Abstract
Prenatal health behaviors can strongly influence risk of poor pregnancy birth outcomes. Although stress has been implicated in structuring the likelihood that individuals will engage in various prenatal health behavior patterns, no studies to date have examined life stress exposure occurring across the entire lifespan, and few have investigated how different types of stressors are comparatively associated with these outcomes. To address these issues, we interviewed 164 women at one of two large Midwestern, urban hospitals after delivering their first infant. We used the Stress and Adversity Inventory (STRAIN) to assess women’s lifetime stress exposure severity and ordinary least squares regression models to examine associations between participants’ life stress exposure and prenatal health behaviors. As hypothesized, greater lifetime stress exposure was associated with engaging in more negative prenatal health behaviors and fewer positive prenatal health behaviors while controlling for relevant sociodemographic factors and current perceived stress levels. These effects were stronger for negative versus positive health behaviors, and they differed substantially as a function of stressor type, exposure timing, and primary life domain. Stressors occurring over the life course thus have negative consequences for prenatal health behaviors, but these effects are not uniform across different types of life stress exposure.
1 |. INTRODUCTION
Prenatal health behaviors play an important role in shaping risk of pregnancy health and birth outcomes (Centers for Disease Control [CDC], 2016; Lobel et al., 2008). Whereas negative health behaviors, such as poor diet and alcohol use, have been found to be associated with low birth weight and preterm birth, positive health behaviors, such as maintaining a healthy diet and taking folic acid, have been found to decrease birth defects (CDC, 2016; Goldenberg & Culhane, 2007; Hankin, 2002). Despite an abundance of research on prenatal health behaviors, though, it remains unclear how different types of stressors occurring across the life course are associated with prenatal health behaviors. More specifically, studies on stress and health behaviors have not incorporated measures of lifetime stress exposure, nor have they generally examined how associations between stress exposure and prenatal health behaviors might differ as a function of the specific types of stressors that individuals experienced and when they were exposed (Malat, Jacquez, & Slavich, 2017; Slavich, 2019). In the present study, therefore, we examined associations between mothers’ exposure to a variety of different stressors across the life course and their prenatal health behaviors, using a sophisticated interview-based system for assessing lifetime stress exposure.
Negative prenatal health behaviors can increase risk for poor maternal and infant health, but positive health behaviors also have the potential to exert countervailing effects by directly protecting against pregnancy complications and poor birth outcomes, such as low birth weight and preterm birth (CDC, 2016; Lobel et al., 2008). Therefore, it is important to understand how psychosocial factors like stress exposure are related to negative as well as positive health behaviors. In general, a positive relationship has been found between negative prenatal health behaviors and stress, whereby chronic stress (i.e., prolonged stress that typically lasts 4 weeks or longer) is associated with engaging in more negative health behaviors (Auerbach, Lobel, & Cannella, 2014). For instance, pregnancy-specific stress has been linked to engaging in more negative health behaviors, such as smoking and poor diet. Likewise, experiencing early adversity in childhood has been associated with negative health behaviors during pregnancy, including smoking and alcohol use (Chung et al., 2010). Lastly, researchers have found that women who experience chronic stressors, such as domestic violence and poverty, are more likely to smoke, use substances, and have a poor diet (Copper et al., 1996; Shah & Shah, 2010).
Despite this body of work, we know of no studies that have examined stressors occurring over the entire lifespan in relation to health behaviors and pregnancy outcomes. Indeed, studies on this topic appear to have only considered pregnancy-specific stress, childhood stress exposure, or chronic stress during specific life periods (Lu & Halfon, 2003; Malat et al., 2017). Additionally, examining how such effects differ by the specific timing or type of stressors experienced is critical for refining theory on this topic, but research on health behaviors—and, indeed, on health in general—has rarely adopted a stressor characteristics perspective to investigate how documented effects might differ as a function of stressor type, exposure timing, or life domain (e.g., health, housing, etc.; Slavich, 2016, 2019, 2020).
The ecobiodevelopmental framework provides a theoretical rationale for examining chronic stress and prenatal health behaviors through a life course perspective. According to this framework, chronic stress occurring over the lifespan can change the brain’s architecture in ways that make it increasingly difficult for individuals to adapt to future adversity in a healthy manner (Shonkoff et al., 2012). Specifically, the prefrontal cortex suppresses amygdala responses when individuals encounter a stressful experience allowing for more adaptive responses (Shonkoff et al., 2012). However, chronic stress and elevated cortisol levels alter this process, thereby inhibiting the adaptive response to stress (Shonkoff et al., 2012). Individuals who experience chronic stress throughout their lives, not just at specific time points, are more likely to adopt unhealthy behaviors (e.g., alcohol consumption, poor diet, and physical inactivity) as a primary coping mechanism (Shonkoff et al., 2012). While it is important to understand the impact of chronic stress on prenatal health behaviors, Macmillan (2001) noted that life course research is limited in that it typically only examines ongoing experiences and does not consider how more time-limited events might also shape future functioning and wellbeing. For instance, it is likely that being a victim of a significant crime might impact the life course trajectory and shape future functioning to a similar degree as chronic experiences of stress (Macmillan, 2001). This is a critical gap in research on this topic, as adverse, acute life events not only produce immediate psychological distress, but also increase the likelihood of recurring distress throughout adulthood and engaging in more risky behaviors later in life (Macmillan, 2001). Therefore, investigating associations between stress exposure across the lifespan and prenatal health behaviors creates the potential to examine the cumulative impact of acute stressors (i.e., time-limited stressful life events) and chronic stressors (i.e., difficulties that persist in the everyday lives of women) across many systemic factors (e.g., community and social relationships; Lu & Haflon, 2003; Slavich, 2016).
In addition to the cumulative impact that acute and chronic stress has on health behaviors, research has found that perceptions of current stress and the timing of life stress exposure likely moderate the effects of stress on health and health behaviors (Keller et al., 2011; Lee, 2013; Lu & Halfon, 2003; Macmillan, 2001). Regarding current stress levels, research has found that the perceptions of current stress contribute to its impact on health and health-risk behaviors (Ng & Jeffery, 2003; Senn, Walsh, & Carey, 2014). However, the present literature on stress and health behaviors is limited in that it typically utilizes self-report checklist measures of early life stress and does not examine the perceptions of stressors across the lifespan, which could be critical for understanding how different life stressors shape human health and functioning (Slavich, 2016). Related to the timing of stressor exposure, in turn, there is a wealth of research on sensitive or critical periods, which highlights how the impact of stress exposure on later behavior is especially strong during certain developmental periods (Eiland & Romeo, 2013; Epel et al., 2018; Gee & Casey, 2015). For instance, the transition into and out of adolescence are considered critical or sensitive periods in which acute and chronic stress can impact developmental trajectories and increase a person’s risk of engaging in negative health behaviors in adulthood (Eiland & Romeo, 2013). However, no studies have examined the timing of stressors in addition to the cumulative impact of these stressors across the lifespan to take critical or sensitive periods into consideration.
To summarize, existing research has indicated associations between stress and prenatal health behaviors, but little is known about how both acute and chronic stressors occurring across the lifespan influences these behaviors, which is important for refining theory and future research on this important topic. Furthermore, to our knowledge, no studies have assessed the timing and perceptions of stress exposure in addition to the cumulative impact that life stress has on prenatal health behaviors. In the present study, therefore, we examined associations between life stress and prenatal health behaviors in a diverse sample of women who recently gave birth using an interview-based system for assessing stress that included the entire lifespan and that assessed several different types of stress exposure, the timing of stress exposure, and the perceived severity of the stressors experienced. Based on the research reviewed above, we hypothesized that greater perceived lifetime stress severity would be associated with more negative prenatal health behaviors and fewer positive prenatal health behaviors, above and beyond levels of current stress burden and sociodemographic factors that could confound results. In addition, consistent with prior research (e.g., Slavich, Stewart, Esposito, Shields, & Auerbach, 2019; Sturmbauer, Shields, Hetzel, Rohleder, & Slavich, 2019), we hypothesized that these effects would differ as a function of stressor type, exposure timing, and primary life domain.
2 |. METHOD
2.1 |. Participants
Participants were 164 women who had recently delivered an infant at one of two large, urban hospitals—a university hospital and a nonprofit acute care facility—and who were admitted to the postpartum care unit. They were drawn from a larger sample of 200 patients and selected for having complete data relating to their lifetime stress exposure, prenatal health behaviors, and current levels of stress. To be included, women had to be 18 to 35 years old, speak English, and have delivered their first infant at the participating hospital. These criteria were based on research indicating that there are unique risks associated with being pregnant over the age of 35 and spacing pregnancies too far apart or too close together (American Congress of Obstetrician & Gynecologist, 2011; World Health Organization, 2013). Women were excluded from the study if they did not speak English, had more than one child, were younger than 18 or older than 35 years old, or did not complete all study measures.
Eligible women were identified by postpartum nursing staff and approached in their private hospital room by a graduate research assistant. Mothers who delivered babies in the neonatal intensive care unit were not recruited, as nurses often indicated that these mothers were experiencing high levels of stress and required more extensive rest times. To respect designated rest times, data collection took place during visiting hours; therefore, visitors were often present during the consent and interview process. Often, the babies were in the room with the women and needed to be taken care of during various points of the interview by their mothers or incoming providers. Patient care was the top priority. Research assistants thus exited the room every time a provider was with a participant. The typical length of stay in the postpartum care unit was 48 to 96 hr, depending on the method of delivery. If data collection was interrupted, a research assistant attempted to follow up with the participant on a subsequent day.
As shown in Table 1, a majority of participants in the final sample were White (67%) and Black/African American (25.6%). Nearly all participants were insured (97%) and in a domestic partnership. Regarding annual household income, there was substantial variability, with 21.8% of participants making less than $20,000 and 51.1% making more than $70,000. The sample was also very diverse in terms of highest level of household education, with 23% of participants reporting having a high school degree or less, 17% having some college credit but no degree, 34% having an associate or bachelor’s degree, and 26% having received a master’s, doctorate, or professional degree.
TABLE 1.
Demographics characteristics of the sample (N = 164)
| Variable | n (%) |
|---|---|
| Household income | |
| Less than $10,000 | 27 (16.3) |
| $10,000–$19,000 | 9 (5.5) |
| $20,000–$29,000 | 14 (8.4) |
| $30,000–$39,000 | 6 (3.6) |
| $40,000–$49,000 | 7 (4.2) |
| $50,000–$59,000 | 6 (3.6) |
| $60,000–$69,000 | 10 (6.0) |
| $70,000–$79,000 | 10 (6.0) |
| $80,000–$89,000 | 12 (7.2) |
| $90,000–$99,000 | 14 (8.4) |
| More than $100,000 | 49 (29.5) |
| Household education | |
| Eighth grade or less | 1 (0.6) |
| Ninth to 12th grade, no diploma | 14 (8.4) |
| High school graduate/GED | 22 (13.3) |
| College credit, but no degree | 28 (17.1) |
| Associate degree | 12 (7.2) |
| Bachelor’s degree | 44 (26.5) |
| Master’s degree | 29 (17.7) |
| Doctorate or professional degree | 14 (8.4) |
| Ethnicity | |
| White/Caucasian | 110 (67.1) |
| Black/African American | 42 (25.6) |
| Hispanic/Latino | 4 (2.4) |
| Asian | 4 (2.4) |
| Other | 4 (2.4) |
| Marital status | |
| Single | 56 (34.1) |
| Married or domestic partnership | 103 (62.8) |
| Divorced | 4 (2.4) |
| Other | 1 (.6) |
| Health insurance | |
| Uninsured | 5 (3.0) |
| Employer-sponsored coverage | 114 (69.5) |
| Medicaid | 42 (25.6) |
| Direct purchase | 1 (0.6) |
Given the unpredictable nature of the hospital setting, 26 women (13%) were not able to complete all study measures because of various interruptions. In addition, nine participants (4.5%) were excluded because they reported items on the prenatal health behavior measure as “not applicable.” Therefore, a prenatal health behavior score could not be computed for them. These data issues resulted in 164 patients with complete data with respect to lifetime stress exposure, prenatal health behaviors, and current levels of stress. Analyses comparing included participants with complete data and those who were excluded because of missing data revealed statistically significant differences with respect to employment status, health insurance type, household income, and household education level (ps < .01). Specifically, participants who did not complete all measures were more likely to be unemployed and enrolled in Medicaid and to report lower annual household incomes (i.e., <$20,000) and less education (i.e., highest education level was a high school diploma or less). Many of these differences were also observed between hospitals. For instance, at the university hospital, 46% of participants had employed–sponsored insurance and 45% had Medicaid, whereas at the nonprofit acute care facility, 81% of participants had employed– sponsored insurance and 9% had Medicaid (ps < .01).
2.2 |. Procedures
Participants were recruited for the study by graduate student researchers while they were in the postpartum care unit. On average, they took approximately 1 hour to complete the study measures (see below) and were given a $30 gift card for their participation. Study data were managed using Research Electronic Data Capture (Harris et al., 2009), and the University of Cincinnati’s Institutional Review Board approved all study procedures.
2.3 |. Measures
2.3.1 |. Stress and Adversity Inventory
Our focal independent variables were derived from the Stress and Adversity Inventory for Adults (Adult STRAIN; Slavich & Shields, 2018), which is a sophisticated online interviewing system for assessing individuals’ exposure to a variety of acute and chronic stressors occurring across the lifespan (see https://www.strainsetup.com). The STRAIN assesses for the presence of 55 major life stressors in total, including 26 acute life events (e.g., car accident, relationship break-up, death of a loved one) and 29 chronic difficulties (e.g., financial difficulties, feeling unsafe in your neigh-borhood, receiving unfair treatment due to race or ethnicity), which are known to have significant implications for health (Dohrenwend, Raphael, Schwartz, Stueve, & Skodol, 2013; Shields & Slavich, 2017). For each stressor that is endorsed, participants are asked a series of tailored follow-up questions that determine the stressor’s severity, frequency, timing, and duration. Stressor severity, or the degree to which participants endorsed acute or chronic stressors as being stressful, is determined by asking, “At its worst, how stressful or threatening was this for you?” Responses are in turn provided on a Likert-type scale, ranging from 1 (Very slightly or not at all) to 5 (Extremely).
Research has shown that the STRAIN has very good concurrent and discriminant validity and excellent test–retest reliability over 2–4 weeks (rs = .904–.919) for the main lifetime stress exposure indices (see Slavich & Shields, 2018). In addition, the STRAIN has been shown to predict a number of different health-related outcomes, including sleep difficulties (Slavich & Shields, 2018), memory and executive function (Goldfarb, Shields, Daw, Slavich, & Phelps, 2017; Shields, Moons, & Slavich, 2017; Slavich & Shields, 2018), metabolic activity (Kurtzman et al., 2012; Olvera Alvarez et al., 2019), biological reactivity to acute stress (Lam, Shields, Trainor, Slavich, & Yonelinas, 2019), diurnal cortisol levels and quality of life in women diagnosed with ovarian cancer (Cuneo et al., 2017), biological aging (Mayer et al., 2019), depression and fatigue in women diagnosed with breast cancer (Bower, Crosswell, & Slavich, 2014; Dooley, Slavich, Moreno, & Bower, 2017), and self-reported mental and physical health in the general population (Cazassa, Oliveira, Spahr, Shields, & Slavich, 2020; Shields, Moons, et al., 2017; Toussaint, Shields, Dorn, & Slavich, 2016). In the present study, we computed one of the STRAIN’s primary outcomes—the cumulative severity of all lifetime stressors experienced—for use in the primary models predicting women’s prenatal health behaviors.
2.3.2 |. Prenatal Health Behaviors Scale
Women’s prenatal health behaviors were assessed using the Prenatal Health Behaviors Scale (PHBS; Lobel et al., 2008), which examines a broad range of pregnancy-relevant health behaviors. Participants are asked how often they participated in certain prenatal health behaviors using the following Likert-type scale: 0 (never), 1 (almost never), 2 (sometimes), 3 (fairly often), and 4 (very often). The scale consists of 24 items, including 10 positive health behaviors (i.e., exercising, sleeping enough, eating dairy, taking vitamins, eating enough food, stretching muscles, eating high-fiber foods, drinking enough fluids, eating a balanced diet, and taking medicine as prescribed by your doctor) and 14 negative health behaviors (i.e., eating fatty or oily foods, smoking cigarettes, eating snack foods instead of regular meals, standing for long periods of time, drinking caffeine, lifting heavy objects, over-stretching, drinking alcohol, smoking marijuana, eating more food than needed, skipping a meal, using hard drugs, and taking store-bought medicines). Factor analysis identified seven health behavior subscales: cigarette smoking, caffeine consumption, healthy eating, prenatal vitamin use, exercise, physical strain, and unhealthy eating. Prior research has shown that the PHBS is valid and reliable (DeLuca & Lobel, 1995; Lobel et al., 2008; Park, Moore, Turner, & Adler, 1997). For the present study, we created a negative health behavior score by summing all of the negative health practices and a positive health behavior score by summing all the positive health practices.
2.3.3 |. Sociodemographic variables
In keeping with prior research, we also assessed and included several sociodemographic control variables in the main models. Extant research suggests that lower socioeconomic status (i.e., education and income) is associated with higher stress levels (Cohen, Doyle, & Baum, 2006) and negatively associated with health-promoting behaviors (CDC, 2011). Therefore, we considered the role that household income and highest level of education in the household might play in structuring women’s lifetime stress exposure and health behaviors. Furthermore, research has found that racial/ethnic minorities are exposed to greater amounts of stress, which can possibly lead to higher rates of negative health behaviors based on the ecobiodevelopmental framework (Guyll, Matthew, & Bromberger, 2001; Lu & Halfon, 2003; Primm et al., 2010). Therefore, we also included respondents’ self-identified race to understand its relation to women’s lifetime stress exposure severity and prenatal health behaviors. Age was also included as a covariate because women who are older have more years during which they can experience major life stressors, which is a potential confound. Lastly, we assessed and controlled for women’s relationship status (i.e., partnered or not), as this has been determined to be an important factor that is associated with engagement in prenatal health behaviors (Fuller, 2010).
2.3.4 |. Perceived Stress Scale
Finally, given that current stress levels have the potential to influence the recall of past stressful life events (Hoscheidt, LaBar, Ryan, Jacobs, & Nadel, 2014), we assessed participants’ levels of perceived stress during the past month using the Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983). The PSS consists of 10 items, including four questions related to positive thoughts and feelings about stress and six questions related to negative thoughts and feelings about stress. Responses are in turn provided using the following Likert-type scale: 0 (never), 1 (almost never), 2 (sometimes), 3 (fairly often), and 4 (very often) (Cohen et al., 1983). Prior research has shown that the PSS has good reliability and validity, and that it is correlated with other measures of stress, health behaviors, smoking status, and help-seeking patterns (Cohen et al., 1988). The internal consistency of the PSS in the present study was very good (α = .85). To calculate participants’ total PSS score, we reversed-scored items indicating less stress and then summed the items to obtain a total score, with higher scores indicating more perceived stress.
2.4 |. Data analysis
Analyses were conducted in four steps. First, we computed descriptive statistics for the STRAIN, PHBS, PSS, and relevant demographic variables. Next, we used Pearson’s correlations to examine zero-order correlations between the predictor and outcome variables. Third, to examine associations between the severity of women’s lifetime stress exposure and their prenatal health behaviors, we estimated a series of ordinary least squares regression models.1 In these models, we considered negative and positive health behaviors as separate outcomes. The PHBS score was regressed onto women’s lifetime stress exposure severity in Model 1. Women’s self-identified age, race, income, and education were added in Model 2; marital status was added in Model 3; and women’s current levels of perceived stress were added in Model 4. Finally, we applied a stressor characteristics perspective to examine whether the stress–health behavior associations observed differed by the specific type, timing, and primary life domain of the stressors experienced. To do so, we conducted general linear regressions with the two different outcome variables (i.e., negative prenatal health behaviors and positive prenatal health behaviors) and the predictors being the timing, type, and domain variables mentioned above.
3 |. RESULTS
3.1 |. Preliminary analyses
As shown in Table 2, greater lifetime stress exposure severity was positively associated with women’s negative health behaviors score (p < .01) and negatively associated with women’s positive health behaviors (p < .01), as assessed by the PHBS. Current perceived stress levels, as assessed by the PSS, were positively associated with women’s negative health behaviors (p < .01) and negatively associated with women’s positive health behaviors (p < .01). Lastly, women’s current perceived stress levels were positively associated with their lifetime stress exposure severity levels (p < .01).
TABLE 2.
Associations between women’s lifetime stress exposure severity, prenatal health behaviors, and sociodemographic characteristics (N = 164)
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Negative prenatal health behaviors | ||||||||||
| 2. Positive prenatal health behaviors | −.24** | |||||||||
| 3. Lifetime stress exposure severity | .40** | −.25** | ||||||||
| 4. Age | −.19* | .15* | −.03 | |||||||
| 5. Household income | −.24** | .35** | −.25** | .61** | ||||||
| 6. Household education | −.30** | .34** | −.22** | .54** | .74** | |||||
| 7. Ethnicity: African American | .13 | −.28** | .07 | −.27** | −.56** | −.53** | ||||
| 8. Ethnicity: Other | −.10 | .02 | −.01 | −.02 | −.05 | .04 | −.16* | |||
| 9. Marital status | −.25** | .24** | −.21** | .50** | .62** | .63** | −.47** | .07 | ||
| 10. Current perceived stress | .36** | −.38** | .41** | −.30** | −.30** | −.23** | .07 | −.00 | −.20** | |
| Mean | 18.26 | 29.76 | 35.15 | 26.55 | 4.26 | 3.30 | .26 | .07 | .63 | 13.64 |
| Standard deviation | 5.84 | 5.73 | 28.02 | 4.55 | 2.19 | 1.38 | .43 | .26 | .48 | 6.57 |
| Range | 8–38 | 10–39 | 0–123 | 18–36 | 1–7 | 1–5 | 0–1 | 0–1 | 0–1 | 2–31 |
p < .05
p < .01
3.2 |. Primary analyses
3.2.1 |. Negative prenatal health Behaviors
Next, we tested our primary hypotheses involving associations between women’s lifetime stress exposure severity and their prenatal health behaviors. As hypothesized, and as shown in Table 3, women who experienced more severe stressors over the life course engaged in more negative health behaviors during pregnancy (see Model 1: p < .0001, β = .40). Including sociodemographic characteristics attenuated the strength of association between women’s lifetime stress exposure severity and their prenatal health behaviors, but this effect still remained significant while adjusting for these covariates (see Model 2: p < .0001, β = .36; and Model 3: p < .0001, β = .35). Finally, Model 4 (p < .001, β = .27) revealed that the association between women’s lifetime stress exposure severity and negative health behaviors during their pregnancy persisted even while adjusting for all of the sociodemographic characteristics measured and women’s current perceived stress levels. As such, the association observed between lifetime stress exposure and women’s prenatal health behaviors cannot be accounted for by potential sociodemographic confounds or reporting biases attributable to participants’ current levels of perceived stress burden.
TABLE 3.
Hierarchical regression analysis for lifetime stress exposure severity predicting negative prenatal health behaviors (N = 164)
| Variable | B | SE (B) | β | R2 | ΔR2 | p |
|---|---|---|---|---|---|---|
| Model 1 | .16 | .000 | ||||
| Lifetime stress exposure severity | .08 | .01 | .04 | .000 | ||
| Model 2 | .22 | .06* | .000 | |||
| Lifetime stress exposure severity | .07 | .01 | .36 | .000 | ||
| Age | −.13 | .12 | −.10 | .25 | ||
| Household income | .09 | .33 | .03 | .75 | ||
| Household education | −.87 | .47 | −.19 | .07 | ||
| Ethnicity: African American | −.24 | 1.22 | −.01 | .83 | ||
| Ethnicity: Other | −2.06 | 1.63 | −.09 | .20 | ||
| Model 3 | .22 | .07* | .000 | |||
| Lifetime stress exposure severity | .07 | .01 | .35 | .000 | ||
| Age | −.12 | .12 | −.09 | .30 | ||
| Household income | .12 | .339 | .04 | .70 | ||
| Household education | −.77 | .48 | −.18 | .11 | ||
| Ethnicity: African American | −.33 | 1.23 | −.02 | .78 | ||
| Ethnicity: Other | −2.01 | 1.64 | −.09 | .22 | ||
| Marital status | −.60 | 1.19 | −.05 | .61 | ||
| Model 4 | .25 | .15** | .000 | |||
| Lifetime stress exposure severity | .05 | .01 | .27 | .001 | ||
| Age | −.05 | .12 | −.04 | .65 | ||
| Household income | .21 | .33 | .08 | .52 | ||
| Household education | −.81 | .48 | −.19 | .09 | ||
| Ethnicity: African American | −.12 | 1.22 | −.01 | .91 | ||
| Ethnicity: Other | −1.84 | 1.61 | −.08 | .25 | ||
| Marital status | −.73 | 1.17 | −.06 | .53 | ||
| Current perceived stress | .17 | .07 | .19 | .01 |
Abbreviation: SE, standard error.
p < .05.
p < .01.
3.2.2 |. Positive prenatal health behaviors
Turning next to women’s positive health behaviors, as hypothesized, and as shown in Table 4, greater lifetime stress exposure severity was negatively associated with positive prenatal health behaviors (see Model 1: p < .001, β = −.25). Consistent with the results reported above, this finding was attenuated, but still significant, while controlling for relevant sociodemographic characteristics (see Model 2: p < .03, β = −.16; and Model 3: p < .03, β = −.16). In contrast with negative prenatal health behaviors, however, lifetime stress exposure severity was not significantly associated with positive prenatal health behaviors while also controlling for current levels of perceived stress burden in addition to the other demographic covariates (see Model 4: p = .64, β = −.03).
TABLE 4.
Hierarchical regression analysis for perceived lifetime stress severity predicting positive prenatal health behaviors (N = 164)
| Variable | B | SE (B) | β | R2 | ΔR2 | p |
|---|---|---|---|---|---|---|
| Model 1 | .06 | .001 | ||||
| Lifetime stress exposure severity | −.1.21 | .37 | −.25 | .001 | ||
| Model 2 | .17 | .07* | .000 | |||
| Lifetime stress exposure severity | −.03 | .01 | −.16 | .03 | ||
| Age | −.09 | .12 | −.07 | .44 | ||
| Household income | .48 | .33 | −.18 | .15 | ||
| Household education | .66 | .47 | −.16 | .16 | ||
| Ethnicity: African American | −.1.29 | 1.23 | −.09 | .29 | ||
| Ethnicity: Other | .06 | 1.64 | .00 | .96 | ||
| Model 3 | .17 | .08* | .000 | |||
| Lifetime stress exposure severity | −.03 | .01 | −.16 | .03 | ||
| Age | −.08 | .12 | −.06 | .48 | ||
| Household income | .49 | .34 | .18 | .15 | ||
| Household education | .69 | .49 | .16 | .15 | ||
| Ethnicity: African American | −1.34 | 1.25 | −.10 | .28 | ||
| Ethnicity: Other | .09 | 1.65 | .00 | .95 | ||
| Marital status | −.30 | 1.20 | −.02 | .79 | ||
| Model 4 | .25 | .20** | .000 | |||
| Lifetime stress exposure severity | −.00 | .01 | −.03 | .64 | ||
| Age | −.19 | .12 | −.15 | .10 | ||
| Household income | .35 | .32 | .13 | .27 | ||
| Household education | .76 | .47 | .18 | .10 | ||
| Ethnicity: African American | −1.67 | 1.19 | −.12 | .16 | ||
| Ethnicity: Other | −.16 | 1.58 | −.00 | .91 | ||
| Marital status | −.09 | 1.15 | −.00 | .93 | ||
| Current perceived stress | −.28 | .07 | −.32 | .000 |
Abbreviation: SE, standard error.
p < .05.
p < .01.
3.2.3 |. Stressor characteristics analyses
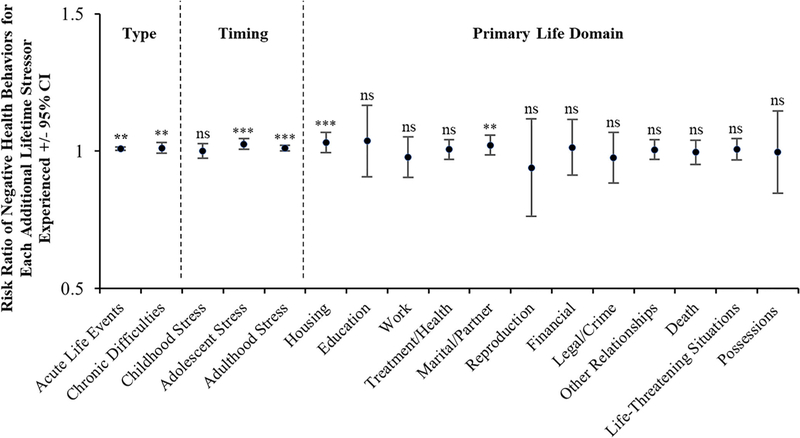
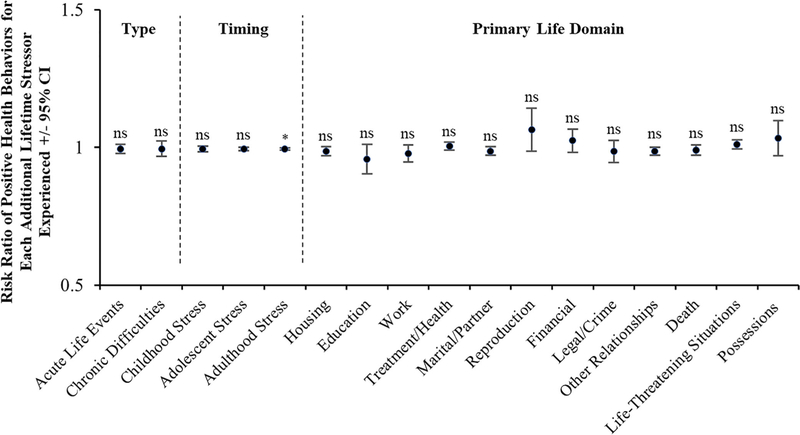
To better understand how different stressor characteristics are related to participants’ negative and positive prenatal health behaviors, supplemental analyses were conducted using Poisson Regression. As depicted in Figure 1, for negative health behaviors, effects were relatively stronger for stressors occurring in adolescence and adulthood versus childhood and for stressors occurring in the life domains of housing and marital/partner. As depicted in Figure 2, for positive health behaviors, effects were not significant across stressor type, timing, and primary life domain with the exception of adulthood stress exposure, which was negatively associated with engaging in positive health behaviors (i.e., more stress = fewer positive health behaviors).
FIGURE 1.
Likelihood of engaging in negative prenatal health behaviors by stressor type, timing, and primary life domain. Risk of engaging in more negative prenatal health behaviors differed substantially by stressor timing and primary life domain. Regarding stress exposure timing, risk was greater for women experiencing stress during adolescence and adulthood versus childhood. Additionally, risk was greater for women experiencing stressors in the life domains of housing and marital/partner. ** p < .01; *** p < .001; CI, confidence interval; ns, not significant. (N = 164)
FIGURE 2.
Likelihood of engaging in positive prenatal health behaviors by stressor type, timing, and primary life domain. Risk of engaging in positive prenatal health behaviors did not differ by stressor type or across the primary life domains assessed by the STRAIN. With respect to timing of stress exposure, however, a negative association was observed, whereby greater life stress exposure occurring during adulthood was associated with fewer positive prenatal health behaviors. * p < .05; CI, confidence interval; ns, not significant. (N = 164)
4 |. DISCUSSION
Despite substantial interest in the role that life stress plays in shaping health behaviors, to our knowledge, no studies to date have examined how acute and chronic stressors occurring across the lifespan are associated with women’s negative and positive health behaviors during the critically important time of pregnancy. In addition, relatively few studies in general have investigated whether the effects of life stress on health-related outcomes differ as a function of the perceptions, specific types, or timing of stressors experienced (Monroe & Slavich, 2020). In addressing this question, we found that more severe lifetime stress exposure was associated with engaging in more negative prenatal health behaviors, such as smoking and overeating. In addition, we found that more severe lifetime stress exposure was negatively associated with engaging in positive prenatal health behaviors, such as exercising regularly and eating a balanced diet. In both instances, these effects were not uniform across the different lifetime stress exposure indices produced by the STRAIN but rather differed substantially as a function of the specific stressors that participants experienced and the timing of these exposures. Considered together, then, these results indicate that stress occurring over the lifespan can accumulate to impact prenatal health behaviors, which are in turn known to impact birth outcomes, but that these effects differ by the specific type and timing of mother’s stress exposure.
Based on these results, it may be important to assess stress occurring over the entire lifespan. Moreover, when promoting maternal and infant health through health behaviors, it may be important to consider how these associations may be structured by lifetime stress exposure. Future studies examining the association between life stress exposure and negative prenatal health behaviors should also consider how documented effects might differ across different stressor types (e.g., housing, education, work stressors) and time points (e.g., childhood, adolescent, adulthood stress). Put simply, studies examining only one type of stress, or stress occurring over only a limited period of time, might obscure important information that has the potential to help refine theories describing how different types of stress exposure affect prenatal health behaviors and health (Slavich, 2019).
Notably, the present results were robust while controlling for current perceived stress levels, as well as participants’ education and income levels, indicating that the findings are not due to possible demographic confounding factors or to current stress levels that could cause reporting biases. Additionally, because experiencing more severe stressors over the life course may have impeded women’s educational and income attainment, which were controlled for in the models, these effects represent conservative estimates of the effect of lifetime stress exposure severity on negative prenatal health behaviors. For example, education was significantly associated with negative prenatal health behaviors, but income was not. This is an important distinction to continue to examine in future research, as it likely has critical implications for interventions. Specifically, the present results indicate that education is a social determinant of negative prenatal health behaviors. Theorists suggest that education is likely the most fundamental sociodemographic factor related to health because it impacts future opportunities, attainment, and access to information (Adler & Newman, 2002). Although broader interventions aimed at reducing the effects of lifetime stress exposure on negative health behaviors should consider education level as a potential risk and protective factor, a prospective study design is necessary to make informed conclusions about the effect that lifetime stress exposure has on prenatal health behaviors.
More broadly, prior research has shown that negative health outcomes are influenced primarily by individuals’ subjective perceptions of the severity of stressors that they have experienced (e.g., Slavich & Cole, 2013). Consequently, how women perceive stressors in their lives may partly determine the degree to which such stressors impact their health behaviors. To the extent that this is true, one intervention strategy could involve providing stress reduction resources or interventions, such as cognitive behavior therapy or mindfulness-based stress reduction, to women experiencing moderate-to-high levels of lifetime stress exposure so as to reduce the severity of their negative stress perceptions. As alluded to above, however, longitudinal studies are needed to examine this possibility further.
Lastly, we examined whether the effects of lifetime stress exposure on both positive and negative prenatal health behaviors differed across the various types of stress assessed by the STRAIN, including type (i.e., chronic and acute), timing (i.e., childhood, adolescent, and adulthood), and primary life domain (i.e., housing, education, work, treatment/health, marital/partner, reproduction, financial, legal/crime, other relationships, death, life-threatening situations, and possessions). Consistent with a stressor characteristics perspective, risk of engaging in negative prenatal health behaviors varied by the specific primary life domain and timing of stressors experienced (e.g., Lam et al., 2019; Slavich & Shields, 2018; Slavich et al., 2019). Specifically, likelihood of engaging in negative prenatal health behaviors was more strongly associated with adulthood and adolescent versus childhood stressors. Regarding life domains, risk of engaging in negative prenatal health behaviors was greatest for women who endorsed stress related to housing or marital/partner. Related to positive prenatal health behaviors, risk did not differ by stressor type or primary life domain. However, within the timing domain, there was a significant, negative association for women experiencing stress in adulthood, but not for stress in childhood or adolescence.
These results can help us move beyond the knowledge that stress is associated with health behaviors overall and toward a more nuanced and refined understanding of specific types of stress that have the greatest impact on negative prenatal health behaviors, which in turn increase risk for poor birth outcomes. Doing so will also help to identify women who are at the greatest risk for experiencing negative prenatal health behaviors and poor birth outcomes. Finally, this information may help inform intervention strategies and policies aimed at reducing this risk by targeting stress-related processes.
Despite these contributions to the stress and health behavior literature, several limitations of the present study should be noted. First, as mentioned previously, the present study design was cross-sectional, and experiences of lifetime stress exposure were collected retrospectively. Although efforts were made to control for this approach (e.g., by increasing recall of stressful life events by having participants fill out a life chart of all the places that they have ever lived), definitive conclusions cannot be drawn to impact policy or intervention based on these data alone. Second, as a result of the cross-sectional study design, it was not possible to collect information about women’s health behaviors prior to the stressors that were reported. Consequently, additional research is needed to confirm the hypothesized temporal precedence of life stressors contributing to subsequent changes in prenatal health behaviors.
Third, 34 participants did not complete measures of lifetime stress, perceived stress, and prenatal health behaviors, and were therefore excluded from the study. These women differed from those who were included with respect to income and education level, employment status, and insurance type, and as a result, the diversity of the sample with respect to relevant demographic factors may have been impacted. Missing data were largely due to the busy nature of the hospital setting (e.g., interruptions due to medical care, nursing, etc.). Therefore, future studies utilizing a maternal sample should consider interviewing women after they have left the hospital in order to reduce distractions and help ensure study completion.2
Relatedly, based on the demographic information collected, participants excluded from the study likely would have different perceptions and experiences of stress compared with women who were included in the study, which decreased the diversity of the sample. Prior research has demonstrated that lower sociodemographic status is associated with chronic stress due to a number of factors, including greater trauma exposure and lack of resources (Auerbach et al., 2014; Gee, Walsemann, & Brondolo, 2012). Therefore, it is possible that if the excluded participants were included in the present sample, we would have observed higher lifetime stress exposure levels and possibly more engagement in negative prenatal health behaviors. To address this issue, future studies should aim to recruit socially diverse samples that include individuals exhibiting the full range of sociodemographic variability.
In conclusion, data from the present study indicate that greater lifetime stress exposure severity is associated with engaging in more negative and fewer positive prenatal health behaviors. The results for negative health behaviors were robust to full adjustment for all potential confounding factors, including current perceived stress levels and the sociodemographic factors assessed (i.e., age, education, income, ethnicity, and marital status), whereas the results for positive health behaviors were robust to all statistical adjustments except for current perceived stress level. In addition, we found that these effects differed substantially depending on the specific types and timing of the stressors experienced. The findings are thus consistent with the ecobiodevelopmental framework (Shonkoff et al., 2012) and provide preliminary support for applying a life course approach to better understand factors affecting prenatal health behaviors (Malat et al., 2017).
As a result of these findings, it may be useful to assess the lifetime stress exposure levels of expecting women as they may be associated with women’s health and, in turn, the health of the newborn, although longitudinal studies are needed to examine this issue further. Ultimately, understanding the cumulative impact that lifetime stressors have on prenatal health behaviors, and identifying women who may be particularly susceptible to this influence, is an important goal for promoting infant and maternal health, especially because women’s health behaviors have been found to predict offspring health (CDC, 2016; Lobel et al., 2008). If the present results are replicated in future studies, the resulting data could help inform the design and implementation of interventions aimed at decreasing lifetime stress levels in order to help promote positive prenatal health behaviors and infant and maternal health.
ACKNOWLEDGMENTS
The authors thank the research team and hospital staff for their commitment and dedication to this project, including Dr. Elizabeth Kelly. The authors also acknowledge the graduate student interviewers for their assistance with data collection, including Shaonta Allen, Chamyia Fambro, Aaryn Green, Angelica Hardee, Lynnissa Hillman, Neslihan James-Kangal, Ainsley Lambert, Quiera Lige, and Caravella McCuisitian. This study was supported by March of Dimes, Grant Number 134267 \ 22-FY14–470, an Institutional Clinical and Translational Science Award, and National Institutes of Health/National Center for Research Resources Grant Number 5UL1RR026314–03. George Slavich was supported by a Society in Science—Branco Weiss Fellowship, NARSAD Young Investigator Grant #23958 from the Brain & Behavior Research Foundation, and National Institutes of Health grant K08 MH103443. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or any of the other funding bodies.
Funding information
This study was supported by March of Dimes, Grant/Award Number: 134267 \ 22-FY14–470; Institutional Clinical and Translational, Science Award, and National Institutes of Health/National Center for Research Resources, Grant/Award Number: 5UL1RR026314–03; George Slavich was supported by a Society in Science—Branco Weiss Fellowship, NARSAD Young, Investigator, Grant/Award Number: 23958; National Institutes of Health, Grant/Award Number: K08 MH103443
Footnotes
CONFLICT OF INTEREST
The authors report no conflicts of interests.
We tested the fit of ordinary least squares (OLS) linear models against generalized linear models assuming a Poisson distribution. In five of eight pairs of models, OLS fit better (Akaike information criterion [AIC] improvements >2.0); in two of eight pairs of models, OLS and Poisson regressions were equivalent (AIC improvements <2.0), and in one of eight pairs of models, OLS fit worse (AIC difference = −4.6). However, the significance levels of all coefficients were equivalent between OLS and Poisson regression for this pair of models. Therefore, for parsimony in presenting the results and because OLS regression fit better than Poisson regression in the majority of models, we present all models as OLS regressions.
Because individuals with some missing data differed in some ways from individuals without missing data, we conducted analyses using maximum likelihood to estimate the missing data and compared those results to the results from models using listwise deletion. All significance levels were equivalent between the two sets of analyses for each model, and all coefficient values were virtually identical. Therefore, our method of handling missing data does not appear to have influenced the results.
REFERENCES
- Adler NE, & Newman K (2002). Socioeconomic disparities in health: Pathways and policies. Health Affairs, 21(2), 60–76. [DOI] [PubMed] [Google Scholar]
- American Congress of Obstetricians and Gynecologists. (2011). Pre-pregnancy counseling. Retrieved from: https://www.acog.org/ClinicalGuidance-and-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Prepregnancy-Counseling.
- Auerbach M, Lobel M, & Cannella D (2014). Psychosocial correlates of health-promoting and health-impairing behaviors in pregnancy. Journal of Psychosomatic Obstetrics and Gynecology, 35(3), 76–83. [DOI] [PubMed] [Google Scholar]
- Bower JE, Crosswell AD, & Slavich GM (2014). Childhood adversity and cumulative life stress risk factors for cancer-related fatigue. Clinical Psychological Science, 2(1), 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazassa MJ, Oliveira MdS., Spahr CM., Shields GS., & Slavich GM. (2020). The Stress and Adversity Inventory for Adults (Adult STRAIN) in Brazilian Portuguese: Initial validation and links with executive function, sleep, and mental and physical health. Frontiers in Psychology, 10: 3083 10.3389/fpsyg.2019.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2011). National center health interview survey 2011 data release. Retrieved from: https://www.cdc.gov/nchs/nhis/nhis_2011_data_release.htm.
- Centers for Disease Control and Prevention. (2016). Infant mortality statistics from the 2013 period linked birth/infant death data set. Retrieved from: http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_09.pdf. [PubMed]
- Chung EK, Nurmohamed L, Mathew L, Elo IT, Coyne JC, & Culhane JF (2010). Risky health behaviors among women’s-to-be: The impact of adverse childhood experiences. Academic Pediatrics, 10(4), 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S (1988). Psychosocial models of the role of social support in the etiology of physical disease. Health Psychology, 7(3), 269–297. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, & Baum A (2006). Socioeconomic status is associated with stress hormones. Psychosomatic Medicine, 68(3), 414–420. [DOI] [PubMed] [Google Scholar]
- Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, … Jones P (1996). The preterm prediction study: Maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. American Journal of Obstetrics and Gynecology, 175 (5), 1286–1292. [DOI] [PubMed] [Google Scholar]
- Cuneo MG, Schrepf A, Slavich GM, Thaker PH, Goodheart M, Bender D, … Lutgendorf SK (2017). Diurnal cortisol rhythms, fatigue and psychosocial factors in five-year survivors of ovarian cancer. Psychoneuroendocrinology, 84, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca RS, & Lobel M (1995). Conception, commitment, and health behavior practices in medically high-risk pregnant women. Women’s Health: Research on Gender, Behavior, and Policy, 1, 257–271. [PubMed] [Google Scholar]
- Dohrenwend BP, Raphael KG, Schwartz S, Stueve A, & Skodol A (2013). The structured event probe and narrative rating method for measuring stressful life events In Goldberger L & Breznitz S (Eds.), Handbook of stress: Theoretical and clinical aspects (pp. 174–199). New York: Free Press. [Google Scholar]
- Dooley LN, Slavich GM, Moreno PI, & Bower JE (2017). Strength through adversity: Moderate lifetime stress exposure is associated with psychological resilience in breast cancer survivors. Stress and Health, 33, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, & Romeo RD (2013). Stress and the developing adolescent brain. Neuroscience, 249, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, & Mendes WB (2018). More than a feeling: A unified view of stress measurement for population science. Frontiers in Neuroendocrinology, 49, 146–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller TD (2010). Relationship status, health, and health behavior: An examination of cohabiters and commuters. Sociological Perspectives, 53 (2), 221–245. [Google Scholar]
- Gee DG, & Casey BJ (2015). The impact of developmental timing for stress and recovery. Neurobiology of Stress, 1, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee GC, Walsemann KM, & Brondolo E (2012). A life course perspective on how racism may be related to health inequities. American Journal of Public Health, 102(5), 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, & Culhane JF (2007). Low birth weight in the United States. The American Journal of Clinical Nutrition, 85(2), 584S–590S. [DOI] [PubMed] [Google Scholar]
- Goldfarb EV, Shields GS, Daw ND, Slavich GM, & Phelps EA (2017). Low lifetime stress exposure is associated with reduced stimulus-response memory. Learning and Memory, 24, 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyll M, Matthews KA, & Bromberger JT (2001). Discrimination and unfair treatment: relationship to cardiovascular reactivity among African American and European American women. Health Psychology, 20(5), 315–325. [DOI] [PubMed] [Google Scholar]
- Hankin JR (2002). Fetal alcohol syndrome prevention research. Alcohol Research and Health, 26(1), 58–65. [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoscheidt SM, LaBar KS, Ryan L, Jacobs WJ, & Nadel L (2014). Encoding negative events under stress: High subjective arousal is related to accurate emotional memory despite misinformation exposure. Neurobiology of Learning and Memory, 112, 237–247. [DOI] [PubMed] [Google Scholar]
- Keller A, Litzelman K, Wisk LE, Maddox T, Cheng ER, Creswell PD, & Witt WP (2011). Does the perception that stress affects health matter? The association with health and mortality. Health Psychology, 31(5), 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman L, O’Donovan A, Koslov K, Arenander J, Epel ES, & Slavich GM (2012). Sweating the big stuff: Dispositional pessimism exacerbates the deleterious effects of life stress on metabolic health. European Journal of Psychotraumatology, 3. [Google Scholar]
- Lam JCW, Shields GS, Trainor BC, Slavich GM, & Yonelinas AP (2019). Greater lifetime stress exposure predicts blunted cortisol but heightened DHEA responses to acute stress. Stress and Health, 35, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Joo EJ, & Choi KS (2013). Perceived stress and self-esteem mediate the effects of work-related stress on depression. Stress and Health, 29(1), 75–81. [DOI] [PubMed] [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, & Meyer BA (2008). Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology, 27(5), 604–615. [DOI] [PubMed] [Google Scholar]
- Lu M, & Halfon N (2003). Racial and ethnic disparities in birth outcomes: A life course perspective. Maternal and Child Health Journal, 7(1), 13–30. [DOI] [PubMed] [Google Scholar]
- Macmillan R (2001). Violence and the life course: The consequences of victimization for personal and social development. Annual Review of Sociology, 27, 1–22. [Google Scholar]
- Malat J, Jacquez F, & Slavich GM (2017). Measuring lifetime stress exposure and protective factors in life course research on racial inequality and birth outcomes. Stress, 20, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer SE, Prather AA, Puterman E, Lin J, Arenander J, Coccia M, … Epel ES (2019). Cumulative lifetime stress exposure and leukocyte telomere length attrition: The unique role of stressor duration and exposure timing. Psychoneuroendocrinology, 104, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, & Slavich GM (2020). Major life events: A review of conceptual, definitional, measurement issues, and practices In Harkness KL & Hayden EP (Eds.), The Oxford handbook of stress and mental health (pp. 7–26). New York: Oxford University Press. [Google Scholar]
- Ng DM, & Jeffery RW (2003). Relationships between perceived stress and health behaviors in a sample of working adults. Health Psychology, 22(6), 638–642. [DOI] [PubMed] [Google Scholar]
- Olvera Alvarez HA, Provencio-Vasquez E, Slavich GM, Laurent JGC, Browning M, McKee-Lopez G, … Spengler JD (2019). Stress and health in nursing students: The nurse engagement and wellness study. Nursing Research, 68, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CL, Moore PJ, Turner RA, & Adler NE (1997). The roles of constructive thinking and optimism in psychological and behavioral adjustment during pregnancy. Journal of Personality and Social Psychology, 73(3), 584–592. [DOI] [PubMed] [Google Scholar]
- Primm AB, Vasquez MJT, Vasquez MJT, Mays RA, Sammons-Posey D, McKnight-Eily LR, … Perry GS (2010). The role of public health in addressing racial and ethnic disparities in mental health and mental illness. Preventing Chronic Disease, 7(1), A20. [PMC free article] [PubMed] [Google Scholar]
- Senn TE, Walsh JL, & Carey MP (2014). The mediating roles of perceived stress and health behaviors in the relation between objective, subjective, and neighborhood socioeconomic status and perceived health. Annals of Behavioral Medicine, 48(2), 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS, & Shah J (2010). Maternal exposure to domestic violence and pregnancy and birth outcomes: A systematic review and meta-analyses. Journal of Women’s Health, 19(11), 2017–2031. [DOI] [PubMed] [Google Scholar]
- Shields GS, Moons WG, & Slavich GM (2017). Better executive function under stress mitigates the effects of recent life stress exposure on health in young adults. Stress, 20, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, & Slavich GM (2017). Lifetime stress exposure and health: A review of contemporary assessment methods and biological mechanisms. Social and Personality Psychology Compass, 11(8), e12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, … Committee on Early Childhood, Adoption, and Dependent Care. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–e246. [DOI] [PubMed] [Google Scholar]
- Slavich GM (2016). Life stress and health: A review of conceptual issues and recent findings. Teaching of Psychology, 43(4), 346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM (2019). Stressnology: The primitive (and problematic) study of life stress exposure and pressing need for better measurement. Brain, Behavior, and Immunity, 75, 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM (2020). Psychoneuroimmunology of stress and mental health In Harkness KL & Hayden EP (Eds.), The Oxford handbook of stress and mental health (pp. 519–546). New York: Oxford University Press. [Google Scholar]
- Slavich GM, & Cole SW (2013). The emerging field of human social genomics. Clinical Psychological Science, 1, 331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Shields GS (2018). Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): An overview and initial validation. Psychosomatic Medicine, 80, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Stewart JG, Esposito EC, Shields GS, & Auerbach RP (2019). The Stress and Adversity Inventory for Adolescents (Adolescent STRAIN): Associations with mental and physical health, risky behaviors, and psychiatric diagnoses in youth seeking treatment. Journal of Child Psychology and Psychiatry, 60, 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmbauer SC, Shields GS, Hetzel EL, Rohleder N, & Slavich GM (2019). The Stress and Adversity Inventory for Adults (Adult STRAIN) in German: An overview and initial validation. PLoS One, 14(5), e0216419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint L, Shields GS, Dorn G, & Slavich GM (2016). Effects of lifetime stress exposure on mental and physical health in young adulthood: How stress degrades and forgiveness protects health. Journal of Health Psychology, 21(6), 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2013). Birth spacing — report from a WHO technical consultation. Retrieved from: https://apps.who.int/iris/bitstream/handle/10665/73710/RHR_policybrief_birthspacing_eng.pdf