Abstract
Background
As the survival rate of cancer patients increases, the clinical importance of rehabilitation provided by healthcare professionals also increases. However, the evidence supporting the relevance of rehabilitation programs is insufficient. This study aimed to review the literature on effectiveness in physical function, quality of life (QOL) or fatigue of supervised physical rehabilitation in patients with advanced cancer.
Methods
A systematic review and meta-analysis was conducted following the Cochrane guidelines. We narratively described the results when meta-analysis was not applicable or appropriate. Literature databases including Ovid-MEDLINE, Ovid-EMBASE, and the Cochrane Library, as well as several Korean domestic databases, were searched up to June 2017 for studies that investigated the effectiveness of supervised physical rehabilitation programs on physical function, QOL or fatigue in patients with advanced cancer. The quality of the selected studies was evaluated independently by paired reviewers.
Results
Eleven studies with 922 participants were finally selected among 2,459 articles. The meta-analysis revealed that after physical exercise, the physical activity level and strength of patients with advanced cancer increased significantly. The QOL showed a statistically significant improvement after physical rehabilitation according to the European Organization for Research and Treatment of Cancer version C30. Though some of measurements about cardiovascular endurance or strength in several studies were not able to be synthesized, each study reported that they were significantly improved after receiving rehabilitation.
Conclusion
Supervised physical rehabilitation for patients with advanced cancer is effective in improving physical activity, strength, and QOL. However, more trials are needed to prove the effectiveness of supervised exercise and to strengthen the evidence.
Keywords: Neoplasm, Rehabilitation, Exercise, Systematic review
Graphical Abstract
INTRODUCTION
With an increasing trend in the number of cancer survivors, increasingly more people experience considerable pain, fatigue, or physical disability, affecting their quality of life (QOL).1 These individuals experience impairments in daily life activities as a result of adverse effects or sequelae associated with treatment, or as they reach the terminal stage of their disease.2
The concept of “cancer rehabilitation” was developed in the 1970s to allow patients to achieve optimal physical, social, psychological, and vocational functioning within the limits imposed by their disease and its treatment.3 Cancer rehabilitation can be classified according to its purpose, components, and setting or according to the disease stage and can be provided by an interdisciplinary team of physicians, nurses, physical therapists, psychologists, and counselors to meet individual patient needs.4 Cancer rehabilitation has a role in patient-centered care as defined by the Institute of Medicine, and patient-centered care includes “providing care that is respectful of and responsive to individual patient preferences, needs, and values, and ensuring that patient values guide all clinical decisions,” with a focus on improving the physical function or QOL of individual patients with cancer.5
Several systematic reviews and guidelines have provided evidence of the effectiveness of physical rehabilitation for cancer patients. Exercise provides physiological and psychological benefits for cancer survivors during the rehabilitation period.6 Physical activity was reported to be associated with reduced body mass index and body weight, increased peak oxygen consumption and peak power output, and improved QOL in patients with cancer.7 Evidence-based clinical guidelines suggest that exercise has benefits for the QOL and physical fitness of patients with cancer, with no harmful effects.8
In many organized clinical settings, these rehabilitation programs are closely supervised by athletic trainers or physical therapists. Patients with advanced cancer experience a symptom cluster consisting of fatigue, pain, and anorexia, which should be controlled and improved through rehabilitation programs supervised by experts. Therefore, patients with advanced cancer are required to take supervised rehabilitation but it is not widely conducted in hospitals. Some studies have shown that the beneficial effects of exercise on physical function or QOL were more pronounced in the supervised setting than in the non-supervised setting.9,10 However, few studies have focused on patients with advanced cancer who need structured rehabilitation provided by experts. Cancer rehabilitation as a part of clinical management is still underutilized because of the low perception of its importance by oncologists or patients; reimbursement issues; lack of equipment, facilities, or experts; and the insufficient number or size of published trials.
To date, the clinical effectiveness of supervised physical rehabilitation in advanced cancer remains inconclusive. This systematic review was performed to ascertain the effects of supervised physical rehabilitation on the physical function of patients with advanced cancer. In addition, its effectiveness in improving QOL and fatigue was evaluated.
METHODS
Data sources and search strategy
We conducted a systematic search of articles up to June 2017 in literature databases including Ovid-MEDLINE, Ovid-EMBASE, and the Cochrane Library, as well as Korean database (KoreaMed, KMBASE and KISS). Extensive database searches using the terms including “neoplasm,” “physical therapy,” “exercise,” “exercise therapy,” and “supervised” were performed (Supplementary Table 1). We used a thesaurus according to each database, such as Medical Subject Headings (MeSH) for MEDLINE and EMTREE for EMBASE.
Selection criteria
Articles that met the following criteria were included: 1) included study subjects with advanced cancer within 2 years after cancer treatment; 2) investigated supervised exercise and usual care or no-intervention, respectively; 3) reported at least one predetermined outcome; 4) designed as a randomized controlled trial, cohort study, case-control study, or pre-post study. Articles that met the following criteria were excluded: 1) included patients with hematological cancer; 2) used interventions under the physical exercise category, such as yoga, massage, or psychological rehabilitation; 3) investigated phone or web-based rehabilitation at home or in the community; 4) reported on animal trials or preclinical studies or published as non-original research articles such as reviews, editorials, letters, and comments; and 5) not published in English or Korean and had duplicate subjects (studies using the same outcome indicators and published in duplicate were also excluded).
Four researchers independently conducted the study selection in pairs. First, the reviewers screened the relevance of the articles by reading the titles and abstracts. Then, we obtained the full text of the articles and judged their eligibility for inclusion. Any disagreement was settled by discussion between the two reviewers or in a consensus meeting with consultation with the expert group. The literature selection process was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Cochrane Handbook.11,12
Data extraction and methodological quality assessment
According to a predefined data extraction format, the pairs of researchers extracted information from the selected studies on the patients' characteristics, type of exercise (aerobic or resistance), follow-up period, and outcomes and confirmed the accuracy of the data. Digitizelt software (https://www.digitizeit.de/; last accessed October 26, 2017; Digitizelt, Braunschweig, Germany) was used when data were only presented in graphs.
Two pairs of researchers independently assessed the quality of the selected studies using the Cochrane Risk of Bias tool for randomized studies (RSs) and the Risk of Bias Assessment for Nonrandomized Studies (RoBANS).11 Any disagreement was resolved by discussion and consultation with the expert group.
Outcome measures
Outcome measures were defined through discussion with clinical experts. The primary outcomes were physical function components including physical activity, physical performance, strength, balance, cardiovascular endurance, pulmonary function, and pain. The secondary outcomes were QOL and fatigue.
Data synthesis and analysis
The selected studies were re-categorized into intervention-control studies and before-after studies in accordance with the Methods for the Development of NICE Public Health Guidance and the Study Design Algorithm for Medical Literature of Intervention of the Health Insurance Review and Assessment Service, because of two RSs that compared the effectiveness of the intervention between two exercise groups.13,14
If two or more studies had common outcome measurements, a meta-analysis was conducted. The mean difference (MD) and 95% confidence interval (CI) in each study were calculated to estimate pooled effect sizes. The difference in mean changes between the intervention and control groups after exercise rehabilitation was used to assess the clinical effectiveness in intervention-control studies, whereas the MD between before and after exercise rehabilitation was used for before-after studies (Table 1). A meta-analysis with random-effect models was performed because of heterogeneity in the characteristics of patients and interventions. A publication bias test (funnel plot asymmetry) could not be performed because of the small number of studies (n < 10) available for each type of outcome measure. The heterogeneity of effects was evaluated using Higgins I2 statistics. All statistical analyses were performed using Cochrane RevMan version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, RevMan, Copenhagen, Denmark). Measurements with a P value of < 0.05 were considered statistically significant.
Table 1. Definition of mean difference.
| Intervention-control study | Before-after study |
|---|---|
| Mean difference = (At1 − At0) − (Bt1 − Bt0) | Mean difference = Dt1 − Dt0 |
A = mean in intervention group, B = mean in control group, t1 = after exercise, t0 = before exercise, Dt1 = mean in post-exercise group, Dt0 = mean in pre-exercise group.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of National Evidence-based Healthcare Collaborating Agency (approval NECAIRB #17-006).
RESULTS
Study characteristics
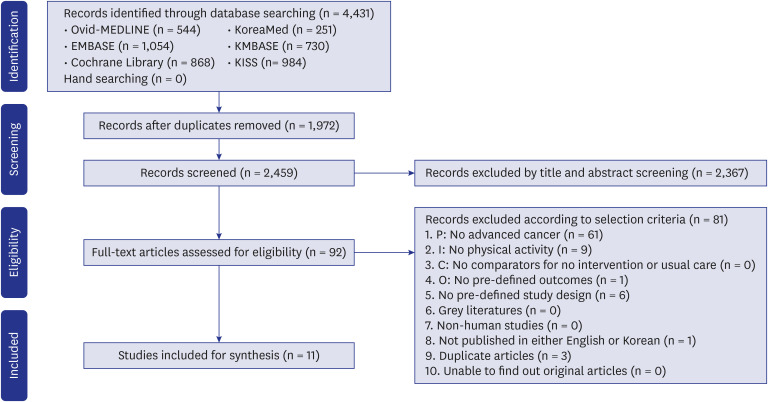
A total of 2,459 articles were retrieved from the databases after deleting duplicates. According to the selection criteria, 92 articles were selected for full review in the third phase of the screening process. All selection steps are presented as PRISMA flowchart (Fig. 1). Finally, 11 studies, including six RSs and five non-randomized studies (NRSs), that consisted of 922 participants were included in our systematic review.15,16,17,18,19,20,21,22,23,24,25
Fig. 1. Flow diagram for identification of eligible studies.
P = patient, I = intervention, C = comparator, O = outcome.
We classified the selected studies as either an “intervention-control” or “before-after” study. For the intervention-control study, four of the six RSs were included.15,16,17,18 The other two RSs compared the exercise groups (cardiovascular vs. resistance and aerobic vs. resistance).19,20 The two studies did not suit the objective of the present study, which was to assess the clinical effectiveness of rehabilitation between exercise and usual care groups. Therefore, the study in each intervention arm was classified as a before-after study.
The before-after study included two RSs with two intervention arms and five NRSs. Among the five NRSs, Loughney et al.21 and Beydoun et al.22 compared the clinical effectiveness among interventions and comparators. However, these studies were not designed as RSs. In the study of Beydoun et al.,22 only clinical results of “face to face group” were reported. Therefore, the two studies were classified to before-after studies. The other NRSs were originally designed as before-after studies. Finally, the before-after studies included four intervention arms in two RSs and five intervention arms in NRSs (Table 2).
Table 2. Characteristics of included studies.
| First author, year, country | Study design/No. of participants (drop out) | Classified study types | Age, mean (SD or range) | Cancer type, No. (%) | Status | Intervention | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|
| When | Where/by whom | How | |||||||
| Jastrzębski, 2015, Poland15 | RS/pulmonary rehabilitation: 12 (5) vs. no exercise: 12 (0) | Intervention–control | 59.0 (7) | Lung (100) | Stage III or IV; earlier stage but unsuitable for surgery or radiation | On chemotherapy | Hospital/not reported | Four 2-week rehabilitation cycles including Nordic Walking, aerobic exercises and respiratory exercises, etc. | Cardiovascular endurance, quality of life, pulmonary function, others |
| Jensen, 2014, Germany19 | RS/resistance: 13 (2) vs. aerobic: 13 (3) | 1. Before–after (resistance) | 55.0 (13.1) | Colon (28.6), Cholangiocellular (19.0), rectal (23.8), etc. | Metastases in other organs | On palliative chemotherapy | Outpatients clinic/not reported | 1. Resistance: 45 min, twice a week, 24 sessions over 12 weeks | Physical activity, strength, quality of life, others |
| 2. Before–after (aerobic) | 2. Aerobic: 45 min on a bicycle ergometer twice a week for 12 weeks | ||||||||
| Rief, 2014, Germany16 | RS/resistance: 30 (0) vs. passive physical therapy: 30 (0) | Intervention–control | I: 61.3 (10.1) | Lung (33.3), prostate (23.3), breast (18.3), etc. | Metastases in spinal bone | On radiotherapy | Hospital/physiotherapist | 30 min, 5 days per week for the two-week RT period | Pain |
| C: 64.1 (10.9) | |||||||||
| Cormie, 2013 Australia17 | RS/resistance: 10 (2) vs. usual care: 10 (3) | Intervention–control | I: 73.1 (7.5) | Prostate (100) | Metastases in bone | On medication | Exercise clinic/accredited exercise physiologist | 60 min, twice-weekly sessions for 12 weeks | Physical activity, cardiovascular endurance, physical performance, strength, balance, quality of life, pain, fatigue, others |
| C: 71.2 (6.9) | |||||||||
| Litterini, 2013, USA20 | RS/cardiovascular exercise: 32 (3) vs. resistance: 34 (11) | 1. Before–after (cardiovascular exercise) | 62.4 (13.5) | Breast (21.2), colorectal (13.6), lung (16.7), etc. | Metastases in other organs | On chemotherapy (36.4), chemotherapy and radiotherapy (28.8), none (16.7), etc. | Hospital-based fitness facility/oncology-trained exercise specialists (a doctor of physical therapy, personal trainers) | 1. Cardiovascular: 30–60 min, twice weekly for 10 weeks with bicycle, treadmill, Nustep (TRS 4000), etc. | Physical performance, pain, fatigue |
| 2. Before–after (resistance) | 2. Resistance: 30–60 min, twice weekly for 10 weeks with Circuit weight training equipment, etc. | ||||||||
| Oldervoll, 2011, Norway18 | RS/physical exercise: 121 (43) vs. usual care: 110 (25) | Intervention–control | I: 62.6 (11.3) | Gastrointestinal (31.6), breast (22.0), lung (16.5), etc. | Incurable and metastatic cancer | On chemotherapy (20.7), hormonal therapy (17.4), radiotherapy (7.4), etc. | Outpatient clinic/physiotherapist | 50–60 min, two exercise sessions per week for an 8-week with circuit training and stretching and relaxation | Cardiovascular endurance, physical performance, strength, fatigue |
| C: 62.2 (10.7) | |||||||||
| Loughney, 2017, UK21 | NRS/exercise: 23 (0) vs. usual care: 10 (0) | Before–after (exercise) | 64.0 (45–82) | Rectal | Locally advanced, no distant metastasis | Between neoadjuvant chemotherapy and surgery | Hospital/not reported | 40 min, 3 sessions per week for a 6-week | Physical activity |
| Quist, 2015, Denmark23 | NRS/group training: 114 (43) | Before–after | 66.0 (31–88) | Lung | Stage IIIb–IV | On chemotherapy | Hospital/research physiotherapist | 90 min, twice weekly for a 6-week with cycling and strength training | Cardiovascular endurance, strength, quality of life, pulmonary function, others |
| Beydoun, 2014, Australia22 | NRS/at home exercise: 255 (30), face to face exercise: 396 (17), man plan support: 208 (28) | Before–after (face to face exercise) | 71.0 (8) | Prostate (100) | Relapsed or metastatic | On androgen deprivation therapy | Hospital/accredited exercise physiologist | A 10-week exercise programme consisting of two group sessions per week | Cardiovascular endurance, strength, physical performance, others |
| Cormie, 2014, Australia24 | NRS/resistance: 20 (5) | Before–after | 70.0 (9.8) | Prostate (85.0), breast cancer (15.0) | Metastases in bone | On medication | Exercise clinic/accredited exercise physiologist | 60 min, twice-weekly sessions for 12 weeks | Physical activity, cardiovascular endurance, physical performance, strength, balance, quality of life, pain, fatigue, others |
| van den Dungen, 2014, Netherland26 | NRS/exercise: 26 (4) | Before–after | 54.5 (8.9) | Breast (26.9), gastrointestinal (30.8), other (42.3) | Incurable metastasized, recurrent or progressive | On palliative anticancer therapy | Physical therapy center/physical therapist | 12-min, twice-weekly for a 6-week, resistance and aerobic interval training | Cardiovascular endurance, strength, quality of life, fatigue, others |
SD = standard deviation, C = comparator, I = intervention, NRS = non-randomized study, RS = randomized study.
In five studies a single type of cancer, such as prostate, lung, or rectal, was the subject of research.15,17,21,22,23 In other studies, the types of cancer were mixed. The patients' ages were between 54.5 and 73.1 years. All patients had metastases, and three studies included patients with bone or spinal metastases.16,17,24 Most participants in the included studies were receiving treatment or ahead of surgery. For the outcomes of studies, their measurements were various, so we classified them to 6 categories of outcomes (Supplementary Table 2).
Risk of bias assessment of the selected studies
The risk of bias in the six RSs was assessed using the Cochrane Risk of Bias tool.11 “Random sequence generation” and “selective reporting” showed approximately low risks of bias across studies. By contrast, “incomplete outcome data” showed high risks of bias. “Allocation concealment” and “blinding of the participants and personnel” showed unclear risks of bias.
The risks of bias in the five NRS were assessed using the revised RoBANS tool.25 The results were as follows: “blinding of outcome assessment” and “measurement of exposure” presented a high or unclear risk of bias, whereas “outcome evaluation” presented a low risk of bias. “Participant comparability,” “selection of participants,” and “selective outcome reporting” presented relatively low risks of bias. The risks of bias in each study were described in Supplementary Fig. 1.
Primary outcome: physical function
Although the outcome measures were reported with slightly different names in each study, we performed a meta-analysis of these measures by assigning a single indicator for similar or identical outcomes through consultations with clinical and statistical experts (Table 3 and Supplementary Fig. 2).
Table 3. Result of meta-analysis.
| Outcome | Type | Measurement | Included studies | No. of I (A)/C (B) | Hetero geneity | Mean difference (95% CI) | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | P-value | ||||||||
| Primary outcomes (physical function) | |||||||||
| Physical activity | BA | Total energy expenditure (kcal/day) | Jensen et al.19; Loughney et al.21 | 44/49 | 0 | 0.74 | 88.22 (−92.64, 269.08) | 0.34 | |
| BA | Step count (n/day) | 58 | 0.12 | 13.04 (−5.31, 31.39) | 0.16 | ||||
| BA | MET | 0 | 0.79 | 0.29 (0.07, 0.51) | 0.01 | ||||
| BA | Sleep duration (h/day) | 8 | 0.30 | 0.60 (−0.04, 1.23) | 0.07 | ||||
| BA | Lying down (h/day) | 0 | 0.95 | 0.23 (−1.00, 1.46) | 0.72 | ||||
| Physical performance (SPPB) | BA | Total score | Litterini et al.20 (cardiovascular); Litterini et al.20 (resistance) | 52/66 | 0 | 0.85 | 0.60 (−0.15, 1.36) | 0.12 | |
| BA | Balance score | 0 | 0.90 | 0.16 (−0.06, 0.39) | 0.15 | ||||
| BA | Gait score | 0 | 0.75 | 0.12 (−0.10, 0.34) | 0.28 | ||||
| BA | Chair stands score | 0 | 0.72 | 0.31 (−0.16, 0.78) | 0.20 | ||||
| Strength | BA | Leg press (kg) | Cormie et al.24; Jensen et al.19 (resistance); Quist et al.23; van den Dungen et al.26 | 119/130 | 0 | 0.41 | 12.18 (6.00, 18.35) | 0.0001 | |
| BA | Knee extensor (kg) | Jensen et al.19(resistance); Quist et al.23 | 82/44 | 0 | 0.35 | 2.43 (−0.45, 5.31) | 0.10 | ||
| BA | Bench press (kg) | Quist et al.23; van den Dungen et al.26 | 93/97 | 0 | 0.78 | 4.81 (0.85, 8.77) | 0.02 | ||
| BA | Abdominal crunch (kg) | 0 | 0.81 | 6.48 (2.01, 10.96) | 0.005 | ||||
| BA | Back (kg) | Jensen et al.19(resistance); Quist et al.23 | 82/44 | 0 | 0.74 | 5.17 (1.60, 8.75) | 0.005 | ||
| Cardiovascular endurance | BA | 6MWD (m) | Quist et al.23; van den Dungen et al.26 | 93/97 | 0 | 0.92 | 32.60 (−3.13, 68.34) | 0.07 | |
| Pain | IC | VAS (cm) | Cormie et al.17; Rief et al.16 | 40/40 | 0 | 0.32 | −0.32 (−1.20, 0.56) | 0.48 | |
| BA | VAS (cm) | Cormie et al.24; Litterini et al.20 (cardiovascular); Litterini et al.20 (resistance) | 67/86 | 0 | 0.70 | −0.02 (−0.61, 0.56) | 0.93 | ||
| Secondary outcomes (QOL, fatigue) | |||||||||
| QOL (SF-36) | IC | Physical function | Cormie et al.17; Jastrzebski et al.15 | 22/18 | 0 | 0.96 | 0.32 (−10.30, 10.94) | 0.95 | |
| IC | Role physical | 0 | 0.41 | 3.06 (−8.25, 14.37) | 0.60 | ||||
| IC | Bodily pain | 0 | 0.96 | −2.27 (−12.52, 7.98) | 0.66 | ||||
| IC | General health | 0 | 0.92 | −0.90 (−10.09, 8.28) | 0.85 | ||||
| IC | Vitality | 0 | 0.61 | 7.68 (−4.73, 20.10) | 0.23 | ||||
| IC | Social functioning | 0 | 0.32 | 3.52 (−6.36, 13.39) | 0.49 | ||||
| IC | Role emotional | 0 | 0.79 | 5.20 (−6.80, 17.19) | 0.40 | ||||
| IC | Mental health | 0 | 0.74 | 3.45 (−6.93, 13.83) | 0.51 | ||||
| IC | Physical health composite | 0 | 0.88 | 0.56 (−8.78, 9.90) | 0.91 | ||||
| IC | Mental health composite | 0 | 0.81 | 1.91 (−9.31, 13.14) | 0.74 | ||||
| BA | Physical function | Cormie et al.24; van den Dungen et al. 26 | 37/46 | 0 | 0.77 | 2.33 (−2.90, 7.57) | 0.38 | ||
| BA | Role physical | 0 | 0.52 | 2.98 (−3.39, 9.34) | 0.36 | ||||
| BA | Social functioning | 0 | 0.76 | 3.19 (−1.99, 8.37) | 0.23 | ||||
| BA | Vitality | 0 | 0.42 | 3.27 (−2.18, 8.71) | 0.24 | ||||
| QOL (EORTC-QLQ-30) | BA | Global health status/QOL | Jensen et al.19 (aerobic); Jensen et al.19 (resistance); van den Dungen et al.26 | 43/52 | 0 | 0.58 | 10.28 (2.53, 18.02) | 0.009 | |
| BA | Physical functioning | 0 | 0.98 | 2.74 (−1.47, 6.96) | 0.20 | ||||
| BA | Role functioning | Jensen et al.19 (aerobic); Jensen et al.19 (resistance) | 21/26 | 0 | 0.72 | 18.83 (5.20, 32.46) | 0.007 | ||
| BA | Emotional functioning | 0 | 0.64 | 8.95 (−5.69, 23.59) | 0.23 | ||||
| BA | Cognitive functioning | 0 | 0.35 | 7.15 (−8.52, 22.81) | 0.37 | ||||
| BA | Social functioning | Jensen et al.19 (aerobic); Jensen et al.19 (resistance); van den Dungen et al.26 | 43/52 | 0 | 0.80 | 6.62 (−3.01, 16.25) | 0.18 | ||
| BA | Fatigue | 50 | 0.13 | −15.21 (−28.46, −1.97) | 0.02 | ||||
| BA | Nausea, vomiting | Jensen et al.19 (aerobic); Jensen et al.19 (resistance) | 21/26 | 0 | 0.91 | −1.24 (−13.26, 10.78) | 0.84 | ||
| BA | Pain | 0 | 0.60 | 1.49 (−16.59, 19.56) | 0.87 | ||||
| BA | Dyspnoea | 0 | 0.75 | −5.26 (−22.47, 11.95) | 0.55 | ||||
| BA | Insomnia | 0 | 0.86 | −5.90 (−21.21, 9.40) | 0.45 | ||||
| BA | Appetite loss | 0 | 0.61 | −5.33 (−22.84, 18.18) | 0.66 | ||||
| BA | Constipation | 0 | 1.00 | 0.00 (−10.92, 10.92) | 1.00 | ||||
| BA | Diarrhoea | 0 | 0.84 | −8.24 (−27.61, 11.13) | 0.40 | ||||
| BA | Financial difficulties | 0 | 0.42 | −0.76 (−22.92, 21.40) | 0.95 | ||||
| Fatigue | BA | VAS (mm) | Litterini et al.20 (cardiovascular); Litterini et al.20 (resistance); van den Dungen et al.26 (ESAS); van den Dungen et al.26 (VAS) | 96/118 | 0 | 0.76 | −4.67 (−10.38, 1.04) | 0.11 | |
6MWD = 6-minute walk distance, A = after, B = before, BA = before-after study, C = comparator, CI = confidence interval, EORTC-QLQ-C30 = European Organization for Research and Treatment of Cancer quality of life questionnaire version C30, ESAS = Edmonton Symptom Assessment System, I = intervention, IC = intervention-comparator study, MET = metabolic equivalent of task, SF-36 = 36-item Short-Form Health Survey, SPPB = Short Physical Performance Battery, VAS = visual analogue scale, QOL = quality of life.
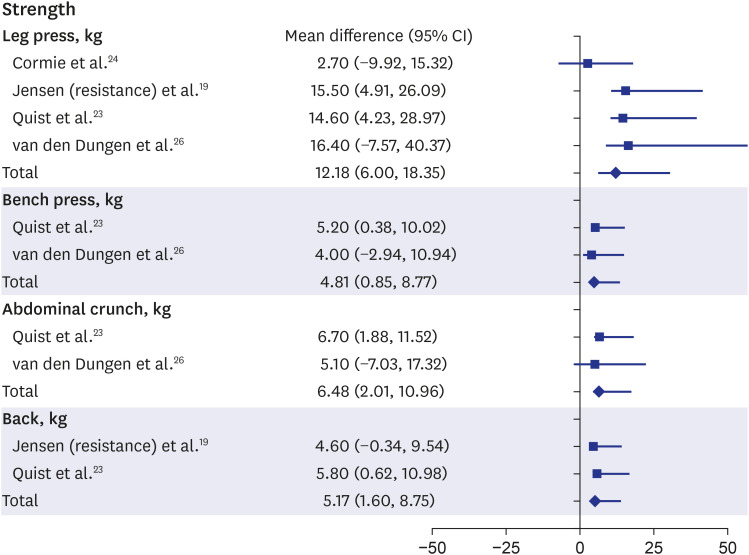
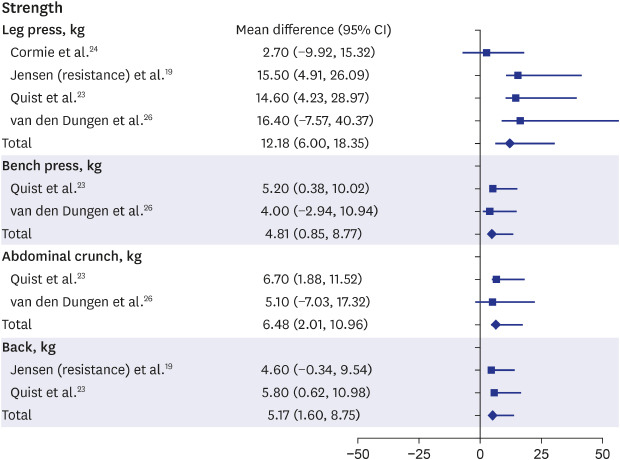
Among the physical function outcomes, “strength” and “physical activity” were improved. The meta-analysis of the articles showed that most strength measurements, including those from leg press, bench press, abdominal crunch, and back extension, showed significant improvements after rehabilitation in patients with advanced cancer (leg press: MD, 12.18, 95% CI, 6.00–18.35; bench press: MD, 4.81, 95% CI, 0.85–8.77; abdominal crunch: MD, 6.48, 95% CI, 2.01–10.96; back: MD, 5.17, 95% CI, 1.60–8.75) (Fig. 2).19,23,24,26 In two articles, knee or knee extensor strengths was also stronger after rehabilitation than before rehabilitation, but the difference was not significant (MD, 2.43; 95% CI, −0.45–5.31; P = 0.1).19,23 Although the other strength measurements, including those from knee push-ups, handgrip, or vertical row, were not synthesized, improvements after rehabilitation were significant.17,18,19,22,23,24,26
Fig. 2. Forest plot of “strength”.
CI = confidence interval.
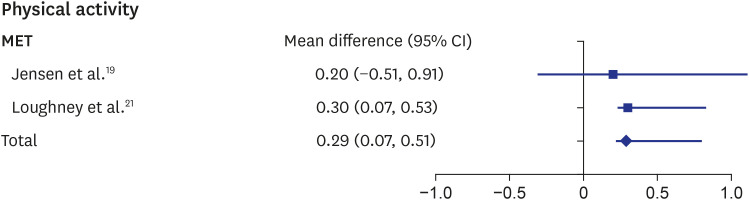
The metabolic equivalent of task (MET) is a measurement for evaluating the amount of physical activity. The MET values 1.3 and 1.8 correspond to “standing” and “doing light activities,” such as studying and note taking, respectively. The rehabilitation result in patients with advanced cancer of the two synthesized studies showed a significant increase in the MET (MD, 0.29; 95% CI, 0.07–0.51) (Fig. 3).19,21 Several measurements such as Godin leisure-time exercise, active energy expenditure, or steps per day were used for evaluating physical activity in several studies; however, the results were not consistent.17,19,21,24
Fig. 3. Forest plot of “MET”.
CI = confidence interval, MET = metabolic equivalent of task.
Other outcomes, including physical performance, cardiovascular endurance, and pain, were synthesized as Short Physical Performance Battery score, 6-minute walking distance, and visual analogue scale (VAS) score, respectively; however, the results of the meta-analysis were not statistically significant.
For instance, the patients could walk 32.6 m on average within 6 minutes after rehabilitation in two articles which was longer than the distance walked before rehabilitation but without statistical significance (MD, 32.6; 95% CI, −3.13–68.34; P = 0.07).23,26 Several studies that were not synthesized reported an explicit improvement in cardiovascular endurance.15,17,18,22,24
Balance and pulmonary function were used as measurements of physical function in the intervention-control and before-after studies; however, they were not synthesized because of the different types and inconsistent results of studies.15,17,23,24
Secondary outcome: QOL
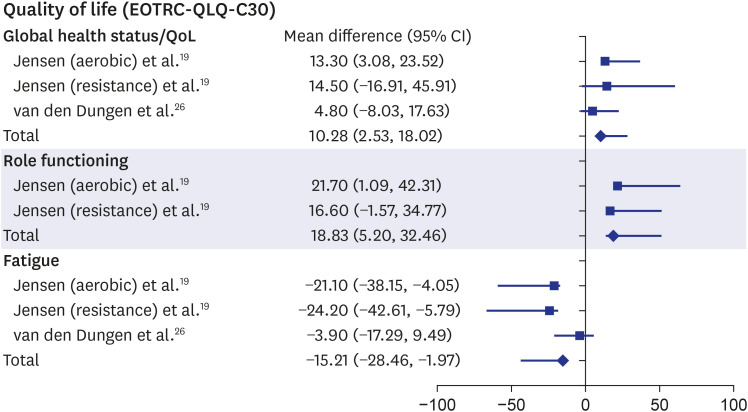
The QOL of patients with advanced cancer significantly improved after physical rehabilitation. In the before-after studies, “global health status/QOL,” a subcategory in the European Organization for Research and Treatment of Cancer quality of life questionnaire version C30 (EORTC-QLQ-C30), substantially increased after rehabilitation (MD, 10.28; 95% CI, 2.53–18.02).19,26 Role functioning, which is related to work or leisure life, was also significantly improved (MD, 18.83; 95% CI, 5.20–32.46).19 Fatigue in the EORTC-QLQ-C30 showed an apparent reduction after physical exercise (MD, −15.21; 95% CI, −28.46−1.97)19,26 (Fig. 4). However, the other subscales of the EORTC-QLQ-C30 or those of the 36-item Short-Form Health Survey (SF-36) in the intervention-control or before-after studies were not significantly improved.15,17,24,26.
Fig. 4. Forest plot of “QOL”.
QOL = quality of life, CI = confidence interval, EORTC-QLQ-C30 = European Organization for Research and Treatment of Cancer quality of life questionnaire version C30.
Secondary outcome: fatigue
Fatigue was synthesized as a VAS score in the before-after studies.20,26 The meta-analysis results showed that fatigue in patients with cancer had a tendency to improve after rehabilitation; however, the improvement was not significant (MD, −4.67; 95% CI, −10.38–1.04). Three articles measured fatigue with other tools, but its improvement was also not significant.17,18,24
DISCUSSION
The main goal of the present systematic review was to determine the effect of supervised physical rehabilitation on the physical function outcomes of patients with advanced cancer, as well as on their QOL and fatigue level. This study reviewed and analysed quantitatively four intervention-control studies and seven before-after studies. Our review showed significant improvement of muscle strength and physical activity and the tendency of relieving the symptom of fatigue after a physical rehabilitation program that was structured or supervised by health professionals. Although some of the quantitative estimates showed no statistically significant results, the several studies showed potential effectiveness in terms of strength and cardiovascular endurance.
In our meta-analysis, before-after studies showed significantly improved physical function outcomes in patients with advanced cancer in terms of strength and physical activity. From the perspective of physical function, previous systematic reviews also showed similar results, although a meta-analysis was not performed. Concerning the physical activity of patients with advanced cancer managed with palliative treatments, vitality and fitness levels improved in eight studies.2 Another systematic review study reported that > 80% of the 25 selected studies showed the effectiveness of aerobic or resistance training on the physical function of patients with advanced cancer.27
Physical rehabilitation, including exercise, is known to positively affect the QOL outcome.9 In our meta-analysis, we could not find a significant change in QOL in the intervention-control studies. However, in the before-after studies, an overall tendency of improvement was observed, and some subcategories showed significant positive effects in the meta-analysis. A previous systematic review without a meta-analysis reported a similar finding. Nineteen studies reported QOL improvements after exercise intervention in patients with advanced cancer.28 Various types of QOL measurement tools are available. The general measurement tool SF-36 showed no significant difference, but the cancer-type-specific tool EORTC-QLQ-30 showed significant differences in our study outcomes. Fong et al.7 reported QOL improvement after physical exercise in cancer survivors but used SF-36, which is not a cancer-specific measurement tool, to evaluate QOL.
Among the cancer treatment-related symptoms, fatigue is the most common and disabling adverse effect.27,28 In our review, fatigue showed a tendency to improve with exercise rehabilitation. The EORTC subscale results in the before-after studies showed significant improvement in fatigue. Similarly, Heywood et al.27 qualitatively analyzed studies and reported a tendency of improvement of fatigue in patients with advanced cancer. One previous systematic review on cancer-related fatigue that did not limit the cancer stage or type analyzed 127 effective sizes. This study found a significant effect during and after the primary cancer treatment; however, the cancer stage was associated with the effect. Although all patients showed an improvement in fatigue, exercise intervention was more beneficial in the non-metastatic stage than in the metastatic stage.29
Several reasons can be assumed for the limited quantitative effect of physical rehabilitation in patients with advanced cancer. The study duration was not long enough to identify the long-term effect, particularly to show the effect of interventions supervised or structured by healthcare professionals. Furthermore, the number of clinical trials for analysis was inadequate. Due to the limited number, the primary outcomes such as physical activity, physical performance, and strength were not derived equally from both before-after studies and intervention-control studies. For this reason, we should conclude the results of meta-analysis carefully. Heterogeneity across studies was also a limitation to interpret the results of this review. The heterogeneity of physical rehabilitation assessed and differences in patients' characteristics could affect the outcomes of physical rehabilitation. If the selected studies reported the common scale for patients' performance status or the number of included studies was enough to conduct sub-group analysis according to patients' characteristics or types of physical rehabilitation assessed, the issue on heterogeneity might be mitigated. Although the rate of survival from cancer has relatively increased recently owing to improvements in cancer treatment,30 RSs on advanced cancer are rare because of ethical issues and the difficulty in controlling other factors in the intervention environment.31 Moreover, the outcome measurement tools and non-standardized therapies are diverse. Because of the heterogeneity of the intervention elements, such as methods, duration, and intensity, several previous systematic reviews were conducted without meta-analyses.2,27,28 However, we attempted to perform a meta-analysis limited to identical or similar measurements, which has its own advantages and disadvantages. For instance, we could easily identify the level of effectiveness with a meta-analysis, but only a few studies could be included because of the aforementioned heterogeneity.
Across studies, “blinding of the participants and personnel” or “blinding of outcome assessment” showed unclear risks of bias. Physical rehabilitation, which is the intervention of our study, has limitation to perform “blinding of the participants and personnel.” Meanwhile, primary outcomes are objective values, which are physical activities, physical performance, strength, and cardiovascular endurance, so unclear risks of bias for blinding might be less likely to affect results of this study.
The strength of this review lies in its thorough search strategy. First, this study focused on patients with advanced cancer who experience considerable symptoms of physical, psychological, or cognitive impairments and disabilities, as well as impaired QOL.32 Most previous systematic reviews on cancer rehabilitation focused on a specific cancer type, early-stage cancer, or cancer survivors. Peddle-McIntyre et al.33 analyzed the effectiveness of exercise training for patients with advanced cancer and concluded that exercise enhanced exercise capacity and QOL but the cancer type was only limited to lung cancer. Therefore, the usefulness of supervised rehabilitation was difficult to generalize for patients with advanced cancer.6,34,35,36 Second, only physical rehabilitation such as resistance or aerobic exercise that was structured or supported by health professionals in hospital related facilities were included in this review. Although some studies have reported the effectiveness of exercise on physical function or QOL in a supervised setting, these were not limited to patients with advanced cancer.10,36,37 Some previous studies investigated the effectiveness of exercise for patients with advanced cancer but the setting of intervention was not informed clearly or mixed (home or hospital-based).27,28,33 Our study focused on the effectiveness of physical rehabilitation by experts. Because patients with advanced cancer experience a symptom cluster consisting of fatigue, pain, and anorexia, which should be controlled and improved during a rehabilitation program provided by experts. Moreover, the previous studies included various types of intervention such as yoga, nutrition counselling, psychologist's intervention or Qigong but our study excluded other intervention except for exercise to delete the effect by non-physical components in rehabilitation programs.27,28,33 Third, we conducted quantitative analyses. Our study demonstrated the effects of physical activity on various health outcomes in the quantitative analysis when a meta-analysis is appropriate. These results are not different from findings of previous similar studies for patients with advanced cancer. Heywood et al.27 and Dittus et al.28 reported the improvement of physical function or QOL on advanced cancer patients after exercise but their results were not synthesized quantitatively. In addition, few studies have focused on patients with advanced cancer who need structured rehabilitation provided by experts. Although some outcomes in our study were not synthesized due to lack of included studies, our study was the first study to investigate the clinical effectiveness of supervised physical rehabilitation in patients with advanced cancer through meta-analysis.
In conclusion, exercise intervention for patients with advanced cancer is effective in terms of improving physical activity, strength, and QOL. It is meaningful that a structured exercise intervention provided by healthcare providers can improve the physical function and QOL of patients with advanced cancer. However, we did not obtain strong evidence owing to the small number of studies and the variety of outcome measurements. In addition, selected literatures on physical function were mostly before-after studies. Therefore, more comparative studies on standardized outcome measures and the long-term effects of exercise in patients with advanced cancer are needed.
Footnotes
Funding: This study was supported by National Evidence-based Healthcare Collaborating Agency (NECA-C-18-001).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yang J, Yu SY, Yang EJ.
- Formal analysis: Yang J, Choi J.
- Investigation: Yang J, Choi M, Chung SH, Sim SH, Kim YJ, Choi J, Jo A, Kang M, Yu SY, Yang EJ.
- Methodology: Choi M, Yang J.
- Writing - original draft: Yang J, Yu SY.
- Writing - review & editing: Yang J, Yu SY, Yang EJ, Choi MY.
SUPPLEMENTARY MATERIALS
PICOTS-SD
Outcomes by defined classification
Quality assessment results.
Forest plot.
References
- 1.Silver JK, Raj VS, Fu JB, Wisotzky EM, Smith SR, Kirch RA. Cancer rehabilitation and palliative care: critical components in the delivery of high-quality oncology services. Support Care Cancer. 2015;23(12):3633–3643. doi: 10.1007/s00520-015-2916-1. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht TA, Taylor AG. Physical activity in patients with advanced-stage cancer: a systematic review of the literature. Clin J Oncol Nurs. 2012;16(3):293–300. doi: 10.1188/12.CJON.293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cromes GF., Jr Implementation of interdisciplinary cancer rehabilitation. Rehabil Couns Bull. 1978;21(3):230–237. [Google Scholar]
- 4.Okamura H. Importance of rehabilitation in cancer treatment and palliative medicine. Jpn J Clin Oncol. 2011;41(6):733–738. doi: 10.1093/jjco/hyr061. [DOI] [PubMed] [Google Scholar]
- 5.Council IM, Sciences CL, Medicine I, Board NC, Simone JV, Hewitt M. Ensuring Quality Cancer Care. Washington, D.C.: National Academies Press; 1999. [PubMed] [Google Scholar]
- 6.Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev. 2010;36(2):185–194. doi: 10.1016/j.ctrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, Petrella T, et al. Exercise for people with cancer: a clinical practice guideline. Curr Oncol. 2017;24(1):40–46. doi: 10.3747/co.24.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Stout NL, Baima J, Swisher AK, Winters-Stone KM, Welsh J. A systematic review of exercise systematic reviews in the cancer literature (2005–2017) PM R. 2017;9:S347–84. doi: 10.1016/j.pmrj.2017.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. Methods for the Development of NICE Public Health Guidance. London: NICE; 2006. [PubMed] [Google Scholar]
- 14.Health Insurance Review & Assessment Service. HIRA's Guideline for Undertaking Systematic Reviews. Seoul: HIRA; 2013. [Google Scholar]
- 15.Jastrzębski D, Maksymiak M, Kostorz S, Bezubka B, Osmanska I, Młynczak T, et al. Pulmonary rehabilitation in advanced lung cancer patients during chemotherapy. In: Pokorski M, editor. Respiratory Health. Advances in Experimental Medicine and Biology, Vol. 861. 2015. pp. 57–64. [DOI] [PubMed] [Google Scholar]
- 16.Rief H, Welzel T, Omlor G, Akbar M, Bruckner T, Rieken S, et al. Pain response of resistance training of the paravertebral musculature under radiotherapy in patients with spinal bone metastases--a randomized trial. BMC Cancer. 2014;14:485. doi: 10.1186/1471-2407-14-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(4):328–335. doi: 10.1038/pcan.2013.22. [DOI] [PubMed] [Google Scholar]
- 18.Oldervoll LM, Loge JH, Lydersen S, Paltiel H, Asp MB, Nygaard UV, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011;16(11):1649–1657. doi: 10.1634/theoncologist.2011-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen W, Baumann FT, Stein A, Bloch W, Bokemeyer C, de Wit M, et al. Exercise training in patients with advanced gastrointestinal cancer undergoing palliative chemotherapy: a pilot study. Support Care Cancer. 2014;22(7):1797–1806. doi: 10.1007/s00520-014-2139-x. [DOI] [PubMed] [Google Scholar]
- 20.Litterini AJ, Fieler VK, Cavanaugh JT, Lee JQ. Differential effects of cardiovascular and resistance exercise on functional mobility in individuals with advanced cancer: a randomized trial. Arch Phys Med Rehabil. 2013;94(12):2329–2335. doi: 10.1016/j.apmr.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Loughney L, West MA, Dimitrov BD, Kemp GJ, Grocott MP, Jack S. Physical activity levels in locally advanced rectal cancer patients following neoadjuvant chemoradiotherapy and an exercise training programme before surgery: a pilot study. Perioper Med (Lond) 2017;6:3. doi: 10.1186/s13741-017-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beydoun N, Bucci JA, Chin YS, Spry N, Newton R, Galvão DA. Prospective study of exercise intervention in prostate cancer patients on androgen deprivation therapy. J Med Imaging Radiat Oncol. 2014;58(3):369–376. doi: 10.1111/1754-9485.12115. [DOI] [PubMed] [Google Scholar]
- 23.Quist M, Adamsen L, Rørth M, Laursen JH, Christensen KB, Langer SW. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced-stage lung cancer undergoing chemotherapy. Integr Cancer Ther. 2015;14(4):341–349. doi: 10.1177/1534735415572887. [DOI] [PubMed] [Google Scholar]
- 24.Cormie P, Galvão DA, Spry N, Joseph D, Taaffe DR, Newton RU. Functional benefits are sustained after a program of supervised resistance exercise in cancer patients with bone metastases: longitudinal results of a pilot study. Support Care Cancer. 2014;22(6):1537–1548. doi: 10.1007/s00520-013-2103-1. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Lee Y, Seo H, Jang B, Son H, Kim S, et al. Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS): Development and Validation of a New Instrument; 19th Cochrane Colloquium; 2011 Oct 19–22; Madrid. London: Cochrane; 2011. [Google Scholar]
- 26.van den Dungen IA, Verhagen CA, van der Graaf WT, van den Berg JP, Vissers KC, Engels Y. Feasibility and impact of a physical exercise program in patients with advanced cancer: a pilot study. J Palliat Med. 2014;17(10):1091–1098. doi: 10.1089/jpm.2013.0638. [DOI] [PubMed] [Google Scholar]
- 27.Heywood R, McCarthy AL, Skinner TL. Efficacy of exercise interventions in patients with advanced cancer: a systematic review. Arch Phys Med Rehabil. 2018;99(12):2595–2620. doi: 10.1016/j.apmr.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124–132. doi: 10.1016/j.ypmed.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3(7):961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20(10):1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daher M. Ethical issues in the geriatric patient with advanced cancer ‘living to the end’. Ann Oncol. 2013;24(Suppl 7):vii55–vii58. doi: 10.1093/annonc/mdt262. [DOI] [PubMed] [Google Scholar]
- 32.Spence RR, Heesch KC, Brown WJ. A systematic review of the association between physical activity and colorectal cancer risk. Scand J Med Sci Sports. 2009;19(6):764–781. doi: 10.1111/j.1600-0838.2009.00992.x. [DOI] [PubMed] [Google Scholar]
- 33.Peddle-McIntyre CJ, Singh F, Thomas R, Newton RU, Galvão DA, Cavalheri V. Exercise training for advanced lung cancer. Cochrane Database Syst Rev. 2019;2:CD012685. doi: 10.1002/14651858.CD012685.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe SS, Tan M, Faily J, Watanabe SM, Courneya KS. Physical activity in advanced cancer patients: a systematic review protocol. Syst Rev. 2016;5:43. doi: 10.1186/s13643-016-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keilani M, Hasenoehrl T, Baumann L, Ristl R, Schwarz M, Marhold M, et al. Effects of resistance exercise in prostate cancer patients: a meta-analysis. Support Care Cancer. 2017;25(9):2953–2968. doi: 10.1007/s00520-017-3771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pergolotti M, Williams GR, Campbell C, Munoz LA, Muss HB. Occupational therapy for adults with cancer: why it matters. Oncologist. 2016;21(3):314–319. doi: 10.1634/theoncologist.2015-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PICOTS-SD
Outcomes by defined classification
Quality assessment results.
Forest plot.