Introduction
Behçet's disease (BD) is a variable vessel vasculitis.1 It affects both the arterial and venous systems.1 BD is mainly characterized by mucocutaneous, articular, ocular, gastrointestinal, and neurologic involvement.2 Vascular involvement is present in up to 40% of BD patients.3,4 It is a significant cause of morbidity and mortality. Vascular involvement occurs primarily in the form of lower extremity deep venous thrombosis (DVT).3 Although this process is thought to be caused by thromboinflammation, the complex crosstalk between inflammation and thrombosis has not been completely elucidated.
Thrombosis is defined as the formation of a clot in a blood vessel.5 It is worth mentioning the Virchow's triad here to understand the main contributors to thrombosis. Virchow's triad consists of three components as endothelial damage, stasis of blood flow, and hypercoagulability.5 In BD, endothelial damage could result from neutrophilic inflammation in the vessel wall (vasculitis) and subsequent recruitment of platelets (discussed below). Recruitment of both neutrophils and platelets could cause a stasis in the blood flow contributing to the formation of thrombosis further. Regarding hypercoagulability, different parameters such as endothelial factors (like von Willebrand factor, vascular endothelial growth factor, or endothelin 1), procoagulant factors (like factor V Leiden or prothrombin mutations), or anticoagulant factors (like protein C and S) were studied in BD.6 However, the data about these were inconsistent and not uniformly confirmed. The results of the recent studies suggest a dysregulation in the fibrinolysis phase contributing to hypercoagulability (discussed below).7,8
Venous inflammation and neutrophil hyperfunction are the hallmarks of BD pathogenesis.9,10,11 Veins are a primary location for inflammation in BD patients, although the etiology of venous predilection remains unknown. Both Seyahi et al.11 and Alibaz-Oner et al.9 demonstrated that vein wall thickness increased among BD patients, even in the absence of evident vascular involvement. As the authors of these studies mentioned, this increased thickness could result from vascular inflammation and could be the precursor for venous thrombosis.9,11
Neutrophil hyperactivation is a central process in BD-related inflammation. The hyperactivated neutrophils produce reactive oxygen species (ROS).8,12 ROS production at the site of inflammation (especially veins in BD) causes endothelial dysfunction and tissue injury.8 Besides, the generation of ROS is one of the main molecular pathways leading to NETosis.13 NETosis is a programmed form of neutrophil necrosis.14 During NETosis, neutrophils die by excreting fibrillary networks called neutrophil extracellular traps (NETs) which contain host nuclear material and neutrophilic granule proteins.14 The structure of NETs differs between different diseases but there are some markers that could be used to follow NETosis in BD such as peptidylarginine deiminase 4 (PAD4).15 PAD4 promotes a key event in NETosis which is citrullination of arginine residues on chromatin.16 Neutrophils from BD patients were shown to be more prone to NETosis even in the absence of stimuli in vitro, and the amount of NETs was higher in BD patients with vascular involvement compared to the ones without vascular involvement.10 In their in-vitro model of thrombin generation, Le Joncour et al.10 have shown that platelet-thrombin-NETs axis promotes intravascular coagulation and vascular dysfunction. In addition to promoting the thrombin generation, NETs also cause endothelial dysfunction via decreasing cell proliferation and increasing apoptosis.15 Moreover, data from previous studies showed that NET-associated thrombi could be located in both veins and arteries.17,18,19
The velocity of thrombin generation has also been shown to increase in BD patients.10,20 Le Joncour et al.10 have shown that the velocity and peak of thrombin generation are positively correlated with NET markers in serum, and these are decreased with the addition of a NET-disrupting enzyme, DNAase, to the medium. In other diseases such as sepsis, NETs are also key triggers of thrombus.21 Thromboinflammation in sepsis is a more studied subject, and we can understand the complex crosstalk between inflammatory cells and platelets using the data from sepsis. In sepsis, microorganism itself and the associated inflammation activate platelets through released NETs and cytokines. The activated platelets degranulate and release proinflammatory cytokines promoting the inflammation further. In the same lines, Maugeri et al.22 demonstrated that activated platelets presented HMGB1 (high mobility group box 1) to neutrophils and induced autophagy and NET generation in neutrophils. HMGB1 is a critical molecule for this interaction since HMGB1 (-/-) platelets failed to induce NETosis.
Moreover, platelets form aggregates that adhere to the endothelium and cause damage. Both NETs and aggregated platelets trigger the thrombosis cascade in the presence of endothelial damage.21 These data also underline the role of platelets in thromboinflammation.
Another possibly affected process in BD is fibrinogen carbonylation. Fibrinogen carbonylation significantly affects its polymerization and causes resistance to plasmin-induced lysis.7,8 Enhanced fibrinogen carbonylation has been reported in BD patients.7 The results of the study by Becatti et al.7 suggest that fibrinogen carbonylation is primarily associated with neutrophil-derived ROS rather than lymphocyte- or monocyte-derived ROS. ROS-induced fibrinogen carbonylation may partially explain the reason why thrombosis is more responsive to immunosuppressive treatment rather than anti-thrombotics alone.
Scientific Hypothesis and Its Limitations
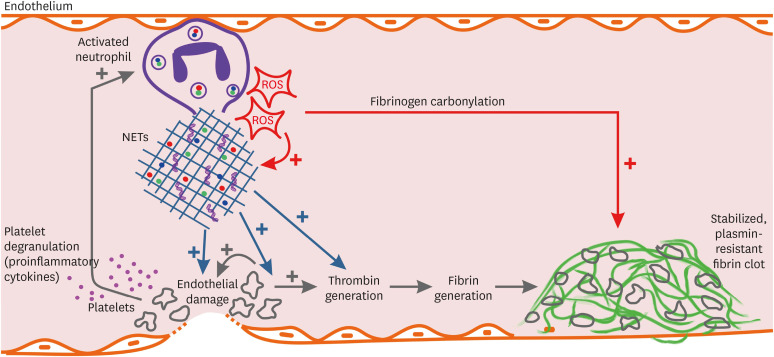
The primary problem in BD is venous inflammation, mainly mediated by neutrophils. These hyperactivated neutrophils produce ROS, which triggers NETosis at the site of inflammation (Fig. 1). NETosis promotes thrombin generation, causes endothelial damage, and activates platelets. Activated platelets degranulate, release proinflammatory cytokines, form aggregates and cause augmented inflammation and endothelial damage which in turn activates thrombosis. Moreover, ROS produced by neutrophils also causes fibrinogen carbonylation, which makes the thrombus more resistant to plasmin-induced lysis. This seems like a vicious circuit in which inflammation triggers thrombosis and vice versa. This hypothesis partially explains the thromboinflammation process in BD; however, there are still unknown parts. For instance, we know that NETosis is a main mechanism in some other diseases such as systemic lupus erythematosus and ANCA-associated vasculitis (AAV). However, thrombotic complications are not the primary aspect of the disease phenotype in SLE or AAV. One of the reasons for this difference is that BD is primarily affecting veins. But the actual reason for predominant venous inflammation in BD remains unknown.
Fig. 1. Schematic representation of thromboinflammation in Behçet's disease. Activated neutrophils produce ROS, which induces NETosis. NETs cause endothelial damage, activate platelets, and induce thrombin generation. Activated platelet degranulate and release pro-inflammatory cytokines that stimulate neutrophils further. Platelet aggregation also damages endothelium and induces thrombin generation, which finally leads to the formation of the fibrin clot. Besides, ROS produced by neutrophils modifies fibrinogen, which makes the fibrin clot more resistant to plasmin-induced lysis.
ROS = reactive oxygen species, NETs = neutrophil extracellular traps.
This hypothesis also underscores the significance of colchicine in treatment/prevention of thrombosis in BD. Colchicine is one of the oldest anti-inflammatory drugs and its main mechanism of action is preventing microtubule elongation by binding to tubulin monomers.23,24 Thus, it interferes with both neutrophil chemotaxis and NETosis.25 Safi et al.15 have recently demonstrated that NETs formation in BD neutrophils was inhibited by colchicine. In addition, in vitro studies have shown that colchicine decreased expression of surface markers of platelet activity and inhibited leukocyte-platelet aggregation.26,27 Previously, in several case studies, its efficacy was reported in treatment of thrombus in BD.28,29,30
How to Test This Hypothesis
A prospective and longitudinal study could be designed to test this hypothesis. BD patients without vascular involvement could be followed up prospectively. ROS, NET markers (such as PAD4), and fibrinogen carbonylation could be measured at different time points where thrombosis is screened as well. In addition, platelet-neutrophil crosstalk could be analyzed by evaluating the expression of HMGB1 molecule. This type of study will give the possibility to evaluate the dynamic change of these markers in relation to the onset of thrombosis. Targeting ROS or NETs in BD treatment could be another strategy to approve the main point of the presented hypothesis. We know that colchicine, anti-TNF (tumor necrosis factor), and anti-interleukin 6 drugs, which have been already used in BD treatment, cause a decrease in NET formation.10 Potential other medicines such as N-acetyl-cysteine, PAD4 inhibitor, or DNase I that directly target circulating NETs could also be tried.31,32,33 However, the efficacy and safety data for novel NET inhibitors are deficient. Thus, we should be cautious while determining new therapy approaches that seem to target pathogenesis in BD.
Ethical Implications
Suggested thromboinflammation mechanisms include complex interactions between inflammatory and thrombotic mediators and cells. Thus, it is difficult to estimate the output of targeting one player (e.g., NETosis) in BD treatment. At first, it would be better to analyze the dynamic changes of all these parameters in relation to thrombosis (laboratory and clinical) without any novel intervention except the conventional treatment of BD. Colchicine, included in the conventional treatment of BD, could be a start point for testing the hypothesis safely. Comparing the thrombosis parameters and NET markers longitudinally in BD patients treated with colchicine and without colchicine could provide significant clues to the pathogenesis. We should also keep in mind that other factors have the potential to contribute to the thrombosis process. For instance, testosterone could activate neutrophils, which could be a reason for the more severe disease course in males compared to females.34 Testosterone might also affect the occurrence of thrombosis. These should be taken into account while designing studies.
Conclusion
Behçet's disease is a complex autoinflammatory disease and thrombosis is a significant aspect of the disease. Neutrophilic venous inflammation is the probable primary mechanism in BD. Hyperactive neutrophils in vessels produce ROS and NETs that augment inflammation, cause endothelial damage, and trigger thrombosis cascade. Platelets are recruited to the site of damage, degranulate, and aggregate, which further induces inflammation and thrombosis. The produced thrombus also gets more resistant to plasmin-induced lysis through fibrinogen carbonylation with ROS produced by hyperactive neutrophils. Although our current knowledge sheds light on some parts of this complex mechanism, there are still missing pieces in this puzzle.
Footnotes
Disclosure: The author has no potential conflicts of interest to disclose.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Batu ED. Diagnostic/classification criteria in pediatric Behçet's disease. Rheumatol Int. 2019;39(1):37–46. doi: 10.1007/s00296-018-4208-9. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Carballeira M, Solans R, Larrañaga JR, García-Hernández FJ, Rios-Fernández R, Nieto J, et al. Venous thrombosis and relapses in patients with Behçet's disease. Descriptive analysis from Spanish network of Behçet's disease (REGEB cohort) Clin Exp Rheumatol. 2018;36(6) Suppl 115:40–44. [PubMed] [Google Scholar]
- 4.Yazici H, Seyahi E, Hatemi G, Yazici Y. Behçet syndrome: a contemporary view. Nat Rev Rheumatol. 2018;14(2):119. doi: 10.1038/nrrheum.2018.3. [DOI] [PubMed] [Google Scholar]
- 5.Kushner A, West DW, Pillarisetty LS. Virchow Triad. Treasure Island, FL: StatPearls; 2020. [PubMed] [Google Scholar]
- 6.La Regina M, Gasparyan AY, Orlandini F, Prisco D. Behcet's disease as a model of venous thrombosis. Open Cardiovasc Med J. 2010;4(1):71–77. doi: 10.2174/1874192401004020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becatti M, Emmi G, Silvestri E, Bruschi G, Ciucciarelli L, Squatrito D, et al. Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behçet disease. Circulation. 2016;133(3):302–311. doi: 10.1161/CIRCULATIONAHA.115.017738. [DOI] [PubMed] [Google Scholar]
- 8.Emmi G, Becatti M, Bettiol A, Hatemi G, Prisco D, Fiorillo C. Behçet's syndrome as a model of thrombo-inflammation: the role of neutrophils. Front Immunol. 2019;10:1085. doi: 10.3389/fimmu.2019.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alibaz-Oner F, Ergelen R, Mutis A, Erturk Z, Asadov R, Mumcu G, et al. Venous vessel wall thickness in lower extremity is increased in male patients with Behcet's disease. Clin Rheumatol. 2019;38(5):1447–1451. doi: 10.1007/s10067-019-04470-z. [DOI] [PubMed] [Google Scholar]
- 10.Le Joncour A, Martos R, Loyau S, Lelay N, Dossier A, Cazes A, et al. Critical role of neutrophil extracellular traps (NETs) in patients with Behcet's disease. Ann Rheum Dis. 2019;78(9):1274–1282. doi: 10.1136/annrheumdis-2018-214335. [DOI] [PubMed] [Google Scholar]
- 11.Seyahi E, Gjoni M, Durmaz EŞ, Akbaş S, Sut N, Dikici AS, et al. Increased vein wall thickness in Behçet disease. J Vasc Surg Venous Lymphat Disord. 2019;7(5):677–684.e2. doi: 10.1016/j.jvsv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 13.Sørensen OE, Borregaard N. Neutrophil extracellular traps - the dark side of neutrophils. J Clin Invest. 2016;126(5):1612–1620. doi: 10.1172/JCI84538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkon KB. Review: Cell death, nucleic acids, and immunity: inflammation beyond the grave. Arthritis Rheumatol. 2018;70(6):805–816. doi: 10.1002/art.40452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safi R, Kallas R, Bardawil T, Mehanna CJ, Abbas O, Hamam R, et al. Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behçet's disease. J Dermatol Sci. 2018;92(2):143–150. doi: 10.1016/j.jdermsci.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducroux C, Di Meglio L, Loyau S, Delbosc S, Boisseau W, Deschildre C, et al. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke. 2018;49(3):754–757. doi: 10.1161/STROKEAHA.117.019896. [DOI] [PubMed] [Google Scholar]
- 18.Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82(2):223–232. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 19.Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenböck A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015;116(7):1182–1192. doi: 10.1161/CIRCRESAHA.116.304944. [DOI] [PubMed] [Google Scholar]
- 20.Mejía JC, Espinosa G, Tàssies D, Reverter JC, Cervera R. Endogenous thrombin potential in Behçet's disease: relationship with thrombosis and anticoagulant therapy. Clin Exp Rheumatol. 2014;32(4) Suppl 84:S67–71. [PubMed] [Google Scholar]
- 21.Guo L, Rondina MT. The era of thromboinflammation: Platelets are dynamic sensors and effector cells during infectious diseases. Front Immunol. 2019;10:2204. doi: 10.3389/fimmu.2019.02204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maugeri N, Campana L, Gavina M, Covino C, De Metrio M, Panciroli C, et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost. 2014;12(12):2074–2088. doi: 10.1111/jth.12710. [DOI] [PubMed] [Google Scholar]
- 23.Gasparyan AY, Ayvazyan L, Yessirkepov M, Kitas GD. Colchicine as an anti-inflammatory and cardioprotective agent. Expert Opin Drug Metab Toxicol. 2015;11(11):1781–1794. doi: 10.1517/17425255.2015.1076391. [DOI] [PubMed] [Google Scholar]
- 24.Batu ED, Arici ZS, Bilginer Y, Özen S. Current therapeutic options for managing familial Mediterranean fever. Expert Opin Orphan Drugs. 2015;3(9):1063–1073. [Google Scholar]
- 25.Angelidis C, Kotsialou Z, Kossyvakis C, Vrettou AR, Zacharoulis A, Kolokathis F, et al. Colchicine pharmacokinetics and mechanism of action. Curr Pharm Des. 2018;24(6):659–663. doi: 10.2174/1381612824666180123110042. [DOI] [PubMed] [Google Scholar]
- 26.Reddel CJ, Pennings GJ, Curnow JL, Chen VM, Kritharides L. Procoagulant effects of low-level platelet activation and its inhibition by colchicine. Thromb Haemost. 2018;118(4):723–733. doi: 10.1055/s-0038-1636915. [DOI] [PubMed] [Google Scholar]
- 27.Shah B, Allen N, Harchandani B, Pillinger M, Katz S, Sedlis SP, et al. Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: a pilot study in healthy subjects. Inflammation. 2016;39(1):182–189. doi: 10.1007/s10753-015-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krumb E, Lefebvre C, Peeters A, Hermans C. Cerebral venous thrombosis revealing Behçet's disease in a Moroccan patient: a case report and literature review. SAGE Open Med Case Rep. 2018;6:2050313X18767053. doi: 10.1177/2050313X18767053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nonaka D, Takase H, Machii M, Ohno K. Colchicine therapy for deep vein thrombosis in a patient with vascular-type Behçet disease: a case report. Medicine (Baltimore) 2020;99(16):e19814. doi: 10.1097/MD.0000000000019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yilmaz S, Cimen KA. Pulmonary artery aneurysms in Behçet's disease. Rheumatol Int. 2010;30(10):1401–1403. doi: 10.1007/s00296-009-1092-3. [DOI] [PubMed] [Google Scholar]
- 31.Garcia RJ, Francis L, Dawood M, Lai ZW, Faraone SV, Perl A. Attention deficit and hyperactivity disorder scores are elevated and respond to N-acetylcysteine treatment in patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65(5):1313–1318. doi: 10.1002/art.37893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64(9):2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, et al. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186(7):4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yavuz S, Akdeniz T, Hancer V, Bicakcigil M, Can M, Yanikkaya-Demirel G. Dual effects of testosterone in Behcet's disease: implications for a role in disease pathogenesis. Genes Immun. 2016;17(6):335–341. doi: 10.1038/gene.2016.28. [DOI] [PubMed] [Google Scholar]