Abstract
A central feature of diabetic wounds is the persistence of chronic inflammation, which is partly due to the prolonged presence of pro-inflammatory (M1) macrophages. Using in vivo and in vitro analyses, we have tested the hypothesis that lncRNA GAS5 (Growth Arrest-Specific 5) is dysregulated in diabetic wounds. We have assessed the contribution of GAS5 to the M1 macrophage phenotype, as well as the functional consequences of knocking down its expression. We found that expression of GAS5 is significantly increased in diabetic wounds and in cells isolated from diabetic wounds. Hyperglycemia induced GAS5 expression in macrophages in vitro. Overexpression of GAS5 in vitro promoted macrophage polarization towards an M1 phenotype by upregulating STAT1. Of most significance in our judgment, GAS5 loss-of-function enhanced diabetic wound healing. These data indicate that the relative level of lncRNA GAS5 in wounds plays a key role in the wound-healing response. Reductions in the levels of GAS5 in wounds appeared to enhance healing by promoting transition of M1 macrophages to M2 macrophages. Thus, our results suggest that targeting lncRNA GAS5 may provide a therapeutic intervention for correcting impaired diabetic wound healing.
Keywords: Diabetic wounds, Long non-coding RNA, GAS5, Macrophage polarization, STAT1
Introduction
Diabetes has reached epidemic proportions in the United States as well as globally, and impaired diabetic wound healing is a significant and growing clinical problem(Prevention, 2014). In 2014, more than 70,000 lower extremity amputations were performed in diabetic patients(Prevention, 2014), and nearly 85% of these non-traumatic amputations were preceded by a diabetic wound (Boulton et al., 2005). Despite the enormous impact of these wounds on both individuals and society, effective therapies are lacking. Thus, acquiring the capacity to correct diabetes impaired wound healing has far reaching consequences, both with respect to patient outcomes as well as on healthcare expenditures.
The impaired healing of diabetic wounds has been shown to be multifactorial (Blumberg et al., 2012). A central pathogenic feature of diabetic wounds is the persistence of chronic inflammation (Pierce, 2001), which is partly due to the sustained presence of pro-inflammatory (M1) macrophages in diabetic wounds (Wetzler et al., 2000) (Khanna et al., 2010, Tang et al., 2013). Macrophages are essential in all phases of wound healing. Early in the response to injury, macrophages are polarized to the M1 phenotype. M1 macrophages produce pro-inflammatory cytokines, and stimulate the production of reactive oxygen species (ROS) to promote clearance of bacteria and debris from the wound (Landen et al., 2016, Wynn and Vannella, 2016). After the initial inflammatory phase, macrophages transition to an M2 phenotype. M2 macrophages are associated with resolution of the inflammatory response and promotion of wound remodeling and closure (Landen et al., 2016, Wynn and Vannella, 2016). In diabetic wounds, persistence of the M1 macrophage phenotype and failure to transition to the M2 phenotype are associated with delayed healing (Wicks et al., 2014). The underlying mechanism, however, has remained far from clear. We report here our recent progress toward elucidating the mechanism of delayed healing of diabetic wounds.
We have uncovered a specific role of a class of non-coding RNAs having a length longer than 200 nucleotides (lncRNAs). The expression of lncRNAs is tissue- and cell-type specific, and is associated with developmental status (Kung et al., 2013). LncRNAs may exert their functions either by binding to DNA or to RNA in a sequence specific manner, or by binding to proteins. This multiplicity of binding strategies suggests that lncRNAs can affect expression of their target genes at epigenetic, transcriptional, post-transcriptional, translational, or post-translational levels (Kung et al., 2013). LncRNAs have been found to be differentially expressed in various types of cancer including leukemia, breast cancer, hepatocellular carcinoma, colon cancer, and prostate cancer; their expression is also dysregulated in cardiovascular diseases, neurological disorders, and immune-mediated diseases (Wapinski and Chang, 2011). Of particular interest to our efforts is that a possible role of lncRNAs in diabetic wounds is now emerging (Reddy et al., 2014, Zgheib et al., 2017).
A particular lncRNA, growth arrest specific 5 (GAS5), has been widely studied in in various types of cancer such as breast, prostate, lung, and colorectal cancer (Pickard and Williams, 2015). GAS5 has been identified as tumor suppressor, and is functionally involved in inhibiting cell proliferation and promoting cellular apoptosis (Pickard and Williams, 2015). Like many tumor suppressors, GAS5 displays reduced expression in cancer cells. GAS5 has no known function in wound healing.
Thus, we hypothesized that lncRNA plays a role in delayed healing of diabetic wounds. We hypothesized that lncRNA GAS5 was dysregulated in diabetic wounds, and that dysregulated GAS5 may be partly responsible for the persistence of M1 macrophages in diabetic wounds. We further hypothesized that GAS5 promotes the M1 phenotype through regulating signal transducer and activator of transcription 1 (STAT1), the crucial transcriptional regulator in M1 macrophage polarization. If correct, then we would expect that reducing the GAS5 level would have a functional effect on diabetic wound healing.
Results
GAS5 expression is significantly higher in diabetic wounds and human diabetic skin
We first checked the expression of GAS5 in unwounded skin (D0 in Figure 1a), there was no significant difference between non-Db and Db unwounded skin on the expression of GAS5. To investigate whether GAS5 plays a role in diabetic wounds, we compared the expression of GAS5 in non-diabetic and diabetic wounds at day 3 and day 7 after injury. Results from real-time qPCR showed that the expression of GAS5 was significantly up-regulated in diabetic wounds compared to non-diabetic wounds at both day 3 and day 7 after injury (Figure 1a). The increased expression of GAS5 in diabetic wounds suggested that it might play a role in the wound healing process. We also examined the expression of GAS5 in human non-diabetic and diabetic skin. Similar to the results seen in mouse diabetic wounds, GAS5 expression was significantly up-regulated in human diabetic skin compared to human non-diabetic skin (Figure 1b).
Figure 1. GAS5 expression is significantly higher in diabetic wounds and human diabetic skin.
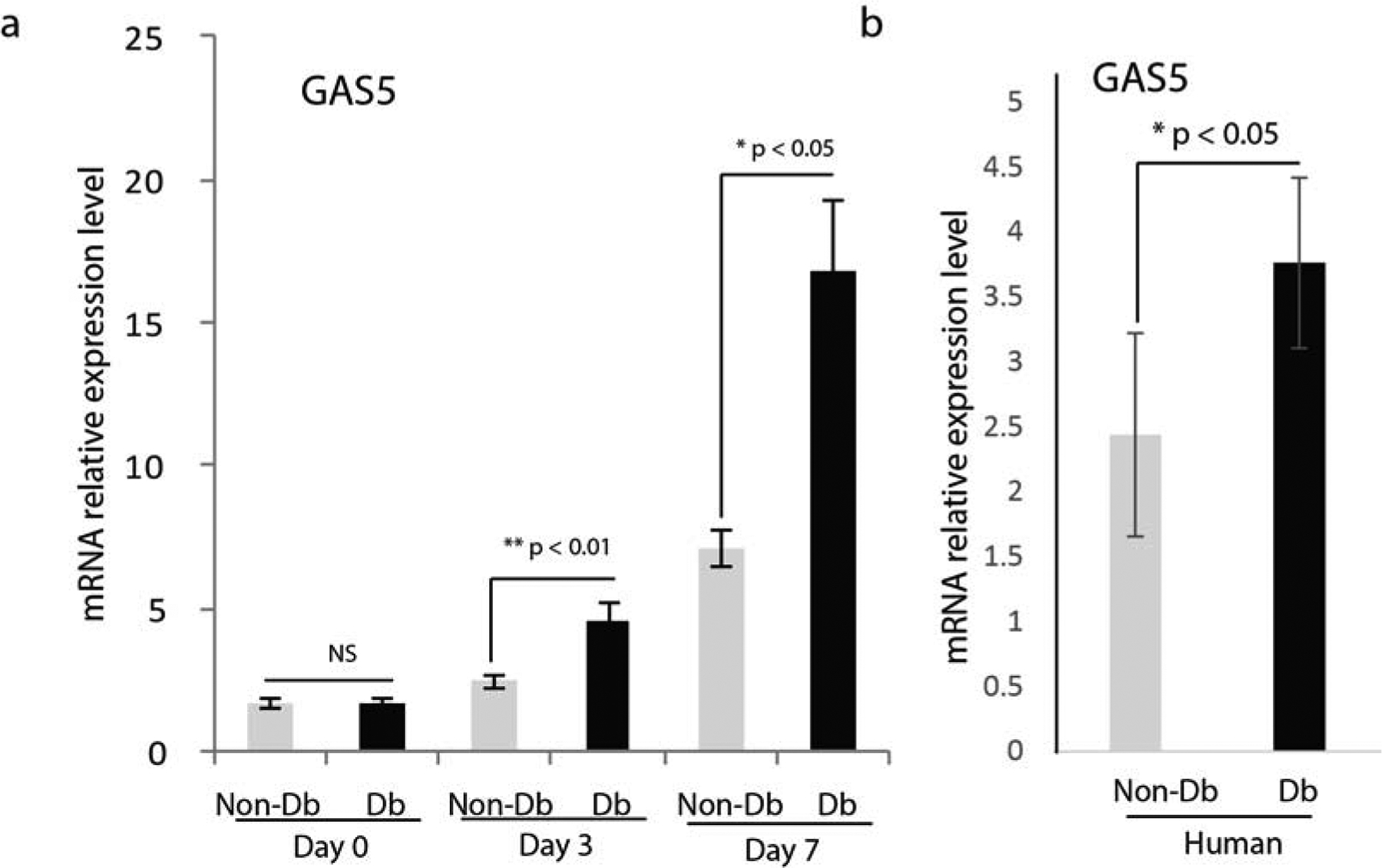
(a) Realtime qPCR analysis of GAS5 mRNA expression in mouse diabetic and non-diabetic dermal skin (D0) and wounds at day 3 (mean±SD, n=5 per group) and day 7 (mean±SD, n=5 per group) after injury. (b) Realtime qPCR analysis of GAS5 mRNA expression in human non-diabetic and diabetic skin.
GAS5 expression is significantly higher in diabetic fibroblasts, induced by hyperglycemia in fibroblasts and macrophages, and highly expressed in M1 macrophage
We then assessed GAS5 expression at the cellular level. Dermal fibroblasts were isolated from non-diabetic or diabetic age/gender-matched mouse skin. Results of real-time qPCR showed that the expression of GAS5 was significantly up-regulated in diabetic fibroblasts compared to non-diabetic fibroblasts (Figure 2a).
Figure 2. GAS5 expression in fibroblasts and macrophages under normal glucose or hyperglycemic conditions.
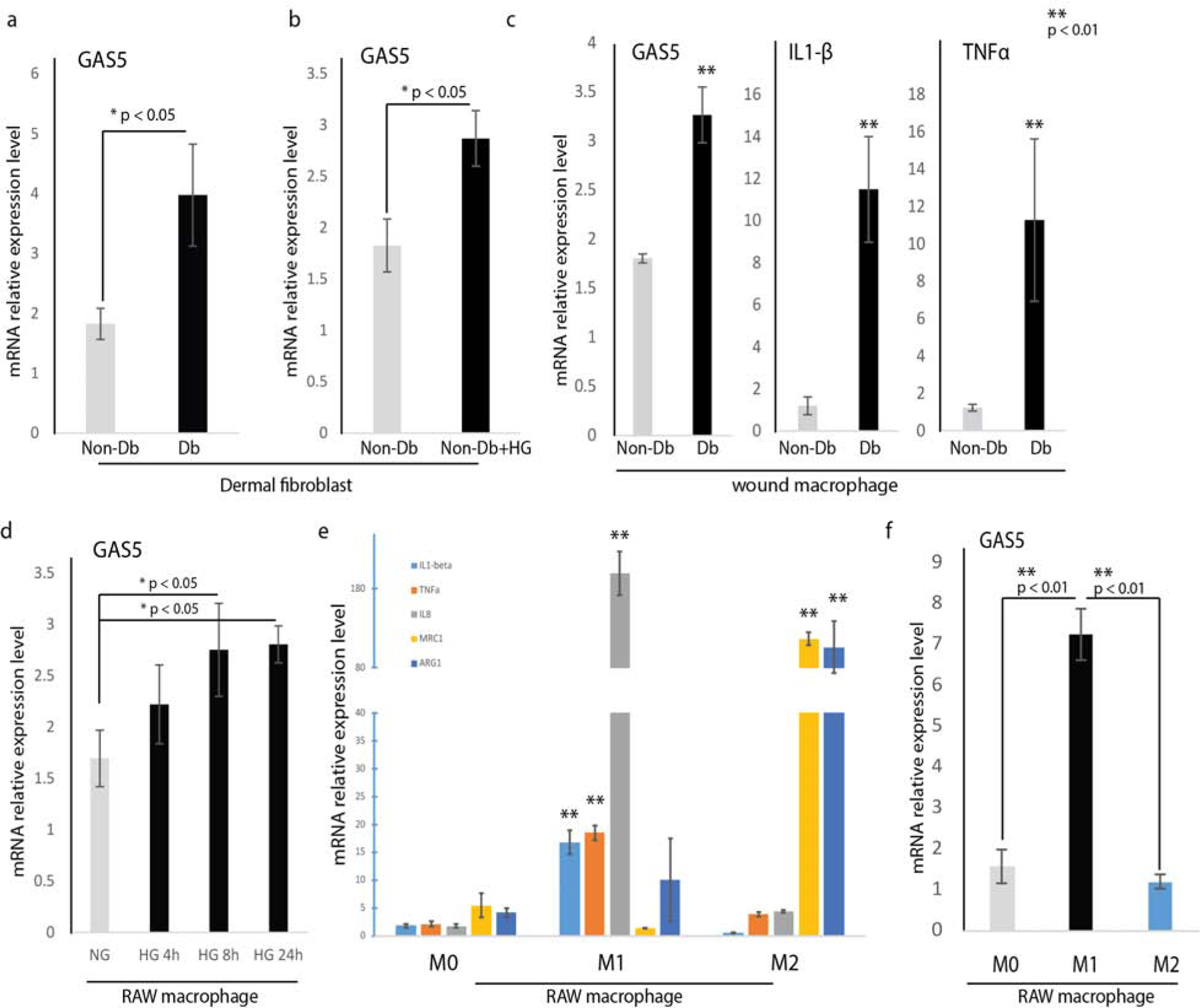
(a) Realtime qPCR (mean±SD, n=3 per group) analysis of GAS5 gene expression in diabetic dermal fibroblasts and non-diabetic dermal fibroblasts. (b) GAS5 expression in high glucose (25 mM D-glucose)-treated non-diabetic dermal fibroblasts compared to normal glucose (5 mM D-glucose)-treated non-diabetic dermal fibroblasts. (c) RNA analyses by RT-qPCR showed significantly increased GAS5, IL1-β and TNFα gene expression in mouse diabetic and non-diabetic wound macrophages (mean+ SD, n=3 per group). (d) GAS5 expression in RAW cells treated with high glucose conditions (25 mM D-glucose) for 4, 8, or 24 hours. (e) RAW macrophages were treated with LPS (10 pg/ml) and IFN-r (20 ng/ml) for 24 h to generate M1 macrophages, or IL-4 (20 ng/ml) for 24 h to generate M2 macrophages after overnight serum starvation. RNAs were isolated and the expressions of M1 markers IL1-β, TNFα, IL8 and M2 markers MRC1, Arg1were determined by realtime qPCR. n = 3; mean ± SD; ** P < 0.01 compared with untreated normal RAW macrophages. (f) Levels of GAS5 in M1 and M2 macrophages were determined by realtime qPCR. U6 was used as an internal control. n = 3; mean ± SD; ** P < 0.01 compared with untreated normal RAW macrophages.
Given that diabetic cells are chronically exposed to hyperglycemic conditions, we asked whether hyperglycemia affected GAS5 expression in non-diabetic fibroblasts. To do this, we cultured non-diabetic fibroblasts in low (5 mM) or high (25 mM) glucose media for 24 hours, and found that culture of non-diabetic fibroblast in high glucose media significantly induced GAS5 gene expression compared to dermal fibroblasts cultured in low glucose media (Figure 2b).
We isolated wound macrophages from non-diabetic and diabetic wounds at day3 after injury according to protocols (Brubaker et al., 2011, Kimball et al., 2017). We routinely obtain 105 wound macrophages per 100 mg wounds, and have confirmed that these cells are ~98% macrophages based on F4/80 and CD11b staining. Results of RT-qPCR analysis indicated that GAS5 was up-regulated in diabetic wound macrophages similar to diabetic fibroblasts; the pro-inflammatory marker genes IL1-β and TNFα were also highly induced in diabetic wound macrophages (Figure 2c). These studies provide evidence that GAS5 and M1 marker genes are also dysregulated in diabetic wound macrophages.
We further analyze the expression of GAS5 in RAW macrophage. First, we asked whether hyperglycemia altered GAS5 expression in macrophages. After culture of RAW macrophages in low (5 mM) or high (25 mM) glucose media for 4, 8, or 24 hours, Realtime results of qPCR demonstrated that GAS5 was significantly induced by hyperglycemia in a time-dependent manner (Figure 2d). Then we asked whether the expression of GAS5 is altered in different macrophage phenotype. To answer this question, RAW were treated with either LPS or IL-4 to generate M1 and M2 macrophages, respectively. As shown in Figure 2e, IL1-β, TNF-α and IL8 were highly expressed in M1 macrophages while MRC1 and ARG1 were highly expressed in M2 macrophages, confirming the M1 and M2 activating states respectively. Results from realtime qPCR analysis indicated that GAS5 was highly expressed in M1 macrophages but at a lower level in M2 macrophages (Figure 2f).
Overexpression of GAS5 induces a pro-inflammatory macrophage phenotype, and increased expression of M1 maker genes
During the wound healing process, Macrophages can adopt different phenotypes (M1 or M2) in response to environment stimuli. To test whether GAS5 plays a role in macrophage polarization, RAW macrophages were transfected with a pcDNA expressing either GAS5 or pcDNA3.1 (control). Our results showed that GAS5 gene expression was increased in the GAS5-transfected cells, confirming successful transfection of the RAW cells with pGAS5 (Figure 3a). Next, we measured expression of M1 and M2 marker genes following transfection with either pGAS5 or pcDNA3.1. We found that that GAS5 overexpression induced expression of M1 marker genes iNOS, IL1 beta, and TNFa compared to RAW macrophages treated with the empty vector control (Figure 3b), but did not affect expression of M2 marker genes (Arg1 and Mrc1) (Figure 3c). We further compared GAS5 overexpression to LPS+IFNγ-induced M1 macrophage. M1 marker genes iNOS, IL1 beta, and TNFa showed a similar tendency, although higher in LPS+IFNγ-induced M1 macrophage (Figure 3b, comparing the red to the black bar). Compared to IL4-induced M2 macrophage, GAS5 overexpression showed no effect on M2 marker genes (Arg1 and Mrc1) (Figure 3c, comparing the red to the black bar). These data suggest that GAS5 is a positive inducer for pro-inflammatory macrophages. Furthermore, we applied flow cytometric analysis using cell surface markers CD11b and CD86 to identify M1 macrophages. Our results showed that RAW cells transfected with pGAS5 had significantly more CD11b and CD86 double positive cells compared to RAW macrophages transfected with the empty vector control (28.4%+2.03% vs 3.15%+1.08%, n=3, p < 0.001) (Figure 3d). These data provided additional evidence that GAS5 induced macrophages to assume the M1 phenotype.
Figure 3. Effects of overexpression of GAS5 on induction of the pro-inflammatory macrophage phenotype, and expression of M1 macrophage marker genes.
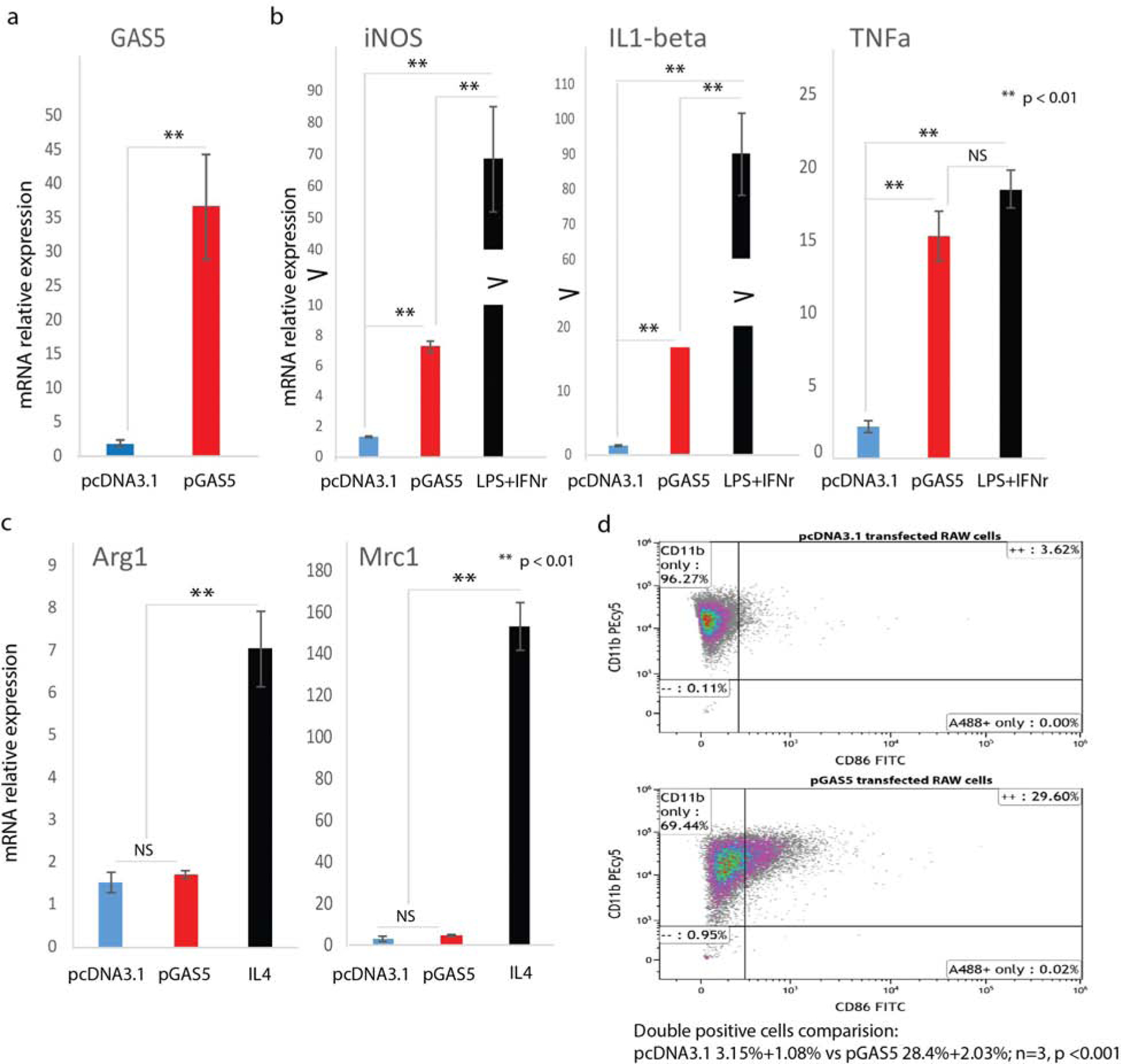
(a) Overexpression of GAS5 mRNA was achieved by plasmid transfection and confirmed by RT-qPCR (mean±SD, n=3 per group). GAS5 gene expression was assessed in RAW macrophages transfected with pGAS5 or transfected with pcDNA3.1. (b) Expression of M1 marker genes iNOS, IL1-beta, and TNFa following transfection of RAW macrophages with pGAS5 (mean±SD, n=5 per group). (c) Expression of M2 marker genes Arg1 and Mrc1 following transfection of RAW macrophages with pGAS5 (mean±SD, n=5 per group). (d) Flow cytometric analysis of M1 macrophage surface markers CD11b and CD86 (28.4%+2.03% vs 3.15%+1.08%, n=3, p < 0.001) in RAW cells overexpressing GAS5.
GAS5 induces STAT1 in macrophages
Macrophage polarization is a dynamic process involving continuous adaptation to environmental stimuli and activation of transcription factors. STAT1 has been considered a crucial inducer of the M1 macrophage phenotype(Kovarik et al., 1998, Ohmori and Hamilton, 2001). We asked whether GAS5 overexpression induced the M1 macrophage phenotype through up-regulating STAT1. Results from realtime qPCR analysis indicated that expression of STAT1 mRNA was significantly higher in GAS5 overexpressing RAW macrophages than in empty vector transfected RAW macrophages (Figure 4A); there was no change in expression of STAT3 or STAT6 mRNA, which are essential to M2 macrophage polarization (Figure 4a). In another set of experiments, we transfected RAW macrophages with increasing amounts of the pGAS5 expression vector (0, 4 ng, 40 ng and 400 ng), and evaluated STAT1 gene expression. We found that STAT1 expression increased in a dose-dependent manner in response to increasing amounts of pGAS5 used to transfect macrophages (Figure 4b). We also evaluated STAT1 protein expression by western blot. We found that both pSTAT1 and total STAT1 were significantly induced by GAS5 overexpression compared to empty vector or no treatment group (Figure 4c). We assessed pSTAT3 and total STAT3, and found no significant change between groups (Figure 4c). We also found no change in expression of pERK, a key component in the MEK/ERK signaling pathway, nor in expression of ARG1, an M2 macrophage marker (Figure 4c). Densitometry analysis was presented in Figure 4d, also indicating that overexpression of GAS5 significantly induced the expression of STAT1, and produced no significant change for pERK and ARG1. These data suggest that GAS5 is a positive regulator of STAT1 expression.
Figure 4. GAS5 induces STAT1 expression in macrophages.
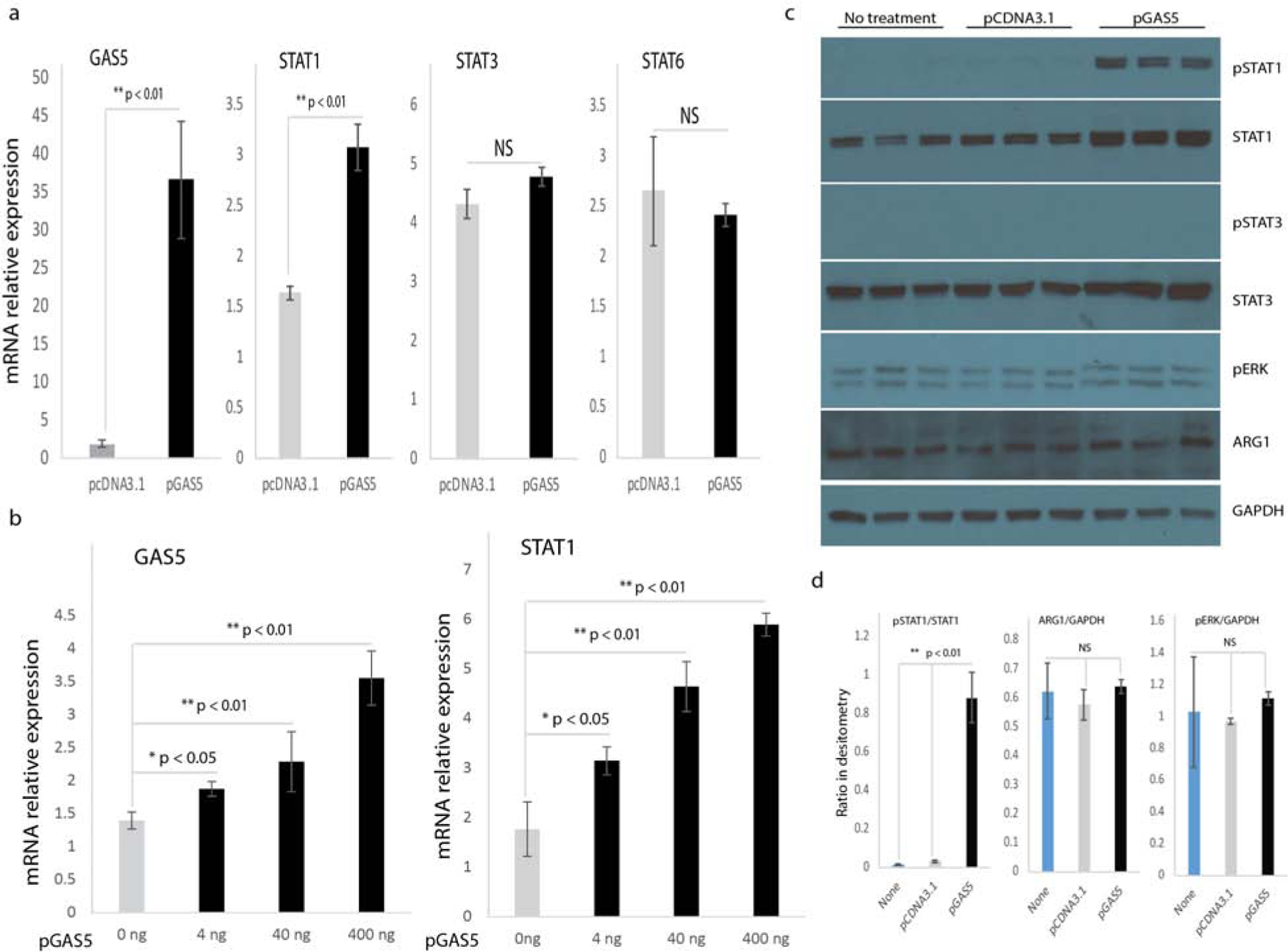
(a) STAT1, STAT3, and STAT6 gene expression in RAW macrophages transfected with pGAS5 or pcDNA3.1 (n=3, p < 0.05). (b) GAS5 and STAT1 gene expression in macrophages transfected with increasing amounts of pGAS5. (c) Western blot analysis of pSTAT1, STAT1, pSTAT3, STAT3, pERK, and ARG1 following transfection of RAW macrophages with pGAS5. GAPDH served as an internal loading control. (d) Quantification of relative intensities for the ratio of pSTAT1/STAT1, ARG1/GAPDH, and pERK/GAPDH from western blot.
STAT1 is required for expression of M1 marker genes
We asked whether STAT1 expression was essential for expression of the M1 macrophage phenotype. For this experiment, we knocked down STAT1 expression in RAW macrophages by transfection with STAT1 siRNA (Figure 5a). We then assessed expression of M1 marker genes, and found that expression of iNOS, IL1 beta, and TNFa was significantly reduced in RAW macrophages following STAT1 knockdown (Figure 5b), whereas expression of M2 marker genes (Arg1 and Mrc1) was unchanged (Figure 5c). These data indicate that STAT1 was required for display of the M1 macrophage phenotype. These data is also consistent with previous reports that suggest Stat1 to be a prime focus for immunological stimuli in a macrophage proinflammatory response (Kovarik et al., 1998, Ohmori and Hamilton, 2001).
Figure 5. STAT1 is required for expression of M1 marker genes.
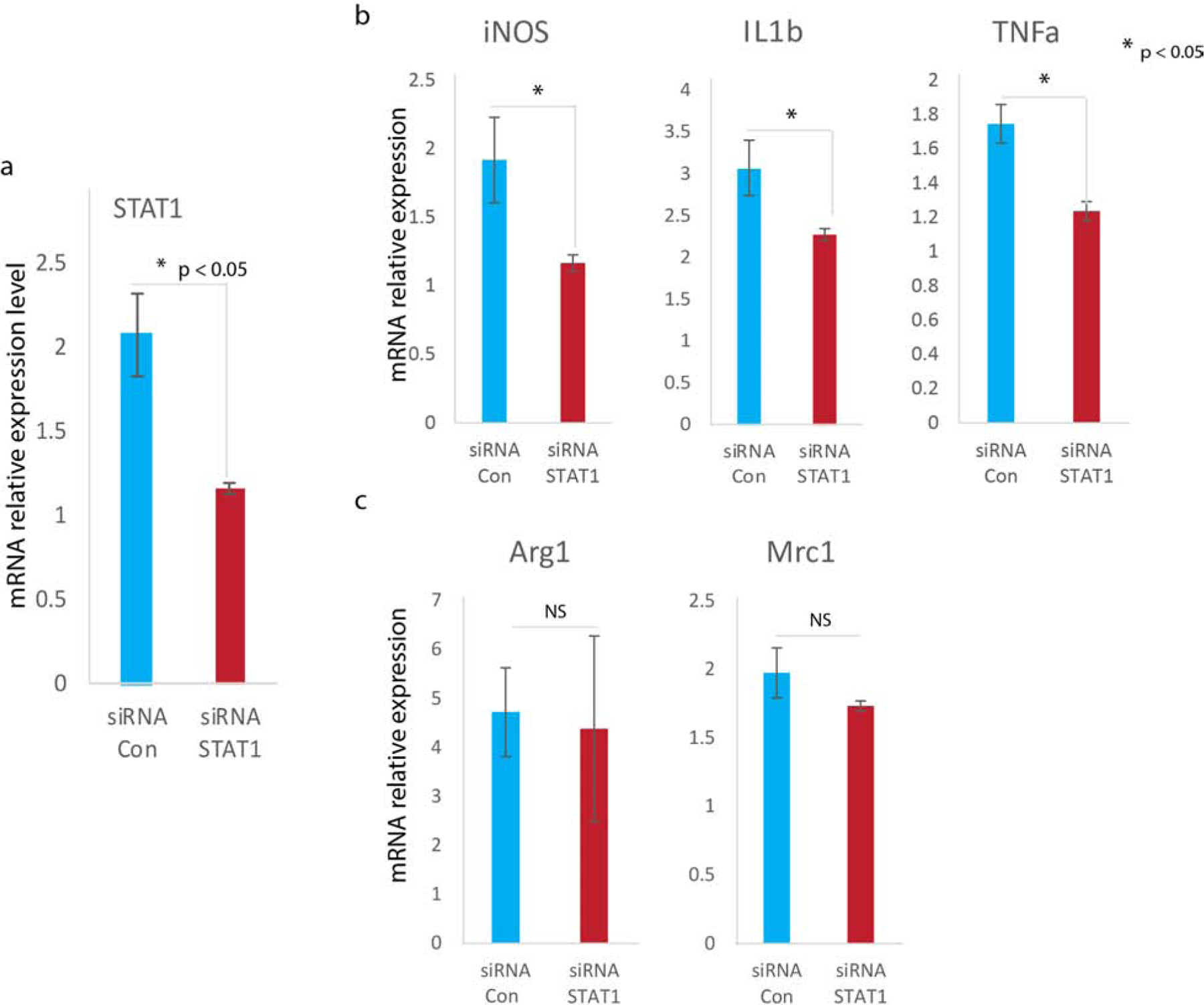
(a) Knock down of STAT1 gene expression by siRNA transfection assessed by RT-qPCR (mean±SD, n=3 per group). (b) Expression of M1 marker genes iNOS, IL1-beta, and TNFa following STAT1 siRNA transfection (mean±SD, n=5 per group). (c) Expression of M2 marker genes Arg1, Mrc1 following STAT1 siRNA transfection (mean±SD, n=5 per group).
Knockdown of GAS5 enhances diabetic wound healing.
To achieve in vivo knockdown of GAS5, we designed and constructed a Lenti-shRNA to specifically knock down GAS5 expression. We monitored GAS5 expression 24 hours after shRNA-mediated knockdown in RAW macrophages. Lenti-shGAS5 transfectants exhibited significantly lower GAS5 expression than did Lenti-shCon transfectants. Lower GAS5 expression resulted in a reduction of expression of IL6 and TNFa (Figure 6 b1 to b3). To confirm in vivo knockdown of GAS5, we harvested diabetic wound macrophages at day 3 after injury. GAS5 gene expression analysis indicated that diabetic wound macrophage infected with Lenti-shGAS5 has 50% less GAS5 expression compared to diabetic wound macrophage infected with Lenti-GFP control (Figure 6 b4). To evaluate the functional consequences of GAS5 knockdown, we treated wounds with lentivirus. Full-thickness excisional 8 mm wounds were created in Db and non-Db mice, and were immediately treated with 106 plaque-forming units of a lentiviral construct containing shGAS5 or GFP (n=5 per group). We monitored wound healing over the course of 16 days. Initial wound size was calculated immediately after wounding, and wound closure was assessed over time as the percentage of initial wound area. Figure 6a indicated the wound healing effect of shGAS5 topical administration on excision wound model on different days after injury. By day 8 after wounding, Db wounds treated with lenti-shGAS5 exhibited a decrease in wound surface area compared to Db wounds treated with Lenti-GFP, indicating that knockdown of GAS5 in diabetic wounds enhanced wound healing (Figure 6c). Epithelial gap analysis was performed on day 8 wounds H&E slide. Quantitative analysis revealed that the epithelial gap of Lenti-shGAS5 treated diabetic wounds (4.20 ± 0.05 mm) was significantly smaller than that of lenti-GFP treated diabetic wounds (4.81 ± 0.05 mm, n=5, P < 0.001) (Figure 6d, e). Full wound closure was achieved at 14 days in lenti-shGAS5 treated diabetic wounds and at 16 days in Lenti-GFP treated diabetic wounds (Figure 6c).
Figure 6. Diabetic wound healing in vivo following knockdown of GAS5.

(a) The wound healing effect of lentivirus mediated knockdown of GAS5 on 8 mm excisional wounds as it heals at each time point (mean±SD, n=5 per group). (b) Knockdown of GAS5 gene expression was achieved by short hairpin RNA (shRNA) technology and assessed by RT-qPCR (mean±SD, n=3 per group). A scramble sequence shRNA (sh-Con) was used as a negative control. GAS5 was confirmed to knockdown by shGAS5 (b1), IL6 (b2) and TNFα (b3) expression following transfection of RAW macrophages with sh-GAS5 (mean±SD, n=3 per group) was significantly reduced. GAS5 expression analysis in diabetic wound macrophage treated with Lenti-sh-GAS5 or Lenti-GFP (b4, mean±SD, n=3 per group). (c) Wound size change during the healing process of initial 8 mm wounds in non-Db and Db mice treated by injection of different lenti-virus constructs. Non-Db wounds: Lenti-GFP, blue; Lenti-shGAS5, gray; Db wounds: Lenti-GFP, orange; Lenti-shGAS5, yellow. (d) The edge of the encroaching epithelium was indicated by lines and marked with epithelial gap. Upper image was Db wounds treated with Lenti-GFP at day 8 after injury; bottom image was Db wounds treated with Lenti-shGAS5 at day 8 after injury. Scale bar =1mm. (E) Epithelial gap of diabetic wounds treated with Lenti-GFP (gray bar) was significantly higher to diabetic wounds treated with Lenti-shGAS5 (black bar) (mean+ SD, n=5, ** p <0.01).
Discussion
LncRNAs are the largest class of non-coding transcripts in the human genome, and have been widely recognized as important regulators in development and in various human diseases (Rao et al., 2017, Wu and Du, 2017). In the clinic, specific lncRNAs have been used as biomarkers for prognosis and diagnosis (Chandra Gupta and Nandan Tripathi, 2017, Viereck and Thum, 2017). However, the role of lncRNAs in diabetic wound healing has remained unclear. Recently, the pathway of GAS5 in human diabetic foot ulcer and the role of c-Myc was studied in fibroblasts and keratinocytes. Sawaya et al. identified the induction of GAS5 expression by statins as a mechanism to accelerate epithelialization in porcine partial thickness wounds (Sawaya et al., 2018). In esophageal squamous cell carcinoma, GAS5 is transcriptionally activated by IFNs via the JAK-STAT pathway, and plays an antitumor role (Huang et al., 2018). Here, we report a previously unreported function of GAS5, a known tumor suppressor in various cancers, in regulating macrophage polarization. We observed that GAS5 was highly expressed in diabetic wounds and wound-derived cells, prompting us to examine a possible role for GAS5 in wound healing. In hyperglycemic conditions, GAS5 was significantly induced in both dermal fibroblasts and macrophages, indicating that up-regulated GAS5 expression in diabetic wounds may result from hyperglycemia. We then forced GAS5 expression in macrophages, and found that over-expression of GAS5 resulted in significantly increased expression of M1 macrophage marker mRNAs (iNOS, TNFa, and IL1-Beta), but having no effect on expression of M2 macrophage marker RNAs (Arg1, and Mrc1), which suggested that GAS5 promoted display of the pro-inflammatory macrophage phenotype. Mechanistically, GAS5 overexpression promoted expression of the M1 macrophage phenotype through significantly induced STAT1 expression, a critical transcription factor for M1 activation. These results suggest a link between an lncRNA and the macrophage M1 phenotype in diabetic wounds.
Normal wound repair follows an orderly and well-defined sequence of events that requires the interaction of many cell types and growth factors; healing is divided into inflammatory, proliferative, and remodeling phases (Xu et al., 2012). In diabetic wound healing, this complex orchestration of the wound healing processes and orderly appearance of its phases are disrupted. While the etiology is multifactorial, increased and persistent inflammation, a consequence of imbalance between pro-inflammatory and anti-inflammatory cytokines (Khanna et al., 2010), has been implicated as a central feature of impaired wound healing and other complications in diabetics. Macrophages are essential for each phase of wound healing, and represent a major source of cytokines found in wounds (Boniakowski et al., 2017). Analysis of macrophages from diabetic wounds has indicated that they are dysfunctional, including significant impairment in efferocytosis activity (Khanna et al., 2010). Macrophages in diabetic wounds can produce high levels of pro-inflammatory cytokines, which prevent transition from pro-inflammatory (M1) macrophages to anti-inflammatory (M2) macrophages (Goren et al., 2007). Therefore, identification of diabetic wound-associated lncRNAs, and analysis of their functions may provide new treatment options in diabetic wound healing. In our study here, we found that the significant up-regulation of GAS5 in diabetic wounds was associated with increased expression of iNOS, IL1-beta, and TNFa mRNAs, marker genes for M1 macrophages, indicating that GAS5 plays a role in promoting the M1 macrophage phenotype.
We then assessed the mechanisms by which GAS5 promoted the M1 macrophage phenotype. STAT signaling is a central pathway in controlling macrophage M1-M2 polarization (Lawrence and Natoli, 2011). The transcription factor STAT1 has been described as a key component in promoting M1 macrophages (Kovarik et al., 1998, Ohmori and Hamilton, 2001), in various circumstances such as in sodium azide-treated human THP-1 and mouse RAW macrophages (Lawrence and Natoli, 2011), in iron-treated RAW macrophages (Gan et al., 2017), in HIV infected macrophages (Appelberg et al., 2017), and in osteopontin-treated mouse macrophages (Guo et al., 2017). T cell Ig mucin-3 (Tim-3) has been demonstrated to be involved in the development of tumor-promoting M2 macrophages through inhibition of STAT1 (Jiang et al., 2016). Moreover, STAT1 deletion results in a shift from Th1 to Th2, and defective classical macrophage activation (Leopold Wager et al., 2014). We found that overexpression of GAS5 increased the expression of STAT1 mRNA and protein, indicating that GAS5 exerts its effect on macrophage polarization via regulating the expression of STAT1. We also noticed that overexpression of GAS5 had no effect on STAT3 or STAT6, which are critical transcription factors for the M2 macrophage phenotype.
Our in vivo data indicate that GAS5 loss-of-function enhanced diabetic wound healing. In this experiment, we knocked down GAS5 expression levels in wounds through Lentivirus mediated shRNA technology. Overall wound closure in diabetic wounds treated with Lenti-sh-GAS5 was improved by 2 days. Importantly, at day 8, there is a 40% reduction by the Lenti-sh-GAS5 treated diabetic wounds, and this acceleration in wound closure is exceptional and of clinical relevance (Hobizal and Wukich, 2012). In short, modulation of lncRNA GAS5 level has beneficial and functional effects in wound healing.
These findings provide evidence that the lncRNA GAS5 is involved in the regulation of macrophage polarization, and suggest a potential role of lncRNA GAS5 in the pathogenesis of impaired diabetic wound healing. The clear implication of our findings is that GAS5 might be therapeutically targeted to correct the impairment in diabetic wound healing.
Materials & Methods
Animal studies
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver - Anschutz Medical Campus, and experimental protocols followed the guidelines described in the NIH Guide for the Care and Use of Laboratory Animals. In these experiments, we used 10 week old, female, genetically diabetic C57BKS.Cgm/Leprdb/J (Db) mice and heterozygous, non-diabetic (non-Db), age-matched female controls from the Jackson Laboratory (Bar Harbor, ME). Mice were anesthetized with inhaled isoflurane. Each mouse was shaved and depilated before wounding. The dorsal skin was swabbed with alcohol and Betadine (Purdue Pharma, Stamford, CT). Each mouse received a single, full-thickness dorsal wound (including panniculus carnosum) with an 8-mm punch biopsy (Miltex Inc, York, PA). All wounds were dressed with Tegaderm (3M, St Paul, MN), which was subsequently removed on postoperative day 2. Postoperatively, the mice received a subcutaneous injection of an analgesic, Banamine (Schering-Plough Animal Health Corp., Union, NJ). A full-thickness skin sample, centered on the wound, was harvested 3 and 7 days after surgery (n=5 per timepoint).
Human samples
Human skin samples at least 5 × 5 cm were collected postmortem from the anterior portion of the lower extremity of individuals with and without diabetes. The specimens were collected within eight hours of death through the National Disease Research Interchange with IRB approval._Patient consent for experiments was not required for deidentified human tissue left over from surgery. Samples were obtained from patients who were 45 to 65 years of age who had no known comorbid malignancy or history of radiation or chemotherapy. The skin samples were immediately flash frozen in liquid nitrogen.
Cell culture and Reagents
RAW 264.7 macrophages (ATCC, USA) were maintained in DMEM (Gibco, USA) supplemented with 10% (v/v) FBS (Gibco, USA), 100 μg/ml streptomycin, and 100 U/ml penicillin, and incubated at 37°C and an atmosphere of 5% CO2. Cell s were serum-starved overnight in DMEM with 1% FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin before being treated with 5 mM D-glucose (LG) DMEM, 25 mM D-Glucose (HG), for 24 hours as described (Jia et al., 2015). RAW macrophages were treated with LPS (10 pg/ml) and IFN-r (20 ng/ml) for 24 h to generate M1 macrophages, or IL-4 (20 ng/ml) for 24 h to generate M2 macrophages after overnight serum starvation. RAW cells were analyzed at passage 2 to 3 in this study.
Dermal fibroblast and wound macrophage isolation and analysis
Dermal fibroblasts were isolated as described (Gallucci et al., 2006). Briefly, skin from adult non-diabetic and diabetic mice were placed dermal side down to allow fibroblast outgrowth. Dermal fibroblasts were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics. Cultures were maintained in a humidified incubator in an atmosphere of 5% CO2, 95% air. Dermal fibroblasts were phenotyped by gross morphology with the classical long spindle shape of fibroblasts. Wound macrophages will be harvested as described (Brubaker et al., 2011), 105 wound macrophages can routinely obtained per 100 mg wounds, and have confirmed that these cells are ~98% macrophages based on F4/80 and CD11b staining.
GAS5 overexpression and transfection
To overexpress GAS5, a pGAS5 plasmid was constructed by GeneCopoeia (Rockville, MD). The GAS5 gene was amplified by a primer designed according to the murine Lethe sequence (NR_002840.2). The empty pcDNA3.1 vector was used as a control. In 6 well culture plates, the RAW cells were transfected with various dose of either pGAS5 or empty pcDNA3.1 using Lipofectamine 2000 (Invitrogen Life Technologies). After 2h transfection of RAW cells, the medium was replaced with fresh DMEM supplemented with 10% (v/v) FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin. Cells were processed 24h following transfection for western blot or gene expression analysis.
Real time quantitative PCR
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s established protocol. RNA was converted into cDNA using the SuperScript First-Strand Synthesis System (Invitrogen, Life Technologies). Primers and probes for mouse GAS5, STAT1, STAT3, STAT6, iNOS, IL1-beta, and TNFa were acquired from Applied Biosystems TaqMan gene expression assay (Applied Biosystems, Foster City, CA). Quantitative PCR was performed on a BIO-RAD CFX96 according to the manufacturer’s instructions. Quantitative values of genes of interest are normalized based on 18s rRNA content or GAPDH expression. Samples (n=5 per group) were amplified in triplicate and results were averaged for each individual sample. The ΔΔCT method was used to calculate relative gene expression. Results are reported as mean ± SD.
Western blot analysis
Cell lysates were prepared in standard NP-40 lysis buffer (Abcam, USA) supplemented with proteinase and phosphatase inhibitors. Protein lysates were quantified using the Pierce BCA protein assay kit (Thermo Fisher Scientific, USA). Equal masses of total protein were separated on 4–12% SDS-polyacrylamide mini-gels, and blotted onto PVDF membranes (Millipore, USA). Membranes were subsequently blocked, incubated with primary antibodies, and incubated with secondary antibodies according to WesternBreeze Chromogenic Kit (Thermo Scientific, USA). Alkaline phosphatase was detected on the PVDF membranes using a ready-to-use BCIP/NBT substrate (Thermo Scientific, USA) for ready visualization of enzyme-linked antibodies. Rabbit anti-STAT1(149943), anti-phospho-STAT1(76493), anti-STAT3(91393), anti-phospho-STAT3 (9131), anti-phospho-ERK(19762), and anti-GAPDH (2118) antibodies were obtained from Cell Signaling (USA). Quantification of relative intensities was achieved by ImageJ analysis.
Flow cytometry
Flow cytometry was performed as described (Mirza et al., 2014). Briefly, cells were suspended to a concentration of 1–5 × 106 cells/mL in ice cold PBS, 10% FCS. Conjugated primary antibodies anti-CD11b (ab25533) and anti-CD86 (ab77276) (1 μg/ml) were added to 100 μl cell suspension, and incubated overnight in the dark at 4°C. After washing, cells were analyzed on a Beckman Coulter Gallios flow cytometer.
Statistical analysis
Results are expressed as mean ± SD for 3 to 5 independent experiments. Statistically significant differences in gene expression between two groups were assessed by Student’s T-test, ANOVA with an appropriate post hoc test was be used for multiple comparisons. P<0.05 was considered to be statistically significant.
Acknowledgments
Dr. Junwang Xu is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis. The research presented in this article was supported by NIH R01 GM128660-01A1to J Xu.
Abbreviations:
- lncRNAs
long non-coding RNAs
- GAS5
growth arrest specific 5
- STAT1
signal transducer and activator 1
- Arg1
Arginase-1
- MRC1
mannose receptor C-type1
- IL
Interleukin-
- iNOS
inducible nitric oxide synthase
- TNFα
tumor necrosis factor alpha
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement
No datasets were generated or analyzed during the current study.
Conflict of Interest
The authors have declared that no competing interests exist.
References
- Appelberg KS, Wallet MA, Taylor JP, Cash MN, Sleasman JW, Goodenow MM. HIV-1 Infection Primes Macrophages through STAT Signaling to Promote Enhanced Inflammation and Viral Replication. AIDS research and human retroviruses 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg SN, Berger A, Hwang L, Pastar I, Warren SM, Chen W. The role of stem cells in the treatment of diabetic foot ulcers. Diabetes research and clinical practice 2012;96(1):1–9. [DOI] [PubMed] [Google Scholar]
- Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J Immunol 2017;199(1):17–24. [DOI] [PubMed] [Google Scholar]
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366(9498):1719–24. [DOI] [PubMed] [Google Scholar]
- Brubaker AL, Schneider DF, Palmer JL, Faunce DE, Kovacs EJ. An improved cell isolation method for flow cytometric and functional analyses of cutaneous wound leukocytes. Journal of immunological methods 2011;373(1–2):161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra Gupta S, Nandan Tripathi Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int J Cancer 2017;140(9):1955–67. [DOI] [PubMed] [Google Scholar]
- Gallucci RM, Lee EG, Tomasek JJ. IL-6 modulates alpha-smooth muscle actin expression in dermal fibroblasts from IL-6-deficient mice. J Invest Dermatol 2006;126(3):561–8. [DOI] [PubMed] [Google Scholar]
- Gan ZS, Wang QQ, Li JH, Wang XL, Wang YZ, Du HH. Iron Reduces M1 Macrophage Polarization in RAW264.7 Macrophages Associated with Inhibition of STAT1. 2017;2017:8570818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren I, Muller E, Schiefelbein D, Christen U, Pfeilschifter J, Muhl H, et al. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol 2007;127(9):2259–67. [DOI] [PubMed] [Google Scholar]
- Guo H, Mi Z, Bowles DE, Bhattacharya SD, Kuo PC. Osteopontin and protein kinase C regulate PDLIM2 activation and STAT1 ubiquitination in LPS-treated murine macrophages. J Biol Chem 2017;292(3):1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobizal KB, Wukich DK. Diabetic foot infections: current concept review. Diabet Foot Ankle 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li Y, Lu Z, Che Y, Sun S, Mao S, et al. Long non-coding RNA GAS5 is induced by interferons and plays an antitumor role in esophageal squamous cell carcinoma. Cancer Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zheng Z, Wang Y, Zhou Q, Cai W, Jia W, et al. SIRT1 is a regulator in high glucose-induced inflammatory response in RAW264.7 cells. PLoS One 2015;10(3):e0120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhou T, Xiao Y, Yu J, Dou S, Chen G, et al. Tim-3 promotes tumor-promoting M2 macrophage polarization by binding to STAT1 and suppressing the STAT1-miR-155 signaling axis. Oncoimmunology 2016;5(9):e1211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 2010;5(3):e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball AS, Joshi AD, Boniakowski AE, Schaller M, Chung J, Allen R, et al. Notch Regulates Macrophage-Mediated Inflammation in Diabetic Wound Healing. Frontiers in immunology 2017;8:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarik P, Stoiber D, Novy M, Decker T. Stat1 combines signals derived from IFN-gamma and LPS receptors during macrophage activation. EMBO J 1998;17(13):3660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics 2013;193(3):651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cellular and molecular life sciences: CMLS 2016;73(20):3861–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature reviews Immunology 2011;11(11):750–61. [DOI] [PubMed] [Google Scholar]
- Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Wormley FL, Jr. STAT1 signaling is essential for protection against Cryptococcus neoformans infection in mice. J Immunol 2014;193(8):4060–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes 2014;63(3):1103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. Journal of leukocyte biology 2001;69(4):598–604. [PubMed] [Google Scholar]
- Pickard MR, Williams GT. Molecular and Cellular Mechanisms of Action of Tumour Suppressor GAS5 LncRNA. Genes 2015;6(3):484–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GF. Inflammation in nonhealing diabetic wounds: the space-time continuum does matter. Am J Pathol 2001;159(2):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention CfDCa. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. US Department of Health and Human Services, Atlanta, GA: (2014) 2014. [Google Scholar]
- Rao A, Rajkumar T, Mani S. Perspectives of long non-coding RNAs in cancer. Molecular biology reports 2017;44(2):203–18. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Chen Z, Park JT, Wang M, Lanting L, Zhang Q, et al. Regulation of inflammatory phenotype in macrophages by a diabetes-induced long noncoding RNA. Diabetes 2014;63(12):4249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya AP, Pastar I, Stojadinovic O, Lazovic S, Davis SC, Gil J, et al. Topical mevastatin promotes wound healing by inhibiting the transcription factor c-Myc via the glucocorticoid receptor and the long non-coding RNA Gas5. J Biol Chem 2018;293(4):1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 2013;62(2):618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viereck J, Thum T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ Res 2017;120(2):381–99. [DOI] [PubMed] [Google Scholar]
- Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends in cell biology 2011;21(6):354–61.21550244 [Google Scholar]
- Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 2000;115(2):245–53. [DOI] [PubMed] [Google Scholar]
- Wicks K, Torbica T, Mace KA. Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound. Seminars in immunology 2014;26(4):341–53. [DOI] [PubMed] [Google Scholar]
- Wu T, Du Y. LncRNAs: From Basic Research to Medical Application. International journal of biological sciences 2017;13(3):295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016;44(3):450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu W, Zhang L, Dorset-Martin W, Morris MW, Mitchell ME, et al. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes 2012;61(11):2906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgheib C, Hodges MM, Hu J, Liechty KW, Xu J. Long non-coding RNA Lethe regulates hyperglycemia-induced reactive oxygen species production in macrophages. PLoS One 2017;12(5):e0177453. [DOI] [PMC free article] [PubMed] [Google Scholar]


