Abstract
This abstract was presented as a quickshot presentation at the 2020 Academic Surgical Congress.
Background:
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is an endovascular adjunct to hemorrhage control. Success relies upon institutional support and focused training in arterial access. We hypothesized that hospitals with higher REBOA volumes will be more successful than low-volume hospitals at aortic occlusion with REBOA.
Methods:
This is a retrospective study from the American Association for the Surgery of Trauma (AAST) Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) Registry from 11/2013–01/2018. Patients ≥ 18 years who underwent REBOA were included. Successful placement of REBOA catheters (defined as hemodynamic improvement with balloon inflation) was compared between high-volume (≥80 cases; 2 hospitals), mid-volume (10–20 cases; 4 hospitals), and low-volume (<10 cases; 14 hospitals) hospitals, adjusting for patient factors.
Results:
Of 271 patients from 20 hospitals, 210 patients (77.5%) had successful REBOA placement. Most patients were male (76.0%) and sustained blunt trauma (78.1%). CPR was ongoing at time of REBOA placement in 34.5% of patients. Inpatient mortality was 67.4%, unchanged by hospital volume. Multivariable logistic regression found increased odds of successful REBOA placement at high-volume vs. low-volume hospitals (OR 7.50, 95% CI 2.10–27.29, p=0.002) and mid-volume vs. low-volume hospitals (OR 7.82, 95% CI 1.52–40.31, p = 0.014) and decreased odds among patients undergoing CPR during REBOA placement (OR 0.10, 95% CI 0.03–0.34, p<0.001) when adjusting for age, sex, mechanism of injury, pre-hospital CPR, CPR on admission, transfer status, hospital location of REBOA placement, GCS ≤ 13, and injury severity.
Conclusion:
Hospitals with higher REBOA volumes were more likely to achieve hemodynamic improvement with REBOA inflation. However, mortality and complication rates were unchanged. Independent of hospital volume, ongoing CPR is associated with a decreased odds of successful REBOA placement.
Keywords: REBOA, trauma, volume, mortality
INTRODUCTION
Multiple studies have evaluated the effect of hospital and procedural volume on outcomes for a variety of procedures, including emergency general surgery operations (1), pancreaticoduodenectomies (2), robotic lobectomies (3), open abdominal aortic aneurysm repair (4), and emergency department thoracotomy (5), finding improved outcomes at higher-volume hospitals (6). Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) is rapidly gaining widespread use throughout the United States (7–9) and as more nuanced indications for its use are identified, it is likely to be used in large trauma centers as well as smaller, community hospitals. The effects of hospital volume of REBOA procedures on outcomes of patients undergoing REBOA are unknown.
Placing a REBOA catheter involves gaining common femoral arterial access and inserting a sheath through which the REBOA device is inserted and advanced into one of two ideal landing zones for aortic occlusion. Which zone is selected is determined by the physician’s suspicion for the most likely source of hemorrhage. Once inflated, REBOA provides time-sensitive hemorrhage control while the patient proceeds to definitive treatment. Successful aortic occlusion with REBOA begins with identifying patients who may benefit, mobilizing the trauma team to perform the procedure, and getting the patient to definitive hemorrhage control. This requires coordination between trauma surgeons and emergency medicine physicians, as well as operating room and emergency department nurses and technicians.
Given these multiple institutional factors, we hypothesized that success of REBOA placement will depend on the frequency of REBOA utilization at an institution and that successful aortic occlusion will be higher at high REBOA-volume versus low REBOA-volume hospitals.
METHODS
This is a retrospective study of data from the American Association for the Surgery of Trauma (AAST) Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) Registry, approved by the AAST Multicenter Trials Committee. The AORTA registry is a multi-institutional registry developed to prospectively collect data on patients undergoing aortic occlusion via open and endovascular techniques. Data was collected from November 2013 to January 2018 from collaborating hospitals in the United States. At the time of this analysis twenty hospitals had contributed to the registry via a secure online portal. All contributing hospitals obtained individual local Institutional Board Review (IRB) approval for waivers of consent prior to submitting de-identified data. This study was approved by our local IRB (1182061–1).
The primary outcome of interest was successful aortic occlusion with REBOA, which was defined as hemodynamic improvement with balloon inflation. We excluded patients with incomplete data which did not address this question, and patients below 18 years of age. Based on natural breaks in the data, hospitals were stratified into three levels based on REBOA volume: high-volume hospitals (defined as ≥ 80 cases during the study period); mid-volume hospitals (defined as 10–20 cases during the study period), and low-volume hospitals (defined as <10 cases during the study period). Rates of hemodynamic improvement with balloon inflation were compared between high-volume hospitals, mid-volume hospitals, and low-volume hospitals, adjusting for patient factors. Secondary outcomes included mortality and REBOA-related complications.
Univariate analyses (t-test and chi-square tests) and multivariable logistic regressions were performed to compare successful REBOA placement by hospital REBOA case volume. Analyses were performed in Stata 145.2 (College Station, TX).
RESULTS
From the AORTA registry, 308 patients were identified as having undergone REBOA during the study period. Of these, 33 patients were excluded for incomplete data regarding key variables, two were excluded because the REBOA balloon was never inflated, and two were excluded for age less than 18 years old (Figure 1).
Figure 1:

Study Population
A total of 271 patients who met the inclusion criteria from 20 hospitals were included in this study (Table 1). The median age was 50 (range 18–91, mean 43) years old. Most patients (n=206, 76.0%) were male and had sustained blunt trauma (n=201, 78.1%). Almost 10% of patients arrived via transfer (n=26, 9.8%). Injury severity score (ISS) ranged from 2–75, with a median ISS of 34 (mean 36). Nearly one-third (n=77, 30.0%) of patients had cardiopulmonary resuscitation (CPR) prior to arriving at the hospital, and one-third (n=92, 34.5%) of patients were undergoing CPR during REBOA placement. Hemoglobin on admission ranged from 5–17.3 g/dL (median=11).
Table 1.
Demographics and outcomes between low-, mid-, and high REBOA-volume hospitals
| Variable | Total (n=271) |
Low-volume hospitals (n=46, 17.0%) |
Mid-volume hospitals (n=58,21.4%) |
High-volume hospitals (n=167, 61.6%) |
P-value |
|---|---|---|---|---|---|
| Age - mean (SD) | 42.5 (17.9) | 41.8 (19.1) | 40.2 (15.4) | 43.5 (18.4) | 0.236 |
| Penetrating trauma -n(%) | 59 (21.9%) | 12 (26.1%) | 10 (17.2%) | 37 (22.4%) | 0.540 |
| Female sex - n (%) | 65 (24.0%) | 13 (28.3%) | 19 (32.8%) | 33 (19.8%) | 0.103 |
| First SBP (mmHg) - mean (SD) | 88 (49) | 102 (48) | 80 (48) | 87 (50) | 0.966^ |
| SBP at initiation of REBOA (mmHg) -mean (SD) | 53 (43) | 67 (44) | 62 (44) | 46 (40) | 0.694^ |
| Ongoing CPR during REBOA placement - n (%) | 92 (34.5%) | 12(26.1%) | 17 (30.4%) | 63 (38.2%) | 0.240 |
| SBP after REBOA (mmHg) - mean (SD) | 98 (54) | 95 (48) | 100 (58) | 98 (55) | 0.500^ |
| Change in SBP before and after REBOA - mean change in SBP (SD) | 45 (47) | 24 (47) | 40 (43) | 52 (47) | 0.891^ |
| Successful REBOA placement - n (%) | 210 (77.5%) | 32 (69.6%) | 47 (81.0%) | 131 (78.4%) | 0.339 |
| Any complication -n(%) | 198 (73.1%) | 35 (76.1%) | 36 (62.1%) | 127 (76.1%) | 0.104 |
| In-hospital mortality - n (%) | 174 (67.4%) | 30 (68.2%) | 28 (54.9%) | 116 (71.2%) | 0.096 |
| Other complications* - n (%) | 36 (13.3%) | 7 (15.2%) | 10 (17.2%) | 19 (11.4%) | 0.481 |
Includes extremity ischemia, distal embolization, hematoma, pseudoaneurysm, or need for patch angioplasty, arterial bypass, amputation. Does not include death.
P-value: Bartlett’s test for equal variance. If not noted, P-value refers to chi-squared analyses.
SBP = Systolic Blood Pressure; SD = Standard Deviation
While most patients underwent percutaneous placement of REBOA (using either external landmarks, 39.48% or ultrasound, 28.78%), 73 patients (26.94%) had the REBOA catheter inserted via a cut-down to facilitate direct visualization of catheter insertion into the femoral artery. Two patients underwent fluoroscopic-guided REBOA placement (0.74%) and in 11 patients the method of access was not reported (4.06%). A majority of catheters were placed in zone 1 (n=180, 68.2%), while 79 (29.9%) were placed in zone 3 and only five were placed in zone 2 (1.9%). The primary performer of REBOA insertion was most commonly the trauma/acute care surgery attending (n=228, 91.9%), but others included trauma/acute care surgery fellow (n=9, 3.6%), surgery resident (n=6, 2.4%), vascular surgery attending (n=3, 1.2%), interventional radiology attending (n=1, 0.4%) and emergency medicine attending (n=1, 0.4%).
Successful REBOA placement (defined as physician-reported hemodynamic improvement with balloon inflation) was identified in 210 (77.5%) of patients. Patient hemodynamics were similar at low, mid, and high REBOA-volume hospitals, with similar admission systolic blood pressure (SBP) recordings (mean 88 mmHg overall), SBP at initiation of REBOA (mean 53 mmHg), and post-REBOA initial SBP (mean 98 mmHg), with a mean change of 45 mmHg before and after REBOA balloon inflation. When comparing patients with successful REBOA placement to patients with unsuccessful REBOA placement, the SBP following REBOA inflation improved by a mean of 57 mmHg (SD 44 mmHg, SE 3.04) from the pre-inflation SBP in the successful group, compared to a mean improvement of 3 mmHg (SD 32 mmHg, SE 4.1) in the unsuccessful group (p<0.001).
Thirty-six patients (13.3%) had a complication; these adverse events included extremity ischemia (n=12, 4.4%), distal embolism (n=17, 6.3%), hematoma (n=4, 1.5%), and pseudoaneurysms (n=2, 0.7%). Acute kidney injury occurred in 19.56% of patients. Interventions for these adverse events included patch angioplasty (n=9, 3.3%), arterial bypass (n=1, 0.4%), and amputation (n=4, 1.5%). One-third of patients with a complication died while in the hospital (n=12, 34.3%).
Outcomes by Hospital REBOA Volume
Two hospitals were identified as high-volume with ≥ 80 cases. Four hospitals were identified as mid-volume with 10–20 cases. Fourteen hospitals were identified as low-volume, with < 10 cases. No institution had case volumes between 21–79 cases (Figure 2).
Figure 2:
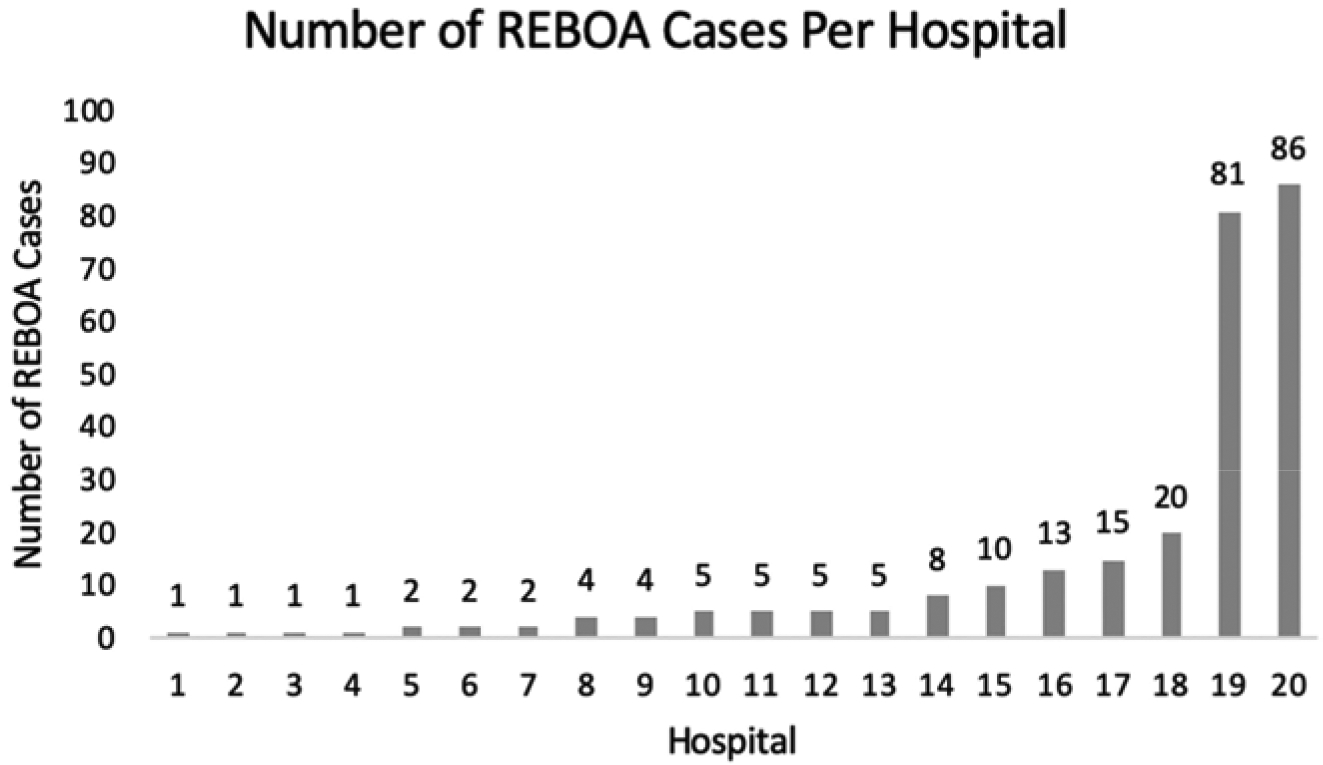
Distribution of REBOA cases performed per hospital during the study period. (Low-volume hospitals: 0–9 REBOAs performed; mid-volume hospitals: 10–20 REBOAs performed; high-volume hospitals: >80 REBOAs performed.)
On univariate analyses, there was no difference in patient demographics (age, mechanism of injury, sex) between patients treated at low-volume, mid-volume, or high-volume hospitals (Table 1). There was also no difference in percentage of patients who were undergoing CPR at the time of REBOA placement, had successful REBOA placement, died while in the hospital, or had a complication (Table 1). Low REBOA-volume hospitals had a longer median time from admission to start of REBOA placement (low-volume 45 minutes, mid-volume 17 minutes, high-volume 11.5 minutes p<0.00001) and to aortic occlusion (low-volume 45 minutes, mid-volume 36 minutes, high-volume 23 minutes, p=0.00027).
Multivariable logistic regression found odds of successful REBOA placement were increased among patients at high REBOA-volume vs. low REBOA-volume hospitals (OR 7.50, 95% CI 2.10–27.29, p=0.002) and mid REBOA-volume vs. low REBOA-volume hospitals (OR 7.82, 95% CI 1.52–40.31, p = 0.014) when adjusting for age, sex, mechanism of injury, pre-hospital CPR, CPR on arrival, CPR ongoing during REBOA placement, transfer status, hospital location of REBOA placement (OR vs. ED), injury severity score, and high GCS (14–15) vs. low GCS (≤ 13) (Table 2).
Table 2.
Multivariable logistic regression predicting successful REBOA placement
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Low-Volume Hospitals | Reference | |
| Mid-Volume Hospitals | 7.82 (1.52–40.31) | 0.014 |
| High-Volume Hospitals | 7.50 (2.06–27.29) | 0.002 |
| Age 18–26 | Reference | |
| Age 27–40 | 0.52 (0.14–1.87) | 0.315 |
| Age 41–58 | 0.37 (0.09–1.45) | 0.152 |
| Age 59+ | 0.46 (0.10–2.10) | 0.314 |
| Female sex | 2.73 (0.93–8.07) | 0.069 |
| Penetrating trauma | 1.58 (0.43–5.82) | 0.491 |
| Pre-hospital CPR | 1.54 (0.32–7.55) | 0.592 |
| CPR on arrival | 0.26 (0.05–1.47) | 0.128 |
| CPR ongoing during REBOA placement | 0.10 (0.03–0.34) | <0.001 |
| Transferred from another hospital | 2.23 (0.29–16.85) | 0.438 |
| REBOA placed in OR (vs. ED) | 0.83 (0.22–3.15) | 0.788 |
| ISS score 2–29 | Reference | |
| ISS score 30–41 | 0.45 (0.14–1.47) | 0.186 |
| ISS score 42–75 | 0.51 (0.15–1.78) | 0.290 |
| GCS ≤ 13 | Reference | |
| GCS 14–15 | 1.03 (0.28–3.91) | 0.962 |
CPR Ongoing During REBOA Placement
As mentioned, a substantial proportion (n = 92, 34.5%) of patients were undergoing CPR at the time of REBOA placement. Odds of successful REBOA placement were decreased in patients who had CPR ongoing during REBOA placement (OR 0.10, 95% CI 0.03–0.34, p<0.001) when adjusting for age, sex, mechanism of injury, pre-hospital CPR, CPR on admission, transfer status, hospital location of REBOA placement, injury severity score, and high GCS (14–15) vs. low GCS (≤ 13). In-hospital mortality for patients undergoing CPR at time of REBOA placement was 91.30% (n = 84/92), while in-hospital mortality for patient not undergoing CPR at time of REBOA placement was 50.86% (n = 75/175, p = <0.00001). When looking at outcomes basedon zone of balloon inflation, CPR was associated with worse outcomes, with 91.89% of patients undergoing zone 1 REBOA inflation under CPR died, compared to a mortality rate of 60.19%for Zone 1 patients not under CPR. Similar mortality differences were observed for zone 3 REBOA placements, with 88.24% mortality while undergoing CPR and 36.07% mortality if not undergoing CPR.
Subgroup analysis of patients undergoing CPR at time of REBOA placement showed no difference in rate of successful REBOA placement by hospital volume on multivariable logistic regression (OR for mid-versus low-volume hospital 4.50, 95% CI 0.40–50.69, p=0.223; OR for high-versus low-volume hospital 5.97, 95% CI 0.80–44.60, p=0.082) (Table 3). For the subset of patients not undergoing CPR at time of REBOA placement, high REBOA-volume hospitals had an increased odds of successful REBOA placement compared to low REBOA-volume hospitals (OR 8.86, 95% CI 1.47–53.61, p=0.017) (Table 4).
Table 3.
Subgroup analysis: Multivariable logistic regression predicting successful REBOA placement for patients under CPR
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Low-Volume Hospitals | Reference | |
| Mid-Volume Hospitals | 4.50 (0.40–50.69) | 0.223 |
| High-Volume Hospitals | 5.97 (0.80–44.60) | 0.082 |
| Age 18–26 | Reference | |
| Age 27–40 | 1.76 (0.35–8.89) | 0.494 |
| Age 41–58 | 0.75 (0.12–4.54) | 0.751 |
| Age 59+ | 2.01 (0.16–25.10) | 0.586 |
| Female sex | 6.04 (1.18–30.79) | 0.031 |
| Penetrating trauma | 1.85 (0.29–11.76) | 0.512 |
| Transferred from another hospital | 6.35 (0.41–98.76) | 0.187 |
| REBOA placed in OR (vs. ED) | 1.01 (0.16–7.00) | 0.966 |
| ISS score 2–29 | Reference | |
| ISS score 30–41 | 0.39 (0.07–2.27) | 0.294 |
| ISS score 42–75 | 0.87 (0.15–5.05) | 0.398 |
| GCS ≤ 13 | Reference | |
| GCS 14–15 | 0.32 (0.03–4.50) | 0.398 |
Table 4.
Subgroup analysis: Multivariable logistic regression predicting successful REBOA placement for patients not under CPR
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Low-Volume Hospitals | Reference | |
| Mid-Volume Hospitals | Omitted, low sample size | |
| High-Volume Hospitals | 8.86 (1.47–53.61) | 0.017 |
| Age 18–26 | Reference | |
| Age 27–40 | 0.05 (0.00–1.03) | 0.052 |
| Age 41–58 | 0.18 (0.01–7.14) | 0.361 |
| Age 59+ | 0.13 (0.01–2.67) | 0.187 |
| Female sex | 1.17 (0.16–8.79) | 0.880 |
| Penetrating trauma | 0.62 (0.04–9.07) | 0.728 |
| Transferred from another hospital | 0.35 (0.02–5.43) | 0.451 |
| REBOA placed in OR (vs. ED) | 1.19 (0.10–10.43) | 0.990 |
| ISS score 2–29 | Reference | |
| ISS score 30–41 | 0.09 (0.01–1.38) | 0.083 |
| ISS score 42–75 | 0.11 (0.01–1.66) | 0.112 |
| GCS ≤ 13 | Reference | |
| GCS 14–15 | 3.08 (0.35–26.87) | 0.31 |
Mortality
Over two-thirds of these patients (n=174, 67.4%) died in the hospital. In-hospital mortality for patients with successful REBOA placement was 59.80%, while 90.16% of patients with unsuccessful REBOA placement died (p = 0.00001). Mortality also differed by zone of REBOA inflation, with 72.78% of patients undergoing Zone 1 placement dying in-hospital, compared to 46.84% of patients undergoing Zone 3 placement. On univariate analysis, admission Glasgow Coma Scale (GCS) of 14–15 was associated with a mortality rate of 33.96%, while GCS of ≤ 13 was associated with a mortality rate of 78.40% (p < 0.00001).
Multivariable logistic regression found odds of mortality were decreased at mid-volume vs. low-volume hospitals (OR 0.13, 95% CI 0.02–0.75, p = 0.023) and in patients with GCS 14–15 (OR 0.09, 95% CI 0.03–0.34, p < 0.001) when adjusting for age, sex, mechanism of injury, pre-hospital CPR, CPR on arrival, CPR ongoing during REBOA placement, transfer status, hospital location of REBOA placement (OR vs. ED), injury severity score, and high GCS (14–15) vs. low GCS (≤ 13). High-volume hospitals had a trend towards decreased odds of mortality which did not reach significance (OR 0.21, 95% CI 0.04–1.01, p = 0.052). Odds of mortality were increased among older patients, patients under CPR on arrival, and in patients with higher ISS scores (Table 5). The regression analysis was repeated after adding in successful REBOA placement into the model, which was not associated with a decreased odds of mortality (OR 0.33, 95% CI 0.04–2.70, p = 0.304). After adjusting for successful REBOA placement, the strongest predictor of mortality was CPR on arrival (OR 85.70, 95% CI 3.10–2372.27, p = 0.009); hospital volume was not a statistically significant predictor of mortality (Table 6).
Table 5:
Multivariable logistic regression predicting mortality
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Low-Volume Hospitals | Reference | |
| Mid-Volume Hospitals | 0.13 (0.02–0.75) | 0.023 |
| High-Volume Hospitals | 0.21 (0.04–1.01) | 0.052 |
| Age 18–26 | Reference | |
| Age 27–40 | 7.52(1.77–32.04) | 0.006 |
| Age 41–58 | 12.01 (2.44–59.10) | 0.002 |
| Age 59+ | 30.76(4.75–199.12) | <0.001 |
| Female sex | 1.41 (0.40–4.95) | 0.593 |
| Penetrating trauma | 0.50 (0.13–1.85) | 0.297 |
| Pre-hospital CPR | 0.84 (0.10–7.43) | 0.876 |
| CPR on arrival | 62.11 (2.48–1558.50) | 0.012 |
| CPR ongoing during REBOA placement | 2.40 (0.46–12.59) | 0.303 |
| Transferred from another hospital | 0.31 (0.05–1.86) | 0.201 |
| REBOA placed in OR (vs. ED) | 1.37 (0.35–5.34) | 0.654 |
| ISS score 2–29 | Reference | |
| ISS score 30–41 | 5.94 (1.70–20.67) | 0.005 |
| ISS score 42–75 | 6.48 (1.64–25.66) | 0.008 |
| GCS ≤ 13 | Reference | |
| GCS 14–15 (compared to 3–13) | 0.09 (0.03–0.34) | <0.001 |
Table 6:
Multivariable logistic regression predicting mortality when adjusting for successful REBOA placement
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Successful REBOA deployment | 0.33 (0.04–2.70) | 0.304 |
| Low-Volume Hospitals | Reference | |
| Mid-Volume Hospitals | 0.16 (0.03–1.02) | 0.052 |
| High-Volume Hospitals | 0.25 (0.05–1.31) | 0.101 |
| Age 18–26 | Reference | |
| Age 27–40 | 6.81 (1.57–29.53) | 0.01 |
| Age 41–58 | 11.24 (2.25–56.26) | 0.003 |
| Age 59+ | 28.73 (4.34–188.93) | <0.001 |
| Female sex | 1.54 (0.44–5.42) | 0.506 |
| Penetrating trauma | 0.48 (0.13–1.82) | 0.281 |
| Pre-hospital CPR | 0.76 (0.09–6.40) | 0.797 |
| CPR on arrival | 85.70 (3.10–2373.27) | 0.009 |
| CPR ongoing during REBOA deployment | 1.27 (0.17–9.50) | 0.817 |
| Transferred from another hospital | 0.28 (0.05–1.71) | 0.167 |
| REBOA placed in OR (vs. ED) | 1.31 (0.34–5.09) | 0.698 |
| ISS score 2–29 | Reference | |
| ISS score 30–41 | 5.59 (1.59–19.65) | 0.007 |
| ISS score 42–75 | 5.88 (1.47–23.45) | 0.012 |
| GCS ≤ 13 | Reference | |
| GCS 14–15 (compared to 3–13) | 0.09 (0.03–0.34) | <0.001 |
Subgroup analysis of patients not undergoing CPR on arrival showed a decreased odds of mortality in mid-vs. low-volume hospitals (OR 0.12, 95% CI 0.02–0.81, p = 0.029) but not at high-volume vs. low-volume hospitals (OR 0.21, 95% CI 0.04–1.13, p = 0.07) when adjusting for successful REBOA placement, hospital volume, age, sex, mechanism of injury, pre-hospital CPR, CPR during REBOA placement, transfer status, location of REBOA placement, ISS score, and high GCS (14–15) vs. low GCS (≤ 13) (Table 7). Subgroup analysis of patients undergoing CPR on arrival was not performed due to small sample sizes.
Table 7:
Subgroup analysis: Multivariable logistic regression predicting mortality for patients not under CPR on arrival
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Successful REBOA placement | 0.56 (0.06–5.62) | 0.624 |
| Low-Volume Hospitals | Reference | |
| Mid-Volume Hospitals | 0.12 (0.02–0.81) | 0.029 |
| High-Volume Hospitals | 0.21 (0.04–1.13) | 0.07 |
| Age 18–26 | Reference | |
| Age 27–40 | 5.66 (1.24–25.83) | 0.025 |
| Age 41–58 | 10.81 (2.06–56.73) | 0.005 |
| Age 59+ | 23.30 (3.54–153.34) | 0.001 |
| Female sex | 1.30 (0.36–4.72) | 0.687 |
| Penetrating trauma | 0.62 (0.15–2.50) | 0.501 |
| Pre-hospital CPR | 1.07 (0.09–13.28) | 0.96 |
| CPR ongoing during REBOA placement | 1.88 (0.21–17.02) | 0.576 |
| Transferred from another hospital | 0.69 (0.10–4.63) | 0.703 |
| REBOA placed in OR (vs. ED) | 1.10 (0.28–4.31) | 0.894 |
| ISS score 2–29 | Reference | |
| ISS score 30–41 | 8.04 (2.11–30.66) | 0.002 |
| ISS score 42–75 | 6.72 (1.65–27.32) | 0.008 |
| GCS ≤ 13 | Reference | |
| GCS 14–15 (compared to 3–13) | 0.09 (0.02–0.33) | <0.001 |
Overall, the most common location of in-hospital mortality among all patients was the ICU (40.46%). The location of death did not differ significantly between low, mid, and high REBOA-volume hospitals (p=0.53). In the low-volume group, the majority of deaths occurred in the ICU (46.67%), followed by the OR (33.33%) and the ER (20%). In the mid-volume group, most died in the ICU (46.43%) followed by the ER (32.14%) and the OR (21.43%). In the high-volume group, the ICU was the location of the majority of deaths (37.07%), followed by the ER (34.83%) and the OR (26.72%), with one patient dying in the Interventional Radiology suite and one location unreported.
DISCUSSION
This multi-institutional registry study is the first to look at hospital REBOA volume as it relates to successful aortic occlusion with REBOA as determined by resultant improvement in the patient’s hemodynamic status. Using data collected from twenty hospitals placing REBOA catheters around the country, we found that odds of successful REBOA placement were increased at high and mid REBOA-volume hospitals compared to low REBOA-volume hospitals. Additionally, odds of successful REBOA placement were lower in patients undergoing CPR at the time of REBOA placement. Hospital REBOA volume did not predict mortality after adjusting for successful REBOA placement, with the strongest predictor of mortality identified as patients under CPR on arrival to the hospital
The steps of inserting a REBOA catheter can be added to a trauma surgeon’s repertoire. There are several training courses available aimed at teaching the use of REBOA (10–13), including the Advanced Surgical Skills for Exposure in Trauma (ASSET) course, the Basic Endovascular Skills for Trauma (BEST) course, the Endovascular Trauma Resuscitation and Management (EVTM) Workshops, and the Advanced Endovascular Skills Course for Trauma Surgeons (10–12). As surgeons and trauma teams gain experience in using REBOA, they will become more facile with its placement. However, successful REBOA use depends not only on surgeon training, but multiple systems issues, from supply and restocking to training of emergency department and operating room staff in assisting with REBOA placement (13). These mechanisms are not intuitive to groups who rarely, if ever, see endovascular techniques. Thus, it is not surprising that low REBOA-volume hospitals have lower rates of hemodynamic improvement with REBOA placement. This is supported by our finding that low-volume and mid-volume hospitals take longer than high-volume hospital to both initiate and complete aortic occlusion via REBOA, which may be due to delay in deciding to proceed with REBOA placement or difficulty in placing the REBOA catheter. This delay may also be due to resorting to REBOA as a ‘last ditch’ effort in hospitals with less REBOA experience. At lower-volume hospitals, it is possible that technical problems with placing the REBOA may more frequently result in aborting the procedure. Additionally, in the exsanguinating patient, aortic occlusion is unlikely to improve hemodynamics if appropriate resuscitation is not simultaneously underway, and this may be an additional contributing factor.
However, the number of REBOA procedures a hospital must perform for increased rates of procedural success is as yet unknown. As with patients undergoing emergency department thoracotomy, appropriate patient selection is paramount for success (5). Institutional REBOA success is unlikely to increase simply by performing the procedure more frequently, but by better understanding for which patients in which endovascular aortic occlusion may be life-saving. Higher REBOA-volume hospitals may have more experience in identifying patients who may benefit from REBOA and in placing REBOA, including troubleshooting and minimizing technical difficulties. The end result is that higher volume hospitals may expect higher rates of success in improving the patient’s hemodynamics with use of the REBOA catheter. As experience gathers and lower-volume hospitals become higher-volume hospitals, they may also hope to see improvements in their success rates with REBOA. In this study, we did not find a difference in complication rates or in-hospital mortality by hospital REBOA volume. However, the time period analyzed here represents a distinct time period in the adoption curve of REBOA around the United States, with many hospitals early in their use of REBOA, with our results most likely representing the institutional learning curve rather than the skillset or technical ability of individual surgeons. Identifying characteristics of surgeons and hospital systems with high levels of REBOA success could be used to improve existing REBOA training courses. Hospitals with the greatest success likely represent multiple contributing factors, including surgeon skill and familiarity with endovascular techniques, a well-developed REBOA algorithm, easy access to necessary equipment, and ancillary support (14). Additionally, the role of training courses for surgical providers in REBOA placement is well-established, with emphasis on simulation training for both attending physicians and trainees (15–18).
We also found that patients undergoing CPR had decreased odds of successful REBOA placement and increased mortality rates. There are likely multiple reasons for this finding. Aortic occlusion in the patient in extremis is a time-sensitive procedure, requiring femoral arterial access as the first step (19). More rapid arterial access has been associated with improved survival among patients undergoing REBOA placement (20). Time needed for arterial access depends on the training of the provider, with more rapid access by surgeons compared to field medics (12), and by attending physicians compared to residents (16). Time to arterial access has been found to be increased among patients undergoing CPR (19), which is likely multifactorial: percutaneous access by landmarks or ultrasound is impeded by the constant motion of chest compressions, and the patient in arrest is cardiovascularly depleted, making arterial access more difficult. In these situations, a cut-down for femoral vascular access may be required. Further research is needed in this subset of patients to determine the optimal treatment approach, although prior studies show an improved survival rate in patients in traumatic arrest undergoing REBOA compared to ED thoracotomy (21). Additionally, patients undergoing Zone 3 REBOA inflation had lower mortality rates compared to those undergoing Zone 1 REBOA placement, supporting the use of REBOA for pelvic sources of hemorrhage in particular.
This study is limited by its retrospective data collection and limited in the data points available for analysis. There are likely additional hospitals that perform REBOA but do not participate in the AAST AORTA Registry. Successful REBOA placement was defined as improvement in hemodynamic status of the patient, but failure to improve hemodynamics with REBOA inflation may reflect the patient’s physiologic decline and imminent demise rather than inability of the trauma team to successful deploy REBOA. Additionally, improvement in hemodynamics was not defined in the AORTA registry and some hospitals may interpret hemodynamic improvement differently than others, although we have attempted to corroborate this by evaluating the change in systolic blood pressure before and after balloon inflation. Finally, participating institutions have different criteria and algorithms for REBOA placement, and this may affect patient selection and the results obtained.
CONCLUSION
Hospitals with high REBOA volumes were more likely to achieve hemodynamic improvement with REBOA inflation. However, mortality and complication rates were unchanged. Independent of hospital REBOA volume, ongoing CPR is at time of REBOA placement is associated with a decreased likelihood of successful REBOA placement.
Acknowledgements:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 for author CMT. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding Source:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 for author CMT. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest:
Megan Brenner: Prytime Medical Inc. Clinical Advisory Board Member
Jeremy Cannon: Prytime Medical Inc. supplied simulation equipment
M. Chance Spalding: Prytime Medical Inc. Speakers Bureau
Charles J. Fox: Prytime Medical Inc. Clinical Advisory Board member
Ernest E. Moore: Research support from Haemonetics, Instrumentation Laboratory, Stago, Thrombo Therapeutics
References
- 1.Mehta A, Efron DT, Canner JK, et al. Effect of Surgeon and Hospital Volume on Emergency General Surgery Outcomes. J Am Coll Surg. 2017;225(5):666–675.e2. [DOI] [PubMed] [Google Scholar]
- 2.Kagedan DJ, Goyert N, Li Q, et al. The Impact of Increasing Hospital Volume on 90-Day Postoperative Outcomes Following Pancreaticoduodenectomy. J Gastrointest Surg. 2017;21(3):506–515. [DOI] [PubMed] [Google Scholar]
- 3.Tchouta LN, Park HS, Boffa DJ, Blasberg JD, Detterbeck FC, Kim AW. Hospital Volume and Outcomes of Robot-Assisted Lobectomies. Chest. 2017;151(2):329–339. [DOI] [PubMed] [Google Scholar]
- 4.Zettervall SL, Schermerhorn ML, Soden PA, et al. The effect of surgeon and hospital volume on mortality after open and endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;65(3):626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumas RP, Seamon MJ, Smith BP, et al. The epidemiology of emergency department thoracotomy in a statewide trauma system: Does center volume matter?. J Trauma Acute Care Surg. 2018;85(2):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27. [DOI] [PubMed] [Google Scholar]
- 7.Moore LJ, Martin CD, Harvin JA, Wade CE, Holcomb JB. Resuscitative endovascular balloon occlusion of the aorta for control of noncompressible truncal hemorrhage in the abdomen and pelvis. Am J Surg. 2016;212(6):1222–1230. [DOI] [PubMed] [Google Scholar]
- 8.Brenner M REBOA and catheter-based technology in trauma. J Trauma Acute Care Surg. 2015;79(1):174–5. [DOI] [PubMed] [Google Scholar]
- 9.Dubose JJ, Scalea TM, Brenner M, et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409–19. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie CF, Garofalo E, Shackelford S, et al. Using an Individual Procedure Score Before and After the Advanced Surgical Skills Exposure for Trauma Course Training to Benchmark a Hemorrhage-Control Performance Metric. J Surg Educ. 2015;72(6):1278–89. [DOI] [PubMed] [Google Scholar]
- 11.Brenner M, Hoehn M, Pasley J, Dubose J, Stein D, Scalea T. Basic endovascular skills for trauma course: bridging the gap between endovascular techniques and the acute care surgeon. J Trauma Acute Care Surg. 2014;77(2):286–91. [DOI] [PubMed] [Google Scholar]
- 12.Borger van der burg BLS, Maayen RCLA, Van dongen TTCF, et al. Feasibility Study Vascular Access and REBOA Placement: From Zero to Hero. J Spec Oper Med. 2018;18(4):70–74. [DOI] [PubMed] [Google Scholar]
- 13.Zakaluzny SA, Beldowicz BC, Salcedo ES, Dubose JJ, Moore LJ, Brenner M. Guidelines for a system-wide multidisciplinary approach to institutional resuscitative endovascular balloon occlusion of the aorta implementation. J Trauma Acute Care Surg. 2019;86(2):337–343. [DOI] [PubMed] [Google Scholar]
- 14.Celso B, Tepas J, Langland-orban B, et al. A systematic review and meta-analysis comparing outcome of severely injured patients treated in trauma centers following the establishment of trauma systems. J Trauma. 2006;60(2):371–8. [DOI] [PubMed] [Google Scholar]
- 15.Brede JR, Lafrenz T, Krüger AJ, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in non-traumatic out-of-hospital cardiac arrest: evaluation of an educational programme. BMJ Open. 2019;9(5):e027980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borger van der burg BLS, Hörer TM, Eefting D, et al. Vascular access training for REBOA placement: a feasibility study in a live tissue-simulator hybrid porcine model. J R Army Med Corps. 2019;165(3):147–151. [DOI] [PubMed] [Google Scholar]
- 17.Qasim Z, Bradley K, Panichelli H, Robinson J, Zern SC. Successful Interprofessional Approach to Development of a Resuscitative Endovascular Balloon Occlusion of the Aorta Program at a Community Trauma Center. J Emerg Med. 2018;54(4):419–426. [DOI] [PubMed] [Google Scholar]
- 18.Keller BA, Salcedo ES, Williams TK, et al. Design of a cost-effective, hemodynamically adjustable model for resuscitative endovascular balloon occlusion of the aorta (REBOA) simulation. J Trauma Acute Care Surg. 2016;81(3):606–11. [DOI] [PubMed] [Google Scholar]
- 19.Romagnoli A, Teeter W, Pasley J, et al. Time to aortic occlusion: It’s all about access. J Trauma Acute Care Surg. 2017;83(6):1161–1164. [DOI] [PubMed] [Google Scholar]
- 20.Matsumura Y, Matsumoto J, Kondo H, et al. Early arterial access for resuscitative endovascular balloon occlusion of the aorta is related to survival outcome in trauma. J Trauma Acute Care Surg. 2018;85(3):507–511. [DOI] [PubMed] [Google Scholar]
- 21.Brenner M, Inaba K, Aiolfi A, et al. Resuscitative Endovascular Balloon Occlusion of the Aorta and Resuscitative Thoracotomy in Select Patients with Hemorrhagic Shock: Early Results from the American Association for the Surgery of Trauma’s Aortic Occlusion in Resuscitation for Trauma and Acute Care Surgery Registry. J Am Coll Surg. 2018;226(5):730–740. [DOI] [PubMed] [Google Scholar]


