Abstract
The inexpensive, well-tolerated, immunomodulatory agent leflunomide, used extensively for the treatment of rheumatoid arthritis, has been shown to produce significant activity against multiple myeloma (MM) in pre-clinical studies. We conducted a phase 1 study (clinicaltrials.gov: NCT02509052) of single-agent leflunomide in patients with relapsed/refractory MM (≥3 prior therapies). At dose levels 1 and 2 (20 mg, 40 mg), no dose-limiting toxicities (DLTs) were observed. At dose level 3 (60 mg), one patient experienced elevated alanine aminotransferase; an additional three patients were enrolled at this dose level without further DLTs. Overall, toxicities were infrequent and manageable. Nine out of 11 patients achieved stable disease (SD), two subjects experiencing SD for nearly one year or longer. The tolerable safety profile of leflunomide, combined with a potential disease stabilization, is motivating future studies of leflunomide, in combination with other MM drugs, or as an approach to delay progression of smoldering MM.
Keywords: leflunomide, clinical trial, relapsed/refractory, multiple myeloma
Introduction
Multiple myeloma (MM) is an incurable plasma cell malignancy in which patients accumulate monoclonal immunoglobulin-producing plasma cells within their bone marrow (BM). Much progress has been made in treating MM with agents from multiple drug classes. Therapeutic agents are used sequentially or in combination. Although the prognosis for MM patients has improved [1,2], MM is associated with a 5-year survival rate of 52% [3], and only about 25% of patients survive for 10 years [4].
Patients who initially respond to therapy almost always develop relapsed/refractory MM (RRMM), defined as disease that is nonresponsive while on salvage therapy, or progressive disease within 60 days of cessation of the most recent prior therapy in patients who have achieved minimal response or better [5]. For multiply treated patients who are refractory to both an immunomodulatory drug and a proteasome inhibitor and have been exposed to an alkylating agent, the progression-free and overall survival was found to be 5 and 13 months, respectively [6]. Similarly, patients refractory to CD38-targeting antibodies experience a median survival of 8.6 months beyond the time refractory status is declared [7]. Furthermore, treatment decision-making in this population is particularly challenging, given the presence of multiple co-morbidities and the toxicities associated with available agents.
Leflunomide, a commercially available oral agent, FDA-approved since 1998 for the treatment of rheumatoid arthritis (RA), offers the potential to improve therapeutic outcomes in MM. When taken, leflunomide is rapidly converted to the active metabolite, teriflunomide, which mediates leflunomide’s pharmacologic activity. The primary clinical mechanism of action in RA is inhibition of de novo pyrimidine synthesis by targeting dihydroorotate dehydrogenase (DHODH), and thus achieving an anti-proliferative effect in B- and T-lymphocytes [8]. A secondary mechanism of action is inhibition of cytokine and growth factor receptor-associated tyrosine kinase activity [8]. Teriflunomide has also been shown to directly inhibit the kinase activity of the EGF receptor [9], and we recently published that leflunomide can regulate c-Myc protein expression and regulate T cell activation markers in a murine myeloma mouse model [10].
The first reports of anti-tumor activity were in mouse xenograft models of glioma and indicated that inhibition of pyrimidine synthesis is insufficient for anti-tumor activity and concomitant inhibition of tyrosine phosphorylation may also play a role [11]. In chronic lymphocytic leukemia (CLL), leflunomide inhibits cell cycle progression in primary leukemic cells in vitro [12]. Dietrich et al. showed that teriflunomide affects proliferation of CLL by blocking DHODH at very low concentrations (3–5 μg/ml) and by additionally inhibiting the JAK/STAT pathway at intermediate concentrations (>10 μg/ml) [13]. In addition, pre-clinical anti-tumor activity of leflunomide has been reported for melanoma [14] and prostate cancer [15].
Teriflunomide inhibited cell growth and induced apoptosis in myeloma cell lines (NCI-H929, OPM-2, RPMI-8226, and U266) at clinically achievable concentrations (50–200 μmol/L) in a time- and dose-dependent manner [16]. In addition, additive effects were seen when teriflunomide was combined with the genotoxic agents melphalan and doxorubicin, and synergistic effects when combined with bortezomib and treosulfan [16].
We have found that leflunomide is active in both glucocorticoid-sensitive and resistant myeloma cell lines [10]. Through a kinase screening assay, we identified that, besides DHODH inhibition, leflunomide directly inhibits the activity of several kinases including the PIM family of serine/threonine kinases. We further found that, in MM cells, PIM kinases positively regulate c-Myc protein levels and that leflunomide impairs this effect. Finally, the anti-MM activity of leflunomide synergizes with the immunomodulatory drug lenalidomide in vitro and in vivo. With pre-clinical data supporting anti-myeloma activity of leflunomide, we conducted a phase 1 clinical trial in RRMM.
Methods
The protocol was formerly designed as a phase 1/phase 2 trial. New preclinical data were cause for us to conclude the trial before beginning the phase 2 portion (see below). The primary objective of this open-label, single-center, phase 1 portion was to determine the maximum tolerated dose/recommended phase 2 dose of leflunomide, when given as a single agent, and to assess the safety and tolerability of leflunomide at each dose level by evaluation of toxicities including: type, frequency, severity, attribution, time course and duration. Leflunomide has been used at up to 40 mg/day in patients with Wegner’s granulomatosis with a safety profile similar to that of the 20 mg/day dose used in RA [17,18]. Doses up to 60 mg/day have been safely used for the treatment of allograft polyoma BK virus in renal allograft patients [19]. Given these findings, three dose levels of leflunomide (dose level 1: 20 mg, dose level 2: 40 mg, and dose level 3: 60 mg), were considered. The starting dose level of daily leflunomide was 20 mg daily, with dose escalation in increments of 20 mg/day, and, in the presence of dose-limiting toxicity, dose de-escalation in decrements of 10 mg/day. The maximum dose tested was 60 mg/day. Intra-patient dose escalation was not permitted. All patients were enrolled at City of Hope. The study was performed in accordance with the provisions of the Declaration of Helsinki and was approved by the institutional review board. All participants gave written informed consent. The study is registered at www.clinicaltrials.gov as #NCT02509052.
Drug administration
Leflunomide administration consisted of a loading dose of 100 mg daily for the first three days, followed by daily dosing, at the assigned dose level, in 28-day cycles. Dose escalation/de-escalation/expansion decisions were based on the dose limiting toxicities (DLTs) occurring during cycle 1. DLT was defined as any of the following toxicities that were at least possibly related to leflunomide and occurred during cycle 1: Any toxicity or series of toxicities resulting in fewer than 21 doses during cycle 1 or extension of cycle 1 by >35 days; grade 4 neutropenia (absolute neutrophil count <500/mm3); grade 3 or 4 febrile neutropenia; grade 4 thrombocytopenia (<25,000/mm3); or grade 3 thrombocytopenia (<50,000/mm3) with bleeding. Also, DLTs included grade 4 non-hematologic toxicity with the exceptions of diarrhea or vomiting that resolves to grade 3 within 48 hours, allergic reaction/hypersensitivity, or electrolyte/metabolic toxicity corrected to <grade 1 or baseline with 48 hours. Grade 3 toxicity despite maximal medical therapy lasting >7 days was also a DLT. Finally, grade 2 alanine aminotransferase, aspartate aminotransferase, total bilirubin lasting >14 days, or grade 3 or higher elevation of ALT (SGPT)/AST (SGOT)/total bilirubin of any duration were considered DLTs. Because of the ability of leflunomide to be reabsorbed through the colon, resulting in a very long half-life (approximately 2 weeks), all participants removed from study treatment received cholestyramine, 8 grams oral suspension three times daily for 11 days, to prevent re-absorption of leflunomide at the end of study treatment.
Inclusion/exclusion criteria
The study enrolled patients with MM who were 18 years of age or older and relapsed or refractory to at least 3 prior lines of therapy, including both a proteasome inhibitor and immunomodulatory drug, and for whom transplantation was not recommended. Induction therapy and stem cell transplant with or without maintenance was considered one regimen. Patients were required to have serum M protein ≥0.5 g/dL, urine M protein ≥200 mg/24 hours, serum free light chain ≥10 mg/dL provided the free light chain ratio is abnormal, or ≥10% plasma cells in the BM; ECOG performance status of 0 to 2, and adequate hematologic (absolute neutrophil count ≥1,000/mm3, platelets ≥50,000/mm3), hepatic (total bilirubin ≤1.5 × upper limit of normal, alanine/aspartate aminotransferase ≤2 × upper limit of normal), and renal (creatinine clearance ≥30 mL/minute) function.
Key exclusion criteria were prior treatment with leflunomide, prior diagnosis of rheumatoid arthritis, prior allogeneic transplantation, pre-existing liver disease, known HIV infection, or acute active infection requiring systemic therapy within 2 weeks prior to enrollment.
Toxicity and disease assessments
Adverse events (AEs) were monitored throughout the study and were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute v4.03. Disease response was assessed using the International Myeloma Working Group (IMWG) uniform criteria, incorporating minimal response (MR) per the European Group for Blood and Marrow Transplantation criteria.
Pharmacokinetics
Pharmacokinetic sampling consisted of trough level monitoring throughout the entire period of active treatment with leflunomide. Four mL of venous blood was collected into a purple-top (EDTA) Vacutainer® tube prior to initiation of drug administration on day 1 of cycle 1 of treatment and then at each follow-up visit thereafter. Plasma was separated from whole blood and analyzed for total (bound and unbound) and free (unbound) teriflunomide concentrations.
Total and free teriflunomide plasma concentrations were determined using a previously described LC-MS/MS method [20]. Briefly, following addition of a deuterated teriflunomide internal standard, plasma (total drug) or ultrafiltered plasma (free drug) samples were prepared by protein precipitation using 3:1 ice cold methanol. The resulting protein-free plasma or ultrafiltered plasma was further diluted 10,000-fold with 0.5 mM ammonium acetate, 0.025% formic acid in 75% methanol, and 10 μl was injected for analysis. The lower limits of quantitation of the assay were 30 μg/ml and 30 ng/ml in plasma and ultrafiltered plasma, respectively.
Pharmacogenomics
All patients were evaluated for germline polymorphisms in the coding exomes for CYP1A2, CYP2C19, and DHODH, the primary routes of hepatic metabolism (CYP1A2 and CYP2C19) and the main molecular target (DHODH) of leflunomide. Peripheral blood mononuclear cells were obtained from blood specimens collected for pharmacokinetic studies and DNA extracted using the QIAamp DNA Blood Mini Kit (Germantown, MD, USA). The extracted DNA was analyzed in the City of Hope Integrative Genomics Core using whole exome sequencing. The FASTQs were aligned to human reference genome (hg19) with BWA v0.7.5a, and duplicates were marked and removed by Picard v1.129. After local realignment of reads around known insertions and deletions (indels) and base quality score recalibration by The Genome Analysis Toolkit (GATK, v3.3–0-g37228af), variants were called using GATK HaplotypeCaller, and variant quality score was recalibrated using GATK VariantRecalibrator. Associations between genotypes and leflunomide pharmacokinetics, toxicity, or response were assessed using one-way ANOVA or Student’s t-test.
Statistical considerations
This phase 1 trial employed a modified rolling six design, a more conservative version of the rolling 6 design of Skolnik et al [21]. In this design, at most 3 patients will be under observation for DLT (in cycle 1) on the current test dose level at any time. Patients who are not evaluable for dose escalation will be replaced. Once each patient completes cycle 1 and passes without a DLT, an additional patient may be accrued on that dose level for up to 6 patients. Once 3 patients have completed cycle 1 with no patient at that dose level experiencing a DLT, the dose can be escalated. Up to 3 additional patients may be treated at the current dose level. Although this design does not require that 6 patients be treated, no more than 6 evaluable patients will be accrued to any dose level during the dose finding portion of this study. If at any time, the dose level has 1 documented DLT with fewer than 6 evaluable patients, accrual will continue until 6 patients are evaluable. Escalation will terminate as soon as two or more patients experience a DLT attributable to the study treatment. MTD will be the highest dose in which ≤1/6 patients experience a DLT.
Patients were evaluable for toxicity if they received any dose of leflunomide. Participants were considered evaluable per dose escalation/de-escalation/expansion criteria if they received at least 75% of leflunomide and were followed for 28 days during cycle 1, or experienced a DLT. All patients were assessed for response.
Maximum total and free teriflunomide plasma concentrations (Cmaxtotal and Cmaxfree) as well as first cycle area under the concentration-time curves for total and free teriflunomide (AUCtotal and AUCfree) were determined for each subject and summarized.
Results
A total of 12 patients were enrolled and treated from 12/2015 to 05/2018; patient, disease, and prior treatment characteristics are provided in Table 1. The median age at the time of enrollment was 68 (range: 48–85); median number of prior therapies is 5 (range: 4–15). Ten (10) patients had prior autologous stem cell transplant. Double refractory (lenalidomide/bortezomib) disease was noted in 8 patients. High-risk cytogenetics was observed in 5 patients, including 2 patients with del17p. All 12 subjects were evaluable for toxicity and DLT assessment.
Table 1.
Patient characteristics. Abbreviation: IMiD, immunomodulatory drug.
| Characteristic (N=12) | Median (range) or N (%) |
|---|---|
| Female/Male | 6 (50%) / 6 (50%) |
| Age at enrollment (years) | 68 (48 – 85) |
| Number of prior lines of therapy | 5 (4–15) |
| ISS staging at diagnosis | |
| Stage I | 3 (25%) |
| Stage II | 3 (25%) |
| Stage III | 3 (25%) |
| Unknown/missing | 3 (25%) |
| Prior autologous transplant | |
| Yes | 10 (83%) |
| No | 2 (17%) |
| Prior radiation | |
| Yes | 4 (33%) |
| No | 8 (67%) |
| Serum electrophoresis and immunofixation | |
| Positive | 11 (92%) |
| IgG κ | 5 (45%) |
| IgG λ | 3 (27%) |
| IgG κ | 2 (18%) |
| Serum unknown, κ | 1 (9.1%) |
| Negative** | 1 (8.3%) |
| Cytogenetic risk* | |
| Low | 4 (33%) |
| Standard | 3 (25%) |
| High | 5 (42%) |
| Del 17p | 2 (40%) |
| Gain 1q | 2 (40%) |
| t(4;14) | 1 (20%) |
| PI refractory | |
| Yes | 10 (83%) |
| No | 2 (17%) |
| IMiD refractory | |
| Yes | 10 (83%) |
| No | 2 (17%) |
| Double refractory (bortezomib/lenalidomide) | |
| Yes | 8 (67%) |
| No | 4 (33%) |
High-risk cytogenetics was defined as Del 17p, Gain 1q, t(4;14), or t(4;16).
One patient had measurable free light chains.
Dose limiting toxicities and recommended phase II dose
Three patients were treated on dose level (DL) 1 (20 mg) and no DLT was observed. One patient experienced grade 2 neutropenia and leukopenia. Following a review of toxicities after 1 cycle at dose 20 mg, authorization to escalation to the 40 mg dose was granted, and 3 patients were enrolled at this dose level. Again, no DLT was observed. Per the modified rolling six design, 3 patients were treated at the dose level of 60 mg. One patient had a DLT with grade 3 elevation of alanine aminotransferase; an additional three patients were enrolled at this dose level without further DLTs. As this dose level was the highest planned in the study, it was the recommended phase II dose (RP2D). Because we obtained preclinical data indicating a synergistic effect between leflunomide and lenalidomide [10], we determined that investigating leflunomide-based combinational therapy was favorable over pursuing a phase 2 study of single-agent leflunomide.
Adverse events
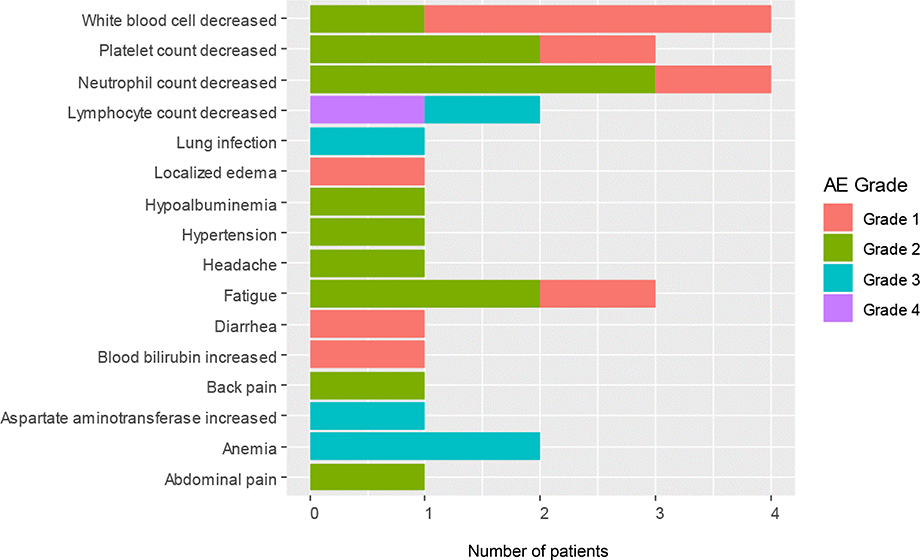
An AE of any grade that was considered at least possibly related to the treatment was reported in Table 2 across all 3 dose levels. There were no treatment-related deaths. There were 4 patients with grade 1 or 2 neutropenia at the 20 and 40 mg dose levels, respectively, and 1 patient with grade 4 lymphopenia at the 40 mg dose. Except for the DLT, most non-hematologic toxicities were ≤grade 2. One patient had grade 3 anemia at the 40 mg dose. At the 60 mg dose, 1 patient was not evaluable for response because of non-compliance and was replaced by another patient. Later, it was determined that the patient experienced grade 3 elevation of alanine aminotransferase, grade 2 neutropenia and thrombocytopenia and grade 3 anemia. The patient died from disease progression. Figure 1 and Supplementary Figure 1 provide the distribution of all grades of selected toxicities deemed to be at least possibly related to administering the drug combination. The most common toxicities were hematologic (Table 2).
Table 2.
Summary of Leflunomide-Related Adverse Events, CTCAE v4.03
| Leflunomide: 20 mg n=3 |
Leflunomide: 40 mg n=3 |
Leflunomide: 60 mg n=6 |
||||
|---|---|---|---|---|---|---|
| Adverse Event^ | Any Grade | Grade ≥ 3 | Any Grade | Grade ≥ 3 | Any Grade | Grade ≥ 3 |
| Hematologic | ||||||
| Anemia | 0 | 0 | 1 | 1 | 1 | 1 |
| Leukopenia | 2 | 0 | 1 | 0 | 1 | 0 |
| Lymphopenia | 0 | 0 | 1 | 1 | 1 | 1 |
| Neutropenia | 2 | 0 | 1 | 0 | 1 | 0 |
| Thrombocytopenia | 1 | 0 | 1 | 0 | 1 | 0 |
| Non-Hematologic | ||||||
| Abdominal pain | 0 | 0 | 0 | 0 | 1 | 0 |
| Back pain | 1 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 1 | 0 |
| Edema | 0 | 0 | 0 | 0 | 1 | 0 |
| Fatigue | 0 | 0 | 1 | 0 | 2 | 0 |
| Headache | 1 | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 1 | 0 | 0 | 0 | 0 | 0 |
| Hypoalbuminemia | 0 | 0 | 1 | 0 | 0 | 0 |
| Increased AST | 0 | 0 | 0 | 0 | 1 | 1* |
| Increased blood bilirubin | 0 | 0 | 0 | 0 | 1 | 0 |
| Lung infection | 0 | 0 | 0 | 0 | 1 | 1 |
: Includes adverse events with an attribution of at least possibly related to leflunomide, worst grade during cycle 1.
: classified as dose limiting
Abbreviations: AST: aspartate aminotransferase; CTCAE: Common Terminology Criteria for Adverse Events
Figure 1.
Distribution of toxicities by grade.
All 12 subjects have completed treatment. Nine patients were removed from study because of disease progression, two from adverse events (bacteremia at 60 mg, possibly related to study drug and angioedema at 40 mg, not related to study drug), and one from noncompliance. Of the eleven patients evaluable for response, the median number of cycles was 3 (range 1– 15). The median follow up was 17 months (range: 4 – 31). Although not all patients were treated at the 60 mg dose, 9 of11 patients achieved stable disease (SD) (Table 3). The median duration of SD among these 9 patients was 119 days (range: 56–429). In the five evaluable patients with high-risk cytogenetics, four of them achieved disease stabilization. Two subjects had SD lasting nearly one year or longer; interestingly, both of these patients were given the lowest planned dose (20 mg) of leflunomide. One of these two patients had a history of indolent, multi-responsive disease. The other patient, however, had an aggressive disease course requiring a prior salvage autologous transplantation. Supplemental Table 1 lists the number of prior treatments, number of completed cycles, best response, duration of response, and vital status for each patient evaluable for response.
Table 3.
Patient outcomes.
| Variable |
Median (range) or N (%) |
|---|---|
| Reason for discontinuation (n=12) | |
| Disease progression | 9 (75%) |
| Non-compliance | 1 (8.3%) |
| Toxicity | 2 (17%) |
| Best response (n=11) | |
| Stable disease (SD) | 9 (82%) |
| SD ≥90 days | 5 (45%) |
| Progressive disease | 2 (18%) |
| Duration of stable disease (n=9) | 119 (56–429) |
Pharmacokinetics
Plasma concentrations of total and free teriflunomide, the active metabolite of leflunomide, were available in all subjects. Because of the long half-life of teriflunomide, steady state concentrations were not achieved until approximately day 100 of treatment. Therefore, it was not possible to determine steady-state teriflunomide pharmacokinetics in all subjects. Table 4 summarizes the maximum total and free drug concentrations (Cmaxtotal and Cmaxfree) and cycle 1 total and free teriflunomide area under the concentration-time curves (AUCtotal and AUCfree) observed in each patient. At the highest dose level tested (60 mg/day), the average Cmaxtotal and Cmaxfree were 429±194 μM and 1.2±0.9 μM, respectively. Furthermore, the average AUCtotal and AUCfree in patients at the highest dose were 5814±2620 μMxday and 11.3±7.6 μMxday, respectively. Consistent with previous reports of high plasma protein binding (i.e., >99.5%), free teriflunomide plasma concentrations were 0.18±0.07% of the total across all dose levels. As illustrated in Supplementary Figure 2, the average plasma teriflunomide AUCfree and AUCtotal values demonstrated dose-proportionality across the leflunomide dose levels tested. Free drug levels displayed similar dose-proportionality (data not shown).
Table 4.
Teriflunomide plasma pharmacokinetic summary
| Patient # | Dose (mg/day) | *Cmaxtotal (µM) | Cmaxfree (µM) | ^AUCtotal (µM x day) | AUCfree (µM x day) | AUCfree/AUCtotal (%) |
|---|---|---|---|---|---|---|
| 1 | 20 | 138.3 | 0.29 | 2110.5 | 4.0 | 0.19 |
| 2 | 20 | 127.9 | 0.24 | 1839.0 | 3.3 | 0.18 |
| 3 | 20 | 212.8 | 0.27 | 2644.7 | 3.0 | 0.11 |
| 4 | 40 | 309.0 | 0.68 | 3325.7 | 5.8 | 0.17 |
| 5 | 40 | 334.0 | 0.73 | 3085.7 | 5.2 | 0.17 |
| 6 | 40 | 533.9 | 1.79 | 4501.2 | 10.6 | 0.23 |
| 7 | 60 | 523.0 | 0.83 | 8689.3 | 13.7 | 0.16 |
| 8 | 60 | 475.7 | 1.79 | 6660.2 | 25.1 | 0.38 |
| 9 | 60 | 695.2 | 2.75 | 3232.2 | 5.7 | 0.17 |
| 10 | 60 | 190.7 | 0.39 | 8728.9 | 11.3 | 0.13 |
| 11 | 60 | 673.4 | 0.95 | 4775.8 | 7.0 | 0.15 |
| 12 | 60 | 339.0 | 0.57 | 2798.3 | 4.9 | 0.17 |
Cmax = maximum plasma concentrations
Area under the concentration-time curve from Cycle 1, Day 1 to Cycle 2, Day 1
Pharmacogenomics
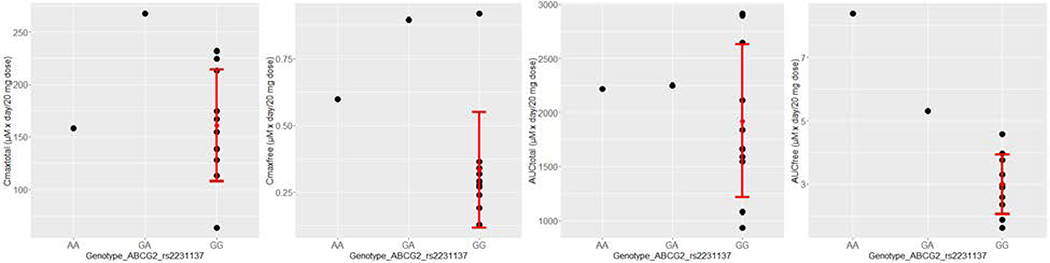
We performed whole exome sequencing using DNA samples from the peripheral blood mononuclear cells of these 12 patients. The coding exons of each sample were sequenced to a minimum depth of 56×, and on average 151,733 variants were identified in these patients. About 7.8% of these variants (11,862 on average) are located in exon region and are non-silencing (missense, nonsense, indels and splicing). Since our main goal of DNA sequencing is to correlate SNPs with pharmacokinetics, we focused on seven SNPs in a few key genes, which have been reported to affect the efficacy and toxicity of leflunomide [22–24], including DHODH, a target of teriflunomide, and CYP1A2, CYP2C19, and ABCG2, which play important roles in drug metabolism and transport. Among the 7 SNPs, rs2231137 (c.34G>A) on the ABCG2 gene has a significant association with the AUCfree (Bonferroni adjusted P value = 0.022, Figure 2). For this SNP, one patient has homozygous variant allele (AA), another patient has heterozygous allele (GA), and the rest of the patients are all with homozygous reference allele (GG).
Figure 2.
Association of rs2231137 on ABCG2 gene with pharmacokinetics data. Each of the four pharmacokinetics metrics (Cmaxtotal, Cmaxfree, AUCtotal, and AUCfree) in plasma following teriflunomide treatment was plotted against the genotype of a mutation (rs2231137) in ABCG2. Each dot represents one patient, with the red error bar representing mean plus and minus the standard deviation. One-way ANOVA test was applied for each PK metric (nominal P = 0.00092 for AUCfree; P = 0.2138 for Cmaxtotal, P = 0.0746 for Cmaxfree, and P = 0.8502 for AUCtotal).
Discussion
Despite the encouraging advances made for the treatment of MM, the prognosis for patients with relapsed/refractory disease remains poor, and new treatments are therefore needed. We have conducted the first phase 1 clinical trial using leflunomide in patients with MM we have found that the agent is not only well tolerated up to doses of 60 mg daily with minimal toxicity but also showed disease stabilization using the same FDA-approved doses for rheumatoid arthritis.
In fact, although the anti-cancer activity of leflunomide as single agent is modest, even FDA-approved drugs or those pending approval, including lenalidomide, daratumumab and elotuzumab, have substantially improved effectiveness when used in combinatorial regimens. In a dose-escalation phase 1 trial of single agent elotuzumab, which targets the SLAMF7 receptor, only 9 of 35 patients with RRMM achieved SD [25]. However, outcomes significantly improved in the ELOQUENT-2 trial, in which elotuzumab plus lenalidomide and dexamethasone were tested versus only lenalidomide and dexamethasone. With a median follow-up of 24.5 months, the overall response rate was 79% versus 66% (p<.001), and the median progression-free survival was 19.4 versus 14.9 months in favor of elotuzumab [26]. Additionally, lenalidomide as monotherapy yielded an overall response rate (ORR) of only 25% in patients with relapsed/refractory disease. When dexamethasone was given to non-responders in the same trial, an additional 29% responded [27]. Further combination with bortezomib led to an ORR of 64% [28].
We have recently published results showing a synergistic effect with leflunomide and lenalidomide preclinically [10], which suggest that the addition of a non-toxic drug to backbone treatment may increase the deepness of response of newly diagnosed patients and may be beneficial in combination with pomalidomide in lenalidomide resistant patients.
Pharmacokinetic results reported here are consistent with previous reports and confirmed the long plasma elimination half-life and high degree of protein binding of the active metabolite teriflunomide [29,30]. Average steady-state plasma teriflunomide concentrations in patients with rheumatoid arthritis taking leflunomide 20 mg/d was 130 μM, and is very similar to the results reported here for patients with MM taking the same dose [30]. An SNP in the ABC G2 gene may influence the pharmacokinetics of teriflunomide in patients with MM. We note the current cohort is small and a larger cohort is needed to confirm this finding. The association with free teriflunomide is consistent with the reported effect of this SNP on teriflunomide absorption and clearance in a group of healthy volunteers in China [31]. Because of an insufficient number of participant samples, we were unable to assess the effects of rs2231142.
Leflunomide may benefit patients other than those with RRMM. Fifty percent of patients with high-risk smoldering myeloma will progress to symptomatic disease within approximately 2 years [32–35]. High-risk patients may benefit from early intervention before symptomatic disease occurs, ideally with limited toxicity. Clinical trials showed an improvement in disease progression as well as overall survival with treatment with lenalidomide [33,36]. Additional studies are ongoing and exploring daratumumab [37], ixazomib/lenalidomide/dexamethasone [38], and elotuzumab/lenalidomide/dexamethasone [39]. The above interventions, however, have potential toxicities that may be disfavored by a population that would otherwise remain untreated. Leflunomide offers an oral, non-toxic, and inexpensive alternative.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health under P30 CA033572 (Biostatistics and Mathematical Modeling, Analytical Pharmacology, Integrated Genomics and Bioinformatics, and Pathology Cores).
This work has been presented in part at the 2016 and 2017 meetings of the American Society of Hematology in San Diego and Atlanta, respectively.
Footnotes
Disclosure of interest
MR is on the speakers’ bureau for Celgene and Takeda. JP is a consultant for Gilead. AK is a consultant for Celgene, serves on the speakers’ bureau for Celgene, Amgen, and Takeda, and has stock ownership in Celgene.
References
- 1.Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia 2009;23:1152–1157. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111:2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 4.Pulte D, Redaniel MT, Brenner H, Jansen L, Jeffreys M. Recent improvement in survival of patients with multiple myeloma: variation by ethnicity. Leuk Lymphoma 2014;55:1083–1089. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KC, Kyle RA, Rajkumar SV, et al. Clinically relevant end points and new drug approvals for myeloma. Leukemia 2008;22:231–239. [DOI] [PubMed] [Google Scholar]
- 6.Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia 2017;31:2443–2448. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breedveld FC, Dayer JM. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Annals of the Rheumatic Diseases 2000;59:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattar T, Kochhar K, Bartlett R, Bremer EG, Finnegan A. Inhibition of the epidermal growth factor receptor tyrosine kinase activity by leflunomide. FEBS Lett 1993;334:161–164. [DOI] [PubMed] [Google Scholar]
- 10.Buettner R, Morales C, Caserta E, et al. Leflunomide regulates c-Myc expression in myeloma cells through PIM targeting. Blood Adv 2019;3:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Shen J, Mall JW, et al. In vitro and in vivo antitumor activity of a novel immunomodulatory drug, leflunomide: mechanisms of action. Biochem Pharmacol 1999;58:1405–1413. [DOI] [PubMed] [Google Scholar]
- 12.Ringshausen I, Oelsner M, Bogner C, Peschel C, Decker T. The immunomodulatory drug Leflunomide inhibits cell cycle progression of B-CLL cells. Leukemia 2008;22:635–638. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich S, Kramer OH, Hahn E, et al. Leflunomide induces apoptosis in fludarabine-resistant and clinically refractory CLL cells. Clin Cancer Res 2012;18:417–431. [DOI] [PubMed] [Google Scholar]
- 14.White RM, Cech J, Ratanasirintrawoot S, et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 2011;471:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hail N Jr., Chen P, Bushman LR. Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention. Neoplasia 2010;12:464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumann P, Mandl-Weber S, Volkl A, et al. Dihydroorotate dehydrogenase inhibitor A771726 (leflunomide) induces apoptosis and diminishes proliferation of multiple myeloma cells. Mol Cancer Ther 2009;8:366–375. [DOI] [PubMed] [Google Scholar]
- 17.Metzler C, Fink C, Lamprecht P, Gross WL, Reinhold-Keller E. Maintenance of remission with leflunomide in Wegener’s granulomatosis. Rheumatology (Oxford) 2004;43:315–320. [DOI] [PubMed] [Google Scholar]
- 18.Metzler C, Miehle N, Manger K, et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford) 2007;46:1087–1091. [DOI] [PubMed] [Google Scholar]
- 19.Josephson MA, Gillen D, Javaid B, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation 2006;81:704–710. [DOI] [PubMed] [Google Scholar]
- 20.Parekh JM, Vaghela RN, Sutariya DK, Sanyal M, Yadav M, Shrivastav PS. Chromatographic separation and sensitive determination of teriflunomide, an active metabolite of leflunomide in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:2217–2225. [DOI] [PubMed] [Google Scholar]
- 21.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. Journal of Clinical Oncology 2008;26:190–195. [DOI] [PubMed] [Google Scholar]
- 22.Pawlik A, Herczynska M, Kurzawski M, Safranow K, Dziedziejko V, Drozdzik M. The effect of exon (19C>A) dihydroorotate dehydrogenase gene polymorphism on rheumatoid arthritis treatment with leflunomide. Pharmacogenomics 2009;10:303–309. [DOI] [PubMed] [Google Scholar]
- 23.O’Doherty C, Schnabl M, Spargo L, et al. Association of DHODH haplotype variants and response to leflunomide treatment in rheumatoid arthritis. Pharmacogenomics 2012;13:1427–1434. [DOI] [PubMed] [Google Scholar]
- 24.Bohanec Grabar P, Rozman B, Tomsic M, Suput D, Logar D, Dolzan V. Genetic polymorphism of CYP1A2 and the toxicity of leflunomide treatment in rheumatoid arthritis patients. Eur J Clin Pharmacol 2008;64:871–876. [DOI] [PubMed] [Google Scholar]
- 25.Zonder JA, Mohrbacher AF, Singhal S, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012;120:552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. New England Journal of Medicine 2015;373:621–631. [DOI] [PubMed] [Google Scholar]
- 27.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood 2006;108:3458–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson PG, Xie WL, Jagannath S, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood 2014;123:1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan V, Charles BG, Tett SE. Population pharmacokinetics and association between A77 1726 plasma concentrations and disease activity measures following administration of leflunomide to people with rheumatoid arthritis. Br J Clin Pharmacol 2005;60:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozman B Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet 2002;41:421–430. [DOI] [PubMed] [Google Scholar]
- 31.Yao X, Wu Y, Jiang J, Chen X, Liu D, Hu P. A population pharmacokinetic study to accelerate early phase clinical development for a novel drug, teriflunomide sodium, to treat systemic lupus erythematosus. Eur J Pharm Sci 2019;136:104942. [DOI] [PubMed] [Google Scholar]
- 32.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 2008;111:785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mateos MV, Hernandez MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med 2013;369:438–447. [DOI] [PubMed] [Google Scholar]
- 34.Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood 2015;125:3069–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakshman A, Rajkumar SV, Buadi FK, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J 2018;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lonial S, Jacobus S, Fonseca R, et al. Randomized Trial of Lenalidomide Versus Observation in Smoldering Multiple Myeloma. J Clin Oncol 2019:JCO1901740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landgren O, Cavo M, Chari A, et al. Updated Results from the Phase 2 Centaurus Study of Daratumumab (DARA) Monotherapy in Patients with Intermediate-Risk or High-Risk Smoldering Multiple Myeloma (SMM). Blood 2018;132.29866817 [Google Scholar]
- 38.Bustoros M, Liu CJ, Reyes K, et al. Phase II Trial of the Combination of Ixazomib, Lenalidomide, and Dexamethasone in High-Risk Smoldering Multiple Myeloma. Blood 2018;132.29866817 [Google Scholar]
- 39.Liu CJ, Ghobrial IM, Bustoros M, et al. Phase II Trial of Combination of Elotuzumab, Lenalidomide, and Dexamethasone in High-Risk Smoldering Multiple Myeloma. Blood 2018;132.29866817 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.