Abstract
We compared the exercise ventilatory response (slope of the ventilation, V̇E and carbon dioxide production, V̇CO2 relationship) in boys and girls with and without obesity. 46 children with obesity (BMI percentile: 97.7±1.4) and 27 children without obesity (BMI percentile: 55.1±22.2) were included and divided into groups by sex (with obesity: 17 girls and 29 boys; without obesity: 13 girls and 14 boys). A 6min constant load cycling test at 45% of peak work rate was performed. The V̇E/V̇CO2 slope was similar (p=0.67) between children with (32.7±4.3) and without (32.2±6.1) obesity; however, it was higher (p=0.02) in girls (35.4±5.6) than boys (32.6±4.9). We also examined a corrected V̇E/V̇CO2 slope for the effects of mechanical dead space (VDM), by subtracting V̇DM from V̇E (V̇Ecorr/V̇CO2 slope). The V̇Ecorr/V̇CO2 slope remained similar (p=0.37) between children with (26.8±3.2) and without obesity (26.1±3.1); however, no sex differences were observed (p=0.13). Therefore, VDM should be accounted for before evaluating the V̇E/V̇CO2 slope, particularly when making between-sex comparisons.
Keywords: Exercise ventilatory response, Obesity, Children, Dead space
1. Introduction
Very little is known about the ventilatory response to exercise (defined as the slope of the relation between the change in pulmonary ventilation, V̇E and change in carbon dioxide production, V̇CO2) in children with obesity. While it is well-known that increases in V̇E are closely matched with increases in V̇CO2 during exercise up to the respiratory compensation point (Sun et al., 2002; Whipp et al., 1984), physiological factors such as altered breathing mechanics (Babb, 1999, 2013; Zavorsky et al., 2007) and/or sex (Bongers et al., 2014; Cooper et al., 2016; Kilbride et al., 2003; Marinov et al., 2000; Neder et al., 2001) could independently influence the V̇E/V̇CO2 slope. With obesity, it is well-known that the extra mass on the chest wall markedly decreases operational lung volumes and increases the propensity for expiratory flow limitation, increases respiratory mechanical impedance (thus, imposing a restriction to ventilation), and alters breathing pattern during exercise (as evidenced by a smaller rise in tidal volume, VT and greater breathing frequency, Fb). The same mechanical ventilatory constraints could lower both ventilatory capacity (Babb, 2013; Babb et al., 2011; DeLorey et al., 2005; Dempsey et al., 1966; Zavorsky et al., 2007) and the ventilatory response to exercise (Babb, 1999, 2013; Zavorsky et al., 2007). Whether these respiratory effects of obesity combine to lower the V̇E/V̇CO2 slope in children with obesity, as compared with children without obesity, remains unknown. Although there is evidence that the V̇E/V̇CO2 slope is remarkably preserved in adults with obesity (Babb et al., 1991; Ofir et al., 2007; Whipp and Davis, 1984), it would be inappropriate to assume that the respiratory effects of obesity in adults would be similar in children. This is because pulmonary physiology is different in children when compared with adults (Lanteri and Sly, 1993; Lazarus et al., 1997) and that children already breathe at very low lung volumes (Bryan and Wohl, 1964). Thus, we would propose that the propensity to approach mechanical ventilatory constraints during exercise may be further exacerbated in those children with obesity, and subsequently result in a lower V̇E/V̇CO2 slope compared with children without obesity.
In addition, the respiratory effects of obesity could be sex-specific. Not only do women have smaller lungs and airways, reduced respiratory muscle mass, and exercise with an altered breathing pattern when compared with men (Dominelli et al., 2019; Kilbride et al., 2003; McClaran et al., 1998; Neder et al., 2003; Sheel and Guenette, 2008), both women and girls also exhibit a greater V̇E/V̇CO2 slope compared with age-matched men and boys (Bongers et al., 2014; Cooper et al., 2016; Kilbride et al., 2003; Marinov et al., 2000; Neder et al., 2001). Thus, it stands to reason that potential effects of obesity on the V̇E/V̇CO2 slope may be confounded by the coexistent physiological influences of sex in children. Although there are currently limited studies examining the V̇E/V̇CO2 slope in children with obesity (Cooper et al., 2016; Kaufman et al., 2007; Marinov et al., 2002; McMurray and Ondrak, 2011; Prado et al., 2009), these studies report conflicting results, and the effects of sex were not taken into account. Considering the evidence that sex alters the V̇E/V̇CO2 slope (Bongers et al., 2014; Kilbride et al., 2003; Marinov et al., 2000), independent of any other physiological factor that is thought to be isolated and under investigation (e.g., respiratory effects of obesity), this highlights the need to clarify the potential influence of sex on the V̇E/V̇CO2 slope in children with obesity. Such information will allow for a better understanding of the factors that may impact exercise tolerance in children (e.g., gas exchange efficiency, dyspnea on exertion, and the oxygen cost of breathing), which is an important consideration given that childhood obesity is a vital national health issue and that regular exercise is an integral modality in the prevention and treatment of obesity (Daniels et al., 2009).
Therefore, the purpose of the present study was to test the hypothesis that the V̇E/V̇CO2 slope would be lower in children with obesity compared with children without obesity, but the V̇E/V̇CO2 slope would be greater in both girls with and without obesity compared with boys with and without obesity. We tested children within a narrow age rage (8–12 y) to negate any confounding influence of potential advancing age-related changes in the V̇E/V̇CO2 slope (Bongers et al., 2014; Nagano et al., 1998), and boys and girls of this age have very similar predicted pulmonary function values (Polgar and Weng, 1979).
2. Methods
The data presented in this article were collected as part of a larger study describing pulmonary function and breathing mechanics during exercise in obese and nonobese children. However, the current article addresses a different question and we repeat only the methods and data essential to the novel findings presented here.
2.1. Participants:
46 children with obesity (29 boys; body mass index [BMI] > 95th percentile for age and sex) and 27 children without obesity (14 boys; BMI within the 16th and 84th percentile for age and sex) children (8–12 y) participated in this study. Children were eligible to participate if they did not have any previous diagnosis of asthma or a history of smoking; were free from cardiopulmonary, neurological, sleep, and/or metabolic diseases; and scored <4 on the Tanner pubertal stage assessment of pubertal status (Tanner, 1962). Prior to all testing, the study purpose and experimental protocols were disclosed in detail. The parent or guardian provided written and informed consent, and all children provided written assent. The experimental procedures were reviewed and approved by the UT Southwestern Institutional Review Board (Reference no: STU 052012–076).
2.2. Study design:
Participants visited the laboratory on 2 separate occasions. On the first visit, participants were required to refrain from consuming a large meal 2 h, and no caffeine 24 h, prior to reporting to the laboratory. Participants underwent pre-participation health screening. Height and weight were recorded using calibrated scales. BMI percentile for age and sex was calculated for each child using specific BMI tables from the Centers of Disease and Control and Prevention (Kuczmarski et al., 2000). Participants also completed a self-report Tanner pubertal stage assessment for assessment of pubertal status (Tanner, 1962) and were excluded from the study if they reported stage 4 or 5. On a separate day, participants performed a submaximal constant load cycling test.
2.3. Submaximal constant load cycling test:
The submaximal constant load cycling test was performed to evaluate the exercise ventilatory response from rest to exercise as we have previously described (Wood et al., 2008a, 2010, 2011). Briefly, participants cycled at a constant load that was equivalent to 45% of peak work rate for 6min on an upright cycle ergometer (Lode Corival, Lode BV, Groningen, the Netherlands), and were asked to maintain a constant cadence of 60 – 80 revolutions per minute. Heart rate was measured via a pulse oximeter placed on the forehead (OxiMax N-595, Nellcor) and pulmonary gas exchange variables including minute ventilation (V̇E), oxygen uptake (V̇O2), carbon dioxide production (V̇CO2), and respiratory exchange ratio (RER) were measured using the Douglas bag technique. Expired volume was measured using a 200 L Tissot spirometer and gas fractions were measured using a mass spectrometer (Perkin-Elmer 1100). End-tidal CO2 (PETCO2) was sampled via a lateral port on the mouthpiece. Flow was measured continuously using an inspiratory pneumotachograph (Hans Rudolph, Model 4813) and a heated expiratory pneumotachograph (Hans Rudolph, model 3850A) connected to a Hans Rudolph valve (Model 2700) via large-bore tubing. Flow signals were combined into a single bidirectional flow signal (Validyne Buffer Amplifier, model BA112) and digitally integrated to yield volume, breathing frequency (Fb) and tidal volume (VT). The ventilatory response to exercise was defined as the slope of the relation between the rest-to-exercise change in V̇E and the rest-to-exercise change in V̇CO2. This calculation has previously been described in more detail (Wood et al., 2008a). We also accounted for the effects of VDM on the V̇E/V̇CO2 slope by subtracting V̇DM (which we determined to equal 0.225 L) from V̇E using the following equation (Cooper et al., 1987; Sun et al., 2002):
V̇Ecorr was substituted for V̇E in the original calculation of the V̇E/V̇CO2 slope to derive a second slope that was independent of the effects of VDM; the V̇Ecorr/V̇CO2 slope. Considering that VDM comprised a large portion of both resting and exercise tidal volume, we subsequently conducted a separate analysis to determine whether VDM would artificially augment the V̇E/V̇CO2 slope and subsequently mask any potential independent effect that obesity or sex may have on the V̇E/V̇CO2 slope in children. The ventilatory equivalents for oxygen (V̇E/V̇O2 ratio) and carbon dioxide (V̇E/V̇CO2 ratio), and the corrected ventilatory equivalents for oxygen (V̇Ecorr/V̇O2 ratio) and carbon dioxide (V̇Ecorr/V̇CO2 ratio) were also calculated.
2.4. Statistical analysis:
Statistical analysis was performed using SAS statistical package v.9.3 (SAS Institute Inc Cary, NY, USA). A two-way analysis of variance was performed to examine whether participant characteristics and pulmonary gas exchange measurements differed between groups (with and without obesity) and sex (boys and girls). Statistical significance was accepted at p < 0.05 and all data are presented as means ± SD.
3. Results
3.1. Participant characteristics:
Participant characteristics are displayed in Table 1. No differences in age were observed (p > 0.05) and, as expected, body weight, BMI and BMI percentile were greater in the children with obesity compared with the children without obesity (p < 0.05). All children had normal pulmonary function (data not shown).
Table 1.
Participant characteristics
| Children without obesity | Children with obesity | |||||
|---|---|---|---|---|---|---|
| Girls (n=13) | Boys (n=14) | All (n=27) | Girls (n=17) | Boys (n=29) | All (n=46) | |
| Age (y) | 10.3 ± 0.9 | 10.4 ± 0.8 | 10.4 ± 0.9 | 9.7 ± 1.4 | 10.2 ± 1.0 | 10.1 ± 1.1 |
| Height (cm) | 146.9 ± 9.2 | 145.8 ± 7.5 | 146.4 ± 8.3 | 146.3 ± 11.4 | 148.0 ± 6.0 | 147.4 ± 8.1 |
| Weight (kg) | 38.7 ± 7.9 | 38.2 ± 5.5 | 38.4 ± 6.7 | 60.3 ± 19.2# | 62.3 ± 14.3# | 61.6 ± 16.0# |
| Body Mass Index (kg/m2) | 17.8 ± 2.0 | 17.8 ± 1.2 | 17.8 ± 1.6 | 27.5 ± 4.7# | 28.1 ± 4.8# | 27.9 ± 4.7# |
| Body Mass Index percentile | 52.9 ± 24.6 | 57.3 ± 20.0 | 55.1 ± 22.2 | 97.7 ± 1.2# | 97.6 ± 1.4# | 97.7 ± 1.4# |
Data are mean ± SD.
Significantly different compared with children without obesity, p < 0.05. No between-sex differences were observed.
3.2. Cardiorespiratory responses at rest and during submaximal constant load exercise:
All children exercised at the same work load and RPE (Table 3, all p > 0.05). Although HR (absolute, and as a % of predicted and maximum) at rest and during exercise was similar in the children with obesity compared with the children without obesity (Table 2 and 3, both p > 0.05), resting and exercise HR were higher in girls compared with boys (Tables 2 and 3, both p < 0.05). Resting and exercise measures of pulmonary gas exchange including V̇E, V̇Ecorr, V̇O2, and V̇CO2 were higher in the children with obesity compared with the children without obesity (Tables 2 and 3, all p < 0.05), with girls having only a lower V̇O2 during exercise compared with boys (Table 2, p < 0.05). With the exception of the V̇E/V̇CO2 ratio at rest (Table 2, p < 0.05), there were no differences in the V̇E/V̇O2, V̇E/V̇CO2, V̇Ecorr/V̇O2, or V̇Ecorr/V̇CO2 ratio in the children with obesity compared with the children without obesity (Tables 2 and 3, all p > 0.05); however, the V̇E/V̇O2, V̇Ecorr/V̇O2, and V̇Ecorr/V̇CO2 ratio were higher in girls compared with boys only during exercise (Tables 2 and 3, all p < 0.05). Although Fb was slightly higher at rest (Table 2, p < 0.05), VT was the same (Table 2, p > 0.05) and both Fb and VT were similar during exercise in the children with obesity compared with the children without obesity (Tables 2 and 3, both p > 0.05); no sex differences were observed (Tables 2 and 3, p > 0.05). PETCO2 did not differ between groups or sex at rest and during exercise (Tables 2 and 3, p > 0.05).
Table 3.
Cardiorespiratory measures during submaximal constant load exercise
| Children without obesity | Children with obesity | |||||
|---|---|---|---|---|---|---|
| Girls (n=13) | Boys (n=14) | All (n=27) | Girls (n=17) | Boys (n=29) | All (n=46) | |
| Work load (W) | 40 ± 7 | 48 ± 12 | 44 ± 10 | 41 ± 8 | 42 ± 6 | 42 ± 7 |
| HR (bpm) | 148 ± 16* | 132 ± 13 | 140 ± 17 | 150± 14* | 141 ± 11 | 144 ± 13 |
| HR (% Predicted) | 69 ± 8* | 62 ± 6 | 65 ± 8 | 69 ± 7* | 66 ± 5 | 67 ± 6 |
| HR (% Maximum) | 78 ± 8* | 70 ± 6 | 73 ± 8 | 78 ± 6* | 75 ± 4 | 76 ± 5 |
| V̇E (L/min) | 29.99 ± 3.27 | 30.61 ± 5.85 | 30.31 ± 4.71 | 33.61 ± 4.96# | 34.03 ± 5.23# | 33.87 ± 5.08# |
| V̇Ecorr (L/min) | 21.79 ± 3.10 | 22.88 ± 5.71 | 22.35 ± 4.59 | 24.40 ± 4.61# | 25.54 ± 4.69# | 25.11 ± 4.65# |
| VT (L) | 0.86 ± 0.20 | 0.94 ± 0.29 | 0.90 ± 0.25 | 0.85 ± 0.18 | 0.93 ± 0.26 | 0.90 ± 0.23 |
| Fb (bpm) | 36.6 ± 9.3 | 34.1 ± 8.3 | 35.3 ± 8.7 | 40.6 ± 9.3 | 38.3 ± 8.7 | 39.1 ± 8.9 |
| V̇O2 (L/min) | 0.80 ± 0.10* | 0.93 ± 0.19 | 0.86 ± 0.16 | 0.92 ± 0.18*# | 1.00 ± 0.17# | 0.97 ± 0.15# |
| V̇CO2 (L/min) | 0.78 ± 0.11 | 0.86 ± 0.22 | 0.82 ± 0.17 | 0.86 ± 0.17# | 0.94 ± 0.13# | 0.91 ± 0.15# |
| V̇E/V̇O2 ratio | 37.9 ± 5.7* | 33.1 ± 4.4 | 35.4 ± 5.5 | 36.6 ± 4.6* | 34.0 ± 3.8 | 35.0 ± 4.2 |
| V̇Ecorr/V̇O2 ratio | 27.3 ± 3.2* | 24.5 ± 2.9 | 25.8 ± 3.3 | 26.3 ± 2.4* | 25.4 ± 2.6 | 25.7 ± 2.5 |
| V̇E/V̇CO2 ratio | 38.5 ± 5.5* | 36.2 ± 5.0 | 3 7.3 ± 5.3 | 39.2 ± 5.2* | 36.3 ± 4.0 | 37.4 ± 4.7 |
| V̇Ecorr/V̇CO2 ratio | 27.7 ± 2.4 | 26.7 ± 2.7 | 27.2 ± 2.6 | 28.1 ± 2.1 | 27.1 ± 3.0 | 27.5 ± 2.7 |
| PETCO2 (Torr) | 40 ± 4 | 41 ± 4 | 40 ± 4 | 39 ± 4 | 40 ± 4 | 40 ± 4 |
| RPE (Borg 6–20 scale) | 11 ± 2 | 11 ± 3 | 11 ± 2 | 11 ± 3 | 12 ± 3 | 11 ± 3 |
Data are mean ± SD. HR: heart rate; V̇E: minute ventilation; V̇Ecorr: minute ventilation corrected for the volume of dead space imposed by the breathing apparatus; VT: tidal volume; Fb: breathing frequency; V̇O2: oxygen uptake; V̇CO2: carbon dioxide production; PETCO2: partial pressure of endtidal CO2; RPE: rating of perceived exertion.
Significantly different compared with children without obesity, p < 0.05;
Significantly different between sexes, p < 0.05.
Table 2.
Cardiorespiratory measures at rest
| Children without obesity | Children with obesity | |||||
|---|---|---|---|---|---|---|
| Girls (n=13) | Boys (n=14) | All (n=27) | Girls (n=17) | Boys (n=29) | All (n=46) | |
| HR (bpm) | 96 ± 9* | 79 ± 18 | 87 ± 16 | 95 ± 11* | 91 ± 10 | 93 ± 10 |
| HR (% Predicted Max) | 44 ± 4* | 37 ± 8 | 40 ± 8 | 44 ± 5* | 42 ± 5 | 43 ± 4.8 |
| HR (% Maximum) | 50 ± 6* | 42 ± 10 | 46 ± 9 | 49 ± 5* | 48 ± 5 | 49 ± 5 |
| V̇E (L/min) | 9.71 ± 1.12 | 9.79 ± 2.17 | 9.49 ± 1.74 | 10.68 ± 1.95# | 11.27 ± 1.77# | 11.05 ± 1.84# |
| V̇Ecorr (L/min) | 5.42 ± 1.01 | 6.20 ± 1.88 | 5.82 ± 1.55 | 6.69 ± 1.98# | 7.05 ± 1.41# | 6.92 ± 1.63# |
| VT (L) | 0.55 ± 0.09 | 0.62 ± 0.13 | 0.59 ± 0.11 | 0.62 ± 0.18 | 0.61 ± 0.13 | 0.62 ± 0.15 |
| Fb (bpm) | 17.0 ± 2.7 | 15.7 ± 2.8 | 16.3 ± 2.8 | 17.7 ± 4.4# | 18.7 ± 3.6# | 18.3 ± 3.9# |
| V̇O2 (L/min) | 0.21 ± 0.02 | 0.23 ± 0.04 | 0.23 ± 0.04 | 0.26 ± 0.05# | 0.28 ± 0.05# | 0.27 ± 0.06# |
| V̇CO2 (L/min) | 0.17 ± 0.02 | 0.20 ± 0.04 | 0.19 ± 0.04 | 0.2 ± 0.05# | 0.24 ± 0.06# | 0.23 ± 0.05# |
| V̇E/V̇O2 ratio | 44.1 ± 6.5 | 41.0 ± 5.9 | 42.5 ± 6.3 | 41.6 ± 6.4 | 40.9 ± 6.5 | 41.2 ± 6.5 |
| V̇Ecorr/V̇O2 ratio | 25.9 ± 6.5 | 25.7 ± 5.2 | 25.8 ± 4.8 | 25.6 ± 4.6 | 25.5 ± 4.6 | 25.5 ± 4.5 |
| V̇E/V̇CO2 ratio | 52.0 ± 3.1 | 49.3 ± 5.0 | 50.6 ± 4.4 | 49.0 ± 7.1# | 46.7 ± 5.5# | 47.6 ± 6.2# |
| V̇Ecorr/V̇CO2 ratio | 30.5 ± 2.5 | 30.8 ± 5.0 | 30.7 ± 3.9 | 29.9 ± 2.9 | 29.0 ± 3.2 | 29.3 ± 3.1 |
| PETCO2 (Torr) | 39.1 ± 3.5 | 38.2 ± 4.3 | 38.6 ± 3.9 | 39.1 ± 3.5 | 40.0 ± 3.5 | 39.7 ± 3.5 |
Data are mean ± SD. HR: heart rate; V̇E: minute ventilation; V̇Ecorr: minute ventilation corrected for the volume of dead space imposed by the breathing apparatus; VT: tidal volume; Fb: breathing frequency; V̇O2: oxygen uptake; V̇CO2: carbon dioxide production; PETCO2: partial pressure of endtidal CO2.
Significantly different compared with children without obesity, p < 0.05;
Significantly different between sexes, p < 0.05.
3.3. Ventilatory response to submaximal constant load exercise:
When V̇E was not corrected for V̇DM, the V̇E/V̇CO2 slope was similar between the children with and without obesity (Figure 1, top left panel, p = 0.67); although, the V̇E/V̇CO2 slope was higher in girls compared with boys (Figure 1, bottom left panel, p = 0.02). These differences were also present when girls with and without obesity were compared with boys with and without obesity (Figure 2, left panel, p < 0.05). When V̇E was corrected for V̇DM, the V̇Ecorr/V̇CO2 slope remained similar between the children with and without obesity (Figure 1, top right panel, p = 0.37); however, the aforementioned sex differences were eliminated (Figures 1 and 2, both bottom right panels, all p > 0.05).
Figure 1.
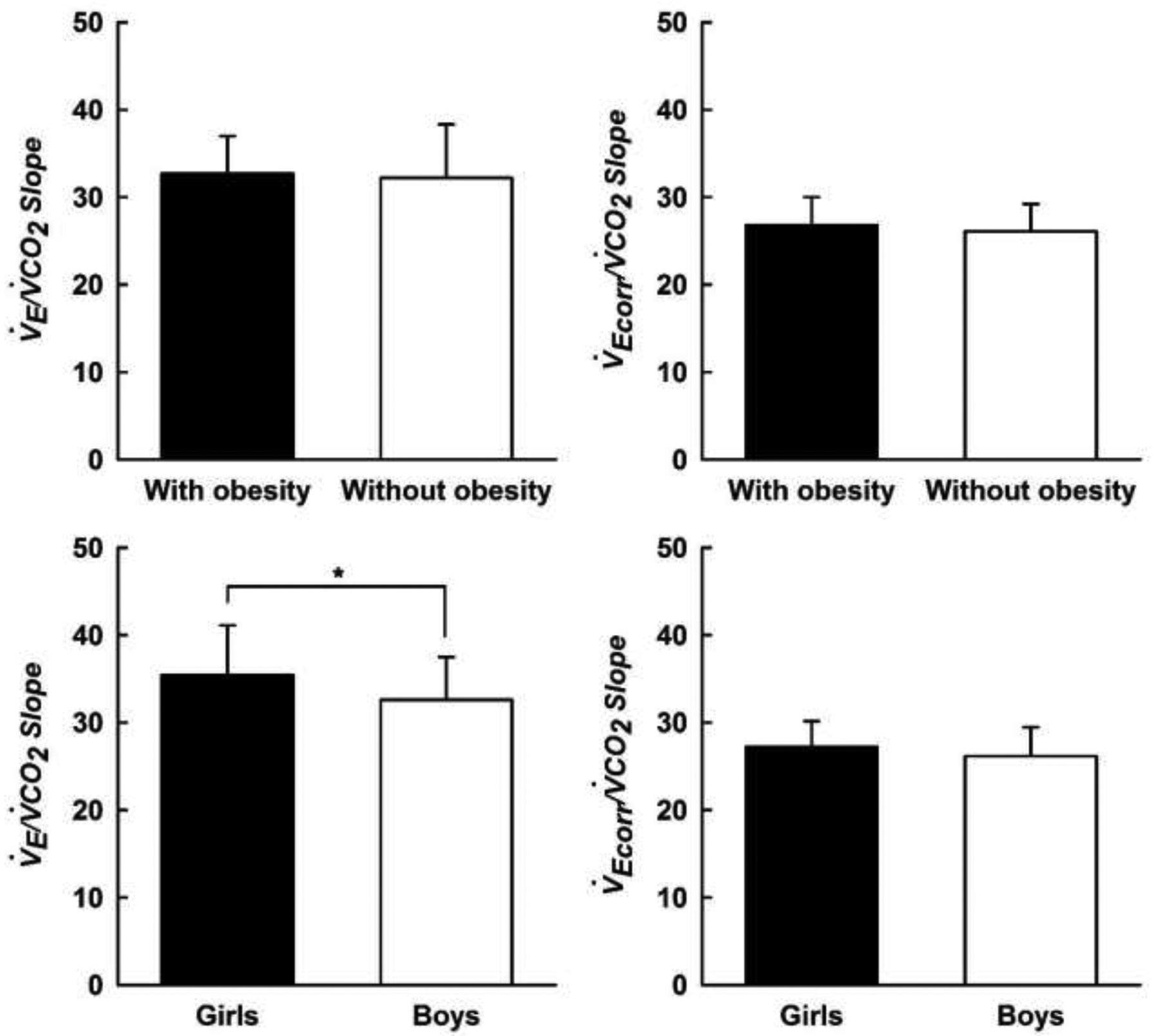
V̇E/V̇CO2 slope (left panel) and V̇Ecorr/V̇CO2 slope (right panel) measured during a submaximal constant load cycling test in children with and without obesity. V̇E/V̇CO2 slope: ventilatory response to exercise; V̇Ecorr/V̇CO2 slope: ventilatory response to exercise corrected for external dead space imposed by the breathing apparatus. Data are means ± SD. *Significantly different between sex, p < 0.05.
Figure 2.
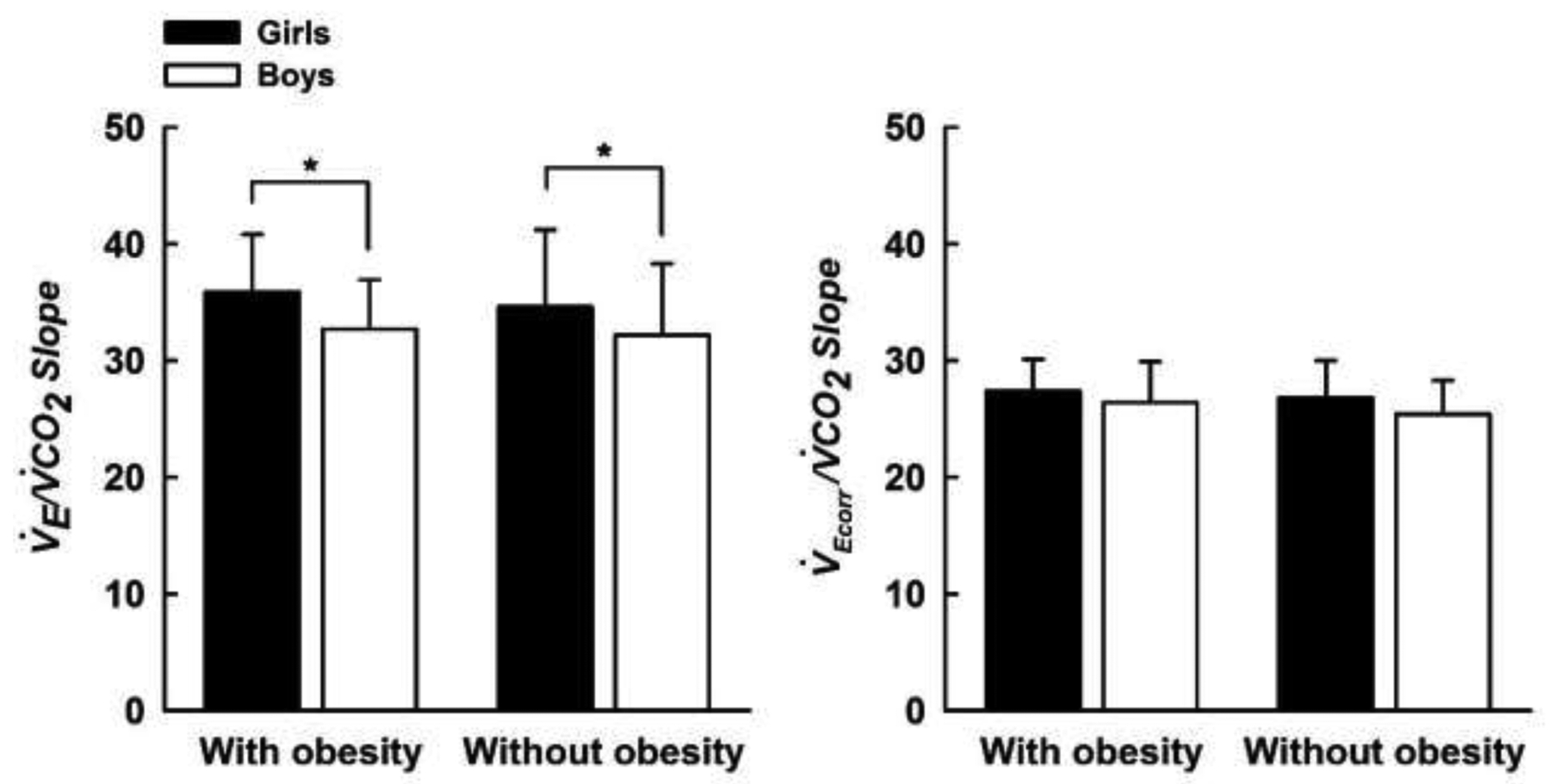
V̇E/V̇CO2 slope (left panel) and V̇Ecorr/V̇CO2 slope (right panel) measured during a submaximal constant load cycling test in boys and girls with and without obesity. V̇E/V̇CO2 slope: ventilatory response to exercise; V̇Ecorr/V̇CO2 slope: ventilatory response to exercise corrected for external dead space imposed by the breathing apparatus. Data are means ± SD. *Significantly different between sex, p < 0.05.
4. Discussion
This study is the first to examine the ventilatory response to moderate-intensity constant load exercise in boys and girls with and without obesity, while accounting for the impact of external dead space imposed by the breathing apparatus. Our findings show that the ventilatory response to exercise is similar between children with and without obesity before, and after, accounting for the impact of external dead space. Furthermore, the ventilatory response to exercise was more pronounced in girls compared with boys; however, these sex-differences were eliminated when the impact of external dead space was removed.
During exercise V̇E was higher in the children with obesity compared with the children without obesity, which is in proportion to the higher metabolic demand of exercise associated with obesity (i.e., a greater V̇O2 in the children with obesity compared with the children without obesity). This is in keeping with others that have examined the respiratory effects of obesity during exercise in adults (Babb, 2013; Ofir et al., 2007; Whipp and Davis, 1984; Wood et al., 2008b). When V̇E was compared with V̇CO2, this relationship yielded a V̇E/V̇CO2 slope that was comparable between children with and without obesity, which is similar to what has been observed in adults (Babb et al., 1991; Ofir et al., 2007; Whipp and Davis, 1984). Even when accounting for the impact of VDM (i.e., V̇Ecorr/V̇CO2 slope) (Furuike et al., 1982; Singleton et al., 1972; Sun et al., 2002), which comprised ~25% of exercising V T, this relationship remained preserved between groups. The preservation of the V̇E/V̇CO2 slope reflects an appropriate increase in the exercise ventilatory response in children with obesity. The fact that PETCO2 was not different between groups at rest and during exercise lends support to this suggestion.
Previous studies have reported conflicting results regarding the exercise ventilatory response in children with obesity, which can make it difficult to generalize these reports into an overall consensus (Cooper et al., 2016; Kaufman et al., 2007; Marinov et al., 2002; McMurray and Ondrak, 2011; Prado et al., 2009). At present, a reason for the dissimilar findings reported in the literature remain unknown; however, it may be owing to prominent methodological considerations including: 1) differing classifications of obesity, 2) large age-ranges (e.g., 6–18 y), 3) examination of the ventilatory response during maximal incremental exercise, 4) and/or lack of a control group comparison (e.g., children without obesity). Indeed, the findings reported in the aforementioned studies (Cooper et al., 2016; Kaufman et al., 2007; Marinov et al., 2002; McMurray and Ondrak, 2011; Prado et al., 2009) may have been confounded by age-related changes in pulmonary function (Bongers et al., 2014; Marinov et al., 2000; Nagano et al., 1998), as well as profound changes in ventilatory and gas exchange responses induced by metabolic acidosis and increasing work rates above the respiratory compensation threshold. In the present study, we included only children with obesity that exhibited a BMI above the 95th percentile and were within a narrow age-range (8–12 y), examined the ventilatory response during moderate constant load exercise (reflecting a primary feedforward exercise stimulus), and our study design comprised an appropriate control group comparison (i.e., children without obesity). These considerations, combined with the fact that we also performed a separate analysis that accounted for the influence of VDM, suggests that our findings may arguably reflect a true representation of the exercise ventilatory response in children with obesity, at least for the level of exercise intensity that was studied.
Similar to others that have examined sex differences in the exercise ventilatory response in children (Marinov et al., 2000; Nagano et al., 1998) and adults (Kilbride et al., 2003), the V̇E/V̇CO2 slope was higher in girls compared with boys in the present study. Interestingly, however, these differences were eliminated when we accounted for the effects of VDM. This was evidenced by both girls and boys exhibiting a similar V̇Ecorr/V̇CO2 slope. These data suggest that VDM may have artificially enhanced the V̇E/V̇CO2 slope in girls, making it appear that sex differences are apparent in the ventilatory response to submaximal exercise, when really the effect of VDM was responsible. Notably, our data are in keeping with that of Cooper et al (Cooper et al., 1987) who also subtracted out V̇DM from V̇E, and reported that the exercise ventilatory response (measured up to the respiratory compensation point) during a maximal incremental cycling test was similar between girls and boys. Therefore, these findings (Cooper et al., 1987), combined with the findings of the present study, suggest that the exercise ventilatory response is similar between boys and girls during incremental and constant load submaximal exercise, and that the VDM should be taken into account before making between-sex comparisons. Moreover, the findings of the present study have important practical implications. Indeed, it may be that previous studies that examined the exercise ventilatory response in children and adults might have incorrectly ascribed differences in the V̇E/V̇CO2 slope to sex differences in respiratory function and/or morphology, when simply the effects of VDM may have been responsible for the apparent differences. Therefore, accounting for VDM reflects a simple methodological consideration that holds strong value for studies that aim to assess respiratory effects of sex during exercise in the future.
At present, the reason why girls may have responded differently to VDM compared with boys is unknown and from our data, we can only speculate on possible mechanisms that may be responsible. Both girls and boys were of similar height and had similar pulmonary function (data not shown); hence, it is unlikely that these factors contributed to the differing response to VDM. Furthermore, studies have demonstrated that added external dead space increases the regulatory set point of the partial pressure of CO2 in the arterial system (PaCO2) (Poon, 1992), and increases ventilatory drive (manifesting as an elevated V̇E/V̇CO2 slope) through a neural mechanism known as short term modulation (STM) (Babb et al., 2010; Wood et al., 2008a, 2009, 2011). Thus, STM serves to maintain isocapnia relative to resting values despite altered requirements for ventilation. A series of studies from our laboratory have separately reported on STM in young (29 ± 3 y) and older (69 ± 3 y) men, as well as young (29 ± 3 y) and older (70 ± 3 y) women (Wood et al., 2008a, 2010, 2011). While exercising at a similar work load (30–50 W) and using the same breathing apparatus as that in the present study (as in the control condition of the aforementioned studies, VDM = 0.225 L), the young women appeared to have a higher mean V̇E/V̇CO2 slope compared with the young men (29.1 ± 5.9 vs. 23.2 ± 5.7), and the older women also appeared to have a higher mean V̇E/V̇CO2 slope compared with the older men (32.4 ± 5.1 vs 29.9 ± 3.8). These separate findings (Wood et al., 2008a, 2010, 2011) seem to corroborate with that described in the present study, and collectively suggest that a given increase in dead space volume may alter resting PaCO2 levels and/or ventilatory drive through STM in a sex-dependant manner. The mechanism(s) responsible for the potential sex difference in STM is unknown (Babb et al., 2010); however, there is some evidence to suggest that circulating levels of female sex hormones may play a role in altering the resting PaCO2 set point and the neural control of breathing in adults (Behan et al., 2003; Schoene et al., 1981; Skatrud et al., 1978). In addition, if chemoreceptor reflex sensitivity to CO2 is enhanced in girls compared with boys, a greater increase in ventilatory drive would be expected for a given increase in dead space volume, thereby generating a greater V̇E/V̇CO2 slope. Although there is evidence that prepubescent boys have a greater chemoreceptor reflex sensitivity to CO2 compared with adult men (Gratas-Delamarche et al., 1993), whether sex differences in the sensitivity to CO2 exist in children remains unknown. Moreover, the girls in the present study exhibited an altered breathing pattern during exercise compared with boys (slightly lower VT and slightly higher Fb). Although differences were not statistically significant, this slight alteration in breathing pattern, combined with the effects of VDM, may have been sufficient to increase the dead space/tidal volume ratio in girls, which would have also contributed to the greater V̇E/V̇CO2 slope observed (Whipp et al., 1984). In light of the above, it is clear that further studies are needed to investigate mechanisms that may explain why girls appear to respond differently to VDM compared with boys.
With regards to breathing pattern, the children with obesity in the present study demonstrated a comparable VT and Fb to the children without obesity during exercise. This is in contrast to what has been previously observed in adults with obesity who tend to exhibit a reduced VT and higher Fb to maintain a given exercise V̇E (Babb, 2013; Ofir et al., 2007; Salvadori et al., 2008). The altered breathing pattern during exercise in adults with obesity can be ascribed to decreased chest wall compliance and mechanical ventilatory constraints secondary to increased fat mass on the chest wall. However, the children in the present study may not have yet developed the total absolute volume of fat mass that could impede upon lung or chest wall movements and/or been sufficiently exposed the effects of obesity to develop obesity-related alterations in breathing pattern.
5. Methodological considerations
Although the inclusion of children between the ages of 8 and 12 years limits the generalization of our findings, it is likely that our data are free from any confounding influence of potential puberty-induced changes in the exercise ventilatory response (Bongers et al., 2014; Nagano et al., 1998). Participants also exercised at 45% of their individual peak work rate. Although the mean level of exercise performed in the present study is typical of routine daily activity (i.e., exercise within the mild-moderate intensity range), it cannot be described whether or not some children were exercising above their individual ventilatory threshold. Given that studies have shown that exercise intensities up to the respiratory compensation threshold has no meaningful effect on the exercise ventilatory response (Marinov et al., 2002; Sun et al., 2002), it is likely that the independent influence of potentially exercising slightly above the ventilatory threshold on the V̇E/V̇CO2 and V̇Ecorr/V̇CO2 slopes was negligible. It is also important to note that normative values of the V̇E/V̇CO2 slope in children have been previously derived using breath-by-breath data from an incremental exercise test up to the ventilatory threshold (Bongers et al., 2014). In the present study, we calculated the ventilatory response to exercise using V̇E and V̇CO2 measured using the Douglas bag technique at rest and during constant-load exercise, as we have previously described (Wood et al., 2008a). Thus, the V̇E/V̇CO2 slopes derived in the present study cannot be directly compared with normative values. Nevertheless, it was not our intention to perform this comparison, but rather compare the ventilatory response to submaximal constant-load exercise (that is typical of routine daily activity) between boys and girls with and without obesity. Furthermore, arterial blood gasses were not collected.
6. Conclusion
We have demonstrated that the exercise ventilatory response is similar between children with and without obesity, even when accounting for the impact of external dead space. Although the exercise ventilatory response was more pronounced in girls compared with boys; these sex-differences were eliminated when the impact of external dead space was taken into account. These findings highlight the importance of accounting for the dead space imposed by the breathing apparatus before evaluating the exercise ventilatory response, particularly when making between-sex comparisons.
Highlights.
The exercise ventilatory response is similar between obese and nonobese children.
The exercise ventilatory response is higher in girls compared with boys.
Sex-differences were eliminated with the removal of external dead space.
Dead space should be removed when assessing the exercise ventilatory response.
Acknowledgments
The authors wish to acknowledge all clinical, nursing, and technical staff that assisted with data collection and analysis.
Grants
This research was supported by the National Institutes of Health (grant no. NIH R01 HL136643)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- Babb TG, 1999. Mechanical ventilatory constraints in aging, lung disease, and obesity: perspectives and brief review. Medicine and science in sports and exercise 31, S12–S22. [DOI] [PubMed] [Google Scholar]
- Babb TG, 2013. Obesity: challenges to ventilatory control during exercise--a brief review. Respiratory physiology & neurobiology 189, 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TG, Korzick D, Meador M, Hodgson JL, Buskirk ER, 1991. Ventilatory response of moderately obese women to submaximal exercise. International Journal of Obesity 15, 59–65. [PubMed] [Google Scholar]
- Babb TG, Wood HE, Mitchell GS, 2010. Short- and long-term modulation of the exercise ventilatory response. Medicine and science in sports and exercise 42, 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TG, Wyrick BL, Chase PJ, DeLorey DS, Rodder SG, Feng MY, Ranasinghe KG, 2011. Weight Loss via Diet and Exercise Improves Exercise Breathing Mechanics in Obese Men. Chest 140, 454–460. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS, 2003. Sex steroid hormones and the neural control of breathing. Respiratory physiology and neurobiology 136, 249–263. [DOI] [PubMed] [Google Scholar]
- Bongers BC, van Brussel M, Hulzebos EHJ, Takken T, 2014. Pediatric norms for cardiopulmonary exercise testing. In relation to sex and age. [Google Scholar]
- Bryan AC, Wohl WEB, (1964). Respiratory mechanics in children, The Handbook of Physiology. [Google Scholar]
- Cooper DM, Kaplan MR, Baumgarten L, Weiler-Ravell D, Whipp BJ, Wasserman K, 1987. Coupling of ventilation and CO2 production during exercise in children. Pediatric Research 21, 568–572. [DOI] [PubMed] [Google Scholar]
- Cooper DM, Leu SY, Taylor-Lucas C, Lu K, Galassetti P, Radom-Aizik S, 2016. Cardiopulmonary Exercise Testing in Children and Adolescents with High Body Mass Index. Pediatr Exerc Sci 28, 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels SR, Jacobson MS, McCrindle BW, Eckel RH, Sanner BM, 2009. American Heart Association Childhood Obesity Research Summit: executive summary. Circulation 119, 2114–2123. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Wyrick BL, Babb TG, 2005. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. Int J Obes (Lond) 29, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Reddan W, Rankin J, Balke B, 1966. Alveolar-arterial gas exchange during muscular work in obesity. Journal of Applied Phsyiology 21, 1807–1814. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Molgat-Seon Y, Sheel AW, 2019. Sex Differences in the Pulmonary System Influence the Integrative Response to Exercise. Exercise and sport sciences reviews 47, 142–150. [DOI] [PubMed] [Google Scholar]
- Furuike AN, Sue DY, Hansen JE, Wasserman K, 1982. Comparison of physiologic dead space/tidal volume ratio and alveolar arterial PO2 difference during incremental and constant work exercise. American Journal of Respiratory and Critical Care Medicine 126, 579–583. [DOI] [PubMed] [Google Scholar]
- Gratas-Delamarche A, Mercier J, Ramonatxo M, Dassonville J, Prefaut C, 1993. Ventilatory response of prepubertal boys and adults to carbon dioxide at rest and during exercise. European Journal of Applied Physiology 66, 25–30. [DOI] [PubMed] [Google Scholar]
- Kaufman C, Kelly AS, Kaiser DR, Steinberger J, Dengel DR, 2007. Aerobic-Exercise Training Improves Ventilatory Efficiency in Overweight Children. Pediatric Exercise Science 19, 82–92. [DOI] [PubMed] [Google Scholar]
- Kilbride E, McLoughlin P, Gallagher CG, Harty HR, 2003. Do gender differences exist in the ventilatory response to progressive exercise in males and females of average fitness? Eur J Appl Physiol 89, 595–602. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, GFlegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL, 2000. CDC growth charts: United States. Advanced Data 314, 1–27. [PubMed] [Google Scholar]
- Lanteri CJ, Sly PD, 1993. Changes in respiratory mechanics with age. Journal of Applied Phsyiology 74, 369–378. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Colditz G, Berkey CS, Speizer FE, 1997. Effects of Body Fat on Ventilatory Function in Children and Adolescents: Cross-Sectional Findings From a Random Population Sample of School Children. Pediatric Pulmonology 24, 187–194. [DOI] [PubMed] [Google Scholar]
- Marinov B, Kostianev S, Turnovska T, 2000. Ventilatory response to exercise and rating of perceived exertion in two pediatric age groups. Acta Physiol Pharmacol Bulg 25, 93–98. [PubMed] [Google Scholar]
- Marinov B, Kostianev S, Turnovska T, 2002. Ventilatory efficiency and rate of percieved exertion in obese and non-obese children performing standardized exercise. Clinical physiology and functional imaging 22, 254–260. [DOI] [PubMed] [Google Scholar]
- McClaran SR, Harms CA, Pegelow DF, Dempsey JA, 1998. Smaller lungs in women affect exercise hyperpnea. Journal of Applied Phsyiology 84, 1872–1881. [DOI] [PubMed] [Google Scholar]
- McMurray RG, Ondrak KS, 2011. Effects of being overweight on ventilatory dynamics of youth at rest and during exercise. Eur J Appl Physiol 111, 285–292. [DOI] [PubMed] [Google Scholar]
- Nagano Y, Baba R, Kuraishi K, Yasuda T, Ikoma M, Nishibata K, Yokota M, Nagashima M, 1998. Ventilatory control during exercise in normal children. Pediatric Research 43, 704–707. [DOI] [PubMed] [Google Scholar]
- Neder JA, Dal Corso S, Malaguti C, Reis S, De Fuccio MB, Schmidt H, Fuld JP, Nery LE, 2003. The pattern and timing of breathing during incremental exercise: a normative study. The European respiratory journal 21, 530–538. [DOI] [PubMed] [Google Scholar]
- Neder JA, Nery LE, Peres C, Whipp BJ, 2001. Reference Values for Dynamic Responses to Incremental Cycle Ergometry in Males and Females Aged 20 to 80. American Journal of Respiratory and Critical Care Medicine 164, 1481–1486. [DOI] [PubMed] [Google Scholar]
- Ofir D, Laveneziana P, Webb KA, O’Donnell DE, 2007. Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol (1985) 102, 2217–2226. [DOI] [PubMed] [Google Scholar]
- Polgar G, Weng TR, 1979. The functional development of the respiratory system from the period of gestation to adulthood. American Journal of Respiratory and Critical Care Medicine 120, 625–695. [DOI] [PubMed] [Google Scholar]
- Poon C-S, 1992. Potentiation of exercise ventilatory response by airway CO2 and dead space loading. Journal of Applied Phsyiology 73, 591–595. [DOI] [PubMed] [Google Scholar]
- Prado DM, Silva AG, Trombetta IC, Ribeiro MM, Nicolau CM, Guazzelli IC, Matos LN, Negrao CE, Villares SM, 2009. Weight loss associated with exercise training restores ventilatory efficiency in obese children. International journal of sports medicine 30, 821–826. [DOI] [PubMed] [Google Scholar]
- Salvadori A, Fanari P, Tovaglieri I, Giacomotti E, Nibbio F, Belardi F, Longhini E, 2008. Ventilation and its control during incremental exercise in obesity. Respiration 75, 26–33. [DOI] [PubMed] [Google Scholar]
- Schoene RB, Robertson HT, Pierson DJ, Peterson AP, 1981. Respiratory drives and exercise in menstrual cycles of athletic and nonathletic women. Journal of Applied Phsyiology 50, 1300–1305. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Guenette JA, 2008. Mechanics of breathing during exercise in men and women: sex versus body size differences? Exercise and Sport Science Reviews 36, 128–134. [DOI] [PubMed] [Google Scholar]
- Singleton GL, Olsen CR, Smith RL, 1972. Correction for Mechanical Dead Space in the Calculation of Physiological Dead Space. Journal of Clinical Investigation 51, 2768–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatrud JB, Dempsey JA, Kaiser DG, 1978. Ventilatory response to medroxy-progesterone acetate in normal subjects: time course and mechanism. Journal of Applied Phsyiology 44, 393–344. [DOI] [PubMed] [Google Scholar]
- Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K, 2002. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med 166, 1443–1448. [DOI] [PubMed] [Google Scholar]
- Tanner JM, 1962. Growth at adolescence. Oxford: Blackwell. [Google Scholar]
- Whipp BJ, Davis JA, 1984. The ventilatory stress of exercise in obesity. American Review of Respiratory Disease 129, S90–S92. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Ward SA, Wasserman K, 1984. Ventilatory responses to exercise and their control in man. American Review of Respiratory Disease 129, S17–S20. [DOI] [PubMed] [Google Scholar]
- Wood HE, Mitchell GS, Babb TG, 2008a. Short-term modulation of the exercise ventilatory response in young men. J Appl Physiol (1985) 104, 244–252. [DOI] [PubMed] [Google Scholar]
- Wood HE, Mitchell GS, Babb TG, 2009. Breathing mechanics during exercise with added dead space reflect mechanisms of ventilatory control. Respiratory physiology & neurobiology 168, 210–217. [DOI] [PubMed] [Google Scholar]
- Wood HE, Mitchell GS, Babb TG, 2010. Short-term modulation of the exercise ventilatory response in older men. Respiratory physiology & neurobiology 173, 37–46. [DOI] [PubMed] [Google Scholar]
- Wood HE, Mitchell GS, Babb TG, 2011. Short-term modulation of the exercise ventilatory response in younger and older women. Respiratory physiology & neurobiology 179, 235–247. [DOI] [PubMed] [Google Scholar]
- Wood HE, Semom TL, Comeau LA, Schwartz B, MacDougall RM, Klocko MN, Babb TG, (2008b). The ventilatory response to exercise does not differ between obese women with and without dyspnea on exertion, Integration in respiratory control. [DOI] [PubMed] [Google Scholar]
- Zavorsky GS, Murias JM, Kim DJ, Gow J, Christou NV, 2007. Poor compensatory hyperventilation in morbidly obese women at peak exercise. Respiratory physiology & neurobiology 159, 187–195. [DOI] [PubMed] [Google Scholar]


