Abstract
It has become clear that hybridization between species is much more common than previously recognized. As a result, we now know that the genomes of many modern species, including our own, are a patchwork of regions derived from past hybridization events. Increasingly researchers are interested in disentangling which regions of the genome originated from each parental species using local ancestry inference methods. Due to the diverse effects of admixture, this interest is shared across disparate fields, from human genetics to research in ecology and evolutionary biology. However, local ancestry inference methods are sensitive to a range of biological and technical parameters which can impact accuracy. Here we present paired simulation and ancestry inference pipelines, mixnmatch and ancestryinfer, to help researchers plan and execute local ancestry inference studies. mixnmatch can simulate arbitrarily complex demographic histories in the parental and hybrid populations, selection on hybrids, and technical variables such as coverage and contamination. ancestryinfer takes as input sequencing reads from simulated or real individuals, and implements an efficient local ancestry inference pipeline. We perform a series of simulations with mixnmatch to pinpoint factors that influence accuracy in local ancestry inference and highlight useful features of the two pipelines. mixnmatch is a powerful tool for simulations of hybridization while ancestryinfer facilitates local ancestry inference on real or simulated data.
Keywords: hybridization, admixture, local ancestry inference, hidden Markov model
Introduction
Since the advent of inexpensive whole genome sequencing, it has become increasingly clear that hybridization is an important part of the evolutionary history of many species. This has made methods to study hybridization fundamental tools in the fields of genetics and evolutionary biology. In addition to methods for inferring the genome-wide history of admixture (Alexander, Novembre, & Lange, 2009; Patterson et al., 2012; Pritchard, Stephens, & Donnelly, 2000), researchers have recently taken advantage of methods that make it possible to infer ancestry at a small spatial scale along the genome (i.e. “local ancestry inference”). Originally applied to study genetic diseases in humans (Hoggart, Shriver, Kittles, Clayton, & McKeigue, 2004; Montana & Pritchard, 2004; Patterson et al., 2004; Winkler, Nelson, & Smith, 2010), applications of local ancestry inference methods have become a cornerstone of studies of genome evolution (Sankararaman et al., 2014; Schumer et al., 2018), population history (Baharian et al., 2016; Corbett-Detig & Nielsen, 2017), and trait evolution (Heliconius Genome, 2012; Jones et al., 2018; Oziolor et al., 2019).
Particularly popular among local ancestry inference methods are methods that use a hidden Markov model (HMM) to infer ancestry as a hidden state based on observations of genotypes or sequencing read counts. For autosomal loci in diploid individuals, there are three possible ancestry states (homozygous parent species 1, homozygous parent species 2, and heterozygous for the two ancestries). Ancestry HMMs allow the probability of a given local ancestry state to be modeled as a function of the observed data in the region, the ancestry state probability at the previous site, and the recombination distance between adjacent ancestry informative sites, among other possible parameters. The output of these methods is typically posterior probabilities for each possible ancestry state at each ancestry informative site along the chromosome (Alexander et al., 2009; Andolfatto et al., 2011; Corbett-Detig & Nielsen, 2017).
As local ancestry methods have been applied in more and more species (e.g. Cande, Andolfatto, Prud’homme, Stern, & Gompel, 2012; R. Li et al., 2018; Sankararaman et al., 2014; Schumer et al., 2014; Slotte et al., 2013), each with their own population genetic properties and demographic histories, simulation tools to evaluate performance have not kept apace. While some studies have carefully modeled the demographic history of hybridizing populations and the impact of this history on the accuracy of ancestry inference (e.g. Sankararaman et al., 2014; Medina, Thornlow, Nielsen, & Corbett-Detig, 2018), this typically requires the development of custom computational pipelines. As a result, many studies do not evaluate the expected performance of local ancestry inference methods and use default parameter sets.
While the majority of tools for local ancestry inference report performance under parameters relevant to human populations (e.g. Maples, Gravel, Kenny, & Bustamante, 2013), these tools are being applied much more broadly. This is concerning because local ancestry inference approaches that perform well in one context may perform poorly in others, as their performance is sensitive to a number of biological and technical variables (Medina et al., 2018; Sankararaman et al., 2014; Schumer, Cui, Rosenthal, & Andolfatto, 2016). Because of the importance of accurate local ancestry inference in evolutionary biology and genetics, simulation tools that allow researchers to systematically evaluate their accuracy and common biases are needed.
Here, we present a hybrid genome simulation pipeline called mixnmatch that can be used to evaluate the accuracy of local ancestry inference under a range of biological and technical parameters. Our pipeline builds on previous simulation tools developed by us and others to implement flexible demographic simulations in both ancestral parental populations and hybrid populations. Species-specific genetic parameters, including base composition and local recombination rates, can be incorporated into simulations. Users also specify a number of technical parameters that impact accuracy, including sequencing depth, sequencing error, and cross-contamination rates.
In addition to simulating admixed genomes with mixnmatch, we provide a paired pipeline called ancestryinfer that implements local ancestry inference on real or simulated data. This pipeline builds off of a previously published HMM (AncestryHMM; Corbett-Detig & Nielsen, 2017) and includes several features that seamlessly integrate its use with raw sequence data, automating and parallelizing steps from mapping Illumina reads to the output of posterior probabilities for ancestry states. Moreover, both pipelines are user-friendly, with parameters defined in a text-editable configuration file and automated and docker-based installation options. Together, mixnmatch and ancestryinfer will make it feasible for users to perform sophisticated simulations to predict accuracy and apply the same approaches when analyzing their data.
Methods & Results
General overview of mixnmatch and ancestryinfer pipelines
The overall structure of mixnmatch is described in Figure 1 and in more detail in Figure S1. First, the mixnmatch pipeline simulates parental haplotypes, either using user-provided genomes or the coalescent simulator macs (Chen, Marjoram, & Wall, 2009), which allows for simulations of complex demographic history (Figure S1; Supporting Information 1). With macs-based simulations, users can still provide one of the parental genomes for use as a base sequence (Figure S1).
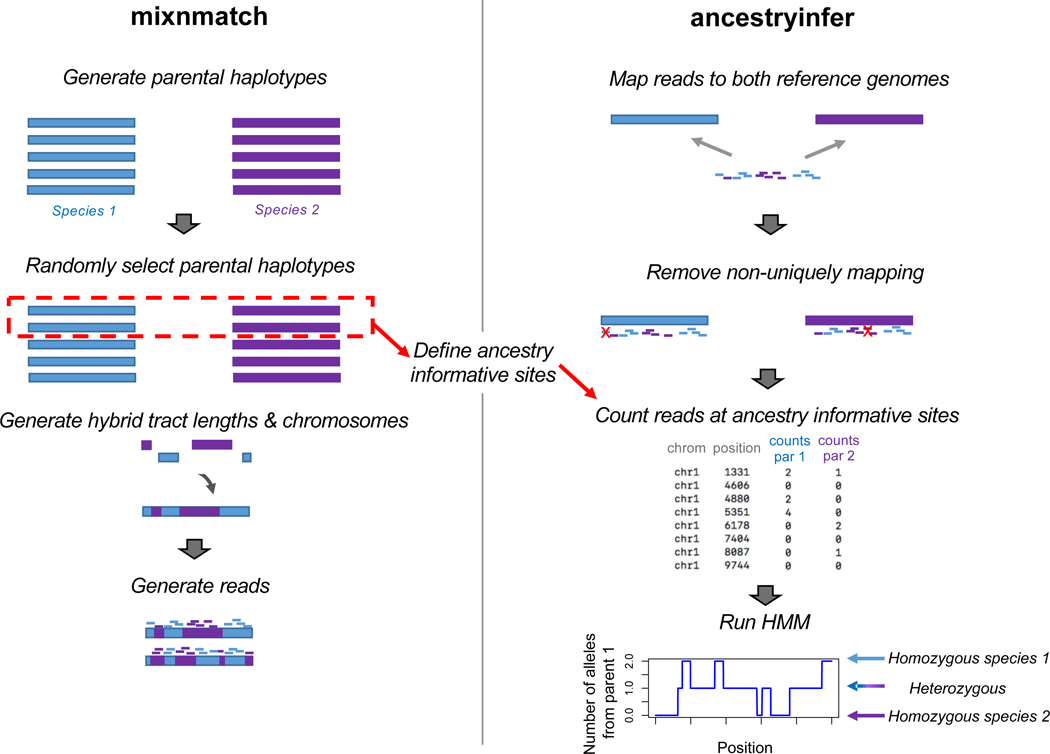
Figure 1.
Schematic showing the major steps in the mixnmatch (left) and ancestryinfer (right) pipelines. mixnmatch can be used to simulate hybrid data under user-specified parental and hybrid demographic scenarios and under a range of technical parameters. A more detailed description of the mixnmatch pipeline and its options is outlined in Figure S1. Simulated Illumina data output by mixnmatch can be input into our automated ancestry inference pipeline, ancestryinfer, as can data from natural and artificial hybrids. The red box indicates the haplotypes chosen from the parental species during mixnmatch to identify ancestry informative sites that can later be used in ancestryinfer. Blue shading indicates tracts and reads derived from parent species 1 and purple indicates tracts and reads derived from parent species 2. Red X’s mark reads that will be removed for not mapping uniquely to both parental genomes.
Next, ancestry tract lengths for hybrid individuals are generated with SELAM (Figure S1; Corbett-Detig & Jones, 2016), using information about the admixture proportion, demographic history of the hybrid population, and number of generations since initial admixture. Based on these tract lengths, hybrid genomes are constructed from previously simulated parental haplotypes. For each individual, reads are generated at user-specified lengths and depth using the wgsim program (H. Li, 2011). If desired, users can simulate cross-contamination between samples during read generation. Together, these features of mixnmatch allow for simulation of experimental design choices and the history of both the parental and hybrid population populations, making it a powerful tool for studies of hybridization.
Output files produced by the mixnmatch simulator include fastq and fasta files for each individual, a bed file indicating true ancestry along the simulated chromosome, and all necessary files for running an efficient local ancestry inference HMM, implemented through our associated pipeline, ancestryinfer (Figure 1, Table S1). ancestryinfer is compatible with real or simulated data. Notably, output files of mixnmatch can be directly input into ancestryinfer, without additional modifications. After running local ancestry inference with ancestryinfer, users can evaluate the performance of local ancestry inference with a provided script that summarizes accuracy by comparing inferred versus true ancestry at each ancestry informative site.
Installation
Users can install the two pipelines using step-by-step instructions provided with mixnmatch and ancestryinfer or by loading a docker image with all dependencies for both pipelines pre-installed (see Appendix 1; user manual). Parameters for each pipeline are set in a text-editable configuration file (examples available on github: https://github.com/Schumerlab/mixnmatch; https://github.com/Schumerlab/ancestryinfer). Instructions for setting parameters can be found in the mixnmatch and ancestryinfer user manuals (see Appendix 1–2). mixnmatch and ancestryinfer can be parallelized with a SLURM resource management system (Appendix 1–2) and the non-parallel version can be run any Linux system or on Mac operating systems. Both pipelines were tested on Centos 7 and MacOS Mojave operating systems.
Description of mixnmatch pipeline
Simulation of parental haplotypes and definition of ancestry informative sites
Generating parental haplotypes is the first step in mixnmatch simulations (Figure S1). An important feature of mixnmatch is the ability to simulate demographic history. This is crucial because the population history of each parental species will influence genetic diversity and the extent of background linkage disequilibrium within species, as well as divergence between species, all of which can impact accuracy in local ancestry inference. To model this, users provide a macs command (Chen, Marjoram, & Wall, 2009) describing the demographic history of the two parental species in the configuration file. mixnmatch executes this command and converts the macs output to nucleotide sequences using the seq-gen program (Rambaut & Grassly, 1997). Users can optionally provide species-specific base composition and transition/transversion ratios in the configuration file, as well as a local recombination map. If provided, this recombination map will be used in the macs simulations of parental haplotypes and in generating hybrid ancestry tracts (see Simulation of hybrid genomes).
One of the simulated sequences from each parental population is set aside as to be used as the reference sequence. The remaining haplotypes are then used to define ancestry informative markers and are later used to generate hybrid chromosomes (see Simulation of hybrid genomes below). Ancestry informative markers are defined as highly differentiated markers among a randomly selected subset of the simulated parental haplotypes. Users specify the required frequency difference between species and the number of parental haplotypes to use to evaluate this in the configuration file. We note that the appropriate choices for these values will depend on the level of divergence and shared polymorphisms between species; users can rely on predictions from population genetic theory (e.g. Wakeley & Hey, 1997) or mixnmatch simulations to explore these parameters. Together, these steps model the impacts of demographic history on the number and distribution of ancestry informative sites, as well as the steps researchers typically use to identify them.
Other options for parental haplotype generation
Although the method of haplotype generation described above incorporates demography, recombination, and incomplete lineage sorting in the parental species, it lacks other complexities of real genome sequences such as repetitive elements and local variation in base composition. To accommodate these additional challenges, we allow users to provide an ancestral sequence to which simulated mutations are added. This option incorporates features of real sequences while modeling mutations and ancestral recombination events using a coalescent framework.
Another biological variable that can impact accuracy in ancestry HMMs is drift between the reference panel used to define ancestry informative sites and the populations that actually contributed to the hybridization event. mixnmatch can simulate drift between the source population and the population used to define ancestry informative sites (using macs). If users choose this option, ancestry informative sites are defined based on the drifted parental population instead of the hybridizing parental population. This generates realistic allele frequency differences between the reference and source parental populations, as well as covariance in allele frequency differences due to linkage. In real data, this could contribute to errors in downstream analysis.
We also provide an option for using mixnmatch with the exact reference genomes and ancestry informative sites that users plan to rely upon in their experiments, if these two genomes are collinear. Instead of a macs command describing parental population history, this option takes the two parental reference genomes and a number of population genetic parameters as input (Figure S1). This approach is described in Supporting Information 1 (see also Schumer et al., 2016). Although this option has some limitations that should be considered (Supporting Information 1), it allows users to evaluate performance on species-specific reference genomes and ancestry informative sites.
Simulation of hybrid genomes
In addition to allowing users to simulate the demographic history of the parental species, mixnmatch models the demographic history of the hybrid population (Figure S1). Any process that influences the distribution of ancestry tract lengths, from bottlenecks to assortative mating, could impact the accuracy of local ancestry inference. To incorporate this, mixmatch uses a previously developed tool (SELAM, Corbett-Detig & Jones, 2016) to model the effects of demography on ancestry tracts. Users specify the mixture proportions contributed from each parent species to the hybrid population and the number of generations since initial admixture in the mixnmatch configuration file. In addition, users can choose to provide a parameter file describing the demographic history of the hybrid population (Appendix 1) as well as selection on hybrids.
Using these tract lengths, mixnmatch next generates hybrid chromosomes. For each ancestry tract, mixnmatch extracts the focal region from a randomly selected parental haplotype of the appropriate ancestry. If users have provided a local recombination map, mixnmatch uses this in converting tract coordinates from genetic to physical distance; otherwise a uniform global recombination rate is used. This process is repeated until an entire haplotype is generated, and two such haplotypes are combined to generate both chromosomes within a diploid hybrid individual. Importantly, this approach introduces variation to the simulation from processes such as incomplete lineage sorting and sampling of a reference panel, both of which can impact downstream accuracy.
The pipeline next simulates reads uniformly from these hybrid chromosomes using the wgsim program (H. Li, 2011), with user specified read lengths, read mate type, coverage, indel and error rates. At the same time contamination between samples can be simulated as might occur during DNA extraction or sequencing library preparation. During this step mixnmatch writes out the true ancestry for each individual at every position along the chromosome, facilitating analysis of accuracy downstream.
The final output of mixnmatch includes all of the files needed for running our paired pipeline for local ancestry inference, ancestryinfer, as well as files needed for other local ancestry inference tools. These include simulated reference genomes, ancestry informative sites and counts for each allele in the parental reference panel, simulated Illumina reads, and bed formatted files containing the true ancestry for each individual (Table S1).
One possible shortcoming of our approach for generating hybrid haplotypes is that it does not model coalescence among samples after admixture, which could generate errors in local ancestry inference not captured by our simulation approach. This is most likely to impact simulations of very small populations or ancient admixture (Corbett-Detig & Nielsen, 2017). We also note that the number of parental haplotypes used to generate the hybrid chromosomes is determined by the total number of parental haplotypes users choose to simulate. Simulating fewer parental haplotypes will decrease mixnmatch runtime, but users should ensure that the total number of parental haplotypes simulated captures most of the genetic variation within the parental populations (e.g. Figure S2; Watterson, 1975).
Description of ancestryinfer: a versatile ancestry inference pipeline
To facilitate local ancestry inference analysis of real and simulated data, we developed a paired pipeline called ancestryinfer. This pipeline automates steps from read mapping to local ancestry inference, and is easy-to-use and parallelizable (Supporting Information 2). While the underlying steps are similar to what users may design independently in their own workflows, this standardized pipeline allows for rapid and repeatable analysis, and includes several important steps that we have found increase accuracy. These are discussed in more detail below and include the ability to use species-specific de novo assemblies, the requirement that reads map uniquely to each reference genome, and the use of a single ancestry informative site per read.
The work flow of the ancestryinfer pipeline (Figure 1) begins with mapping reads from a hybrid individual to both parental references independently with bwa mem (H. Li & Durbin, 2009) and identifying reads that do not map uniquely to either of the parental genomes. These reads are then excluded from the hybrid individual’s bam file using ngsutils (Breese & Liu, 2013). Such reads may fall within repetitive regions of the parental genomes, be impacted by mapping bias, incompleteness of one parental reference, or insertions/deletions that disrupt mapping. These technical issues have received less attention as it relates to their impact on local ancestry inference (Supporting Information 3) but have been shown to have major impacts in other types of analyses such as allele-specific expression (Degner et al., 2009; Stevenson, Coolon, & Wittkopp, 2013). If desired, users can adjust the default mapping quality threshold (30) used at this step in the configuration file.
Next, reads matching each parental allele at ancestry informative sites are counted from a samtools mpileup file (H. Li, 2011) generated for each hybrid individual. Importantly, read counts are used instead of variant calling since variant calling can introduce biases in low-coverage data (Yu & Sun, 2013). There are two options in the pipeline for identifying ancestry informative sites. If the genomes provided by the user are collinear, users can direct ancestryinfer to automatically identify sites that differ between them. Alternatively, users can provide the locations of ancestry informative sites in the coordinate space of one genome and their estimated frequencies in the parental species. The latter option allows users to take advantage of mapping to reference assemblies for both species if they are available.
Counts for each parental allele at ancestry informative sites are subsampled to thin to one ancestry informative site per read if multiple sites occur within one read. This thinning is performed jointly across individuals such that the same site is retained for all individuals in the dataset. We implement this thinning because mismapping can generate clusters of errors and non-independence between sites is not modeled in the HMM. If read lengths differ among samples we recommend specifying the longer read length for thinning. Finally, Ancestry_HMM (Corbett-Detig & Nielsen, 2017; Supporting Information 4) is applied to infer posterior probabilities of each ancestry state at ancestry informative sites along the genome. In addition, ancestryinfer summarizes the intervals over which ancestry transitions occur (Figure S3). With the exception of false switches in ancestry generated by errors, these intervals reflect observed crossover events in hybrids. These crossover intervals can be used to generate recombination maps (see below, Generating a hybrid recombination map using observed ancestry transitions).
If users have run the ancestryinfer pipeline on data simulated by mixnmatch, the accuracy of local ancestry inference can be summarized by running a script provided with the mixnmatch pipeline (Appendix 2). Briefly, the script generates hard-calls at a user-specific posterior probability threshold and compares true and inferred ancestry at each ancestry informative site along the chromosome. The output of this script includes plots summarizing individual-level accuracy, accuracy as a function of tract length (Figure S4), and a file tabulating all accurate and inaccurate calls in individual tracts as well as mean posterior error.
Because the mixnmatch and ancestryinfer pipelines described above make use of several software packages, we encourage users to cite the underlying components when using these tools. We provide a suggested format in Supporting Information 4.
Predicted accuracy of local ancestry inference in simulated data
Basic simulation setup
Using mixnmatch and ancestryinfer, we next tested the accuracy of local ancestry inference with simulated data under a range of biological and technical scenarios. For these simulations, we started with a base parameter set (Table S2) and then systematically modified parameters in individual simulations. For this base parameter set we simulated 200 generations since initial admixture, 50–50 mixture proportions between the two parental species, per site polymorphism rates in each of the parental species of 0.1%, and pairwise sequence divergence between the parental species of 0.5%. We used the first 10 Mb of chromosome 1 from the swordtail fish species Xiphophorus birchmanni as the ancestral sequence, and provided mixnmatch with an inferred recombination map (Schumer et al., 2018) from that same region. We simulated 100 parental haplotypes for each species, which will capture the majority of parental polymorphisms segregating in these populations (Figure S2; Watterson, 1975). We sampled 20 parental haplotypes from both species to define ancestry informative sites and required a frequency difference of 95% between species for a site to be treated as ancestry informative (approximately 5 sites every 2 kb in base simulations). A complete description of these simulations can be found in Supporting Information 5. For this set of parameters, the pipeline required a total of 0.66 CPU hours and was run with 96 Gb of memory for the sequence generation step and 64 Gb of memory for the hybrid genome simulation step. Simulations were performed on Dell C6420 servers with a CentOS 7 operating system on Stanford’s Sherlock High Performance Computing cluster. Simulations with larger genome sizes than explored here (Table S2) are tractable but have higher memory requirements in the parental haplotype generation step. Simulations based on user-provided genomes have much lower memory and time requirements (Supporting Information 1; 0.08 CPU hours when run with 32 Gb of memory for all steps).
In simulations with the base parameter set (Table S2), accuracy of local ancestry inference was high (Figure S4). As expected, shorter ancestry tracts have higher per-basepair error rates (Figure S4). Because tract lengths often differ systematically across the genome and in particular around selected sites (Sedghifar, Brandvain, Ralph, & Coop, 2015; Shchur, Svedberg, Medina, Corbett-Detig, & Nielsen, 2019), this highlights the importance of considering local error rates throughout the genome when analyzing local ancestry data (Supporting Information 5, Figure S5).
Simulations under a range of scenarios
To understand what biological and technical variables impact accuracy in local ancestry inference, we next modified individual parameters in turn. Below we summarize the scenarios we tested that had the greatest impact on accuracy. A full description of our simulations can be found in Supporting Information 5. Although it is assumed in the simulations described below that researchers have information about the history of hybridizing populations, in practice researchers can explore a range of scenarios in simulations. Moreover, results of ancestryinfer are typically robust to parameter misspecification (Supporting Information 5).
Intuitively, with increasing divergence between species, there will be more ancestry informative sites. This is predicted to result in more accurate local ancestry inference. To evaluate this, we performed simulations varying pairwise divergence between the hybridizing species from 0.25% to 1%. We note that these simulations focus on deeper divergence than what has been considered in previous work (Maples et al., 2013; Medina et al., 2018) but span realistic levels of divergence found in naturally hybridizing species (Brandvain, Kenney, Flagel, Coop, & Sweigart, 2014; Schumer et al., 2014; Teeter et al., 2008; Turissini & Matute, 2017). As expected, accuracy increased with higher divergence between the hybridizing species (Figure 2), as did the precision with which the locations of ancestry transitions were identified (Figure S3; consistent with previous results; Medina et al., 2018).
Figure 2.
A) Results of mixnmatch simulations evaluating accuracy under different biological and technical parameters. All simulations start with the same basic parameter set (Table S2) and systematically vary the focal parameter (see Supporting Information 2). Points indicate the mean of individual-level accuracy and whiskers indicate two standard deviations. B) Example local ancestry results for simulated hybrids. Parameter used in this simulation were ~1X average coverage, sequence divergence of 0.5%, within species polymorphism rates of 0.1%, 20 generations since initial admixture, and 35% of the genome derived from parent species 1. C) Example local ancestry results for the first 10 Mb of chromosome 1 for natural hybrids formed X. birchmanni x X. malinche from the Chahuaco falls hybrid population, where per-base coverage and inferred parameter values match those simulated in B. Note the qualitative similarities between C and B in the number of ancestry transitions and the size of the ancestry tracts. In C parent 2 alleles are those derived from the X. malinche parental species.
As the time since initial admixture increases, recombination events in each generation split haplotypes of a given ancestry into smaller and smaller pieces. Since these short tracts will contain fewer ancestry informative sites, this leads to the prediction that local ancestry inference will be more accurate in populations that have hybridized recently, which is indeed what we observed (Figure 2). The point at which accuracy decreases substantially as a result of admixture time will be dependent on biological variables such as admixture proportion, divergence between the parental species, and recombination rates. In the case of the simulations presented here, accuracy fell below 95% by 10,000 generations post-admixture (Table S2). Another important biological scenario that can impact accuracy is when populations experience multiple admixture events; possible approaches to this type of data are discussed in Supporting Information 5.
Following a similar logic, skewed admixture proportions are expected to reduce accuracy of ancestry inference in tracts derived from the “minor” parental species (i.e. the parental species that contributed less to the initial hybridization event). This is because only recombination events that occur in regions heterozygous for ancestry from the two parent species are detectable with ancestry HMMs, and minor parent haplotypes are more frequently found in this state (Gravel, 2012). As expected, we observe that the accuracy of local ancestry inference within minor parent tracts is reduced in simulations with skewed initial admixture proportions (Figure S6).
Ideally, reference panels for defining ancestry informative sites should be derived from the same parental populations that contributed to the admixture event. In practice, this is often not possible since source populations may no longer exist, may be unknown, or may themselves be admixed, making it more sensible to use allopatric populations for a reference panel. However, such populations are also expected to have some level of genetic drift from the admixing populations, which could impact accuracy. In mixnmatch this can be modeled by adding drift to the simulation and specifying which populations to use in defining ancestry informative sites.
To investigate the impact of using reference panels with drift from the hybridizing populations, we used mixnmatch to simulate two additional populations that split from the parental source populations before hybridization and treated these populations as the reference panel (0.4–3Ne generations ago in different simulations, with initial divergence between species occurring 8Ne generations ago; Supporting Information 5). We found that accuracy substantially decreased with increasing drift between the reference population and the source parental populations (Figure 2). Notably, this can be partially remediated by increasing the required frequency difference between the parental populations when defining ancestry informative sites (Supporting Information 5). However, users should be aware of the inherent tradeoffs in accuracy and resolution when performing such filtering (Supporting Information 2, 5).
A common decision that researchers make is how much coverage to collect per sample. Intuitively, early generation hybrids will require less data to accurately infer local ancestry than later generation hybrids because of differences in the distribution of ancestry tract lengths. We simulated genome-wide coverage between 0.05–0.5X with mixnmatch (Supporting information 5). As expected, increased coverage improved accuracy but our results also suggested that beyond a certain level of coverage, improvements in accuracy plateau (Figure 2). However, higher coverage continued to improve the resolution of the locations of ancestry transitions (Figure S7).
In general, we find that the HMM implemented in ancestryinfer (Corbett-Detig & Nielsen, 2017) is not particularly sensitive to user-provided priors for admixture time or admixture proportion, but is somewhat sensitive to recombination rate priors (Supporting Information 5). Providing a local recombination prior in ancestryinfer modestly increases accuracy when the recombination map does not contain errors (Figure S8). However, in practice recombination maps will contain errors that depend on the method used for map construction among other factors (Supporting Information 5). With moderate levels of error in map inference our simulations suggest that users may benefit from providing a uniform recombination prior (Figure S8).
In recent years there has been substantial interest in the ecological and evolutionary genomics community in restriction site associated sequencing (or RAD-seq; Andrews, Good, Miller, Luikart, & Hohenlohe, 2016; Peterson, Weber, Kay, Fisher, & Hoekstra, 2012; Van Tassell et al., 2008) as a low-cost option for generating genomic data. However, RAD-seq data may be suboptiomal for local ancestry inference applications. This is because overdispersion in the spacing between sampled sites, coverage variation, and genealogical biases generated by variants in restriction enzyme cut-sites introduced by this method could all reduce the accuracy of local ancestry inference. To explore this, we generated reads in silico associated with a commonly used enzyme in RAD (EcoRI) but otherwise performed simulations as described above (Supporting Information 6). We found that in the case of ancestryinfer performance with RAD data is poor (Figure S9), likely due to the reliance on fewer ancestry informative sites for inference (Supporting Information 6).
Finally, although we focus on modeling accuracy under neutral demographic scenarios, mixnmatch can also be used to simulate selection on hybrids. The SELAM program that is used to simulate ancestry tract lengths in mixnmatch accommodates selection on hybrid populations (Corbett-Detig & Jones, 2016; Figure S10, Supporting Information 7). This allows users to implement versatile selection scenarios in mixnmatch (Appendix 1), and use it to explore signatures of selection on hybrids. For example, recent work has described the impact of selection against hybrid incompatibilities on the number and distribution of ancestry junctions (Hvala, Frayer, & Payseur, 2018), the impact of selection on ancestry tract lengths (Sedghifar et al., 2015; Shchur et al., 2019), and the local frequency of haplotypes derived from the minor parental species (Sankararaman et al., 2014; Schumer et al., 2018; Vernot & Akey, 2014). We demonstrate the use of this feature of mixnmatch in Supporting Information 7 and Figure S10.
Application to natural and artificial swordtail hybrids
To demonstrate the utility of ancestryinfer with real data, we applied it to data from F1 and F2 hybrids generated from crosses between the swordtail fish species X. birchmanni and X. malinche. These species are closely related, with an estimated pairwise sequence divergence of 0.5% per base pair (Schumer et al., 2018). We constructed libraries using a tagmentation based library preparation protocol and collected low coverage whole-genome sequence data for these libraries (~0.2X coverage per individual, Supporting Information 8, Appendix 3). We used previously collected low-coverage sequence data from 60 individuals of each parental species to estimate allele frequencies at ancestry informative sites (Schumer et al., 2018).
Illumina sequencing data from lab-generated hybrids was input into the ancestryinfer pipeline to infer local ancestry along the 24 swordtail chromosomes (here 150 basepair paired-end data was used). We used the appropriate parameters for mixture proportion, generations since mixture, and global recombination rate based on known information about the cross (see details in Supporting Information 8). We converted posterior probabilities for a given ancestry state into hard calls using a threshold of 0.9 and examined local patterns of ancestry in F1 and F2 hybrids. We also summarized expected ancestry proportions, heterozygosity in ancestry, and the numbers of observed ancestry transitions genome-wide (Figure 3).
Figure 3.
Results of the ancestryinfer pipeline run on F1 (A) and F2 (B) hybrids generated between X. birchmanni and X. malinche. A) As expected, we infer that F1 hybrids have precisely 50% of their genomes derived from each parental species and infer nearly 100% heterozygosity at ancestry informative sites in these individuals. Example local ancestry plots for chromosome 1 for a subset of these F1 hybrids are also shown (right). B) Similarly, genome-wide ancestry distributions and genome-wide ancestry heterozygosity in F2 hybrids follows predicted distributions. Example local ancestry plots for chromosome 1 for a subset of these F2 hybrids are also shown (right).
We find that local and global ancestry patterns in F1 and F2 hybrids mirror expectations for each cross type (Figure 3), and that the results are consistent with extremely low error rates in ancestry inference. For example, estimated homozygosity at ancestry informative sites in F1 hybrids is <0.1% (Figure 3). Importantly, this high level of accuracy is predicted from simulations of early generation hybrids with mixnmatch (Supporting Information 8), suggesting that mixnmatch simulations are capturing important properties of real data.
Generating a hybrid recombination map using observed ancestry transitions
Accurate local ancestry inference has a large number of downstream applications. One such application is inferring the locations of crossovers for the construction of genetic maps (Amores et al., 2014; Rastas, Calboli, Guo, Shikano, & Merilä, 2015; Salomé et al., 2012). As discussed previously, if users specify a posterior probability threshold in the ancestryinfer configuration file, the program will output a bed file containing recombination intervals inferred from observed ancestry transitions in hybrids.
We used the locations of observed ancestry transitions in 139 F2 hybrids that we generated between X. birchmanni and X. malinche (at a posterior probability threshold of 0.9; Supporting Information 8–9) to estimate the recombination rate in 5 Mb windows. We used a large window size due to the spatial scale over which ancestry transitions were localized (lower and upper 5% quantile of intervals genome-wide: 23 kb-667 kb) and because we expected the resulting map to be relatively coarse, given a total of 4038 inferred crossovers genome-wide (average 1.2 per individual per chromosome).
We compared inferred recombination rates in this F2 map to a linkage disequilibrium based recombination map for X. birchmanni that we had previously generated (Schumer et al., 2018). As expected, we observed a strong correlation in estimated recombination rate between the linkage disequilibrium based and crossover maps (R=0.82, Figure 4, Supporting Information 9). Simulations suggest that the observed correlation is consistent with the two recombination maps being indistinguishable, given the low resolution of the F2 map (Supporting Information 9).
Figure 4.
Comparison of F2 crossover recombination map generated with ancestryinfer and previously published linkage disequilibrium map from the X. birchmanni parental species. Relative rates per 5 Mb window for both the linkage disequilibrium map (x-axis) and F2 map (y-axis) are shown by gray dots. The blue line shows the best fit regression line between the maps (R2 = 0.67) and the gray area shows the 95% confidence intervals. Simulations suggest that the observed correlation is consistent with recombination rates being identical across the two maps (Supporting Information 9).
Discussion
With an increasing appreciation that hybridization is a common evolutionary process, there has been renewed interest in local ancestry inference in the fields of genetics and evolutionary biology. Accurate local ancestry information is important for applications from admixture mapping to insights into genome evolution after hybridization. Despite this, there are few simulation tools that have been developed to model the impacts of biological and technical variables on the accuracy of local ancestry inference.
We demonstrated the use of mixnmatch as a flexible tool to predict the accuracy of local ancestry inference under a range of biological scenarios. As expected a prori, our simulations show that the factors with the strongest impact on accuracy include the number of ancestry informative sites that distinguish the hybridizing species, the length of the ancestry tracts containing these sites, and the frequency at which sites are erroneously defined as ancestry informative (either due to genetic drift or high levels of shared polymorphisms; Figure 2). We show how mixnmatch can also help users make important decisions about their projects, such as how much coverage to collect per hybrid individual and how many parental individuals to sequence to define ancestry informative sites.
mixnmatch is primarily designed to allow users to explore demographic and technical parameters that may influence the accuracy of local ancestry inference. However, because it uses the SELAM program to generate ancestry tract lengths (Corbett-Detig & Jones, 2016), it is also possible to implement natural selection during admixture (Figure S10). This will allow users to study the impacts of selection on local ancestry, ancestry junctions, and ancestry tract lengths. We predict that this will be a useful feature of mixnmatch for the many research groups studying selection after hybridization.
Simulated data from mixnmatch can be used to evaluate the accuracy of any ancestry inference program. However, it is designed to pair seamlessly with the ancestryinfer pipeline we describe here, which automates steps from read mapping to local ancestry inference. ancestryinfer has excellent accuracy under a broad range of biological conditions, and is fast and easy to use. At present the mixnmatch and ancestryinfer accommodate admixture between two parental species; simulations and ancestry inference under more complex admixture scenarios will be an important extension for future work.
ancestryinfer is also intended to be an easy-to-use pipeline for local ancestry inference in real data. We demonstrate an application of the ancestryinfer pipeline to real data by using it to identify the locations of crossover events in X. birchmanni x X. malinche F2 hybrids (Figure 3) and construct a recombination map (Figure 4). Other possible uses of ancestryinfer include generating ancestry probabilities for QTL mapping or for studying genome evolution in natural hybrid populations, highlighting the versatile applications of the mixnmatch and ancestryinfer pipelines.
Supplementary Material
Acknowledgements
We thank Peter Andolfatto, Andrés Bendesky, Quinn Langdon, Ben Moran, David Reich, Alisa Sedghifar, and members of the Schumer and Corbett-Detig labs for helpful discussions and feedback on this work. We appreciate help from Patrick Reilly running MSG and from David Turissini running int-HMM. Stanford University and the Stanford Research Computing Center provided computational resources and support for this project. This work was supported by a Hanna H. Gray fellowship and NIH 1R35GM133774 grant to MS and by NIH 1R35GM128932 and an Alfred P. Sloan Fellowship to RC-D.
Footnotes
Data accessibility
Data associated with this manuscript is available on Dryad (doi:10.5061/dryad.rn8pk0p69; Schumer, Powell, & Corbett-Detig, 2019) and the mixnmatch and ancestryinfer pipelines are available on github (https://github.com/Schumerlab/mixnmatch; https://github.com/Schumerlab/ancestryinfer) and dockerhub (https://hub.docker.com/repository/docker/schumer/mixnmatch-ancestryinfer-image)
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/1755–0998.13175
References
- Alexander DH, Novembre J, & Lange K. (2009). Fast model-based estimation of ancestry in unrelated individuals. Genome Research, 19(9), 1655–1664. doi: 10.1101/gr.094052.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores A, Catchen J, Nanda I, Warren W, Walter R, Schartl M, & Postlethwait JH (2014). A RAD-Tag Genetic Map for the Platyfish (Xiphophorus maculatus) Reveals Mechanisms of Karyotype Evolution Among Teleost Fish. Genetics, 197(2), 625–641. doi: 10.1534/genetics.114.164293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfatto P, Davison D, Erezyilmaz D, Hu TT, Mast J, Sunayama-Morita T, & Stern DL (2011). Multiplexed shotgun genotyping for rapid and efficient genetic mapping. Genome Research, 21(4), 610–617. doi: 10.1101/gr.115402.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews KR, Good JM, Miller MR, Luikart G, & Hohenlohe PA (2016). Harnessing the power of RADseq for ecological and evolutionary genomics. Nature Reviews Genetics, 17(2), 81–92. doi: 10.1038/nrg.2015.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharian S, Barakatt M, Gignoux CR, Shringarpure S, Errington J, Blot WJ, … Gravel S. (2016). The Great Migration and African-American Genomic Diversity. PLOS Genetics, 12(5), e1006059. doi: 10.1371/journal.pgen.1006059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandvain Y, Kenney AM, Flagel L, Coop G, & Sweigart AL (2014). Speciation and Introgression between Mimulus nasutus and Mimulus guttatus. PLOS Genetics, 10(6), e1004410. doi: 10.1371/journal.pgen.1004410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese MR, & Liu Y. (2013). NGSUtils: a software suite for analyzing and manipulating next-generation sequencing datasets. Bioinformatics (Oxford, England), 29(4), 494–496. doi: 10.1093/bioinformatics/bts731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande J, Andolfatto P, Prud’homme B, Stern DL, & Gompel N. (2012). Evolution of multiple additive loci caused divergence between Drosophila yakuba and D. santomea in wing rowing during male courtship. PloS One, 7(8), e43888. doi: 10.1371/journal.pone.0043888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GK, Marjoram P, & Wall JD (2009). Fast and flexible simulation of DNA sequence data. Genome Research, 19(1), 136–142. doi: 10.1101/gr.083634.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett-Detig R, & Jones M. (2016). SELAM: simulation of epistasis and local adaptation during admixture with mate choice. Bioinformatics, 32(19), 3035–3037. doi: 10.1093/bioinformatics/btw365 [DOI] [PubMed] [Google Scholar]
- Corbett-Detig R, & Nielsen R. (2017). A Hidden Markov Model Approach for Simultaneously Estimating Local Ancestry and Admixture Time Using Next Generation Sequence Data in Samples of Arbitrary Ploidy. PLOS Genetics, 13(1), e1006529. doi: 10.1371/journal.pgen.1006529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner JF, Marioni JC, Pai AA, Pickrell JK, Nkadori E, Gilad Y, & Pritchard JK (2009). Effect of read-mapping biases on detecting allele-specific expression from RNA-sequencing data. Bioinformatics, 25(24), 3207–3212. doi: 10.1093/bioinformatics/btp579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel S. (2012). Population Genetics Models of Local Ancestry. Genetics, 191(2), 607–619. doi: 10.1534/genetics.112.139808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heliconius Genome C. (2012). Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature, 487(7405), 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggart CJ, Shriver MD, Kittles RA, Clayton DG, & McKeigue PM (2004). Design and Analysis of Admixture Mapping Studies. The American Journal of Human Genetics, 74(5), 965–978. doi: 10.1086/420855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvala JA, Frayer ME, & Payseur BA (2018). Signatures of hybridization and speciation in genomic patterns of ancestry: Genomic ancestry in hybrid zones. Evolution, 72(8), 1540–1552. doi: 10.1111/evo.13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MR, Mills LS, Alves PC, Callahan CM, Alves JM, Lafferty DJR, … Good JM (2018). Adaptive introgression underlies polymorphic seasonal camouflage in snowshoe hares. Science, 360(6395), 1355–1358. doi: 10.1126/science.aar5273 [DOI] [PubMed] [Google Scholar]
- Li H. (2011). A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics, 27(21), 2987–2993. doi: 10.1093/bioinformatics/btr509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, & Durbin R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 25(14). doi: 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Bitoun E, Altemose N, W Davies R, Davies B, & Myers S. (2018). A high-resolution map of non-crossover events in mice reveals impacts of genetic diversity on meiotic recombination. doi: 10.1101/428987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples BK, Gravel S, Kenny EE, & Bustamante CD (2013). RFMix: A Discriminative Modeling Approach for Rapid and Robust Local-Ancestry Inference. American Journal of Human Genetics, 93(2), 278–288. doi: 10.1016/j.ajhg.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina P, Thornlow B, Nielsen R, & Corbett-Detig R. (2018). Estimating the Timing of Multiple Admixture Pulses During Local Ancestry Inference. Genetics, 210(3), 1089–1107. doi: 10.1534/genetics.118.301411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana G, & Pritchard JK (2004). Statistical Tests for Admixture Mapping with Case-Control and Cases-Only Data. The American Journal of Human Genetics, 75(5), 771–789. doi: 10.1086/425281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oziolor EM, Reid NM, Yair S, Lee KM, VerPloeg SG, Bruns PC, … Matson CW (2019). Adaptive introgression enables evolutionary rescue from extreme environmental pollution. Science, 364(6439), 455–457. doi: 10.1126/science.aav4155 [DOI] [PubMed] [Google Scholar]
- Patterson N, Hattangadi N, Lane B, Lohmueller KE, Hafler DA, Oksenberg JR, … Reich D. (2004). Methods for High-Density Admixture Mapping of Disease Genes. The American Journal of Human Genetics, 74(5), 979–1000. doi: 10.1086/420871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N, Moorjani P, Luo Y, Mallick S, Rohland N, Zhan Y, … Reich D. (2012). Ancient Admixture in Human History. Genetics, 192(3), 1065–1093. doi: 10.1534/genetics.112.145037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BK, Weber JN, Kay EH, Fisher HS, & Hoekstra HE (2012). Double Digest RADseq: An Inexpensive Method for De Novo SNP Discovery and Genotyping in Model and Non-Model Species. PLOS ONE, 7(5), e37135. doi: 10.1371/journal.pone.0037135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, & Donnelly P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155(2), 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A, & Grassly NC (1997). Seq-Gen: An application for the Monte Carlo simulation of DNA sequence evolution along phylogenetic frees. Computer Applications in the Biosciences, 13(3). [DOI] [PubMed] [Google Scholar]
- Rastas P, Calboli FCF, Guo B, Shikano T, & Merilä J. (2015). Construction of Ultradense Linkage Maps with Lep-MAP2: Stickleback F2 Recombinant Crosses as an Example. Genome Biology and Evolution, 8(1), 78–93. doi: 10.1093/gbe/evv250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé PA, Bomblies K, Fitz J, Laitinen RAE, Warthmann N, Yant L, & Weigel D. (2012). The recombination landscape in Arabidopsis thaliana F2 populations. Heredity, 108(4), 447–455. doi: 10.1038/hdy.2011.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankararaman S, Mallick S, Dannemann M, Prüfer K, Kelso J, Pääbo S, … Reich D. (2014). The genomic landscape of Neanderthal ancestry in present-day humans. Nature, 507(7492), 354–357. doi: 10.1038/nature12961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankararaman S, Mallick S, Patterson N, & Reich D. (2016). The Combined Landscape of Denisovan and Neanderthal Ancestry in Present-Day Humans. Current Biology, 26(9), 1241–1247. doi: 10.1016/j.cub.2016.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumer M, Cui R, Powell DL, Dresner R, Rosenthal GG, & Andolfatto P. (2014). High-resolution mapping reveals hundreds of genetic incompatibilities in hybridizing fish species. ELife, 3, e02535. doi: 10.7554/eLife.02535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumer M, Cui R, Rosenthal GG, & Andolfatto P. (2016). simMSG: an experimental design tool for high-throughput genotyping of hybrids. Molecular Ecology Resources, 16(1), 183–192. doi: 10.1111/1755-0998.12434 [DOI] [PubMed] [Google Scholar]
- Schumer M, Powell DL, & Corbett-Detig R, 2019. Data availability-Dryad, doi: 10.5061/dryad.rn8pk0p69. [DOI] [Google Scholar]
- Schumer M, Xu C, Powell DL, Durvasula A, Skov L, Holland C, … Przeworski M. (2018). Natural selection interacts with recombination to shape the evolution of hybrid genomes. Science, 360(6389), 656. doi: 10.1126/science.aar3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedghifar A, Brandvain Y, Ralph P, & Coop G. (2015). The Spatial Mixing of Genomes in Secondary Contact Zones. Genetics, genetics.115.179838. doi: 10.1534/genetics.115.179838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchur V, Svedberg J, Medina P, Corbett-Detig R, & Nielsen R. (2019). On the distribution of tract lengths during adaptive introgression. BioRxiv, 724815. doi: 10.1101/724815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte T, Hazzouri KM, Ågren JA, Koenig D, Maumus F, Guo Y-L, … Wright SI (2013). The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nature Genetics, 45, 831. [DOI] [PubMed] [Google Scholar]
- Stevenson KR, Coolon JD, & Wittkopp PJ (2013). Sources of bias in measures of allele-specific expression derived from RNA-seq data aligned to a single reference genome. BMC Genomics, 14(1), 536. doi: 10.1186/1471-2164-14-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter KC, Payseur BA, Harris LW, Bakewell MA, Thibodeau LM, O’Brien JE, … Tucker PK (2008). Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Research, 18(1), 67–76. doi: 10.1101/gr.6757907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turissini DA, & Matute DR (2017). Fine scale mapping of genomic introgressions within the Drosophila yakuba clade. PLOS Genetics, 13(9), e1006971. doi: 10.1371/journal.pgen.1006971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tassell CP, Smith TPL, Matukumalli LK, Taylor JF, Schnabel RD, Lawley CT, … Sonstegard TS (2008). SNP discovery and allele frequency estimation by deep sequencing of reduced representation libraries. Nature Methods, 5(3), 247–252. doi: 10.1038/nmeth.1185 [DOI] [PubMed] [Google Scholar]
- Vernot B, & Akey JM (2014). Resurrecting Surviving Neandertal Lineages from Modern Human Genomes. Science, 343(6174), 1017–1021. doi: 10.1126/science.1245938 [DOI] [PubMed] [Google Scholar]
- Wakeley J, & Hey J. (1997). Estimating Ancestral Population Parameters. Genetics, 145(3), 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson GA (1975). On the number of segregating sites in genetical models without recombination. Theoretical Population Biology, 7(2), 256–276. [DOI] [PubMed] [Google Scholar]
- Winkler CA, Nelson GW, & Smith MW (2010). Admixture Mapping Comes of Age. Annual Review of Genomics and Human Genetics, 11(1), 65–89. doi: 10.1146/annurev-genom-082509-141523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, & Sun S. (2013). Comparing a few SNP calling algorithms using low-coverage sequencing data. BMC Bioinformatics, 14, 274. doi: 10.1186/1471-2105-14-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.