Abstract
The use of liposomes as drug carriers improves the therapeutic efficacy of anti-cancer drugs, while at the same time reducing side effects. Hyaluronic acid (HA) is recognized by the CD44 receptor, which is over-expressed in many cancer cells. In this study, we developed HA-modified liposomes encapsulating 5-fluorouracil (5-FU) and tested them against a CD44 expressing colorectal cell line (HT29) and a non-CD44 expressing hepatoma cell line (HEPG2). The average size of 5-FU-lipo and 5-FU-lipo-HA nanoparticles were 112±28 nm and 144±77 nm respectively. The MTT assay showed selective cancer cell death depending on CD44 expression in a time-dependent manner. Apoptosis assays and cell cycle analysis indicated that G0/G1 arrest occurred. The colony formation study revealed that cells treated with 5-FU-lipo and 5-FU-lipo-HA had reduced colony formation. QRT-PCR study showed that the oncogenic mRNA and miRNA levels were significantly reduced in the 5-FU-lipo-HA treated group, while tumor suppressors were increased in that group. We suggest that optimal targeted delivery and release of 5-FU into colorectal cancer cells, renders them susceptible to apoptosis, cell cycle arrest and decreased colony formation.
Keywords: Colorectal cancer, 5-Fluorouracil, Hyaluronic acid, CD44, Liposomes, Apoptosis, Clonogenicity, Cell cycle, microRNA
Graphical Abstract
1. Introduction
Colorectal cancer is one of the most common cancers in the world, although its prevalence is higher in developed countries (Janout & Kollárová, 2001; Wilmink, 1997; Winawer et al., 1997). Surgery, Chemotherapy, Radiotherapy are common treatment methods for colorectal cancer. Although chemotherapy agents are widely used for many types of cancers, their efficacy and selectivity are limited due to low bioavailability, poor solubility, development of resistance, and non-specific bio-distribution between cancer and normal cells/tissues (Abedi-Gaballu et al., 2018; Arnold et al., 2017; Singh, Palombo, & Sinko, 2008; Watanabe et al., 2012). For example, 5-fluorouracil (5-fluoro-2, 4-pyrimidinedione or 5-FU) is one chemotherapeutic agent that was first reported for colorectal cancer in 1960. It is still widely used to manage colorectal cancer. 5-FU is an antimetabolite and an analogue of the Pyrimidine, Uracil that disrupts DNA replication, but is metabolized through identical pathways to Uracil (Arias, 2008). In addition, 5-FU is an effective treatment for advanced colorectal cancer with poor prognosis and improves patient survival and quality of life (Fanciullino et al., 2007; Zhang, Yin, Xu, & Chen, 2008). Furthermore, both oral administration or intravenous injection of 5- FU have problems such as rapid removal from the blood due to a short biological half-life, and rapid catabolism in the liver leading to the need for larger doses that cause side effects, such as diarrhea, mucositis, hand-foot syndrome and leucopenia (Cheng et al., 2012; Othman et al., 2017). To overcome these deficiencies, drug delivery systems have been developed to deliver 5-FU. Their main purposes are: (a) the protection of 5-FU from the degradation within the body before reaching the desired target sites (Abedi-Gaballu et al., 2018; Ghaffari et al., 2018); (b) to preserve the normal cells/tissues from non-specific cytotoxicity; (c) to allow controlled 5-FU released at predictable rates; (d) to carry a suitable amount of 5-FU to overcome poor drug permeation; (e) to deliver 5-FU to the target tumor site by a passive targeting process; (f) to deliver 5-FU by an active targeting process. The passive targeting process is a strategy whereby nanocarriers that are loaded with drugs can accumulate into specific tissues, relying on the specific properties of the delivery system and the particular pathophysiology of the disease to be treated. This process is well known as the “enhanced permeability and retention” (EPR) effect. Active targeting is, however, another type of delivery in which the nanocarrier is covalently attached to specific ligands, such as hyaluronic acid (HA), folic acid (FA), or transferrin (Tf). These molecular ligands can be recognized by cognate receptors, which are overexpressed on the target tissues or cells. Recognition of ligands by their receptors leads to the gradual accumulation of functionalized nanocarriers at the target site, which eventually enhances the internalization of drugs via increasing ligand-receptor mediated cellular uptake (Adams et al., 2001; Kannagi, Izawa, Koike, Miyazaki, & Kimura, 2004). For instance, HA is a naturally occurring polysaccharide that can specifically bind to the CD44 receptor that is overexpressed by several types of cancer cells. HA has been widely employed as an active targeting moiety for various drug delivery systems (Hayward, Wilson, & Kidambi, 2016). Several nanocarriers, such as solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), polymeric nanoparticles, liposomes, graphene oxide, gold nanoparticles etc, have been developed and used as drug delivery systems. Most of these nanoparticles have been examined for delivery of 5-FU for cancer therapy via passive or active targeting strategies (Amasya, Badilli, Aksu, & Tarimci, 2016; Barzegar Behrooz et al., 2017; Paliwal, Paliwal, Agrawal, & Vyas, 2016; Safwat, Soliman, Sayed, & Attia, 2016; Thomas et al., 2011; Vinothini & Rajan, 2017). Among the mentioned nanoparticles, liposomes have been considered as a potential vehicle for therapeutic agents and imaging agents (Kim, 2016; Malam, Loizidou, & Seifalian, 2009). The present study aimed to prepare liposomes-decorated with HA as nanoparticles for targeted delivery of 5-FU, and to evaluate its anticancer effects on proliferation, apoptosis, cell cycle, and colony formation of CD44 expressing HT-29 colorectal cancer cells.
2. Materials and methods
2.1. Materials
1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP), 1,2-dioleoyl-sn-glycerol-3 phosphoethanolamine (DOPE) and 1-ethyl-3-[3-dimethyl) aminopropyl] carbodiimide hydrochloride (EDAC) were purchased from CAIMAN. DSPE-PEG2000 was provided from Lipoid GmbH (Ludwigshafen Germany). 5-Fluorouracil (2, 4-Dihydroxy-5-fluoropyrimidine, PubChem CID: 24278439), 3-(4, 5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium (MTT) and hyaluronic acid (HA) were obtained from Sigma Aldrich (St. Louis, Missouri, United States). Fetal bovine serum (FBS), penicillin-streptomycin (10,000 U/ml), Roswell Park Memorial Institute 1640 (RPMI), 2-(4-amidinophenyl)-6-indolecarbamidine dihyformaldehyde (DAPI) and trypsin-EDTA (0%25) were provided from Gibco. Dimethyl sulfoxide (DMSO) and phosphate-buffered saline (PBS) was obtained from Merck Company, Germany.
2.2. Methods
2.2.1. Preparation of liposomes and liposome decoration with HA
Liposomal nanoparticles were prepared by incorporating DOPE, DOTAP, and des PEG2000 at a 1:1:1.5 molar ratio using the thin layer film hydration method, which was previously described (Hayward et al., 2016). Briefly, 4.46mg DOPE ( 600μM), 4.19 mg DOTAP (600 μM) and des PEG2000 (9 μM) were dissolved in 10 ml of chloroform, then 1mg 5-FU was added to the lipid solution. In the next step, the organic solvent was removed using a rotary evaporator under vacuum at 37 oC. The resulting thin film was hydrated with 1ml HEPES buffer and sonicated in order to achieve size reduction of the liposomes. After preparing the liposomes, the amine groups of the DOPE phospholipids were covalently conjugated to the carboxylic acid groups of EDC-activated HA to form an amide bond according to a previously described method with minor modification (Zarebkohan et al., 2015). To achieve this, 7mg HA was dissolved in 2.5 ml in water and preactivated with 3 mg EDC for 2h at 37 oC, followed by titration by 1 N HCL to the final pH 4. Next, the activated HA was mixed with prepared formulation liposomes (1:1 molar ratio liposomes: HA).
2.2.2. Size, Zeta Potential, and Morphology of 5FU-Loaded HA-liposomes
The particle size of the liposomes was measured by dynamic light scattering technique (DLS) using a particle size analyzer (Malvern Instruments Ltd, UK) at room temperature. All samples were diluted 10 times with deionized water. In addition, the morphology of 5-FU loaded bare liposome (5-FU-lipo) and 5-FU loaded HA decorated liposome (5-FU-lipo-HA) nanoparticles was investigated using a scanning electron microscope (SEM) (MV2300, Vega Tescan, Czech Republic).
2.2.3. Encapsulation efficiency and 5-FU loading measurement
Encapsulation efficiency (EE) of 5-FU in liposomes and liposomes-HA was measured using the following equation EE = Wo/Wi × 100%, where Wo and Wi are the weight of the drug before and after separation respectively. To evaluate the weight of non-entrapped 5-FU, 1 ml of the formulation was filtered via Amicon® Ultra-Centrifugal filters (MWCO 30 KD, Millipore, USA) for 15 min at 3000rpm. The concentration of unloaded 5-FU was measured via ultraviolet-visible spectroscopy (UV-vis) method. The drug loading (DL) ratio was calculated as the weight of encapsulated 5-FU divided by the weight of lipids.
2.2.4. In vitro 5-FU release assay:
The dialysis bag diffusion technique was employed to evaluate the rate of 5-FU release from 5-FU-lipo and 5-FU-lipo-HA nanoparticles. Briefly, 2ml of each 5-FU loaded liposomes and liposome-HA nanoparticles were separately diluted with 2 ml PBS and then placed into a dialysis bag (MWCO 20 kDa) and immersed into 25 ml of PBS (10 mM, pH7.4 and 5.5), containing 1% (v/v) Tween 80 as a surfactant. The release system was kept at 37oC and 300 rpm. At a predetermined time interval, 1 ml of the samples were withdrawn and replaced with fresh buffer solution. The amount of released 5-FU was measured using UV–Vis spectrophotometry at 266nm.
2.2.5. Cell culture
Human colorectal cancer cells (HT-29) (CD44high) and HEPG2 (CD44low) hepatoma cells were purchased from the Pasteur Institute (Tehran, Iran). The cells were maintained in RPMI 1640 with 10% FBS (GIBCO, Carlsbad, CA, USA) and cultured at 37C with 5% CO2. The cells were sub-cultured 24–48 h later with an initial concentration of 4×104 cells/ml and used in the logarithmic phase in all experiments.
2.2.6. In Vitro Cellular Uptake
To confirm and compare the internalization of bare liposomes and liposome-HA nanoparticles into the HT-29 and HEPG2 cells, rhodamine B (RhoB) was used as a fluorescent marker according to a previous report (Sabzichi et al., 2017). In vitro cellular uptake was performed via encapsulation of RhoB (as a model drug) into the liposomes. To separate, the free RhoB from entrapped RhoB, a purification process was applied using an Amicon® tube (Ultra-30kDa molecular weight cut-off membrane, Millipore, Germany). The fluorescent liposomes were placed into the upper chamber of Amicon® Falcon and centrifuged at 5000 rpm for 15 min. Unloaded RhoB molecules were passed through the filter and gathered in the lower chamber of the Amicon tube. The upper chamber that comprised pure fluorescent liposomes was diluted with PBS and then centrifuged again five times to ensure the total removal of non-entrapped RhoB from the nanoparticles. The cell lines were seeded in six-well plates with 4×105 cells per well for 24 h. After that, the cells were treated with fluorescent dye-labelled liposomes for 1, 3, 6, 12 and 24 hours; they were washed three times with sterile PBS and collected by centrifugation at 1000 rpm for 10min. Finally, quantitative cellular uptake of the formulations was measured using flow cytometry. Moreover, nuclear staining using DAPI was done to image qualitative cell uptake using fluorescence microscopy at 6 and 12 h.
2.2.7. Cell viability assay
The MTT (3-[4, 5-dimethyl-2-thiazolyl]- 2, 5 diphenyltetrazolium bromide) assay was used to measure cell viability after incubation with free 5-FU, 5-FU-lipo and 5-FU-lipo-HA [23]. Briefly, 15×103 HT-29 and HEPG2 cells at passage 3 were seeded into each well of a 96-well plate. Then, the cells were treated with different concentrations (10–70 μg/ml) of free 5-FU and its nanoformulations. After 24, 48 and 72 h, MTT solution (2mg/ml PBS) was added to each well. The incubated medium was replaced with 200 μl of DMSO after 4 h. Then, the absorption of the plates was read at a wavelength of 570 nm using TCAN Elisa Reader.
2.2.8. DAPI staining
DAPI staining is a method that reveals chromatin fragmentation. HT-29 cells were seeded into six-well plates, including 12mm cover-slips at 5 × 105 cells per well. The cultivated cells were treated with free 5-FU, blank liposomes, 5-FU-lipo and 5-FU-lipo-HA over 48 h. Then, the cells were fixed with 5% formaldehyde for 3 h and permeabilized with 0.1% Triton X-100 in PBS for 5 min. Following that, they were stained with DAPI 0.1% for 10 min and finally monitored by a fluorescence imaging system (Cytation 5, Biotek, USA).
2.2.9. Determination of Apoptotic Cells by Flow Cytometry
HT-29 cells at a density of 4×105 cells per well were seeded and incubated overnight in medium containing the IC50 concentrations of free 5-FU, 5-FU-lipo and 5-FU-lipo-HA at 37°C for 48 h. Then, they were detached by diluted trypsin three times, washed twice in PBS, and re-suspended in binding buffer at ice-cold temperature. Finally, the cells were detached and stained using an Annexin V/PI apoptosis detection kit (Exbio, Czech Republic) according to the manufacturer’s protocol. Then, the rate of the apoptosis was analyzed by flow cytometry (MACS Quant 10, Miltenyi Biotech GmbH). The achieved data were analyzed by using FlowJo software package (Treestar, Inc., San Carlos, CA).
2.2.10. Cell cycle assay
HT-29 cells were cultivated at a density of 2×106 cells/well in 6-well plates in 4 groups: blank liposome, free 5-FU, 5-FU-lipo and 5-FU-lipo-HA. The cells were harvested 48 h after the incubation, fixed with 50% ethanol, treated with 5 mg/ml RNase A (Bioneer, Daedeok-gu, Daejeon, Korea), stained with 50 mg/ml propidium iodide, and subsequently analyzed by flow cytometry for DNA synthesis and cell cycle status (MACS Quant 10, Miltenyi Biotech GmbH).
2.2.10. Clonogenicity Assays
The colony formation assay was performed to investigate the effect of the blank liposome, free 5-FU, 5-FU-lipo and 5-FU-lipo-HA on the clonogenicity of HT29 cells. 5×103 HT29 cells were seeded in 6-well plate after incubation and then followed up for two weeks. The colonies were fixed, stained with crystal violet, and then photographed. They were counted manually and plotted as the number of colonies.
2.2.11. qRT-PCR
Total cellular RNA were isolated by the RiboEX reagent after 48 h treatment from 1×106 HT-29 cells (GeneAll, GeneAll biotechnology, Seoul, Korea). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA via thermal cycler system (Bio-Rad, Hercules, CA). qRT-PCR was performed with the Light Cycler 96 instrument (Roche Diagnostics, Mannheim, Germany) using a 2X SYBR green master mix.
The primer sequences were as follows in Table 1. The evaluation of ALDH, KLF4, HMGA2, C-Myc, Nanog, CD44, Kras, miR-181a, miR181b, Let-7, miR-21, miR-155, miR-200c and miR-34c were carried out using an initial denaturation step at 94 °C for 10 min, followed by 45 cycles at 94 °C for 10 sec, and then 60 °C. Beta-actin and U6 were used as reference gene for mRNA and miRNA. The relative expression of miRNA and mRNA measured by 2-ΔΔct.
Table 1.
Primer sequences
| Primers | Sequences (5’–3’) | |
| Beta-actin | F | TCCCTGGAGAAGAGCTACG |
| R | GTAGTTTCGTGGATGCCACA | |
| ALDH | F | AGATGTGGACAAGGCAGTGAA |
| R | ATCCACCAGGTAGGAGATGAC | |
| KLF4 | F | ACCTTCTTCACCCCTAGAGC |
| R | CCCAGTCACAGTGGTAAGGT | |
| HMGA2 | F | TGGGAGGAGCGAAATCTAAA |
| R | TCCCTGGAGAAGAGCTACG | |
| c-Myc | F | CACATCAGCACAACTACGCA |
| R | GCTCCAAGACGTTGTGTGT | |
| Nanog | F | CAATGGTGTGACGCAGGGAT |
| R | TGCACCAGGTCTGAGTGTTC | |
| CD44 | F | CAAGCCACTCCAGGACAAGG |
| R | ATCCAAGTGAGGGACTACAACAG | |
| Kras | F | CGTAGGCAAGATGCCTTGA |
| R | CCTCTTGACCTGCTGTGTCG | |
| miRNA | Target Sequence | |
| miR-181a | AACAUUCAACGCUGUCGGUGAGU | |
| miR-181b | AACAUUCAUUGCUGUCGGUGGGU | |
| Let-7 | UGAGGUAGUAGGUUGUAUAGUU | |
| miR-21 | UAGCUUAUCAGACUGAUGUUGA | |
| miR-155 | UUAAUGCUAAUCGUGAUAGGGGUU | |
| miR-200c | CGUCUUACCCAGCAGUGUUUGG | |
| miR-34c | AGGCAGUGUAGUUAGCUGAUUGC | |
2.2.12. Statistical analyses
All of the obtained parameters were expressed as the mean ± standard deviation (SD). All the experiments were repeated three times independently. Statistical analyses were carried out using a one-way and two-way analysis of variance (one-way and two-way ANOVA) with Dunnett’s multiple comparisons test (Prism, version 6.0, GraphPad software, INC). A p-value less than 0.05 was considered significant.
Results and discussion
3.1. Preparation and characterization of nanoparticles
To investigate the possibility of using HA as a targeting ligand for colorectal cancer, liposomal nanoparticles were firstly prepared using the thin layer film hydration method. Empty liposomes and HA-decorated liposomal nanoparticles were prepared by incorporating DOTAP: DOPE: des PEG at a 1:1:1 molar ratio and DOTAP: DOPE: des PEG: HA with 1:1:1: 0.5 molar ratio, respectively. The optimized ratios of phospholipids of the nanoparticles were obtained according to pilot study results (data not shown). The conjugation of HA to DOPE in the liposomal nanoparticles was done using EDC to facilitate amide linkage formation between the amine group of DOPE and the carboxyl group of the HA and this was confirmed by FTIR spectroscopy. The conjugation of HA with DOPE was confirmed by IR bands at 729–500 cm−1 out of plane N-H wagging, 1644 cm−1 aromatic C=C stretch of HA, and 3454 cm−1 N-H stretch of amide. Therefore, the presence of these bands in HA and DOPE IR spectrum (Figure 1 a and b) indicated that DOPE-HA conjugation had been formed. This result is in accordance with several previous reports showing conjugation of HA to DOPE through covalent amide bond formation (Hayward et al., 2016; Taetz et al., 2009).
Figure 1.
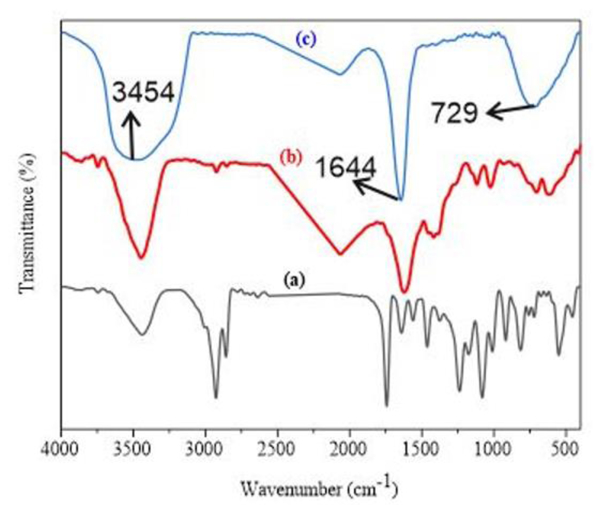
Shows the FTIR spectrum for DOPE (a), HA (b) and DOPE-HA (c).
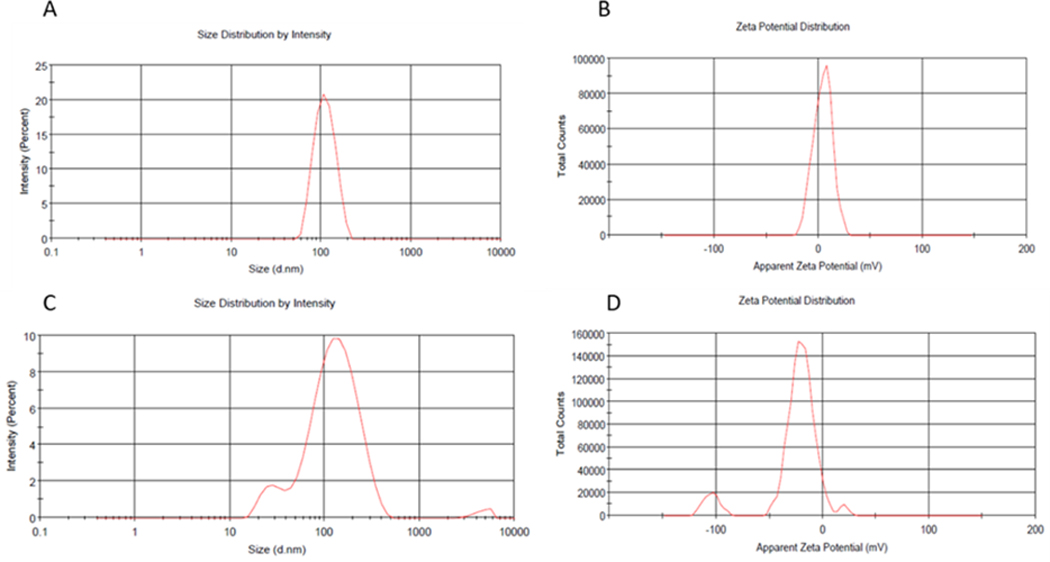
The nanoparticles were prepared and then characterized by measuring their size, zeta potential and PDI. It was reported that the in vivo fate of nanoparticles can be influenced by several parameters such as their size and surface charge. The particle size of bare liposomes and HA-decorated liposomes were 112±28 and 144±77nm with acceptable PDI values of 0.15 and 0.2, respectively (Figure 2 a, b c, d). Therefore, surface functionalization of liposomal nanoparticles with HA increased the particle size in agreement with a report by Ravar et al in 2016 (Ravar et al., 2016). They showed that HA, as a hydrophilic molecule, is able to uptake water and swell, increasing the size several folds higher than the original size in aqueous media (Ravar et al., 2016). They concluded that the increased particle size of HA-modified liposomes is related to the presence of HA. On the other hand, the measured size for the nanoparticles should be suitable for 5-FU delivery due to their appropriate size. Previously, it was reported that nanoparticles with an average size of 70 to 200 nm have longer blood circulation time than free drugs, because they are able to effectively escape clearance by the reticuloendothelial system (RES), whereas if the particle size was larger than 200nm, they can be eliminated by the RES (Ossipov, 2010; T. Yang et al., 2007). The zeta potential was found to be +4mV and −19mV for bare liposomes and HA-decorated liposomes, respectively. These results indicated that the positive charge of bare liposomes had been converted to a negative charge in HA-decorated liposomes due to the presence of excess carboxylic acid of HA on the surface of the liposomes. Overall, these results confirmed the surface functionalization of liposomal nanoparticles with HA (Hayward et al., 2016).
Figure 2.
DLS obtained particle size for 5-FU-lipo (A), zeta potential of 5-FU-lipo (B), particle size of 5-FU-lipo-HA (C), and zeta potential of 5-FU-lipo-HA (D).
The % value for EE and DL were found to be 92±4.54 % and 4.6±0.22 % for bare liposomal nanoparticles, respectively, while these values were calculated to be 91% and 4.21% for HA-liposomes. These results showed that there was no significant difference between EE and DL % for both bare and HA-modified liposomes consistent with several previous studies (Assanhou et al., 2015; Han et al., 2016; Paliwal et al., 2016). SEM was employed to monitor the morphology of nanoparticles. The result showed that both bare and HA-decorated liposomes had a spherical shape (Figure 3 a and b).
Figure 3.
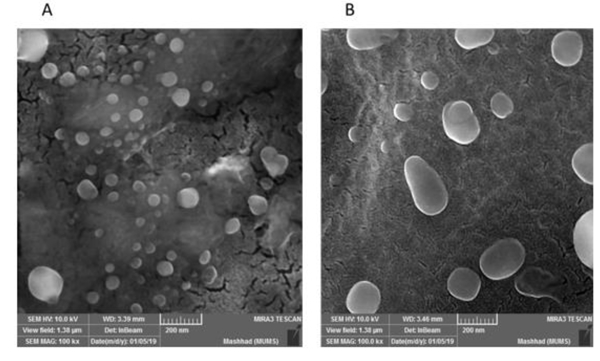
SEM visualized the morphology of (A) 5-FU-lipo and (B) 5-FU-lipo-HA nanoparticles
The amount of 5-FU released from liposomal nanoparticles (bare liposomes and HA-liposomes) was measured after complete separation of non-entrapped 5-FU from the liposomes. Figure4 shows that the release of 5-FU from HA-liposomes was about 40% after 10 h in an acidic environment (pH 5.5 similar to the endosomal pH). Furthermore, at physiological (pH 7.4, similar to blood pH), 5-FU release was about 19% over 10 h. Moreover, in the case of bare liposomes after 2h, 5-FU release at pH 5.5 and pH 7.4 was about 40% and 20%, respectively. According to these results, 5-FU release from bare liposome more rapid than that from HA-decorated liposomes. Therefore, surface modification of liposomes with HA decreased 5-FU release when compared with bare liposomes. This can be explained by the surface covering of the liposomes with HA, which reduces the bilayer fluidity or membrane permeability. Our release profile results are consistent with recent reports. In one study, Tian et al 2018 studied the DOX release profile from bare liposomes and HA-modified liposomes. Their results showed that DOX release from bare and HA-modified liposomes was prolonged and that DOX release from bare liposomes was about two-fold higher in comparison to HA-modified liposomes (Tian, Liu, Li, Garamus, & Zou, 2018). Elsewhere, Qu et al 2015 could successfully modify the surface of nanostructured lipid carrier (NLC) nanoparticles with HA to allow combination dual delivery of 5-FU and cisplatin. Their results showed that NLC modified with HA had significantly lower 5-FU and cisplatin release than the plain NLC nanoparticles (Qu et al., 2015).
Figure4.
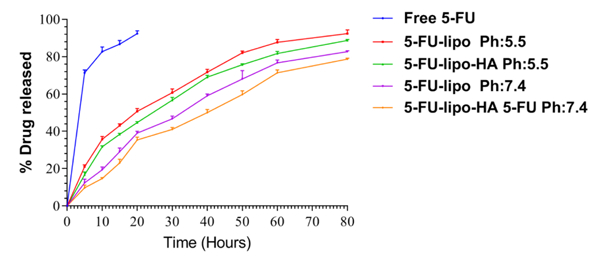
The in vitro release of 5-FU from 5-FU-lipo and 5-FU-lipo-HA nanoparticles at pH = 7.4 and pH = 5.5.
3.2. Cellular uptake of liposomal nanoparticles
The quantitative cellular uptake of bare liposomes and HA-liposomes was measured by flow cytometry on HT-29 cells, which overexpress CD44, and HEPG2 cells, which have low expression of CD44. The fluorescent intensity of RhoB was proportional to the amount of the liposomes that were internalized. HT-29 and HEPG2 cells were incubated at 1, 3, 6, 12 and 24 h with RhoB loaded bare liposomes and HA-liposomes. The results showed that both nanoparticles were successfully internalized into HT-29 cells in a time-dependent manner, but RhoB/HA-liposomes had a significantly higher uptake (P<0.001) than RhB/ bare liposomes. However, the HEPG2 cells incubated with lipo/RhoB and lipo-HA/RhoB also showed cell uptake. However, there was no significant (P> 0.005) difference in HEPG2 cell uptake between targeted (lipo-HA/RhoB) and non-targeted (lipo/RhoB) nanoparticles. The results showed that liposomes decorated with HA had cellular uptake related to CD44 receptors that mediated the endocytosis process (active targeting). It should be mentioned that CD44 receptors are overexpressed on some target cells; therefore, conjugation of HA as their cognate ligand on the surface of liposomes not only increases the specific binding of HA-liposomes to CD44 receptors but also can increase cellular uptake (Paliwal et al., 2016). In a similar study, Hayward et.al compared DOX loaded plain liposomes and HA surface-modified liposomes internalization into glioblastoma multiform cells by flow cytometry. The result of this study showed that the cell uptake of DOX/HA-liposomes was significantly higher than DOX/bare liposomes related to CD44 receptor-mediated endocytosis (Hayward et al., 2016). To further verify cellular uptake of the formulations, the qualitative uptake of RhB loaded bare liposomes and HA-liposomes were monitored by fluorescence microscopy. The studied cells were treated with RhoB loaded liposomes and HA-liposomes for 6 and 12 h. Figure 5 shows that the red colour (RhoB) intensity was increased from 6 to 12 h, demonstrating the time-dependent accumulation of the nanoparticles into the cells. The observed red colour in the cytoplasmic area of the cells revealed that the nanoparticles could successfully enter the cells, while the difference between targeted and non-targeted nanoparticles was shown by the intensity of the red colour. Lipo-HA/RhoB nanoparticles had a stronger fluorescent intensity when compared with RhB/liposomes in HT-29 cells, which may be related to CD44 receptor-mediated internalization. However, this was not observed for HEPG2 cells due to the lower expression of CD44 receptors on the surface of HEPG2 hepatoma cells. It seems that an active targeting process did not play an essential role in HEPG2 uptake of lipo-HA/RhoB nanoparticles (Figure 6). RhoB/HA-liposomes cellular uptake by HT-29 cancer cells occurred based on CD44 receptor-mediated endocytosis (active targeting); however, non-specific internalization could be the main mechanism of cellular uptake for lipo/RhoB and, lipo-HA/RhoB by HEPG2 cancer cells. These results are an agreement with several recent studies (Hayward et al., 2016; Paliwal et al., 2016; Sabzichi et al., 2017).
Figure 5.
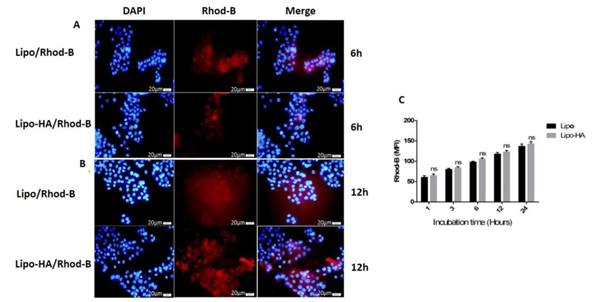
Fluorescence microscopy images of HT29 (CD44high expressed) cancer cells treated with lipo/RhoB and lipo-HA/RhoB nanoparticles (with 1μM concentration of RhoB in each nanoparticle) showing the cellular uptake at 6 and 12 h incubation times (A-B). Uptake of lipo/RhoB and lipo-HA/RhoB nanoparticles measured by flow cytometry (C). Blue and red fluorescence are referred to the DAPI and RhoB respectively (magnification 40x). *p<0.001 versus lipo/RhodB. Data represent mean ± SD (n=3). MFI: Mean fluorescence intensity.
Figure 6.
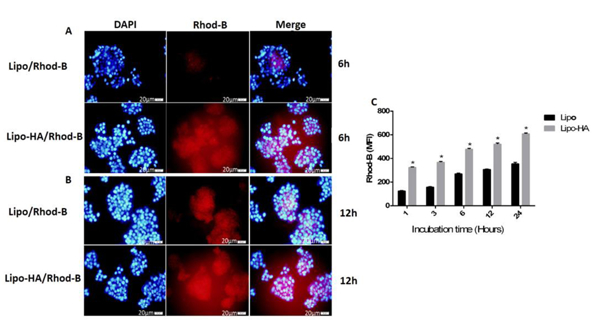
Fluorescence microscopy images of HEPG2 (CD44low expressed) cancer cells treated with lipo/RhoB and lipo-HA/RhoB nanoparticles (with 1μM concentration of RhoB in each preparation) showing the cellular uptake at 6 and 12 h incubation times (A-B). Uptake of lipo/RhoB and lipo-HA/RhoB nanoparticles measured by flow cytometry (C). Blue and red fluorescence are related to the DAPI and RhoB respectively (magnification 40x). The data of each group compared to lipo/RhodB. Data represent mean±SD (n=3). MFI: Mean fluorescence intensity.
3.3. Liposomal formulation effects on the cell cytotoxicity
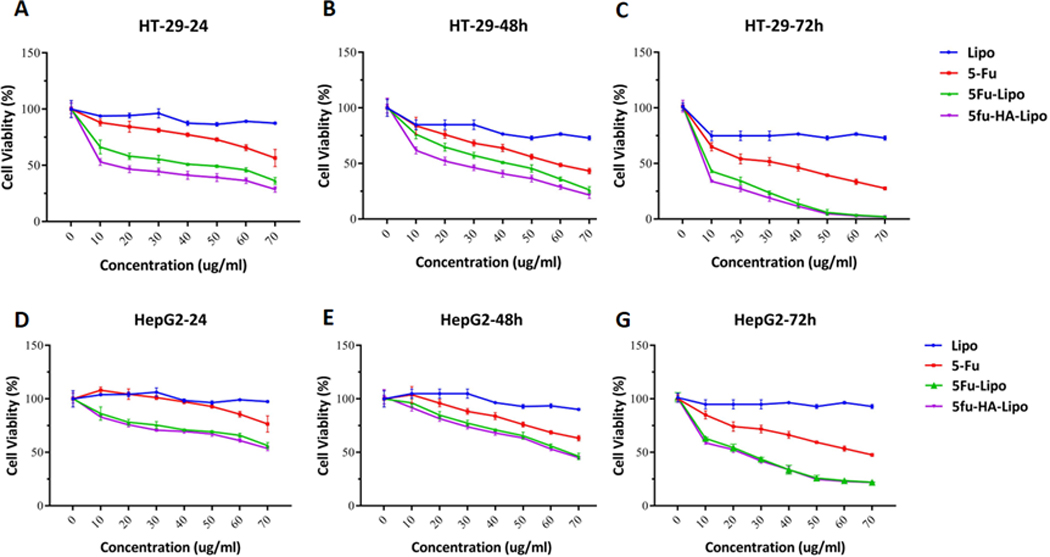
The effects of free 5-FU, 5-FU-lipo and 5-FU-lipo-HA nanoparticles on the HT-29 and HepG2 cell viability were measured using an MTT assay. After 24, 48 and 72 h of treatment, a significant reduction in the cell viability was observed. It was calculated that the IC50 of free 5-FU, was 93, 63, and 35 μg/ml and the IC50 of 5-FU-lipo was 59, 48, and 8 μg/ml and 5-FU-lipo-HA was 44, 25, 4 μg/ml during 24, 48 and 72 h of incubation, respectively (Figure 7A, B, and C) for HT-29 cancer cells. Notably, 5-FU-loaded HA-decorated liposomes showed a higher reduction of cell viability in a time-dependent manner over 24, 48 and 72 h; however, blank liposomes did not show any cell death during 24, 48 and 72 h (Figures 7A, B, and C). On the other hand, the cell cytotoxicity effects of the targeted system (5-FU-lipo-HA) and non-targeted (5-FU-lipo) nanoparticles were tested on the HEPG2 cancer cells, with low expression of CD44 (HA receptor). The result of the MTT assay indicated that both nanoparticles were significantly able to reduce the cell viability in comparison with the control cells. However, there was no significant difference in cytotoxicity between 5-FU-lipo and 5-FU-lipo-HA nanoparticles. The IC50 values of free 5-FU were found to be 100, 70, 40 μg/ml over 24, 48 and 72 h, respectively, while the IC50 values of the non-targeted and targeted liposomes were calculated to be 78, 65, 28 and 65, 55, 22 μg/ml over 24, 48 and 72 h, respectively. Overall, according to the obtained data the 5-FU-lipo-HA nanoparticles had superior cytotoxicity against the HT-29 cells than 5-FU-lipo and free 5-FU. By contrast, there was no significant difference between the effects of 5-FU-lipo and 5-FU-lipo-HA on the cell viability of HEPG2 cells. These results are consistent with the cellular uptake findings, which showed higher cellular uptake of HA-decorated liposome compared to bare liposomes on HT-29 cancer cells. Moreover, it was reported that liposome nanoparticles decorated with HA had higher binding affinity to CD44 receptors; therefore, HA-decorated liposomes are able to recognize CD44 receptors and internalize into the cells by CD44 receptor-mediated endocytosis (Paliwal et al., 2016). On the contrary, bare liposomes also could enter into the cells through non-specific endocytosis. It can be concluded that the active targeting of 5-FU liposomes through HA binding to CD44 receptors resulted in higher cytotoxicity and greater selectivity against HT-29 cancer cells. These results are consistent with a recent study by Paliwal et al. They modified pH-sensitive liposomal nanoparticles with HA to specifically deliver DOX into MCF-7 breast cancer cells (Paliwal et al., 2016). The results of this study showed that HA-modified liposomes loaded with DOX showed higher cytotoxicity than bare liposomes/DOX. It was suggested that this observation was associated with the presence of HA molecules on the surface of the nanoparticles, which generally increase the cellular uptake of DOX (Paliwal et al., 2016). Moreover, the prepared nanoparticles had time-dependent cell cytotoxicity on both cell lines that may be related to the release profile of 5-FU from the nanoparticles (Qu et al., 2015).
Figure 7. Cell toxicity of 5-FU loaded liposomes in colorectal cancer cells.
Represents in vitro cell viability results of blank liposomes, free 5-FU, 5-FU-lipo and 5-FU-lipo-HA in HT-29 and HepG2 cell lines over 24, 48, and 72 h (A-G). The data represent mean±SD (n = 3); *P < 0.05, versus blank liposome treated cells.
3.4. 5-FU-loaded liposome nanoparticles increase apoptosis in colorectal cells
To investigate quantitative apoptosis, HT-29 cells were stained with annexin V/PI after 48 h of incubation with free 5-FU, 5-FU-lipo and 5-FU-lipo-HA. In this study, the cells treated with blank liposomes were considered as a negative control group. As shown in Figure 8, the blank liposome HT-29 cells were negative in this assay. According to the obtained results, free 5-FU showed 7.55±1.09% apoptosis (Pvalue<0.01), while 5-FU-lipo and 5-FU-lipo-HA showed 9.25±0.53% (Pvalue< 0.001) and 14.83±0.3% (Pvalue< 0.0001) apoptosis compare to blank liposome group, respectively (Figure 8A, B). Therefore, 5-FU-lipo-HA nanoparticles are able to induce more apoptosis than 5-FU (Pvalue< 0.05) and 5-FU-lipo (Pvalue< 0.0001). In addition, the rate of apoptosis also was qualitatively considered. DAPI staining assay was used for recognition of nuclear fragmentation in treated HT-29 cells with free 5-FU, 5-FU-lipo and 5-FU-lipo-HA (Figure 8C). From the morphological assessment of DAPI stained cells, free 5-FU resulted in less deformation and nuclear fragmentation, while nuclear fragmentation was increased when the cells were treated with 5-FU-lipo and 5-FU-lipo-HA. This result is in accordance with the Tian et al. study, which reported that surface modification of liposomal nanoparticles with HA could increase the rate of apoptosis relative to DOX/liposome nanoparticles (Tian et al., 2018). Another active targeting drug delivery strategy was developed to deliver 5-FU into HT-29 cancer cells through conjugation of transferrin (Tf) as a ligand on the surface of liposomal nanoparticles. The results of this study showed that 5-FU/Tf-liposomal nanoparticles were able to induce apoptosis more effectively than bare 5-FU/liposomes, which supports our results (Moghimipour et al., 2018).
Figure 8. Cell apoptosis induced by free 5-FU, 5-FU-lipo and 5-FU-lipo-HA nanoparticles in colorectal cancer cells.
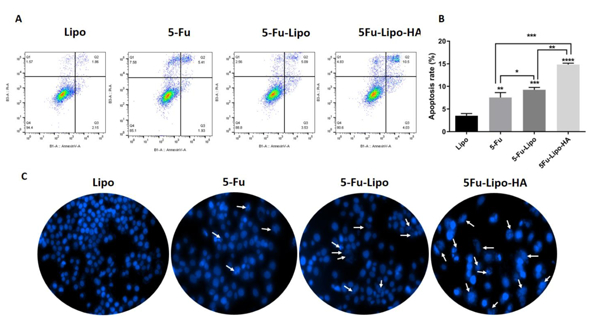
Annexin V/PI staining in free 5-FU, 5-FU-lipo and 5-FU-lipo-HA over 48 h showed the high rate of apoptosis in 5-FU-lipo-HA group (8A and B). Fluorescent images of treated HT-29 cells stained with DAPI (8C). The data represent mean±SD (n = 3); **P < 0.01, ***P < 0.001, and ****P < 0.0001, versus cells with blank liposomes.
3.5. 5-FU-lipo and 5-FU-lipo-HA arrest the cell cycle in the G0/G1 phase
After the synchronization of the cells in different phases, the effects of free 5-FU, 5-FU-lipo and 5-FU-lipo-HA nanoparticles were studied at different phases of the cell cycle via the DAPI staining method. The results showed a significant cell cycle arrest in the G0/G1 phase in 5-FU-lipo (P value<0.01) and 5-FU-lipo-HA (P value<0.001) groups after 48h (Figure 9 A and B). Encapsulation of 5-FU by the liposomes led to cell cycle arrest at the G0 /G1 phase of the cell cycle, significantly more compared to when 5-FU was used alone (Figure 9 B). In addition, flow cytometry analysis confirmed the inhibitory effects of 5-FU-lipo-HA on the proliferation of HT29 cells via stimulation of cell cycle arrest at the G0/G1 phase along with apoptosis. A study in 2012 showed that the cell cycle was arrested at G1-phase when human colorectal cancer cells were treated with PEG-liposomal oxaliplatin (C. Yang, Liu, & Fu, 2012). In another study in 2005, it was revealed that, compared with free 5-FU, liposomal 5-FU demonstrated inhibitory effects on the cell cycle of hepatic cancer cells (Jin et al., 2005). On the other hand, Venkatesan et al, at 2011 showed encapsulation of celecoxib by liposomes showed no difference in cell cycle arrest, and cell cycle analysis showed that apoptosis induced by liposomal celecoxib was somewhat lower than treatment with free celecoxib (Perumal, Banerjee, Das, Sen, & Mandal, 2011).
Figure 9. 5-FU-lipo-HA could arrest the cell cycle in the G0/G1 phase in colorectal cancer cells.
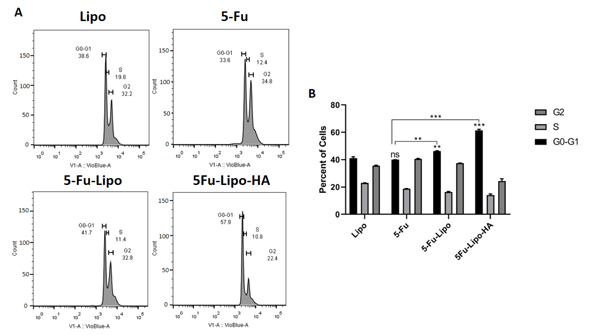
Blank liposomes, free 5-FU, and 5-FU-lipo and 5-FU-lipo-HA treated cells were prepared and stained with the DAPI. The percentage of the cell population in the blank liposomes, free 5-FU, 5-FU-lipo and 5-FU-lipo-HA groups were evaluated in each cell cycle phase. The data represent mean±SD (n = 3); **P < 0.01, and ***P < 0.001 versus blank liposome treated cells.
3.6. 5-FU-lipo and 5-FU-lipo-HA reduce colony formation by HT-29 cells
In order to understand if the free 5-FU, 5-FU-lipo and 5-FU-lipo-HA nanoparticles could decrease the colony-forming ability, a clonogenic assay was performed on HT-29 colorectal cancer cells. The colony number significantly decreased (more than 70 fold) in 5-FU-lipo and 5-FU-lipo-HA treated cells compared with the control, blank liposomes, and free 5-FU groups (Figure 10A and B). In a study, Han and et al. successfully modified the surface of liposomal nanoparticles with HA encapsulating gemcitabine to target CD44 receptors that are overexpressed on the surface of the MCF-7 cell line. The results of this study were in agreement with our results and showed that gemcitabine/HA-liposomal nanoparticles showed colony formation reduction compared with bare gemcitabine/liposome and free gemcitabine (Han et al., 2016). Additionally, we observed that the encapsulation of 5-FU by liposomes led to a much greater reduction of colony formation compared to free 5-FU. In another study in 2005, the inhibition of tumor colony formation with liposomal 5-FU was significantly more than with free 5-FU. This suggests that liposomal compounds may be a valuable agent against tumors (Jin et al., 2005). The results of a study by Baumgartner et al suggested that the anti‐cancer effects of 5‐FU were mediated via colony formation inhibition (Udofot, Affram, & Bridg’ette Israel, 2015).
Figure 10. 5-FU loaded liposome suppressed the clonogenicity in HT-29 colorectal cancer cells.
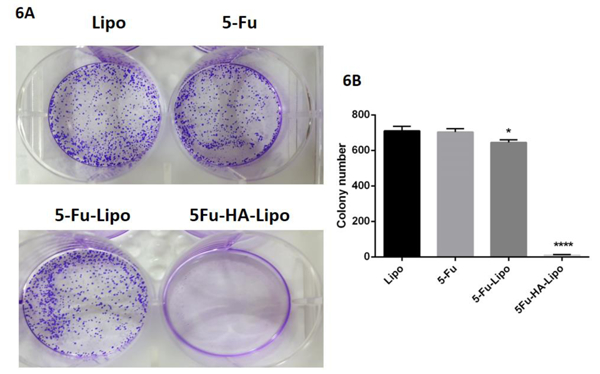
Free 5-FU and 5-FU-lipo and 5-FU-lipo-HA decreased the formation of the colorectal cancer cell colonies (5A and B). Data are presented as means ± SD. (n=3);* p<0.05, and **** p<0.0001versus blank liposome treated cells.
3.7. 5-FU-lipo-HA inhibited the genes and miRNAs related to oncogenesis and increased the expression of tumor suppressors in HT-29 cells
To confirm the effect of delivery of 5-FU via the HA-liposomes on the gene expression and miRNA expression related to oncogenesis, a qRT-PCR assay was performed to measure ALDH, KLF4, HMGA2, cMyc, Nanog, CD44, Kras mRNA, miR-181a, miR-181b, Let-7, miR-21, miR-155, mir-200c and miR-34c. The results showed that the expression of ALDH, KLF4, HMGA2, cMyc, Nanog, CD44 and Kras mRNA were significantly decreased compared with the blank liposome (Figure 11A). Further, miR-181a, miR-181b, Let-7 and miR-200c expression was significantly increased compared with the blank liposome group (Figure 11B). In contrast, a decrease in miR-21 in the 5-FU-lipo-HA group was observed compared to the blank liposome (Figure 11B). Our results did not show any significant changes in miR-155 and miR-34c expression between groups (Figure 11B).
Figure 11. 5-FU-lipo-HA changes the expression of mRNAs and miRNAs in colorectal cancer cells.
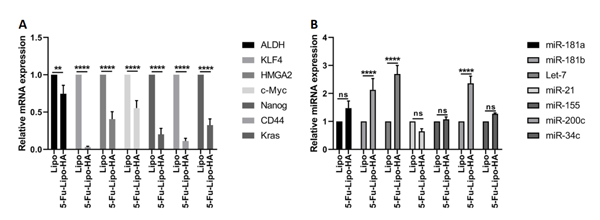
Relative ALDH, KLF4, HMGA2, cMyc, Nanog, CD44, Kras mRNAs, miR-181a, miR-181b, Let-7, miR-21, miR-155, mir-200c and miR-34c were evaluated by the qRT-PCR assay. Data are presented as means ± SD. (n=3);** p<0.01, and **** p<0.0001 versus blank liposome treated cells.
There are some stem cell markers for colon cancers, such as CD44 and ALDH that have a critical role in the formation and development of tumors (Kozovska, Gabrisova, & Kucerova, 2014). Also, Nanog is a transcription factor that is involved in tumorigenesis and is related to the poor outcome of tumors (IV Santaliz‐Ruiz, Xie, Old, Teknos, & Pan, 2014). HMGA2 is a member of the high-mobility group A family, that is associated with tumorigenicity of different malignancies. In addition, it seems that HMGA2 causes the resistance to 5-FU to develop in colon cancer cells (D’Angelo, Mussnich, Arra, Battista, & Fusco, 2017). The relative expression level of these genes in tumor cells could provide useful insights into liposomal 5-FU therapy. Our gene expression results suggested that liposomal 5-FU might provide better treatment of colorectal cancer. In other words, cells which were treated with 5-FU-lipo-HA had lower expression levels of CD44, ALDH, HMGA2 and Nanog compared with the cells treated with free 5-FU. Additionally, our results showed that treatment with 5-FU-lipo-HA nanoparticles could better inhibit the expression levels of oncogenes, such as c-Myc and Kras. We also investigated expression of a tumor suppressor gene, KLF4. It was observed that the expression level of KLF4 mRNA was reduced in HT29 cells after treatment with 5-FU-lipo-HA nanoparticles. Kruppel-like factor 4 (KLF4 or GKLF) is an inhibitor of cell cycle colony formation and migration in colorectal cancer (Zhao et al., 2004). KLF4 can act as inhibitor or inducer of proliferation depending on the type of cell, but in a study on colorectal cancer cells, KLF4 inhibited colony formation, migration and invasion (Dang et al., 2003). In a similar study, Mohan et al investigated apoptosis gene expression cascade in head and neck cancer cell lines, and their results showed that the encapsulation of 5-FU by liposomes had better anti-proliferative effects than free 5-FU on the gene expression (Mohan, Narayanan, Sethuraman, & Krishnan, 2014).
MicroRNAs (miRNA) are a group of small, noncoding RNA molecules that contribute to the developmental control of gene expression. Therefore, any change in miRNA expression can lead to physiological changes, such as proliferation, migration, and apoptosis that are important in malignant cells (Shah, Pan, Fix, Farwell, & Zhang, 2011). In the current study, we investigated the expression level of some miRNAs that are involved in colorectal cancer, after treatment with 5-FU-lipo-HA. Our data showed that miR-181a, miR-181b, Let-7 and miR-200c were overexpressed, while miR-21 was down-regulated after 5-FU treatment. Also, we observed no significant difference in the expression levels of miR-15 and miR-34c. MiR-21 is a miRNA that has been shown to be overexpressed in colorectal cancer in different studies, and a higher miR-21b expression level has been associated with 5-FU resistance(Schetter, Okayama, & Harris, 2012). Therefore, it seems that the down-regulation of miR-21 in the present study could show that the efficacy of 5-FU could be markedly enhanced by encapsulation in liposomes. By contrast, the results of a study in 2006 revealed that miR-181b, Let-7g and miR-200c were overexpressed in colorectal cancer and were correlated with a good response to an oral 5-FU prodrug called S-1. miR-181b was down regulated especially in the patients who responded to S-1 treatment (Nakajima et al., 2006). Rossi et al investigated the effect of 5-FU treatments on some miRNA expressions. Their results showed that 5-FU up-regulated miRNA genes that were previously over-expressed in malignant tissues (Rossi, Bonmassar, & Faraoni, 2007).
Conclusion
In the present study, HA surface-modified liposomes encapsulating 5-FU were prepared for targeting the CD44 receptor in colorectal cancer. The prepared nanoparticles had optimal physicochemical characteristics, such as particle size, zeta potential, and PDI that enables them to be effectively internalized compared to non-targeted nanoparticles. Moreover, the HA-decorated drug delivery systems showed slightly higher (but not markedly) EE and DL % values with sustained 5-FU release compared to bare liposomal nanoparticles, with good cytotoxicity against colorectal cancer cells. Our results also suggested that the targeted delivery system of 5-FU rendered colorectal cancer cells susceptible to apoptosis and arrested the cell cycle in G0/G1 phases. The clonogenicity study revealed that the effective delivery of 5-FU could decrease colony formation in colorectal cancer cells. The molecular experiments also suggested that 5-FU could have a significant effect on the down-regulation of oncogenes and up-regulation of tumor suppressors.
Acknowledgement
We are grateful to Dr. Hamed Hamishehkar for the gift of des PEG2000. Also, we are deeply grateful to Miss Sahar Safaei, and Susan Najafi for their collaboration in cell culture preparation This work was supported financially by the Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran [grant numbers 61294]. Michael R Hamblin was supported by US NIH grants R01AI050875 and R21AI121700.
Funding
Behzad Mansoori was supported by Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran, Grant/Award Number: 61294
Michael R Hamblin was supported by US NIH Grants R01AI050875 and R21AI121700.
Footnotes
Data availability statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
All the authors declare no conflict of interest and Dr Hamblin is on the following Scientific Advisory Boards:
Transdermal Cap Inc, Cleveland, OH
BeWell Global Inc, Wan Chai, Hong Kong
Hologenix Inc. Santa Monica, CA
LumiThera Inc, Poulsbo, WA
Vielight, Toronto, Canada
Bright Photomedicine, Sao Paulo, Brazil
Quantum Dynamics LLC, Cambridge, MA
Global Photon Inc, Bee Cave, TX
Medical Coherence, Boston MA
NeuroThera, Newark DE
JOOVV Inc, Minneapolis-St. Paul MN
AIRx Medical, Pleasanton CA
FIR Industries, Inc. Ramsey, NJ
UVLRx Therapeutics, Oldsmar, FL
Ultralux UV Inc, Lansing MI
Illumiheal & Petthera, Shoreline, WA
MB Lasertherapy, Houston, TX
ARRC LED, San Clemente, CA
Varuna Biomedical Corp. Incline Village, NV
Niraxx Light Therapeutics, Inc, Boston, MA
Dr Hamblin has been a consultant for
Lexington Int, Boca Raton, FL
USHIO Corp, Japan
Merck KGaA, Darmstadt, Germany
Philips Electronics Nederland B.V.
Johnson & Johnson Inc, Philadelphia, PA
Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany
Dr Hamblin is a stockholder in
Global Photon Inc, Bee Cave, TX
Mitonix, Newark, DE.
Reference:
- Abedi-Gaballu F, Dehghan G, Ghaffari M, Yekta R, Abbaspour-Ravasjani S, Baradaran B, . . . Hamblin MR (2018). PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Applied Materials Today, 12, 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, . . . Weiner LM (2001). High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer research, 61(12), 4750–4755. [PubMed] [Google Scholar]
- Amasya G, Badilli U, Aksu B, & Tarimci N (2016). Quality by design case study 1: Design of 5-fluorouracil loaded lipid nanoparticles by the W/O/W double emulsion—Solvent evaporation method. European Journal of Pharmaceutical Sciences, 84, 92–102. [DOI] [PubMed] [Google Scholar]
- Arias J (2008). Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules, 13(10), 2340–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, & Bray F (2017). Global patterns and trends in colorectal cancer incidence and mortality. Gut, 66(4), 683–691. [DOI] [PubMed] [Google Scholar]
- Assanhou AG, Li W, Zhang L, Xue L, Kong L, Sun H, . . . Zhang C (2015). Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials, 73, 284–295. [DOI] [PubMed] [Google Scholar]
- Barzegar Behrooz A, Nabavizadeh F, Adiban J, Shafiee Ardestani M, Vahabpour R, Aghasadeghi MR, & Sohanaki H (2017). Smart bomb AS 1411 aptamer‐functionalized/PAMAM dendrimer nanocarriers for targeted drug delivery in the treatment of gastric cancer. Clinical and Experimental Pharmacology and Physiology, 44(1), 41–51. [DOI] [PubMed] [Google Scholar]
- Cheng M, He B, Wan T, Zhu W, Han J, Zha B, . . . Wang W (2012). 5-Fluorouracil nanoparticles inhibit hepatocellular carcinoma via activation of the p53 pathway in the orthotopic transplant mouse model. PloS one, 7(10), e47115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo D, Mussnich P, Arra C, Battista S, & Fusco A (2017). Critical role of HMGA proteins in cancer cell chemoresistance. Journal of Molecular Medicine, 95(4), 353–360. [DOI] [PubMed] [Google Scholar]
- Dang DT, Chen X, Feng J, Torbenson M, Dang LH, & Yang VW (2003). Overexpression of Krüppel-like factor 4 in the human colon cancer cell line RKO leads to reduced tumorigenecity. Oncogene, 22(22), 3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanciullino R, Giacometti S, Mercier C, Aubert C, Blanquicett C, Piccerelle P, & Ciccolini J (2007). In vitro and in vivo reversal of resistance to 5-fluorouracil in colorectal cancer cells with a novel stealth double-liposomal formulation. British journal of cancer, 97(7), 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari M, Dehghan G, Abedi-Gaballu F, Kashanian S, Baradaran B, Dolatabadi JEN, & Losic D (2018). Surface functionalized dendrimers as controlled-release delivery nanosystems for tumor targeting. European Journal of Pharmaceutical Sciences. [DOI] [PubMed] [Google Scholar]
- Han N-K, Shin DH, Kim JS, Weon KY, Jang C-Y, & Kim J-S (2016). Hyaluronan-conjugated liposomes encapsulating gemcitabine for breast cancer stem cells. International journal of nanomedicine, 11, 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward SL, Wilson CL, & Kidambi S (2016). Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget, 7(23), 34158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IV Santaliz‐Ruiz LE, Xie X, Old M, Teknos TN, & Pan Q (2014). Emerging role of nanog in tumorigenesis and cancer stem cells. International Journal of Cancer, 135(12), 2741–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janout V, & Kollárová H (2001). Epidemiology of colorectal cancer. Acta-Universitatis Palackianae Olomucensis Facultatis Medicae, 5–10. [Google Scholar]
- Jin Y, Li J, Rong L-F, Li Y-H, Guo L, & Xu S-Y (2005). Anti-hepatocarcinoma effects of 5-fluorouracil encapsulated by galactosylceramide liposomes in vivo and in vitro. World Journal of Gastroenterology: WJG, 11(17), 2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R, Izawa M, Koike T, Miyazaki K, & Kimura N (2004). Carbohydrate‐mediated cell adhesion in cancer metastasis and angiogenesis. Cancer science, 95(5), 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S (2016). Liposomal drug delivery system. Journal of Pharmaceutical Investigation, 46(4), 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozovska Z, Gabrisova V, & Kucerova L (2014). Colon cancer: cancer stem cells markers, drug resistance and treatment. Biomedicine & Pharmacotherapy, 68(8), 911–916. [DOI] [PubMed] [Google Scholar]
- Malam Y, Loizidou M, & Seifalian AM (2009). Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends in pharmacological sciences, 30(11), 592–599. [DOI] [PubMed] [Google Scholar]
- Moghimipour E, Rezaei M, Ramezani Z, Kouchak M, Amini M, Angali KA, . . . Handali S (2018). Transferrin targeted liposomal 5-fluorouracil induced apoptosis via mitochondria signaling pathway in cancer cells. Life sciences, 194, 104–110. [DOI] [PubMed] [Google Scholar]
- Mohan A, Narayanan S, Sethuraman S, & Krishnan UM (2014). Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. BioMed research international, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima G, Hayashi K, Xi Y, Kudo K, Uchida K, Takasaki K, . . . Ju J (2006). Non-coding microRNAs hsa-let-7g and hsa-miR-181b are associated with chemoresponse to S-1 in colon cancer. Cancer Genomics-Proteomics, 3(5), 317–324. [PMC free article] [PubMed] [Google Scholar]
- Ossipov DA (2010). Nanostructured hyaluronic acid-based materials for active delivery to cancer. Expert opinion on drug delivery, 7(6), 681–703. [DOI] [PubMed] [Google Scholar]
- Othman M, Zayed G, El-Sokkary G, Ali U, Abdellatif A, & Othman M (2017). Preparation and evaluation of 5-fluorouracil loaded microsponges for treatment of colon cancer. J Cancer Sci Ther, 9, 307–313. [Google Scholar]
- Paliwal SR, Paliwal R, Agrawal GP, & Vyas SP (2016). Hyaluronic acid modified pH-sensitive liposomes for targeted intracellular delivery of doxorubicin. Journal of liposome research, 26(4), 276–287. [DOI] [PubMed] [Google Scholar]
- Perumal V, Banerjee S, Das S, Sen R, & Mandal M (2011). Effect of liposomal celecoxib on proliferation of colon cancer cell and inhibition of DMBA-induced tumor in rat model. Cancer nanotechnology, 2(1–6), 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C-Y, Zhou M, Chen Y. w., Chen M. m., Shen F, & Xu L-M (2015). Engineering of lipid prodrug-based, hyaluronic acid-decorated nanostructured lipid carriers platform for 5-fluorouracil and cisplatin combination gastric cancer therapy. International journal of nanomedicine, 10, 3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravar F, Saadat E, Gholami M, Dehghankelishadi P, Mahdavi M, Azami S, & Dorkoosh FA (2016). Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. Journal of Controlled Release, 229, 10–22. [DOI] [PubMed] [Google Scholar]
- Rossi L, Bonmassar E, & Faraoni I (2007). Modification of miR gene expression pattern in human colon cancer cells following exposure to 5-fluorouracil in vitro. Pharmacological research, 56(3), 248–253. [DOI] [PubMed] [Google Scholar]
- Sabzichi M, Mohammadian J, Mohammadi M, Jahanfar F, Movassagh Pour AA, Hamishehkar H, & Ostad-Rahimi A (2017). Vitamin D-Loaded Nanostructured Lipid Carrier (NLC): A New Strategy for Enhancing Efficacy of Doxorubicin in Breast Cancer Treatment. Nutrition and cancer, 69(6), 840–848. [DOI] [PubMed] [Google Scholar]
- Safwat MA, Soliman GM, Sayed D, & Attia MA (2016). Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. International journal of pharmaceutics, 513(1–2), 648–658. [DOI] [PubMed] [Google Scholar]
- Schetter AJ, Okayama H, & Harris CC (2012). The role of microRNAs in colorectal cancer. Cancer journal (Sudbury, Mass.), 18(3), 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MY, Pan X, Fix LN, Farwell MA, & Zhang B (2011). 5‐fluorouracil drug alters the microrna expression profiles in MCF‐7 breast cancer cells. Journal of cellular physiology, 226(7), 1868–1878. [DOI] [PubMed] [Google Scholar]
- Singh Y, Palombo M, & Sinko PJ (2008). Recent trends in targeted anticancer prodrug and conjugate design. Current medicinal chemistry, 15(18), 1802–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taetz S, Bochot A, Surace C, Arpicco S, Renoir J-M, Schaefer UF, . . . Fattal E (2009). Hyaluronic acid-modified DOTAP/DOPE liposomes for the targeted delivery of anti-telomerase siRNA to CD44-expressing lung cancer cells. Oligonucleotides, 19(2), 103–116. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Kapanen AI, Hare JI, Ramsay E, Edwards K, Karlsson G, & Bally MB (2011). Development of a liposomal nanoparticle formulation of 5-fluorouracil for parenteral administration: formulation design, pharmacokinetics and efficacy. Journal of Controlled Release, 150(2), 212–219. [DOI] [PubMed] [Google Scholar]
- Tian Z, Liu J, Li N, Garamus VM, & Zou A (2018). Hyaluronic acid-coated liposome for active targeting on CD44 expressing tumors. Journal of Nanoparticle Research, 20(9), 235. [Google Scholar]
- Udofot O, Affram K, & Bridg’ette Israel EA (2015). Cytotoxicity of 5-fluorouracil-loaded pH-sensitive liposomal nanoparticles in colorectal cancer cell lines. Integrative cancer science and therapeutics, 2(5), 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinothini K, & Rajan M (2017). Investigation on the use of graphene as a unique drug delivery platform for dissimilar anticancer drugs. Progress in Bioscience and Bioengineering, 1(1). [Google Scholar]
- Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, . . . Ishida H (2012). Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. International journal of clinical oncology, 17(1), 1–29. [DOI] [PubMed] [Google Scholar]
- Wilmink A (1997). Overview of the epidemiology of colorectal cancer. Diseases of the colon & rectum, 40(4), 483–493. [DOI] [PubMed] [Google Scholar]
- Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar M, Mulrow C, . . . Bond J (1997). Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology, 112(2), 594–642. [DOI] [PubMed] [Google Scholar]
- Yang C, Liu H-Z, & Fu Z-X (2012). Effects of PEG-liposomal oxaliplatin on apoptosis, and expression of Cyclin A and Cyclin D1 in colorectal cancer cells. Oncology reports, 28(3), 1006–1012. [DOI] [PubMed] [Google Scholar]
- Yang T, Choi M-K, Cui F-D, Kim JS, Chung S-J, Shim C-K, & Kim D-D (2007). Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. Journal of Controlled Release, 120(3), 169–177. [DOI] [PubMed] [Google Scholar]
- Zarebkohan A, Najafi F, Moghimi HR, Hemmati M, Deevband MR, & Kazemi B (2015). Synthesis and characterization of a PAMAM dendrimer nanocarrier functionalized by SRL peptide for targeted gene delivery to the brain. European Journal of Pharmaceutical Sciences, 78, 19–30. [DOI] [PubMed] [Google Scholar]
- Zhang N, Yin Y, Xu S-J, & Chen W-S (2008). 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules, 13(8), 1551–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Hisamuddin IM, Nandan MO, Babbin BA, Lamb NE, & Yang VW (2004). Identification of Krüppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene, 23(2), 395. [DOI] [PMC free article] [PubMed] [Google Scholar]