Abstract
Systemic therapy for metastatic triple-negative breast cancer (TNBC) still remains challenging because there are no targeted agents or endocrine therapies currently available. The present case report documents the successful use of cisplatin monotherapy to manage a heavily pretreated TNBC patient showing poor response to therapy. The patient was a 51-year-old woman who had already undergone several lines of systemic chemotherapy for widespread TNBC. Although the mutation analysis performed on DNA isolated from blood cells and progressed lesion samples confirmed the tumor to be germline BRCA wild-type, cisplatin monotherapy was administered based on the increasing evidence of safety and efficacy of platinum for breast cancer. After three cycles of cisplatin treatment, the patient’s metastatic lesions dramatically improved without any major toxicity, and she completed 17 cycles with good response. This case study indicates that patients with heavily pretreated TNBC can potentially achieve a good response to cisplatin monotherapy.
Keywords: Antineoplastic agents, Cisplatin, DNA repair, Triple negative breast neoplasms
Introduction
Triple-negative breast cancer (TNBC) is characterized by the absence of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) [1]; TNBC accounts for 15%–20% of all breast cancers [2]. Generally, it has been well known that TNBC appears at a younger age and has a more aggressive clinical course than other breast cancer subtypes [3]. Although several clinical trials for TNBC have suggested a potential survival benefit using targeted agents, such as poly ADP-ribose polymerase (PARP) inhibitors or immune modulating agents, there are limitations in using these agents in daily practice due to domestic guidelines [4,5]. Thus, cytotoxic chemotherapy still remains the main therapeutic strategy for metastatic TNBC along with palliative radiotherapy and/or surgery [6,7]. However, to date, the treatment response of patients with advanced TNBC has been unsatisfactory [8].
In the presence of germline breast cancer susceptibility gene (BRCA) 1/2 mutations, PARP inhibitors or platinum agents can be considered as palliative regimens after the failure of taxanes, anthracyclines, antimetabolites, and microtubule inhibitors. Platinum is a cytotoxic agent and one of the most widely used drugs for treating solid tumors. Cisplatin, a bifunctional DNA cross-linking agent, induces cell apoptosis by causing DNA damage and interfering with the DNA repair mechanism [9]. The side effects of cisplatin include myelosuppression, nephrotoxicity, neurotoxicity, and nausea/vomiting. However, compared to other regimens, including taxanes and anthracyclines, the side effects of cisplatin are generally mild-to-moderate and manageable. While cisplatin is not usually included in the adjuvant or neoadjuvant chemotherapy for breast cancer, cisplatin-based combination therapy has recently been reported to be highly efficacious in metastatic breast cancer patients with a germline BRCA mutation [10,11]. However, the clinical impact of cisplatin monotherapy as a palliative treatment for BRCA-negative metastatic TNBC patients with a poor general condition has not yet been fully evaluated.
When selecting a therapeutic agent for advanced breast cancer with multiple metastases, it is important to carefully determine a regimen with minimal adverse effects to preserve the patients’ quality of life. Therefore, in this case report, we describe our successful experience of administering cisplatin monotherapy to treat a 51-year-old woman who previously underwent multiple systemic chemotherapies for widespread BRCA-negative TNBC.
Case
A 51-year-old woman was transferred from a nearby hospital after receiving heavy pretreatment for disseminated breast cancer. At the time of admission to our hospital in July 2017, the patient’s general condition was poor, with an Eastern Cooperative Oncology Group (ECOG) performance status of 4. She complained of widespread skin metastases with discharge and generalized edema (Fig. 1). According to her medical history, the patient was diagnosed with inoperable advanced TNBC and underwent 10 cycles of palliative chemotherapy with docetaxel and doxorubicin from May to December 2015, resulting in a partial response. However, the treatment was terminated owing to intolerance of the cumulative doxorubicin dose. Thereafter, the patient underwent a palliative modified radical mastectomy in December 2015. With ongoing disease progression, she received palliative radiotherapy and multiple lines of chemotherapy, including paclitaxel, gemcitabine, capecitabine, vinorelbine, and eribulin, before visiting our hospital.
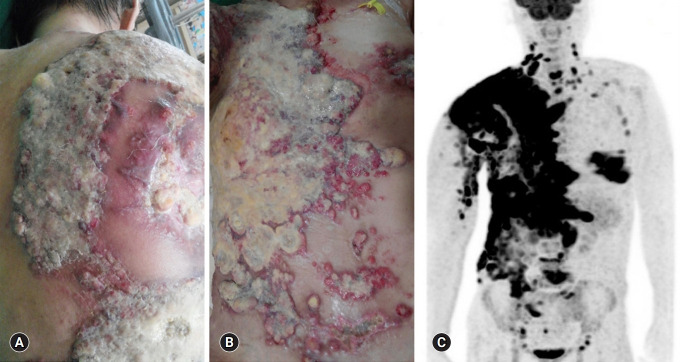
Fig. 1.
Heavily pretreated breast cancer with widespread skin metastases on July 2017. At the time of admission to our hospital, the patient was suffering from severe skin metastases with discharge (A, B). Positron emission tomography-computed tomography shows breast cancer with multiple metastases in the chest wall, liver, bone, and lymph nodes (C). The patient provided written informed consent for publication of clinical details and images.
At the time of admission, the patient’s vital signs were stable and she was alert. Her chest computed tomography (CT) scan demonstrated a diffuse infiltrative mass lesion in the anterior chest wall with bilateral pleural effusion and multiple metastatic lymphadenopathies in both the neck and axilla. The abdomen-pelvis CT scan revealed multiple liver, lymph nodes, and abdominal wall metastases. Tissue biopsy of the right chest wall tumor mass identified the tumor as a triple-negative-type invasive ductal carcinoma (Fig. 2). Cisplatin monotherapy was selected based on the results of several pilot studies, which revealed a remarkable efficacy of cisplatin for TNBC [12,13]. We also took into consideration the patient’s poor general condition and the mild cisplatin-induced toxicity. The patient received 75 mg/m2 of cisplatin every 3 weeks, along with standard hydration and antiemetic prophylaxis. Chemotherapy was administered in an in-patient setting because of the patient’s poor general condition and the extensive dressing care needed for the metastatic skin lesions. After three cycles of cisplatin, the occurrence of skin ulcerative lesions was remarkably decreased. As the patient’s ECOG performance status was dramatically improved to 1, the treatment was continued in an outpatient setting. After six cycles of the regimen, responses were further noted in both the liver and skin lesions (Fig. 3). With regard to adverse events, transient neutropenia and mild nausea were identified, yet both were manageable. The patient recently completed her 17th cycle of cisplatin with a good performance and minimal peripheral neuropathy (Fig. 4).
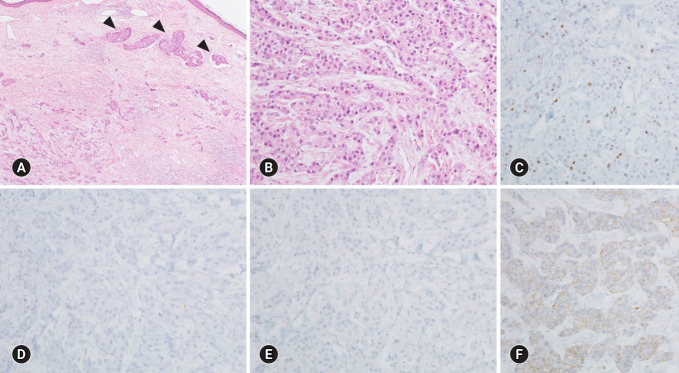
Fig. 2.
Representative histological features and immunohistochemical (IHC) findings of the metastatic carcinoma. The metastatic tumor shows the histologic grade 3, according to the modified Nottingham grading system (tubule formation 3, nuclear pleomorphism 3, and mitotic activities 2), and frequent lymphovascular emboli (A, arrowheads) within the dermal area (A, B). The tumor cells shows 10% Ki-67 labeling index (C), loss of estrogen receptor (D), and progesterone receptor (E). The expression of HER2 was classified as 2+ (F) on IHC, subsequently proven HER2 negativity on HER2 silver in situ hybridization (not shown) (hematoxylin and eosin stain, ×40 [A, B]; IHC stain, ×200 [C−F]).
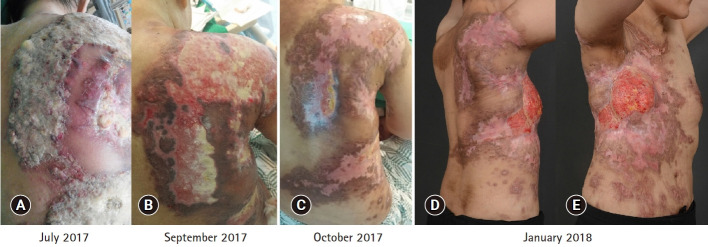
Fig. 3.
Before the cisplatin treatment, the patient complained of widespread skin metastases with discharge and generalized edema (A). She received 75 mg/m2 of cisplatin every 3 weeks, along with standard hydration and antiemetic prophylaxis. After three cycles of cisplatin, skin ulcerative lesions were remarkably decreased (B). After six cycles of the regimen, responses were further noted in skin lesions as well as liver, chest wall, and lymph nodes (C). The patient completed her 17th cycle of cisplatin with dramatic metastatic skin lesion improvement (D, E). The patient provided written informed consent for publication of clinical details and images.
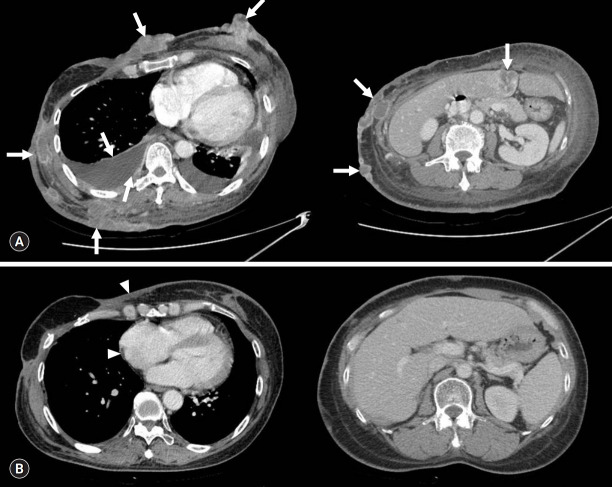
Fig. 4.
Computed tomography (CT) shows the impressive response of cisplatin monotherapy. Before the cisplatin monotherapy, multiple metastases with bilateral pleural effusion (arrows) were found in the chest and abdomen CT (July 2017) (A). Pleural effusion and metastatic lesions were dramatically improved (arrowheads) after the cisplatin treatment (December 2018) (B).
Although she has no family history of breast or ovarian cancer, germline and somatic BRCA mutation tests were performed in the peripheral blood and primary/residual malignant tumor tissues for the potential use of a PARP inhibitor. However, no pathogenic BRCA mutation was identified (Table 1).
Table 1.
Summary of BRCA mutation assay results (no pathogenic mutation was identified)
| Germline (blood) | Breast tumor at diagnosis | Skin lesion prior to cisplatin monotherapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nucleotide | Amino acid | SNP no. | Nucleotide | Amino acid | SNP no. | Nucleotide | Amino acid | SNP no. | |
| BRCA1 | c.5383C>T | p.Leu1795Phe | rs878854958 | ||||||
| c.4837A>G | p.S1613G | rs1799966 | c.4837A>G | p.Ser1613Gly | rs1799966 | c.4837A>G | p.Ser1613Gly | rs1799966 | |
| c.4308T>C | p.S1436= | rs1060915 | c.4308T>C | p.Ser1436= | rs1060915 | c.4308T>C | p.Ser1436= | rs1060915 | |
| c.3548A>G | p.K1183R | rs16942 | c.3548A>G | p.Lys1183Arg | rs16942 | c.3548A>G | p.Lys1183Arg | rs16942 | |
| c.3113A>G | p.E1038G | rs16941 | c.3113A>G | p.Glu1038Gly | rs16941 | c.3113A>G | p.Glu1038Gly | rs16941 | |
| c.2612C>T | p.P871L | rs799917 | c.2612C>T | p.Pro871Leu | rs799917 | c.2612C>T | p.Pro871Leu | rs799917 | |
| c.2311T>C | p.L771 | rs16940 | c.2311T>C | p.Leu771= | rs16940 | c.2311T>C | p.Leu771= | rs16940 | |
| c.2082C>T | p.S694= | rs1799949 | c.2082C>T | p.Ser694= | rs1799949 | c.2082C>T | p.Ser694= | rs1799949 | |
| c.441+36_441+38delCTT | rs147856441 | c.441+36_441+38delCTT | rs147856441 | ||||||
| c.-19-115T>C | rs3765640 | c.-19-115T>C | rs3765640 | ||||||
| BRCA2 | c.1114A>C | p.N372H | rs144848 | c.1114A>C | p.Asn372His | rs144848 | c.1114A>C | p.Asn372His | rs144848 |
| c.3807T>C | p.V1269= | rs543304 | c.3807T>C | p.Val1269= | rs543304 | c.3807T>C | p.Val1269= | rs543304 | |
| c.4563A>G | p.Leu1521= | rs206075 | c.4563A>G | p.Leu1521= | rs206075 | ||||
| c.6513G>C | p.Val2171= | rs206076 | c.6513G>C | p.Val2171= | rs206076 | ||||
| c.7393G>A | p.Ala2465Thr | ||||||||
| c.7397C>T | p.Ala2466Val | rs169547 | c.7397C>T | p.Ala2466Val | rs169547 | ||||
SNP, single-nucleotide polymorphism.
The patient provided written informed consent for publication of clinical details and images.
Discussion
The National Comprehensive Cancer Network (NCCN) guidelines currently suggest platinum monotherapy as an alternative for metastatic TNBC based on the results of two small single arm phase II clinical trials [7,14,15]. In the TBCRC009 trial, although the tolerable response rate to platinum monotherapy was 25.6% in metastatic TNBC, progression-free survival was only 2.9 months, which was a disappointing result with respect to considering platinum as a first-line treatment [14]. However, this case showed that cisplatin monotherapy can be safe and effective for managing heavily pretreated TNBC patients without any major toxicities; thus, cisplatin monotherapy represents a possible palliative therapeutic option, although the current international guidelines do not recommend its routine use, especially for patients without germline BRCA 1/2 mutations [7].
BRCA1 plays a key role in the homologous recombination (HR) DNA repair system by initiating the repair of DNA double-strand breaks and thereby maintaining DNA stability, which makes it a potential predictive biomarker for DNA-damaging agents, such as platinum [16]. Moreover, a specific TNBC subtype harboring BRCA mutations has been shown to be more sensitive to cisplatin than taxane [17]. Nevertheless, in the current case, whole-genome sequencing of the blood and tumor tissue samples showed no pathogenic somatic or germline BRCA mutation. Interestingly, the germline BRCA mutation status was not found to play any predictive role in carboplatin efficacy in the GeparSixto trial, although carboplatin did generate a higher response rate in the TNBC subgroup [18]. Additionally, a preclinical study reported that, similar to the BRCA1-mutant cell type, a non-BRCA1 mutant basal-like cell line had a considerably high sensitivity to cisplatin treatment. Therefore, such an inconsistency in the efficacy of platinum agents against breast cancer with BRCA mutations suggested that BRCA mutation status might be insufficient for showing HR DNA repair abnormalities.
Instead of identifying the existence of BRCA mutations, “BRCAness” can be an alternative explanation for the existence of HR DNA repair abnormalities. For instance, homologous recombination deficiency (HRD) score, which is a measurement of BRCA promoter methylation, loss of heterozygosity, large-scale state transitions, or telomeric allelic imbalance, using next-generation sequencing can indicate BRCAness. In various small-sized retrospective and post hoc analyses, the HRD score has been highly correlated with the response to platinum-based treatment of TNBC patients with or without BRCA mutations [19]. However, in the TNT trial, no statistically significant difference in the response has been identified according to HRD score; long-term studies for estimating the advantages of platinum for TNBC patients are still warranted [20]. Although the DNA repair mechanism is extremely complex, a comprehensive genomic alteration test should be conducted to understand the actual efficacy of DNA-targeting agents for DNA repair and facilitate the exploration of alternative mechanisms underlying cancer progression and resistance to chemotherapeutic agents.
In this case report, a dramatic and durable response to cisplatin monotherapy was observed in a heavily pretreated TNBC patient with a poor performance status. Although platinum agents have shown effective clinical outcomes for BRCA-mutated TNBC, they should also be considered as an option for treating advanced TNBC without BRCA mutations.
Footnotes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010;15(Suppl 5):39–48. doi: 10.1634/theoncologist.2010-S5-39. [DOI] [PubMed] [Google Scholar]
- 2.O’Toole SA, Beith JM, Millar EK, West R, McLean A, Cazet A, et al. Therapeutic targets in triple negative breast cancer. J Clin Pathol. 2013;66:530–42. doi: 10.1136/jclinpath-2012-201361. [DOI] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 4.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 5.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–63. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mersin H, Yildirim E, Berberoglu U, Gulben K. The prognostic importance of triple negative breast carcinoma. Breast. 2008;17:341–6. doi: 10.1016/j.breast.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN) Plymouth Meeting (PA): NCCN; 2019. NCCN guidelines: breast cancer (version 3.2019) [Internet] Available at: https://www.nccn.org. [Google Scholar]
- 8.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 9.Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–71. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirohi B, Arnedos M, Popat S, Ashley S, Nerurkar A, Walsh G, et al. Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol. 2008;19:1847–52. doi: 10.1093/annonc/mdn395. [DOI] [PubMed] [Google Scholar]
- 11.Koshy N, Quispe D, Shi R, Mansour R, Burton GV. Cisplatin-gemcitabine therapy in metastatic breast cancer: improved outcome in triple negative breast cancer patients compared to non-triple negative patients. Breast. 2010;19:246–8. doi: 10.1016/j.breast.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal LS, Mayer IA. Platinum agents in the treatment of early-stage triple-negative breast cancer: is it time to change practice? Clin Adv Hematol Oncol. 2014;12:654–8. [PubMed] [Google Scholar]
- 13.Staudacher L, Cottu PH, Dieras V, Vincent-Salomon A, Guilhaume MN, Escalup L, et al. Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Ann Oncol. 2011;22:848–56. doi: 10.1093/annonc/mdq461. [DOI] [PubMed] [Google Scholar]
- 14.Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–9. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–53. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortesi L, Masini C, Cirilli C, Medici V, Marchi I, Cavazzini G, et al. Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer. 2010;10:90. doi: 10.1186/1471-2407-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zander SA, Kersbergen A, van der Burg E, de Water N, van Tellingen O, Gunnarsdottir S, et al. Sensitivity and acquired resistance of BRCA1;p53-deficient mouse mammary tumors to the topoisomerase I inhibitor topotecan. Cancer Res. 2010;70:1700–10. doi: 10.1158/0008-5472.CAN-09-3367. [DOI] [PubMed] [Google Scholar]
- 18.Hahnen E, Lederer B, Hauke J, Loibl S, Krober S, Schneeweiss A, et al. Germline mutation status, pathological complete response, and disease-free survival in triple negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017;3:1378–85. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22:3764–73. doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med. 2018;24:628–37. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]