1078-0432.CCR-15-1631. [DOI] [PubMed] [Google Scholar]
- 58.Diab A, Haymaker C, Bernatchez C, Andtbacka RHI, Shaheen M, Johnson D, Markowitz J, Puzanov I, Murthy R, Johnson DH, et al. Intratumoral (it) injection of the TLR9 agonist tilsotolimod (imo-2125) in combination with ipilimumab (ipi) triggers durable responses in pd-1 inhibitor refractory metastatic melanoma (rmm): results from a multicenter, phase 1/2 study. Ann Oncol. 2018;29(Suppl 8):viii442–viii466. doi: 10.1093/annonc/mdy289.001. [DOI] [Google Scholar]
- 59.Milhem MM, Long GV, Hoimes CJ, Amin A, Lao CD, Conry RM, Hunt J, Daniels GA, Almubarak M, Shaheen MF, et al. Phase 1b/2, open label, multicenter, study of the combination of SD-101 and pembrolizumab in patients with advanced melanoma who are naïve to anti-PD-1 therapy. J Clin Oncol. 2019;37(Suppl 15):9534–9534. doi: 10.1200/JCO.2019.37.15_suppl.9534. [DOI] [Google Scholar]
- 60.Wiehagen KR, Girgis NM, Yamada DH, Smith AA, Chan SR, Grewal IS, Quigley M, Verona RI. Combination of CD40 agonism and CSF-1R blockade reconditions tumor-associated macrophages and drives potent antitumor immunity. Cancer Immunol Res. 2017;5:1109–1121. doi: 10.1158/2326-6066.CIR-17-0258. [DOI] [PubMed] [Google Scholar]
- 61.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, Yan L, Targan S, Solomon J, Nichol G, et al. Extended dose ipilimumab with a peptide vaccine: Immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17:896–906. doi: 10.1158/1078-0432.CCR-10-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ribas A, Comin-Anduix B, Chmielowski B, Jalil J, de la Rocha P, McCannel TA, Ochoa MT, Seja E, Villanueva A, Oseguera DK, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15:6267–6276. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilgenhof S, Van Nuffel AM, Benteyn D, Corthals J, Aerts C, Heirman C, Van Riet I, Bonehill A, Thielemans K, Neyns B. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 2013;24:2686–2693. doi: 10.1093/annonc/mdt245. [DOI] [PubMed] [Google Scholar]
- 66.Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, Thielemans K, Neyns B. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. 2016;34:1330–1338. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 67.Chick RC, Faries MB, Hale DF, Kemp Bohan PM, Hickerson A, Vreelandc TJ, Myers JW, Cindass JL, Brown TA, et al. Multi-institutional, prospective, randomized, double-blind, placebo-controlled phase IIb trial of the tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine to prevent recurrence in high-risk melanoma patients: A subgroup analysis. J Clin Oncol. 2020;38(Suppl 5):63–63. doi: 10.1200/JCO.2020.38.5_suppl.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der Burg S, Kapiteijn E, Michielin O, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. [DOI] [PubMed] [Google Scholar]
- 69.Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest. 2016;126:3447–3452. doi: 10.1172/JCI87324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foley KC, Nishimura MI, Moore TV. Combination immunotherapies implementing adoptive T-cell transfer for advanced-stage melanoma. Melanoma Res. 2018;28:171–184. doi: 10.1097/CMR.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 72.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94:S3–S9. doi: 10.1002/ajh.25418. [DOI] [PubMed] [Google Scholar]
- 74.Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: Long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pelster MS, Amaria RN. Combined targeted therapy and immunotherapy in melanoma: A review of the impact on the tumor microenvironment and outcomes of early clinical trials. Ther Adv Med Oncol. 2019;11:1758835919830826. doi: 10.1177/1758835919830826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 78.Minor DR, Puzanov I, Callahan MK, Hug BA, Hoos A. Severe gastrointestinal toxicity with administration of trametinib in combination with dabrafenib and ipilimumab. Pigment Cell Melanoma Res. 2015;28:611–612. doi: 10.1111/pcmr.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ribas A, Butler M, Lutzky J, Lawrence DP, Robert C, Miller W, Linette WMGP, Ascierto PA, Kuzel T, Algazi AP, et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol. 2015;33(Suppl 15):3003. doi: 10.1200/jco.2015.33.15_suppl.3003. [DOI] [Google Scholar]
- 80.Shui L, Yang X, Li J, Yi C, Sun Q, Zhu H. Gut microbiome as a potential factor for modulating resistance to cancer immuno-therapy. Front Immunol. 2020;10:2989. doi: 10.3389/fimmu.2019.02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Longo V, Brunetti O, Azzariti A, Galetta D, Nardulli P, Leonetti F, Silvestris N. Strategies to improve cancer immune checkpoint inhibitors efficacy, other than abscopal effect: A systematic review. Cancers (Basel) 2019;11:539. doi: 10.3390/cancers11040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Velez MA, Burns TF, Stabile LP. The estrogen pathway as a modulator of response to immunotherapy. Immunotherapy. 2019;11:1161–1176. doi: 10.2217/imt-2019-0024. [DOI] [PubMed] [Google Scholar]
- 83.Matsushita M, Kawaguchi M. Immunomodulatory Effects of Drugs for Effective Cancer Immunotherapy. J Oncol. 2018;2018:8653489. doi: 10.1155/2018/8653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vivarelli S, Falzone L, Basile MS, Nicolosi D, Genovese C, Libra M, Salmeri M. Benefits of using probiotics as adjuvants in anticancer therapy (Review) World A Sci J. 2019;1:125–135. [Google Scholar]
- 85.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 87.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banna GL, Torino F, Marletta F, Santagati M, Salemi R, Cannarozzo E, Falzone L, Ferraù F, Libra M. Lactobacillus rhamnosus GG: An overview to explore the rationale of its use in cancer. Front Pharmacol. 2017;8:603. doi: 10.3389/fphar.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elkrief A, Derosa L, Kroemer G, Zitvogel L, Routy B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: A new independent prognostic factor? Ann Oncol. 2019;30:1572–1579. doi: 10.1093/annonc/mdz206. [DOI] [PubMed] [Google Scholar]
- 92.Eskiocak B, McMillan EA, Mendiratta S, Kollipara RK, Zhang H, Humphries CG, Wang C, Garcia-Rodriguez J, Ding M, Zaman A, et al. Biomarker accessible and chemically addressable mechanistic subtypes of BRAF melanoma. Cancer Discov. 2017;7:832–851. doi: 10.1158/2159-8290.CD-16-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salemi R, Falzone L, Madonna G, Polesel J, Cinà D, Mallardo D, Ascierto PA, Libra M, Candido S. MMP-9 as a candidate marker of response to BRAF inhibitors in melanoma patients with BRAFV600E mutation detected in circulating-free DNA. Front Pharmacol. 2018;9:856. doi: 10.3389/fphar.2018.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guarneri C, Bevelacqua V, Polesel J, Falzone L, Cannavò PS, Spandidos DA, Malaponte G, Libra M. NFκB inhibition is associated with OPN/MMP 9 downregulation in cutaneous melanoma. Oncol Rep. 2017;37:737–746. doi: 10.3892/or.2017.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 96.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: A potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 98.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019–1031. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 101.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations associated with acquired resistance to PD 1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, et al. Primary resistance to PD 1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7:188–201. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu D, Schilling B, Liu D, Sucker A, Livingstone E, Jerby-Arnon L, Zimmer L, Gutzmer R, Satzger I, Loquai C, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. 2019;25:1916–1927. doi: 10.1038/s41591-019-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tuaeva NO, Falzone L, Porozov YB, Nosyrev AE, Trukhan VM, Kovatsi L, Spandidos DA, Drakoulis N, Kalogeraki A, Mamoulakis C, et al. Translational application of circulating DNA in oncology: review of the last decades achievements. Cells. 2019;8:1251. doi: 10.3390/cells8101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee EY, Kulkarni RP. Circulating biomarkers predictive of tumor response to cancer immunotherapy. Expert Rev Mol Diagn. 2019;19:895–904. doi: 10.1080/14737159.2019.1659728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Napoli S, Scuderi C, Gattuso G, Bella VD, Candido S, Basile MS, Libra M, Falzone L. Functional roles of matrix metallopro-teinases and their inhibitors in melanoma. Cells. 2020;9:E1151. doi: 10.3390/cells9051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Falzone L, Salemi R, Travali S, Scalisi A, McCubrey JA, Candido S, Libra M. MMP-9 overexpression is associated with intragenic hypermethylation of MMP9 gene in melanoma. Aging (Albany NY) 2016;8:933–944. doi: 10.18632/aging.100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moogk D, da Silva IP, Ma MW, Friedman EB, de Miera EV, Darvishian F, Scanlon P, Perez-Garcia A, Pavlick AC, Bhardwaj N, et al. Melanoma expression of matrix metallopro-teinase-23 is associated with blunted tumor immunity and poor responses to immunotherapy. J Transl Med. 2014;12:342. doi: 10.1186/s12967-014-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable