Abstract
Objective:
To examine the relationship between dementia status and receipt of eye care among US Medicare beneficiaries.
Design:
Retrospective claims-based analysis
Participants:
A 20% representative sample of Medicare beneficiaries who received care between January 1, 2006, and December 31, 2015.
Methods:
Dementia was identified from diagnosis codes documented in a beneficiary’s first 3 years of observed Medicare enrollment. Eye care visits were identified from provider specialty codes on each encounter claim. We used multivariable Cox proportional hazards regression models with time-varying covariates to compare likelihood of receiving eye care between beneficiaries with and without dementia. All models were adjusted for potential confounders, including demographics, urban/rural residence, systemic health (Charlson Index), and ocular comorbidities.
Main Outcome Measures:
Hazard ratio and 95% confidence interval (CI) for 1) being seen by any eye care provider (ophthalmologist or optometrist), 2) being seen by an ophthalmologist specifically, and 3) receiving cataract surgery (among beneficiaries with ophthalmologist encounters).
Results:
4,451,200 beneficiaries met inclusion criteria; 3,805,718 (85.5%) received eye care during the study period and 351,127 (7.9%) had diagnosed dementia. 70.4% of beneficiaries with diagnosed dementia saw an eye care provider during the study period and 54.7% saw an ophthalmologist, versus 86.8% and 73.9% of beneficiaries without dementia diagnoses. Compared to those without dementia diagnoses, beneficiaries with diagnosed dementia had lower likelihood of seeing any eye care provider (adjusted HR=0.69, 95%CI=0.69–0.70) and were even less likely to see an ophthalmologist (adjusted HR=0.55, 95%CI=0.55–0.55). Among the subset of beneficiaries who did see ophthalmologists, those with diagnosed dementia were also less likely to receive cataract surgery than beneficiaries without diagnosed dementia (HR=0.62, 95%CI=0.62–0.63) and less likely to receive a cataract diagnosis (18% versus 82%).
Conclusions:
US Medicare beneficiaries diagnosed with dementia are less likely to receive eye care than those without diagnosed dementia. Depending on visual acuity and functional status, this may have implications for injury prevention, physical and cognitive function, and quality of life. Further work is needed to identify barriers to receiving eye care, determine eye care services and settings that provide greatest value to dementia patients, and implement measures to improve access to appropriate eye care.
Keywords: Alzheimer’s disease, Dementia, Aging, Eye Disease, Cataract Surgery, Ophthalmology, Eye Care, Optometry
Introduction
Alzheimer’s disease and related dementias are prevalent among older adults. An estimated one in nine people 65 years or older has Alzheimer’s disease. As the US population ages, the number of older adults with Alzheimer’s disease is expected to grow to 7.1 million by 2025 and is likely to rise as high as 13.8–16 million by 2050.1–3
Eye diseases are also very prevalent among older adults. Cataracts, age-related macular degeneration, and glaucoma, respectively, are present in 70%, 12%, and 8% of US adults over age 80.4–6 Among dementia patients over age 65, cataract is one of the top 10 most prevalent chronic comorbidities,7 and glaucoma is the 18th most common comorbidity.8 Additionally, uncorrected refractive error (unmet need for spectacles) has been reported in dementia patients, especially those in nursing homes or long-term care facilities.9
The treatment of eye disease in patients with Alzheimer’s disease and related dementias raises important challenges. Eye disease can lead to visual impairment, which is associated with reduced quality of life and physical function, as well as cognitive decline among dementia patients.10–15 Treatments that improve or maintain vision may mitigate some of these effects.16–28 For example, in cooperative patients, cataracts can usually be treated quickly and safely with an outpatient surgery performed by an ophthalmologist, resulting in better vision and quality of life, lower risk of injuries such as hip fractures, and possibly reduced cognitive decline among patients with dementia.18–27,29–30 However, if patients are not able to lie still and cooperate with surgery—as may be the case in dementia—general anesthesia may be necessary, increasing risk of systemic complications.
In our previous work, we found that patients with dementia are 47% less likely to undergo cataract surgery, compared to patients without dementia,31 even after adjusting for other potentially confounding factors. However, we did not explicitly examine the source of these differences. One possibility is that lower rates of surgery among dementia patients represent decisions made in discussion with an ophthalmologist, weighing the risks and benefits of potential treatment options. However, barriers to accessing care may also play a role. Patients with dementia may face unique obstacles to receiving eye care, including lower functional status, differences in perceived risks and potential benefits of eye care, and logistical challenges such as unrecognized visual impairment or need for a caregiver for transportation to an eye care provider.32–34
In this study, we examine access to eye care more broadly to provide evidence on the potential sources of these differences. We used national Medicare administrative claims data to examine access to eye care services among beneficiaries with dementia. We evaluated the likelihood of being seen by any eye care provider (optometrist or ophthalmologist) as well as the likelihood of being seen specifically by an ophthalmologist. In addition, because our prior work regarding likelihood of cataract surgery based on dementia status was not limited to beneficiaries who saw an ophthalmologist, in this analysis we explicitly evaluate the likelihood of cataract surgery among the subset of beneficiaries seen by an ophthalmologist. Finally, we specifically evaluated beneficiaries who were institutionalized or had multiple medical comorbidities to determine whether other serious ailments may preclude access to eye care. By examining both access to eye care and treatment decisions conditional on accessing services, our analyses provide insight into the mechanisms by which treatment decisions differ between patients with and without Alzheimer’s disease and related dementias.
Methods
Data Source
Our analysis was performed using administrative insurance claims for a random, national 20% sample of Medicare beneficiaries with Medicare Parts A, B, and D coverage between January 1, 2006, and December 31, 2015. We used Medicare master beneficiary and carrier (Physician/Supplier Part B) files to verify Medicare eligibility, determine demographics and other patient characteristics, and identify patient visits, diagnoses, and procedures. Stanford University Institutional Review Board approval was obtained prior to the study, and the study adhered to the Declaration of Helsinki.
Sample Selection
Using similar methods as reported previously,31 we limited our sample to beneficiaries aged 65 years and older, with at least four years of continuous fee-for-service Medicare coverage. We excluded beneficiaries with noncontinuous enrollment, enrollment in Medicare Advantage plans, and/or residence outside the United States, since observed claims were incomplete for these enrollees. We also excluded beneficiaries with missing values for sex or geographic location (urban/rural) (Figure 1). We used each beneficiary’s first three years of observed plan enrollment as a “lookback period” to determine baseline characteristics and define predictor variables. We limited our analysis to beneficiaries with a minimum of one full year of continuous Medicare enrollment after the lookback period, and followed beneficiaries for a mean of 5.1 years (IQR 3.1–7.0 years). To evaluate incident cataract surgery, we secondarily limited the full study sample to a only those beneficiaries who were seen by an ophthalmologist and had no evidence of prior cataract surgery, based on ICD-9-CM and Current Procedural Terminology (CPT) codes for cataract surgery, pseudophakia, or aphakia in the lookback period (Supplemental Table 1, available at www.aaojournal.org).
Figure 1: Sample Selection Strategy.
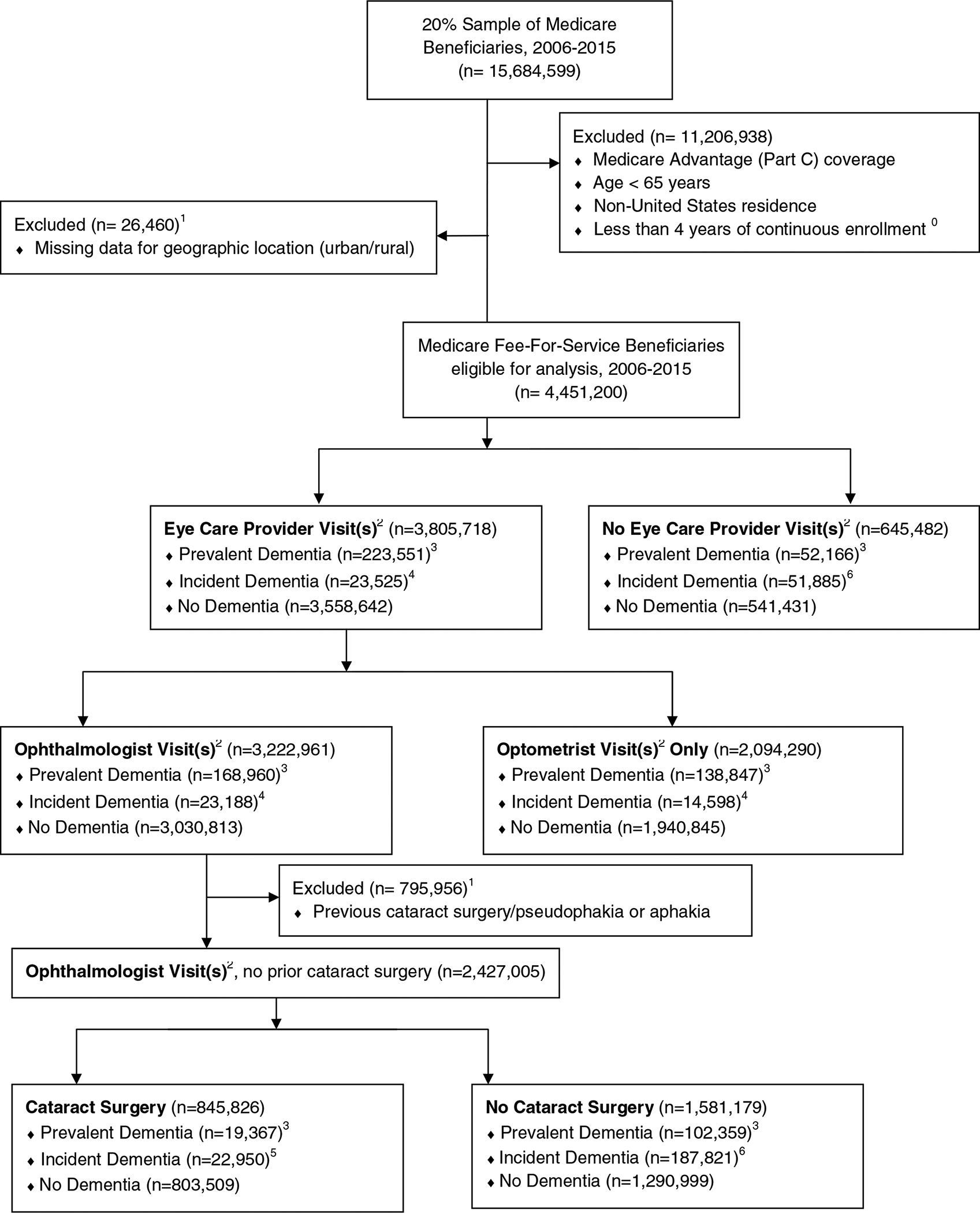
0 coverage ends at the date of deaths if beneficiary died
1 presence of a procedure code for cataract surgery and/or a diagnosis code for pseudophakia or aphakia present in the first 3 years of a given beneficiary’s enrollment period (“lookback period”) and/or missing value associated with key variables
2 based on provider type classification
3 presence of a dementia diagnosis recorded during a beneficiary’s lookback period
4 presence of a new dementia diagnosis first recorded after the lookback period but prior to any eye care provider visit
5 presence of a new dementia diagnosis first recorded after the lookback period but prior to any cataract surgery
6 presence of a new dementia diagnosis first recorded after the lookback period but prior to end of observed enrollment
Outcome
Our primary outcome was optometrist and ophthalmologist visits, collectively considered as eye care provider visits. These were identified from provider specialty codes on each encounter claim during the post-lookback followup period. We additionally performed a subanalysis on the sample of beneficiaries who had observed ophthalmologist visit(s) prior to any cataract surgery; in this analysis our primary outcome was incident cataract surgery.
Predictor Variables
We identified beneficiaries with dementia using International Classification of Diseases, 9th edition, Clinical Modification (ICD-9-CM) diagnosis codes (Supplemental Table 1, available at aaojournal.org). We classified dementia beneficiaries using methods described previously, with “prevalent dementia” defined as dementia documented during the lookback period, “incident dementia” defined as a dementia diagnosis first recorded after the end of lookback period but before receiving eye care services, and “no dementia” defined as no recorded dementia diagnosis or a diagnosis first recorded after receiving eye care after the end of the lookback period.31
Other clinical comorbidities, including depression,35 diabetic retinopathy, age-related macular degeneration, and glaucoma were also identified by presence of ICD-9-CM codes (Supplemental Table 1, available at www.aaojournal.org) in the lookback period for each beneficiary. We used Charlson Comorbidity Index (determined using ICD-9-CM codes and modified to exclude dementia) to account for general systemic health (Supplemental Table 1, available at www.aaojournal.org), and Rural-Urban Commuting Area codes to determine rural versus urban settings for each beneficiary’s geographic residence during the lookback period.36 Baseline institutionalized status (nursing home care) was evaluated based on presence of at least one skilled nursing facility stay during a given beneficiary’s lookback period. Provider specialty codes were used to determine whether beneficiaries were seen at least once by an optometrist or ophthalmologist, or by both during their lookback periods.
Statistical Analysis
Enrollee characteristics were summarized using frequencies and percentages. Consistent with prior methods,31 we used Cox proportional hazards regression models with time-varying covariates to evaluate the likelihood of receiving eye care services after the end of the 3-year lookback period (defined as the analysis index date). Length of follow-up varied among beneficiaries. For those who did not receive eye care during our analysis, the time to receiving eye care was censored by the length of follow-up, and the follow-up period was treated as unobserved censored time. Censoring was more common among beneficiaries with dementia diagnoses (HR for censoring = 3.24 among dementia-diagnosed beneficiaries).
We constructed separate multivariable regression models to estimate hazard ratios for (1) hazard of being seen by any eye care provider (ophthalmologist or optometrist), (2) hazard of being seen specifically by an ophthalmologist (adjusting for any optometrist visits at baseline), and (3) hazard of receiving cataract surgery conditional on seeing an ophthalmologist during the lookback and/or followup period(s). For this analysis of likelihood of receiving cataract surgery, we additionally limited our study sample to beneficiaries who were (1) seen by an ophthalmologist (because only ophthalmologists perform cataract surgery and a visit with an ophthalmologist is necessary for surgery) and (2) did not have evidence of prior cataract surgery in their lookback period (Figure 1). We considered both incident and prevalent dementia as “dementia” beneficiaries in our primary analysis, however, in sensitivity analyses we additionally estimated regression models comparing beneficiaries with incident dementia to those without dementia.
We included potential confounders in the regression models: age, sex, race/ethnicity, urban versus rural residence, first observed year of Medicare data, institutionalized status (nursing home care), Charlson Comorbidity Index, depression, and ocular comorbidities. In Model 1 (evaluating hazard of any eye care provider visits), we also adjusted for previous visits to an eye care provider in the lookback period, and in Models 2 and 3 (evaluating hazard of ophthalmologist visits and cataract surgery, respectively), we adjusted for previous visits to an optometrist in the lookback period since this may influence later likelihood of referral to an ophthalmologist. We tested for interaction effects between dementia and age, sex, institutionalized status, and Charlson Comorbidity Index, respectively. For interaction analyses, Charlson Comorbidity Index was evaluated as a binary variable (<4 versus 4+, since 67% of beneficiaries had a Charlson index of 3 or lower). All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Study Sample
A total of 4,451,200 beneficiaries met initial study inclusion criteria (Figure 1). The study sample was predominantly female and white, and the majority of beneficiaries were urban-dwelling (74.4%). Beneficiaries with a dementia diagnosis represented 7.9% of the study sample (n=351,127), of whom 6.2% (n=275,717) had prevalent dementia identified during the lookback period and 1.7% (n=75,410) had incident dementia reported after the lookback period, excluding beneficiaries for whom dementia was first diagnosed after receiving eye care. Prevalence of dementia in this dataset was 7.4% in whites, 10.6% in blacks, 11.3% in Hispanics, 6.8% in Asians, and 1.5% in Native Americans/Other/Unknown.
In unadjusted comparison, beneficiaries with dementia diagnoses were on average older and more likely to be female, non-white, live in an urban setting, have skilled nursing facility stays, and have other systemic comorbidities, including depression (Table 1). Dementia diagnoses were observed in the highest proportions among beneficiaries over age 85 years, those with depression, and those with skilled nursing facility stays (over 20% of each group had prevalent or incident dementia). Dementia diagnoses were also observed slightly more frequently among beneficiaries who were female (9% of female beneficiaries, versus 6% of male beneficiaries) and those who were black or Hispanic (approximately 11% of beneficiaries, compared to 7% or less among other races/ethnicities).
Table 1.
Baseline Demographics and Clinical Characteristics among Medicare Beneficiaries based on Dementia Status, 2009–20151,2 (N=4,451,200)
| Prevalent Dementia3 N(%) |
Incident Dementia3 N(%) |
Any Dementia N (%) | No Dementia N(%) | p-value4 | |
|---|---|---|---|---|---|
| Number of beneficiaries | 275717 (6.2) | 63954 (1.4) | 339671 (7.6) | 4111529 (92.4) | <0.0001 |
| Age at enrollment (years) | |||||
| 65–74 | 73111 (26.5) | 23307 (36.4) | 96418 (28.4) | 2532630 (61.6) | <0.0001 |
| 75–84 | 136102 (49.4) | 30494 (47.7) | 166596 (49.0) | 1288545 (31.3) | |
| 85–94 | 63049 (22.9) | 9858 (15.4) | 72907 (21.5) | 276800 (6.7) | |
| Over 95 | 3455 (1.3) | 295 (0.5) | 3750 (1.10) | 13554 (0.3) | |
| Gender | <0.0001 | ||||
| Female | 190536 (69.1) | 41495 (64.9) | 232031 (68.3) | 2373125 (57.7) | |
| Male | 85181 (30.9) | 22459 (35.1) | 107640 (31.7) | 1738404 (42.3) | |
| Race / ethnicity | <0.0001 | ||||
| White | 230288 (83.5) | 53840 (84.2) | 284128 (83.6) | 3570491 (86.8) | |
| Black | 28433 (10.3) | 6380 (10.0) | 34813 (10.2) | 294340 (7.2) | |
| Hispanic | 7735 (2.8) | 1514 (2.4) | 9249 (2.72) | 72724 (1.8) | |
| Asian | 5285 (1.9) | 1277 (2.0) | 6562 (1.93) | 89647 (2.2) | |
| Native American, Other, or Unknown | 3976 (1.4) | 943 (1.5) | 4919 (1.45) | 84327 (2.1) | |
| Geographic Location5 | <0.0001 | ||||
| Rural | 58017 (21.0) | 14234 (22.3) | 72251 (21.3) | 1068645 (26.0) | |
| Urban | 217700 (79.0) | 49720 (77.7) | 267420 (78.7) | 3042884 (74.0) | |
| Year of Study Period Entry | <0.0001 | ||||
| 2009 | 242565 (88.0) | 59712 (93.4) | 302277 (89.0) | 3478438 (84.6) | |
| 2010 | 5804 (2.1) | 1113 (1.7) | 6917 (2.04) | 94767 (2.3) | |
| 2011 | 6628 (2.4) | 1068 (1.7) | 7696 (2.27) | 107498 (2.6) | |
| 2012 | 5937 (2.2) | 743 (1.2) | 6680 (1.97) | 104428 (2.5) | |
| 2013 | 7700 (2.8) | 838 (1.3) | 8538 (2.51) | 163635 (4.0) | |
| 2014 | 5670 (2.1) | 425 (0.7) | 6095 (1.79) | 124845 (3.0) | |
| 2015 | 1413 (0.5) | 55 (0.1) | 1468 (0.43) | 37918 (0.9) | |
| Baseline Eye Care Status6 | <0.0001 | ||||
| No Eye Care Visit | 79983 (29.0) | 22721 (35.5) | 102704 (30.2) | 1151138 (28.0) | |
| Optometrist Only | 50981 (18.5) | 9873 (15.4) | 60854 (17.9) | 665191 (16.2) | |
| Ophthalmologist Only | 99594 (36.1) | 23281 (36.4) | 122875 (36.2) | 1733153 (42.2) | |
| Both Optometrist and Ophthalmologist | 45159 (16.4) | 8079 (12.6) | 53238 (15.7) | 562047 (13.7) | |
| Baseline Cataract Surgery7 | <0.0001 | ||||
| Prior cataract surgery | 31332 (11.4) | 7174 (11.2) | 38506 (11.3) | 549929 (13.4) | |
| No evidence of prior cataract surgery | 244385 (88.6) | 56780 (88.8) | 301165 (88.7) | 3561600 (86.6) | |
| Other Clinical Comorbidities | |||||
| Cataract diagnosis | 99225 (36.0) | 21050 (32.9) | 120275 (35.4) | 1658636 (40.3) | <0.0001 |
| Diabetic Retinopathy diagnosis | 8936 (3.2) | 1717 (2.7) | 10653 (3.14) | 125050 (3.0) | <0.0001 |
| Age-related Macular Degeneration diagnosis | 43911 (15.9) | 7637 (11.9) | 51548 (15.2) | 476757 (11.6) | <0.0001 |
| Glaucoma diagnosis | 52613 (19.1) | 8525 (13.3) | 61138 (18.0) | 747167 (18.2) | <0.0001 |
| Depression diagnosis | 39692 (14.4) | 4731 (7.4) | 44423 (13.1) | 112904 (2.7) | <0.0001 |
| Charlson Comorbidity Index8 | <0.0001 | ||||
| 2–3 | 29406 (10.7) | 13340 (20.9) | 42746 (12.6) | 1582514 (38.5) | |
| 4–5 | 99561 (36.1) | 25416 (39.7) | 124977 (36.8) | 1411249 (34.3) | |
| 6–7 | 81746 (29.6) | 15236 (23.8) | 96982 (28.6) | 702426 (17.1) | |
| 8+ | 65004 (23.6) | 9962 (15.6) | 74966 (22.1) | 415340 (10.1) | |
| Skill Nursing stay | 11702 (4.2) | 978 (1.5) | 12680 (3.73) | 30144 (0.7) | <0.0001 |
2006–2009 data were used to define the lookback period and establish baseline beneficiary characteristics; 2015 data was only applicable for regression analysis since beneficiaries were required to have at least one year of enrollment after the end of the “lookback period.”
Results presented with column percentages for all variables except number of beneficiaries (row percentage)
Prevalent dementia was recorded during the initial 3-year lookback period; incident dementia was a dementia diagnosis that was first recorded during the subsequent follow-up period and prior to first eye care visit during the followup period
Chi-square test statistic
Based on rural urban commuting area codes, in which rural areas represent small urban clusters with a population of 49,000 or fewer persons.
Based on receipt of eye care services during beneficiaries’ “lookback period” (first three years of observed plan enrollment)
Beneficiaries who had evidence of baseline/prior cataract surgery during their initial 3-year lookback period were excluded from the study sample used to identify likelihood of cataract surgery in the subsequent followup period.
We omitted the dementia variable from computation of Charlson Index, such that the lowest permitted Charlson Index score was 2.
3,805,718 beneficiaries (85.5% of our overall sample) had eye care visits during the follow-up period. Beneficiaries with diagnosed dementia made up progressively smaller proportions of those who received eye care visits, saw ophthalmologists, and underwent cataract surgery, respectively (Table 2).
Table 2:
Prevalence of Diagnosed Dementia among Medicare Beneficiaries receiving Eye Care, 2009–2015
| Prevalent Dementia1 N(%) |
Incident Dementia1 N(%) |
Any Dementia N (%) |
No Dementia N(%) |
p-value2 | |
|---|---|---|---|---|---|
| Any Eye Care Provider Visit | 171178(5.0) | 63954(1.9) | 235132(6.8) | 3202832(93.2) | <0.0001 |
| Ophthalmologist Visit | 111529(4.0) | 37089(1.3) | 148618(5.3) | 2634179(94.7) | <0.0001 |
| Cataract Surgery3 | 19378(2.3) | 8418(1.0) | 27796(3.3) | 822351(96.7) | <0.0001 |
Prevalent dementia was recorded during the initial 3-year lookback period; incident dementia was a dementia diagnosis that was first recorded during the subsequent follow-up period and prior to first eye care visit during the followup period
Chi-square test statistic
N = 3,437,964 beneficiaries with any eye care provider visit
N = 2,782,797 beneficiaries with ophthalmologist visit
Among the subset of beneficiaries seen by an ophthalmologist (necessary for cataract surgery)
N = 850,147 beneficiaries with cataract surgery
Relationship between Dementia and Likelihood of Any Eye Care Visits
Among beneficiaries with documented dementia, only 70.4% (n=247,076) had any eye care visits (optometrists or ophthalmologists), compared to 86.8% of beneficiaries without dementia diagnoses (n=3,558,642). After adjusting for potential confounders, beneficiaries with incident or prevalent dementia diagnoses were 31% less likely to be seen by an eye care provider, compared to beneficiaries without dementia diagnoses (adjusted HR=0.69, 95%CI:0.69–0.70) (Table 3).
Table 3.
Adjusted Multivariable Cox Regression Models for Likelihood of Receiving Eye Care Among US Medicare Beneficiaries with and without Diagnosed Dementia
| Unadjusted Hazard Ratio (95% Confidence Interval) |
Adjusted Hazard Ratio1 (95% Confidence Interval) |
p-value | |
|---|---|---|---|
| Any Eye Care Provider Visit (n = 4,451,200) | |||
| Incident or Prevalent Dementia, versus No Dementia | 0.79 (0.79–0.79) | 0.69 (0.69–0.70) | <0.0001 |
| Ophthalmologist Visit (n = 4,451,200) | |||
| Incident or Prevalent Dementia, versus No Dementia | 0.63 (0.63–0.64) | 0.55 (0.55–0.55) | <0.0001 |
| Cataract Surgery2 (n = 2,427,005) | |||
| Incident or Prevalent Dementia, versus No Dementia | 0.54 (0.53–0.54) | 0.62 (0.62–0.63) | <0.0001 |
Adjusted for age, sex, race/ethnicity, urban/rural geographic location, year of plan entry, presence of eye care visits at baseline, Charlson Comorbidity Index, skilled nursing facility institutionalization, depression, diabetic retinopathy, age-related macular degeneration, and glaucoma.
Among the subset of beneficiaries seen by an ophthalmologist (necessary for cataract surgery)
Unsurprisingly, ocular comorbidities and baseline eye care visits were each highly associated with having subsequent eye care visits (29–80% higher likelihood in beneficiaries with ocular comorbidities and over 3-fold higher likelihood in beneficiaries with baseline eye care visits). Older beneficiaries were also less likely to receive eye care visits (over 50% lower likelihood among those aged 95 years and older). Other factors showed less marked but still significant associations. Females, white beneficiaries, and those in urban settings were more likely to have eye care visits. Presence of more comorbid systemic conditions, as measured by Charlson Comorbidity Index, was associated with increased likelihood of eye care visits. (Supplemental Table 2, available at www.aaojournal.org).
We found that the effect of age and sex varied based on dementia status. With every 5-year increase in age, beneficiaries with documented dementia were 12% less likely to receive any eye care visits, whereas beneficiaries without documented dementia were only 4% less likely. And female beneficiaries were 14% less likely than males to receive eye care visits if they had a dementia diagnosis, but 12% more likely if they did not have a dementia diagnosis. (Table 4 and Figure 2).
Table 4.
Differential Effects of Age, Sex, Comorbidity, and Institutionalization on Likelihood of Receiving Eye Care based on Dementia Status
| Dementia | No Dementia | |||
|---|---|---|---|---|
| Adjusted Hazard Ratio | 95% Confidence Interval | Adjusted Hazard Ratio | 95% Confidence Interval | |
| Any Eye Care Provider Visit | ||||
| Age (5-year increments) | 0.879 | 0.877–0.882 | 0.961 | 0.961–0.962 |
| Female sex | 0.862 | 0.855–0.870 | 1.116 | 1.113–1.118 |
| Charlson Comorbidity Index >4 | 0.935 | 0.924–0.946 | 1.156 | 1.153–1.159 |
| Skilled nursing facility | 1.093 | 1.069–1.118 | 0.909 | 0.896–0.922 |
| Ophthalmologist Visit | ||||
| Age (5-year increments) | 0.814 | 0.811–0.817 | 0.962 | 0.961–0.963 |
| Female sex | 0.779 | 0.771–0.788 | 1.180 | 1.177–1.183 |
| Charlson Comorbidity Index >4 | 0.938 | 0.924–0.951 | 1.344 | 1.341–1.348 |
| Skilled nursing facility | 0.811 | 0.785–0.837 | 0.872 | 0.858–0.887 |
| Cataract Surgery1 | ||||
| Age (5-year increments) | 0.724 | 0.719–0.729 | 0.925 | 0.923–0.927 |
| Female sex | 0.752 | 0.737–0.767 | 1.037 | 1.033–1.042 |
| Charlson Comorbidity Index >4 | 0.746 | 0.729–0.763 | 1.046 | 1.041–1.051 |
| Skilled nursing facility | 0.925 | 0.852–1.005 | 1.042 | 1.004–1.081 |
among beneficiaries with ophthalmologist visits at baseline
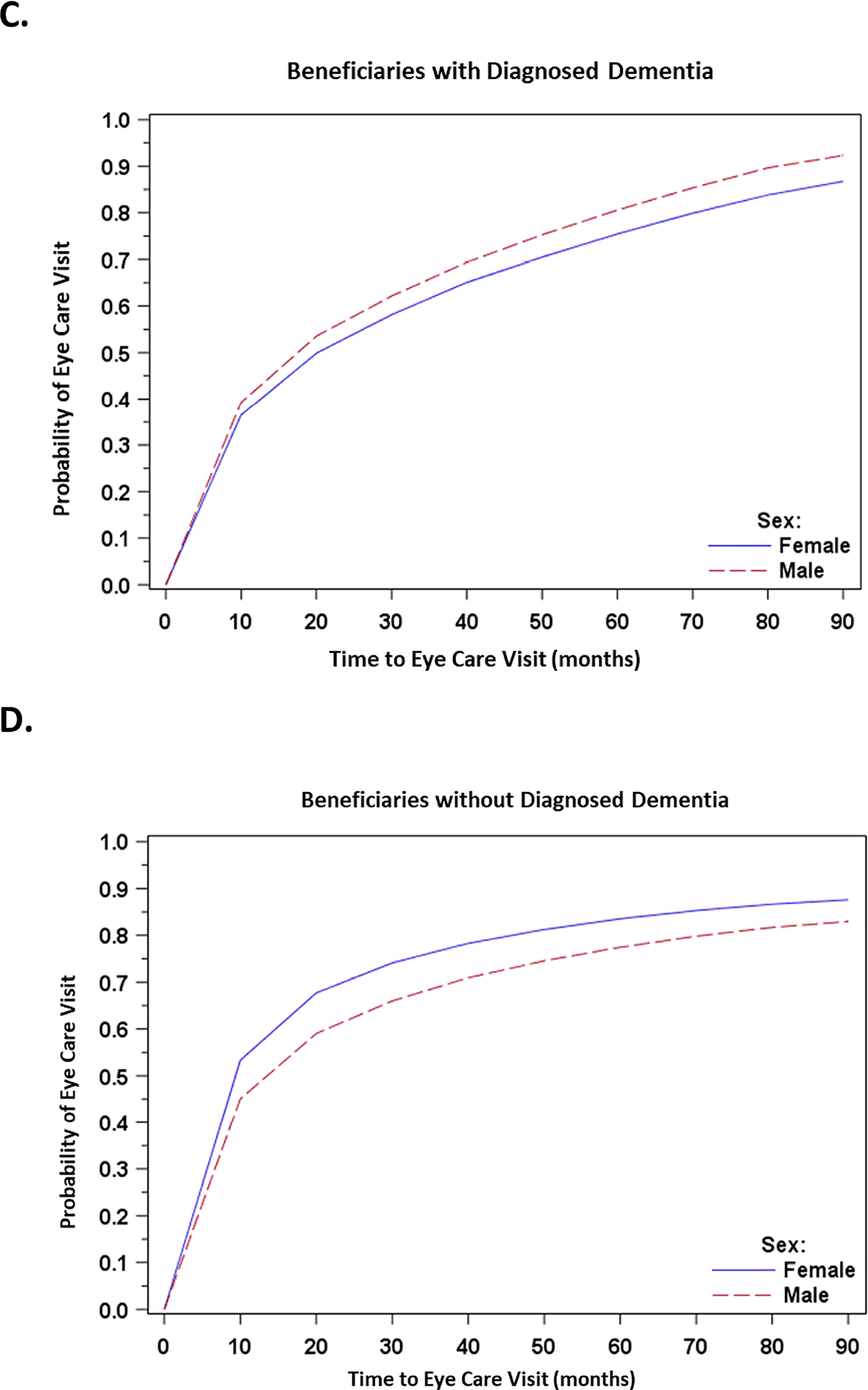
Figure 2: Unadjusted Kaplan-Meier Curves for Likelihood of Eye Care Visits among Beneficiaries with and without Diagnosed Dementia, Stratified by Age and Sex.
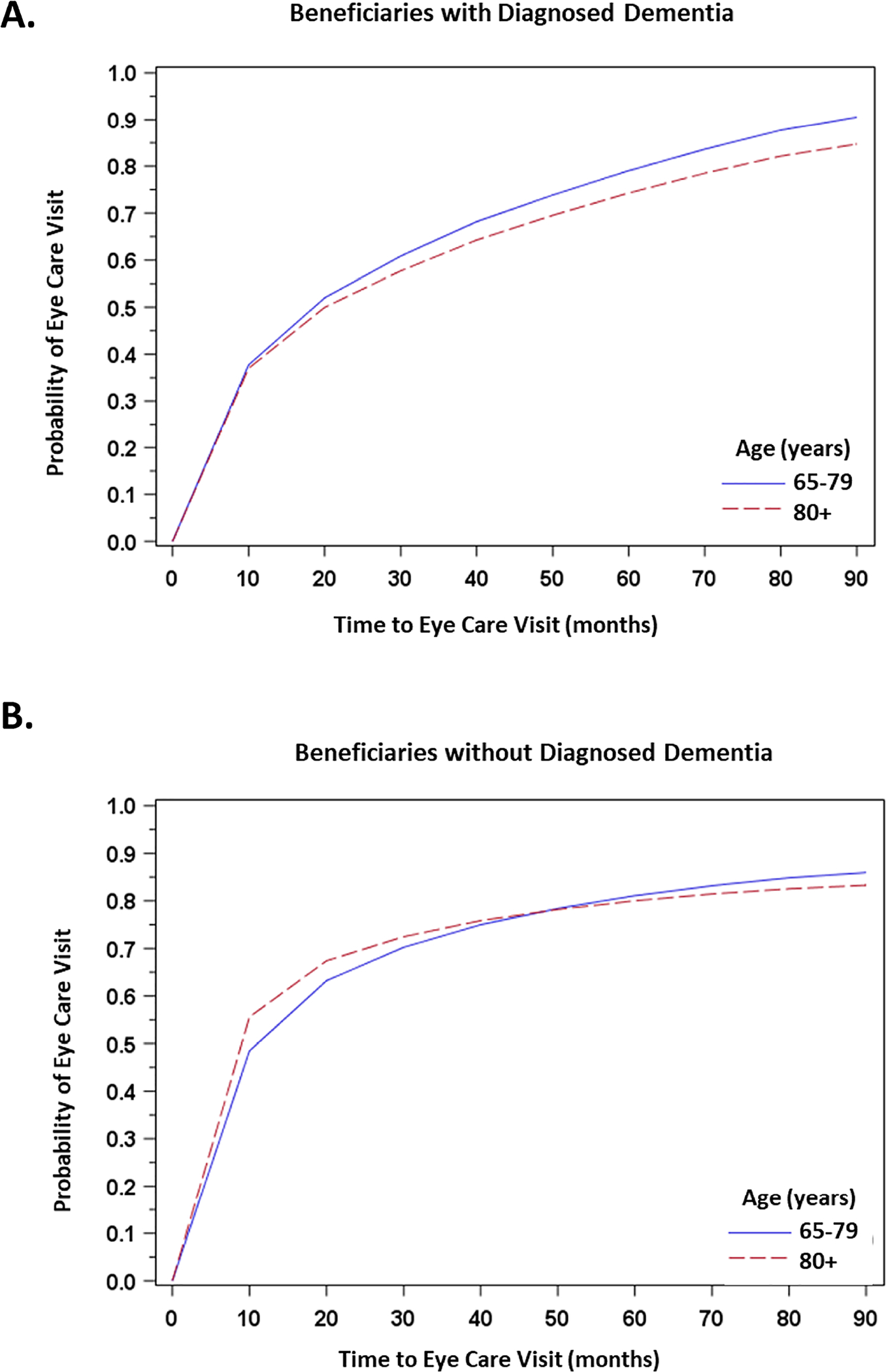
A. Time to Eye Care Visits among Beneficiaries with Diagnosed Dementia, Stratified by Age (65–79 years and 80+ years)
B. Time to Eye Care Visits among Beneficiaries without Diagnosed Dementia, Stratified by Age (65–79 years and 80+ years)
C. Time to Eye Care Visits among Beneficiaries with Diagnosed Dementia, Stratified by Sex (Male and Female)
D. Time to Eye Care Visits among Beneficiaries with Diagnosed Dementia, Stratified by Sex (Male and Female)
Relationship between Dementia and Likelihood of Ophthalmologist Visits
Only 54.7% (n=192,148) of beneficiaries with dementia diagnoses had visits specifically with ophthalmologists. By contrast, 73.9% of beneficiaries without dementia diagnoses (n=3,030,813) received ophthalmologist visits (Figure 1). Beneficiaries diagnosed with dementia were 45% less likely to be seen by an ophthalmologist (adjusted HR=0.55, 95%CI:0.55–0.55) than beneficiaries without dementia diagnoses (Table 3).
The direction of effect for other patient characteristics was similar to findings for likelihood of any eye care provider visits (Supplemental Table 3, available at www.aaojournal.org). We observed even lower likelihood of ophthalmologist visits among older beneficiaries and those in rural settings, while beneficiaries with ocular comorbidities and more systemic comorbidities were more likely to see ophthalmologists. Notably, beneficiaries previously seen by optometrists were over 40% less likely to see ophthalmologists (adjusted HR=0.57, 95% CI:0.57–0.58); however, the difference was slightly less marked among dementia beneficiaries. Beneficiaries with documented dementia who were previously seen by optometrists had 38% lower likelihood of ophthalmologist visits, compared to 43% lower likelihood among beneficiaries without documented dementia who were previously seen by an optometrist (Supplemental Table 4, available at www.aaojournal.org).
There were significant interactions between dementia and age and between dementia and sex. With increasing age, all beneficiaries were less likely to be seen by an ophthalmologist, an effect more pronounced for beneficiaries diagnosed with dementia (approximately 20% lower likelihood vs. less than 4% lower likelihood for beneficiaries without dementia; Table 3). Female beneficiaries diagnosed with dementia were 22% less likely to see an ophthalmologist than males diagnosed with dementia, whereas female beneficiaries without diagnosed dementia were 18% more likely (Table 4).
Relationship between Dementia and Likelihood of Cataract Surgery among Beneficiaries Seen by Ophthalmologists
Among the subset of beneficiaries seen by an ophthalmologist—thus potentially able to undergo cataract surgery—12.7% (n=42,317) of those with dementia diagnoses and 38.4% (n=803,509) of those without dementia diagnoses underwent incident cataract surgery during the study period (Figure 1). Beneficiaries with a dementia diagnosis were 38% less likely to undergo cataract surgery (adjusted HR=0.62, 95%CI:0.62–0.63) than those without a dementia diagnosis (Table 3).
Likelihood of cataract surgery also declined with age, and the lowest likelihood of cataract surgery was observed in the very old, regardless of dementia status (adjusted HR=0.15, 95%CI:0.13–0.17 for beneficiaries age 95 and over, compared to 65–75 years). Female sex, white race/ethnicity, and living in rural settings were each associated with higher likelihood of cataract surgery, as was being seen by an optometrist at baseline (49% higher likelihood of cataract surgery) (Supplemental Table 5, available at www.aaojournal.org). Having more ocular comorbidities was associated with lower likelihood of cataract surgery and, in aggregate, having more systemic comorbidities did not substantially change the likelihood of cataract surgery.
Interestingly, beneficiaries with dementia diagnoses were not only less likely to receive cataract surgery but also less likely to have a cataract diagnosis documented in billing records. Among beneficiaries seen by an ophthalmologist who did not undergo cataract surgery, only 18% of those with documented dementia received a cataract diagnosis, compared to 82% of those without documented dementia.
Eye Care for Beneficiaries with Poor Health or Institutionalized Status
Sicker beneficiaries (those with a higher Charlson Comorbidity Index) were more likely to see any eye care provider and more likely to see an ophthalmologist, but had minimal difference in likelihood of cataract surgery after being seen by an ophthalmologist (Supplemental Tables 2, 3, and 5). Beneficiaries with documented dementia and with a Charlson Index >4 were less likely to see any eye care provider, less likely to see an ophthalmologist, and less likely to have cataract surgery after being seen by an ophthalmologist. By contrast, beneficiaries without documented dementia who had a Charlson Index >4 were more likely to see any eye care provider, more likely to see an ophthalmologist, and slightly more likely to undergo cataract surgery after being seen by an ophthalmologist. (Table 4).
Among beneficiaries with documented dementia, those who were institutionalized in a skilled nursing facility were more likely to see any eye care provider, less likely to see an ophthalmologist, and had no significant difference in likelihood of cataract surgery after being seen by an ophthalmologist. Nondementia beneficiaries who were institutionalized were less likely to see any eye care provider, less likely to see an ophthalmologist, and slightly more likely to undergo cataract surgery after being seen by an ophthalmologist. (Table 4).
Sensitivity Analyses
We separately analyzed likelihood of eye care among the subset of beneficiaries with incident dementia diagnoses, compared to beneficiaries without dementia diagnoses, allowing us to estimate the effect of dementia duration based on time since diagnosis.
We observed a milder reduction in likelihood of any eye care provider visits among beneficiaries with incident dementia diagnoses compared to those without dementia (adjusted HR=0.90, 95% CI:0.89–0.91; Supplemental Table 6, available at www.aaojournal.org). The difference in likelihood of ophthalmologist visits was more substantial; beneficiaries with incident dementia diagnoses were 26% less likely to be seen than those without dementia diagnoses (adjusted HR=0.74, 95%CI:0.73–0.75; Supplemental Table 7, available at www.aaojournal.org). In both models, each additional year after dementia diagnosis conferred a 13% reduction in likelihood of being seen by any eye care provider or specifically by an ophthalmologist.
Among beneficiaries seen by an ophthalmologist, cataract surgery remained less likely among beneficiaries with incident dementia diagnoses compared to those without dementia diagnoses (adjusted HR=0.84, 95%CI:0.82–0.85), and beneficiaries had 15% lower likelihood of cataract surgery with every additional year since dementia diagnosis (Supplemental Table 8, available at www.aaojournal.org).
Discussion
In this analysis of a large national sample of Medicare beneficiaries, we found that patients with documented dementia have lower likelihood of receiving eye care compared to those without documented dementia—approximately one-third less likely to be seen by any eye care provider (optometrist or ophthalmologist), half as likely to be seen specifically by an ophthalmologist, and 40% less likely to undergo cataract surgery even if seen by an ophthalmologist. This has important public health implications. Ophthalmic care is necessary for diagnosis and management of eye disease, and treatments such as cataract surgery improve vision-related quality of life, reduce injury, and may improve cognitive outcomes in dementia.18–27,29–30 Our findings may signal a need to facilitate eye care access for dementia patients.
Eye disease is prevalent among older adults, and guidelines in the US and Canada recommend that patients over age 65 receive complete eye exams every 1–2 years.37–40 Examinations by an eye care provider are essential, due to the need for specialized clinical expertise as well as access to dilating eye drops, exam equipment, and imaging. In particular, ophthalmologist evaluation is necessary for complex or surgical eye diseases such as cataract, wet age-related macular degeneration, and advanced glaucoma. Untreated, these eye diseases have been associated with impaired quality of life, depression, and falls and fractures.15,17–18,41 Furthermore, improving vision, as by spectacle correction or cataract surgery, has been shown to improve quality of life and reduce depression and fall-related fractures.16,18–21,28,42
However, despite evidence of benefit from eye disease treatment, there may be barriers to dementia patients receiving eye care. Reduced functional status or visual impairment that is not reported by patients or noticed by family or caregivers may result in undetected symptoms. Limited availability or lengthy travel distance to the nearest eye care provider, as in rural settings, may limit patients’ ability to receive eye care. Other challenges may include cumbersome referral mechanisms and/or caregiver fatigue.43–44 Also, primary care physicians may be best equipped to discuss treatment recommendations with patients and their family and caregivers due to long-standing relationships. However, suboptimal communication from the eye care provider back to the primary care physician may impair their ability to contribute to discussion of risks and benefits.43
While patients with very advanced dementia (e.g., bed-bound and unable to interact with others) may be less likely to benefit from referral for eye care, in aggregate, its value has been demonstrated among patients with dementia, including improved vision and contrast sensitivity as well as better quality of life and even potential cognitive benefits.28,45 For example, a retrospective analysis found reduced risk of new-onset dementia among older adults who underwent cataract surgery, and other studies have shown improved cognitive scores on postoperative cognitive testing after cataract surgery, including longitudinal studies ranging from 6 months of followup to 13 years of retrospective followup.22–26,30,46
We previously found that beneficiaries with dementia diagnoses are in aggregate less likely to receive cataract surgery compared to those without dementia.31 In this analysis, we found that lower rates of cataract surgery persisted even in the subset of beneficiaries who were seen by an ophthalmologist. Combined with lower likelihood of eye care visits, this suggests that reduced access to eye care providers as well as decision making when receiving eye care both contribute to reduced rates of cataract surgery. Additional factors that may play a role include potential need for a higher-acuity surgical setting, postoperative hospitalization monitoring, and/or deeper sedation or general anesthesia. Associated risks in this population include postoperative cognitive dysfunction or respiratory complications and difficult extubation.31,47–49 These risks may reduce the likelihood that an ophthalmologist will recommend cataract surgery and lower the acceptance rates of cataract surgery by patients or family members acting as power of attorney for patients with dementia. It is also important to note that dementia patients may not benefit from cataract surgery to the same degree as their nondementia counterparts. If a patient with dementia does not drive or do other activities that require high levels of visual function, they may not experience functional impairment from cataract, and potential benefits from cataract surgery may not justify the risks (especially if needing general anesthesia, etc.).
Among beneficiaries seen by an ophthalmologist, those with documented dementia were not only less likely to receive cataract surgery but also substantially less likely to receive a cataract diagnosis compared to those without documented dementia (18% of beneficiaries versus 82% of beneficiaries). However, claims-based cataract diagnosis codes are imprecise at best. Absence of a cataract diagnosis code on billing claims does not necessarily mean that cataracts were not present. We would expect cataract (that is, some lens brunescence) to be nearly-ubiquitous among Medicare-age adults and not to vary based on dementia status.4–7 Lower likelihood of documented cataract diagnoses is thus a revealing finding that suggests cataract may be underdiagnosed in Medicare beneficiaries with dementia. It is possible that dementia patients who are seen by an ophthalmologist have other ocular diagnoses that prompt examination. In such cases, ophthalmologists’ attention may not be focused on cataract evaluation, and other diagnoses may be coded in the billing record instead of cataract. Cataracts may also be less likely to be deemed visually-significant or to warrant surgery in patients with dementia, especially if other ocular comorbidities are present and independently impair vision. It is also possible that eye care providers may be biased against diagnosing a cataract, hoping not to add to the burden of caregivers in arranging for and affording cataract extraction. This is an area that warrants future study.
Our analysis is also notable in that beneficiaries with a documented dementia diagnosis were increasingly less likely to receive eye care with advancing age, more so than beneficiaries without documented dementia. Similarly, greater time since dementia diagnosis was associated with declining likelihood of receiving eye care (13–15% lower with each additional year). It is likely that barriers to eye care manifest more in patients with dementia of longer duration and greater severity. Beneficiaries seen by optometrists were less likely to be referred to an ophthalmologist across the board, possibly because they were screened for eye diseases by the optometrist and ophthalmology referral was not warranted. However, this effect was less strong among beneficiaries with documented dementia, perhaps suggesting that optometrists are more likely to refer dementia patients.
Women with documented dementia were also consistently less likely to receive eye care, whereas those without documented dementia were more likely to receive eye care. Reasons for this are likely multifactorial. Older women are more likely than men to be widowed and to live alone (32% of women versus 18% of men in 2014).50 Thus, older men with dementia may be more likely to have a younger spouse who is able to take on the role of caregiver, including transportation for eye exams and assistance with home care such as eye drop instillation and pre- and post-operative care for eye surgery.50 By contrast, older women with dementia may outlive an older spouse and lack readily-available caregivers.
Finally, our findings suggest that poor health and infirmity may influence eye care differently for dementia and nondementia patients. Results of aggregate and stratified analyses indicate that sicker beneficiaries without documented dementia are more likely to receive eye care, a finding that likely reflects greater contact with the health care system and more opportunities for cataract surgery discussion. By contrast, sicker beneficiaries with a documented dementia diagnosis are less likely to receive eye care, including optometrist visits, ophthalmologist visits, and cataract surgery after being seen by an ophthalmologist. Since most chronic diseases would not be expected to affect dementia patients differently from nondementia patients, there may be additional barriers or need for education regarding potential indications and value of eye care for dementia patients with multiple comorbidities.
We also found that institutionalized beneficiaries who resided in skilled nursing facilities had lower likelihood of being seen by an ophthalmologist, regardless of dementia status. However, institutionalized beneficiaries with documented dementia were more likely to see an optometrist than beneficiaries without documented dementia. Moreover, beneficiaries with documented dementia had no significant difference in likelihood of cataract surgery once seen by an ophthalmologist (whereas nondementia beneficiaries were less likely). We hypothesize that residing in a skilled nursing facility may facilitate identification of potential visual impairment in dementia patients, prompting optometrist consultation, but that there may be barriers to getting these patients to be seen by an ophthalmologist. These might include lack of referrals or difficult access to an ophthalmologist, especially if patients require costly and logistically-challenging special mode transportation to an ophthalmologist’s office but have access to either a closer optometrist or an optometrist able to visit in the skilled nursing facility. However, it appears that if these patients do end up seen by an ophthalmologist, they are equally able to undergo cataract surgery compared to noninstitutionalized patients (more so that their nondementia counterparts). Additional research is needed to further evaluate these considerations.
Strengths and Limitations
Our study is limited by several factors, including coding imprecision or inaccuracies in administrative claims, inability to account for unobserved care obtained outside of Medicare, and underdiagnosis of dementia. Beneficiaries with dementia diagnoses were more likely to be censored than those without dementia diagnoses, however, censoring should not affect the appropriateness of eye care services for remaining enrolled beneficiaries (American Academy of Ophthalmology guidelines, for example, recommend eye exams every 1–2 years among patients aged 65 years and older).37
Some patients may have had cataract surgery prior to becoming a Medicare beneficiary, however, we excluded beneficiaries who had a diagnosis of pseudophakia or aphakia at any point within their first three years of observed data. We also recognize that some beneficiaries may have received eye care or a new dementia diagnosis under different health care plans. However, most beneficiaries use Medicare as their primary insurer (estimated 97.7% of US adults age 65 years and older),51–52 and we excluded Medicare Advantage beneficiaries to minimize unobserved events. Furthermore, some beneficiaries in our non-dementia group may have had dementia, since diagnoses are not always reliably recorded, patients may manifest early signs of dementia before their first diagnosis (up to 40% of patients meeting clinical criteria for probable dementia are undiagnosed), and many patients seen in ophthalmologist offices may have undetected cognitive impairment.53–54 Assuming a fully-representative dementia population with mild and severe disease, underdetection would be expected to bias us away from finding a difference in eye care based on dementia diagnosis, suggesting that our results may actually underestimate the effect of dementia. However, we recognize that underdetection may more selectively involve beneficiaries with mild dementia. It is possible that these beneficiaries with milder dementia and less functional limitation are still receiving eye care, at less reduced rates. Our results may thus overestimate the reduction in likelihood of eye care among beneficiaries with milder dementia.
Additionally, since we are unable to determine visual acuity or functional status from administrative claims data, it is not possible to directly assess the appropriateness of cataract surgery. For example, risks of surgery may outweigh benefits in patients with severe dementia and/or cataracts that do not affect their daily activities, and deferring surgery in these patients may be appropriate. Additional work is needed to investigate disparities in receipt of cataract surgery among patients with visually-significant cataracts. Finally, our results only address receipt of eye care services. Other comorbid neurosensory impairments (e.g., hearing impairment) may also affect cognitive function in dementia.10 It is possible that beneficiaries with dementia may be similarly less likely to be seen by hearing specialists such as otolaryngologists or audiologists, and/or be less likely to use hearing aids (which are costly and require regular battery replacement).
Conclusions and Implications
There are growing numbers of older adults with dementia, who are also at risk for aging-related eye diseases such as cataract, glaucoma, and age-related macular degeneration that substantially affect function and quality of life. Ophthalmic care is important for timely diagnosis and management of these diseases, with potentially beneficial effects on cognitive function and quality of life. However, we find that beneficiaries with documented dementia diagnoses are consistently less likely to receive eye care compared to their non-dementia counterparts in a US Medicare population. While interventions such as cataract surgery may not be indicated in dementia patients (depending on visual acuity and functional status), initial eye care is still important to identify eye disease and enable informed decision-making around risks and benefits for treatment. These findings are robust and timely given an aging population and growing numbers of patients with dementia.
Dementia patients may benefit from measures to improve access to eye care services, including improved referral and coordination between primary care providers, geriatricians, neurologists, and ophthalmologists to enable evaluation for diagnosis and appropriate management of visually-limiting eye disease. Telemedicine initiatives with skilled nursing facilities and rehabilitation facilities, for example, may enable remote interpretation of eye examination data by an ophthalmologist, overcoming some of the logistical barriers of bringing dementia patients to the ophthalmologist’s office. There is a need for further research regarding reasons for differences in receipt of eye care, including barriers to access, as well as further studies of the effect of eye care on dementia patients’ cognitive and physical function and quality of life, and risks and benefits of specific eye disease treatments among patients with different stages of dementia. In addition, research is warranted to determine whether patients with dementia are similarly less likely to visit hearing specialists or receive costly hearing aids. These considerations will collectively be important for point-of-care decision making and developing clinical guidelines for more targeted care.
Supplementary Material
ACKNOWLEDGEMENTS
Financial Disclosure: This work was supported by the National Institute on Aging (R03-AG056453). SP also received departmental support from Research to Prevent Blindness and is a consultant for Acumen, LLC (Burlingame, CA) and Verana Health (San Francisco, CA). VWH also received support from P50-AG047366.
Financial Support: National Institute on Aging R03-AG056453 (SP); departmental support from Research to Prevent Blindness. VWH also received supported from NIH grant P50-AG047366. The funding organizations had no role in the design or conduct of this research. No conflicting relationship exists for any author
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentations: Findings presented at the U13 Workshop on Embedding/Sustaining a Focus on Function in Specialty Research and Care, January 31-February 1, 2019, Washington, DC, USA, and at the Association for Research in Vision and Ophthalmology, May 1, 2019, Vancouver, BC, Canada.
Conflicts of Interest: None
Sponsor’s Role: The sponsor had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the paper.
REFERENCES
- 1.Alzheimer’s Association. 2017 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2017;13:325–373. [Google Scholar]
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrigh HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2011;3:186–191 [DOI] [PubMed] [Google Scholar]
- 3.Prince M, Wimo A, Guerchet M, et al. World Alzheimer Report 2015: The Gobal Impact of Dementia ana analysis of prevalence, incidence, cost and trends. Available at: http://www.alz.co.uk.laneproxy.stanford.edu/research/WorldAlzheimerReport2015.pdf.
- 4.National Eye Institute Statistics and Data, last reviewed July 2018. Available at https://nei.nih.gov/eyedata/. Last accessed 2/13/19
- 5.Tielsch JM, Javitt JC, Coleman A, et al. The prevalence of blindness and visual impairment among nursing home residents in Baltimore. N Engl J Med 1995;332:1205–1209. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P, Hayes P, Wang JJ. Visual impairment in nursing home residents: the Blue Mountains eye study. MJA 1997;166:73–76. [DOI] [PubMed] [Google Scholar]
- 7.Poblador-Plou B, Calderón-Larrañaga A, Marta-Moreno J, et al. Comorbidity of dementia: a cross-sectional study of primary care older patients. BMC Psychiatry. 2014;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayer AU, Ferrari F & Erb C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur Neurol 2002;47:165–168. [DOI] [PubMed] [Google Scholar]
- 9.Koch JM, Datta G, Makhdoom S, Grossberg GT. Unmet Visual Needs of Alzheimer’s Disease Patients in Long-term Care Facilities. J Am Med Dir Assoc 2005;6(4):233–7. [DOI] [PubMed] [Google Scholar]
- 10.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–734. [DOI] [PubMed] [Google Scholar]
- 11.Spierer O, Fischer N, Barak A, Belkin M. Correlation Between Vision and Cognitive Function in the Elderly: A Cross-Sectional Study. Medicine (Baltimore). 2016. January;95(3):e2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SP, Bhattacharya J, Pershing S. Association of Vision Loss with Cognition in Older Adults. JAMA Ophthalmol. 2017. September 1;135(9):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitson HE, Cousins SW, Burchett BM, et al. The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc 2007. June;55(6):885–91. [DOI] [PubMed] [Google Scholar]
- 14.Ward ME, Gelfand JM, Lui LY, et al. Reduced contrast sensitivity among older women is associated with increased risk of cognitive impairment. Ann Neurol. 2018. April;83(4):730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox A, Blaikie A, MacEwen CJ, et al. Visual impairment in elderly patients with hip fracture: causes and associations. Eye (Lond). 2005;19(6):652–656. [DOI] [PubMed] [Google Scholar]
- 16.Groessl EJ, Liu L, Sklar M, Tally SR, Kaplan RM, Ganiats TG. Measuring the impact of cataract surgery on generic and vision-specific quality of life. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2013;22(6):1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodge W, Horsley T, Albiani D, et al. The consequences of waiting for cataract surgery: a systematic review. CMAJ. 2007;176(9):1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng VL, Yu F, Lum F, Coleman AL. Risk of fractures following cataract surgery in Medicare beneficiaries. JAMA. 2012;308(5):493–501. [DOI] [PubMed] [Google Scholar]
- 19.Heemraz BS, Lee CN, Hysi PG, et al. Changes in quality of life shortly after routine cataract surgery. Can J Ophthalmol. 2016;51(4):282–7. [DOI] [PubMed] [Google Scholar]
- 20.Gray CS, Karimova G, Hildreth AJ, et al. Recovery of visual and functional disability following cataract surgery in older people: Sunderland Cataract Study. Journal of Cataract & Refractive Surgery. 2006;32(1): 60–66. [DOI] [PubMed] [Google Scholar]
- 21.Lundström M, Stenevi U, Thorburn W. Quality of life after first- and second-eye cataract surgery: five-year data collected by the Swedish National Cataract Register. J Cataract Refract Surg. 2001;27(10):1553–9. [DOI] [PubMed] [Google Scholar]
- 22.Lerner A, Debanne S, Belkin J, et al. Visual and cognitive improvement following cataract surgery in subjects with dementia. Alzheimers and Dementia. 2014;10(4):S456–457 [Google Scholar]
- 23.Duffy M Alzheimer Research: Cataract Surgery for People with Dementia Improves Vision and Quality of Life. VisionAware Blog. September 24, 2014. Available act https://www.visionaware.org/blog/visionaware-blog/alzheimer-research-cataract-surgery-for-people-with-dementia-improves-vision-and-quality-of-life/12. Last accessed September 25, 2018 [Google Scholar]
- 24.Tamura H, Tsukamoto H, Mukai S, et al. Improvement in cognitive impairment after cataract surgery in elderly patients. J Cataract Refract Surg. 2004;30(3):598–602. [DOI] [PubMed] [Google Scholar]
- 25.Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg 2015;41(6):1241–7. [DOI] [PubMed] [Google Scholar]
- 26.Hall TA, McGwin G Jr, Owsley C. Effect of cataract surgery on cognitive function in older adults. J Am Geriatr Soc. 2005;53(12):2140–4. [DOI] [PubMed] [Google Scholar]
- 27.Ishii K, Kabata T, Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. Am J Ophthalmol. 2008;146(3):404–9. [DOI] [PubMed] [Google Scholar]
- 28.Owsley C, McGwin G Jr, Scilley Ket al. Effect of refractive error correction on health-related quality of life and depression in older nursing home residents. Arch Ophthalmol. 2007;125:1471–1477. [DOI] [PubMed] [Google Scholar]
- 29.Gogate PM, Kulkarni SR, Krishnaiah S, et al. Safety and efficacy of phacoemulsification compared with manual small-incision cataract surgery by a randomized controlled clinical trial: six-week results. Ophthalmology. 2005;112(5):869–74. [DOI] [PubMed] [Google Scholar]
- 30.National Institute on Aging; Gilmore GC, University Hospitals Case Medical Center. Cataract Removal in Alzheimer’s Disease In: ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited October 1, 2016]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT00921297 NLM Identifier: NCT00921297 [Google Scholar]
- 31.Pershing S, Henderson V, Bundorf MK, et al. Differences in Cataract Surgery Rates based on Dementia Status. Journal of Alzheimer’s Disease. 2019;69(2):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller BK, Hejkal T, Potter JF. Barriers to vision care for nursing home residents. J Am Med Dir Assoc. 2001;2(1):15–21. [PubMed] [Google Scholar]
- 33.Stephan A, Bieber A, Hopper L, et al. Barriers and facilitators to the access to and use of formal dementia care: findings of a focus group study with people with dementia, informal carers and health and social care professionals in eight European countries. BMC Geriatr 2018;18(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dal Bello-Haas VP, Cammer A, Morgan D, et al. Rural and remote dementia care challenges and needs: perspectives of formal and informal care providers residing in Saskatchewan, Canada. Rural Remote Health. 2014;14(3):2747. [PubMed] [Google Scholar]
- 35.Verkaik R, Francke AL, van Meijel B, et al. Comorbid depression in dementia on psychogeriatric nursing home wards: which symptoms are prominent? Am J Geriatr Psychiatry. 2009;17(7):565–73. [DOI] [PubMed] [Google Scholar]
- 36.Rural-Urban Commuting Area Codes (RUCA) Documentation. Available at https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/ Last accessed 5/21/2018
- 37.American Academy of Ophthalmology Policy Statement: Frequency of Ocular Examinations. March 2015. Available online at https://www.aao.org/clinical-statement/frequency-of-ocular-examinations Last accessed 2/19/2019
- 38.Prevent Blindness America “How often should I have an eye exam?” Available online at https://www.preventblindness.org/how-often-should-i-have-eye-exam. Last accessed 2/19/2019
- 39.Clinical Practice Guideline Expert Committee. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the periodic eye examination in adults in Canada. Can J Ophthalmol 2007;42:39–45, 158–63. [DOI] [PubMed] [Google Scholar]
- 40.Robinson BE, Stolee P, Mairs K, et al. Review of the Canadian Association of Optometrists. Frequency of eye examinations guideline – summary. An evidence-based approach. Can J Optom 2011;73:15–16. [Google Scholar]
- 41.Slakter JS, Stur M. Quality of life in patients with age-related macular degeneration: impact of the condition and benefits of treatment. Surv Ophthalmol. 2005;50(3):263–73. [DOI] [PubMed] [Google Scholar]
- 42.Fraser ML, Meuleners LB, Lee AH, et al. Vision, quality of life and depressive symptoms after first eye cataract surgery. Psychogeriatr Off J Jpn Psychogeriatr Soc. 2013;13(4):237–243. [DOI] [PubMed] [Google Scholar]
- 43.Wenger NS, Solomon DH, Roth CP, et al. The quality of medical care provided to vulnerable community-dwelling older patients. Ann Intern Med. 2003;139(9):740–7. [DOI] [PubMed] [Google Scholar]
- 44.Thorpe JM, Van Houtven CH, Sleath BL. Barriers to Outpatient Care in Community-Dwelling Elderly with Dementia: The Role of Caregiver Life Satisfaction. Journal of Applied Gerontology. 2009;28(4),436–460. [Google Scholar]
- 45.Friedman DS, Munoz B, Roche KB, et al. Poor uptake of cataract surgery in nursing home residents. The Salisbury eye evaluation in nursing home groups study. Arch Ophthalmol 2005;123:1581–1587. [DOI] [PubMed] [Google Scholar]
- 46.Maharani A, Dawes P, Nazroo J, et al. SENSE-Cog WP group. Cataract surgery and age-related cognitive decline: A 13-year follow-up of the English Longitudinal Study of Ageing. PLoS One. 2018;13(10):e0204833 Published 2018 Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel D, Lunn AD, Smith AD, et al. Cognitive decline in the elderly after surgery and anaesthesia: results from the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Anaesthesia. 2016; 71(10): 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’ Brien H, O’ Leary N, Scarlett S, et al. Hospitalisation and surgery: are there hidden cognitive consequences? Evidence from The Irish Longitudinal study on Ageing (TILDA). Age Ageing. 2018;47(3):408–415. [DOI] [PubMed] [Google Scholar]
- 49.Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interv Aging. 2008;3(2):351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stepler R (2016) Smaller share of women ages 65 and older are living alone: more are living with spouse or children. Washington, D.C.:Pew Research Center, February. [Google Scholar]
- 51.CMS Program Statistics 2016. Medicare Enrollment Section, available online at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2016/2016_Enrollment Last accessed November 28, 2019
- 52.Barnett JC, Berchick ER. Current Population Reports, p60–260, Health Insurance Coverage in the United States: 2016, U.S. Government Printing Office, Washington, DC, 2017. Available online at https://www.census.gov/content/dam/Census/library/publications/2017/demo/p60-260.pdf Last accessed November 28, 2019 [Google Scholar]
- 53.Amjad H, Roth DL, Sheehan OC, et al. Underdiagnosis of Dementia: an Observational Study of Patterns in Diagnosis and Awareness in US Older Adults. J Gen Intern Med. 2018;33(7):1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jefferis JM, Taylor JP & Clarke MP. Does cognitive impairment influence outcomes from cataract surgery? Results from a 1-year follow-up cohort study. Br J Ophthalmol 2015;99:412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


