Abstract
The N-terminal regions of histone proteins (tails) are dynamic elements that protrude from the nucleosome and are involved in many aspects of chromatin organization. Their epigenetic role is well-established, and post-translational modifications (PTMs) present on these regions contribute to transcriptional regulation. While hydrogen/deuterium exchange mass spectrometry (HX-MS) is well-suited for the analysis of dynamic structures, it has seldom been employed to analyze histones due to the poor N-terminal coverage obtained using pepsin. Here, we test the applicability of a dual protease type XIII/pepsin digestion column to this class of proteins. We optimize online digestion conditions using the H4 monomer, and extend the method to the analysis of histones in monomeric states and nucleosome core particles (NCPs). We show that the dual protease column generates many short and overlapping N-terminal peptides. We evaluate our method by performing hydrogen exchange experiments of NCPs for different time points and present full coverage of the tails at excellent resolution. We further employ electron transfer dissociation and showcase an unprecedented degree of overlap across multiple peptides that is several fold higher than previously reported methods. The method we report here may be readily applied to the HX-MS investigation of histone dynamics and to the footprints of histone binding proteins on nucleosomes.
Keywords: histone tails, mononucleosomes, HX-MS, ETD, protease type XIII/pepsin
Histones are highly conserved proteins found primarily in the nucleus that organize into higher order structures with DNA to form nucleosomes, the basic building block of chromatin. Nucleosomes are composed of ~147 base pairs of DNA wrapped around a histone octamer, which is assembled by two H2A/H2B dimers, and an H3/H4 tetramer [1]. The highly basic and largely flexible histone N-termini (tails) extend out of the nucleosome and are readily accessible to enzymes that can add (writers) or remove (erasers) post-translational modifications (PTMs). These PTMs may act in a synergistic or sequential fashion to recruit various histone binding proteins (readers) and remodelers, affecting downstream transcriptional processes [2] and chromatin structure [3]. Nucleosome core particles that include histones and DNA can be reconstituted in vitro, and serve as a useful model system for the study of chromatin biology.
Despite their critical function, the three dimensional structure of the tails and their role in mediating intra- and inter-nucleosome interactions with both DNA and proteins of the transcriptional machinery are largely unexplored. Since the first crystal structure of the octameric histone core was determined [4], a number of high resolution histone (and variant) structures have been reported in complexes with chaperones, nucleosomes and nucleosomal arrays (622 entities in 378 PDB entries, rcsb.org) [5]; in most of these structures, parts of the histone tails could not be resolved due to their high flexibility. Employing histone peptides corresponding to modified segments of the tails has been employed as an alternative approach and has resulted in high resolution complexes (~270 entities). Binding sites or epitopes represented in linear, modified histone peptides however, may be substantially different from the complex nucleosome structure. Analytical methods that will tackle these challenges and will allow for the structural analysis of full histone sequences in a physiological context are therefore highly desirable.
Hydrogen/deuterium exchange-mass spectrometry (HX-MS) is a powerful method for probing highly dynamic regions of proteins (such as histone tails) that may be inaccessible to other methods [6]. In the few published studies that have attempted to probe histone dynamics, the tails were represented poorly, either by the lack of peptides in this region or by the identification of long peptides (~50 amino acids (AAs)); for a detailed background see [7]). In all these studies, a traditional bottom-up approach was employed, where proteins were digested rapidly into peptides using pepsin, a non-specific endoprotease that is functional under HX-MS quenching conditions (pH 2.5). Neprosin, a selective prolyl endoprotease, was reported to improve resolution of the H3 and H4 tails compared to pepsin [8], but has not yet been used for monitoring in-solution histone dynamics. More recently, we improved coverage and redundancy of H2A, H3 and H4 tails further (peptides ~20–25 AAs long) through the use of cathepsin-L and showcased its applicability to the analysis of the in-solution dynamics of H3 and H4 in a monomeric context [7]. Sequence resolution of H3 and H4 was significantly increased using cathepsin-L followed by on-line pepsin digestion, but background signals also increased, necessitating additional washes with high concentrations of guanidine hydrochloride between runs to maintain a low background. This is a known issue with offline, in-solution digestions where a high enzyme:substrate ratio is required, therefore immobilized protease columns are highly preferred.
Recently, a dual protease type XIII/pepsin column became commercially available and it is slowly being adopted for use in HX-MS studies [9,10]. Protease type XIII from Aspergillus saitoi was introduced in the HX-MS field in 2003 [11] and has mainly been used for the digestion of proteins in-solution [12–15], although an immobilized version used for deuterium exchange studies of proteins has also been described [16,17]. Like pepsin, it is a non-specific enzyme and maintains its activity in the presence of reducing and denaturing agents [15] commonly required in HX-MS experiments. Its cleavage preferences at the C-terminal side of basic AAs [14] are complementary to pepsin, enabling improved peptide maps with a high percentage of overlapping fragments [9,10,16]. In this study, we introduce protease type XIII for studying histone tails by HX-MS. We optimize online digestion conditions using the dual protease type XIII/pepsin column and showcase excellent resolution for all core histones either at the peptide level where proteolytic fragments are separated by their mass-to-charge (m/z) ratio (MS1) or at the residue level, where peptides detected in MS1 are fragmented into smaller fragment ions, separated by their (m/z) ratio (MS2).
Experimental Section
Materials
Human recombinant histones (H2A; M2502S, H2B.1; M2505S, H3.1; M2503S and H4; M2504S) were purchased from New England BioLabs Inc (Ipswich, MA). Mononucleosomes, recombinant human (16–0009) containing H2A (P04908), H2B (O60814), H3.1 (P68431), and H4 (P62805) were purchased from EpiCypher (Durham, NC). D; 151882). All other reagents were from Sigma-Aldrich (St. Louis, MO).
Sample preparation
Individual histones (5 μL of 0.2 μg/μL in guanidine HCl, pH 2.5) or mononucleosomes (10 μL of 0.2 μg/μL in guanidine HCl, pH 2.5) were injected to the MS via an automated HDx-3 PAL™ system (LEAP Technologies, Morrisville, NC). Samples were digested online for 2 min at 200 μL/min using an immobilized protease type XIII/pepsin column (w/w, 1:1, NBA2014002, NovaBioAssays, Woburn, MA), operated at 8 °C and were trapped onto an Acclaim™ PepMap™ 300 μ-Precolumn™ (C18, 1 × 15 mm, 163593, Thermo Fisher Scientific, Waltham, MA) using solvent A (0.1% formic acid (v/v)). Peptides were separated onto a Hypersil Gold C18 (1 × 50 mm, 1.9 μM, 25002–051030, Thermo Scientific, Waltham, MA) at 40 μL/min using solvents A and B (0.1% v/v formic acid in acetonitrile). The following gradient was applied: 3% B to 10% B in 0.1 min, to 35% B in 6 min, to 95% B in 0.1 min; kept at 97% B for 1.9 min and returned to initial conditions in 0.9 min. Both the trap and analytical column were operated at 0 °C. For hydrogen exchange (HX) experiments, mononucleosomes (4 μL of 0.4 μg/μL) were mixed with ice-chilled deuterated PBS (36 μL, final D2O content during reaction 90% v/v). Samples were quenched at 60, 600 and 3600 s, with 6 M guanidine HCl (20 μL, final guanidine HCl 2 M) to a final pH 2.2 (0.6% formic acid). Samples were injected as described above and incubated in the loop for 1 min prior to online digestion. Full deuteration controls were prepared by overnight labeling at 37 °C using an Eppendorf™ Thermomixer™ R (Thermo Scientific, Rockford, IL) and injected as described above.
MS data acquisition
MS analysis was carried out on an Orbitrap Fusion™ Lumos™ Tribrid™ Mass Spectrometer (Thermo Fisher Scientific) using a spray voltage of 3.5 kV, capillary temperature 220 °C and vaporizer temperature 50 °C. Full MS scans were acquired in the m/z range 270–1500, with an AGC target 5e5 and 60,000 resolution (at m/z 200). Peptides with charge states 2 to 8 were selected for MS/MS fragmentation using electron transfer dissociation (ETD) [18] or higher-energy C-trap dissociation (HCD) [19]. Parameters were as follows: AGC target 4e5, loop count 12 and isolation window 3 m/z. ETD reaction times were 50 ms for charges 2–3, 25 ms for charge 4 and 20 ms for charges 5–8; HCD collision energy was 28% for all charges. Data were acquired in profile mode and peptides were identified using Spectrum Mill Proteomics Workbench (prerelease version B.06.01.202, Agilent Technologies). Searches were performed using ESI QExactive in the Instrument menu. The fragmentation mode was ETD only for ETD experiments and All for HCD experiments. A non-specific enzyme search was performed using a FASTA file containing sequences of human histones, pepsin and protease type XIII. Peptide and fragment tolerances were at ±20 ppm and peptide FDR at 1%. Further processing, manual validation of the data and deuterium uptake measurements occurred in HDExaminer (Sierra Analytics).
RESULTS AND DISCUSSION
Optimization of digestion conditions
The proteolytic activity of the dual protease column was optimized using the H4 monomer followed by ETD fragmentation [20]. Due to the hydrophilic nature of the tails, we tested varying digestion times (1–3 min) and flow rates (100–300 μL/min) aiming to obtain high N-terminus coverage and repeated measurements of AAs from overlapping peptides (often referred to as redundancy). The detection of many overlapping peptides with overhanging N- and C-terminal residues is highly desirable in HX studies for localizing deuterium content at residue resolution [21], especially when ETD fragmentation is not available (see below). In all conditions, spectra corresponding to intact H4 were not detected, indicating that digestion was complete. The number of tail peptides identified (up to amino acid 30) ranged from 6–11 (Fig. 1A) with the total digestion volume being the largest contributor; it was found that 400–600 μL provided optimum number of peptides and intensities. Using less than 400 μL was insufficient to remove guanidine HCl, resulting in decreased peptide intensities, while using digestion volumes above 600 μL resulted in the loss of shorter peptides during trapping. In this optimal range, increasing the pressure (from 80 bar to 255 bar) by increasing the flow rate (from 100 μL/min to 300 μL/min) had minimal effect in the number of peptides identified, we therefore chose the minimum optimum digestion volume (2 min at 200 μL/min) to prevent back exchange.
Figure 1. Optimization of digestion conditions and peptide identifications.
(A) Effect of flow rate and digestion time on peptide identification for the H4 N-terminus (amino acids 1–30) using ETD. Only peptides that start at amino acid 1 are shown. Black lines indicate the end amino acid of each peptide identified. (B) Total number of unique peptides identified by HCD (white) and ETD (light grey) at optimized digestion conditions (2 min at 200 μL/min). Common peptides are shown in dark grey.
Histone peptide identifications by HCD and ETD
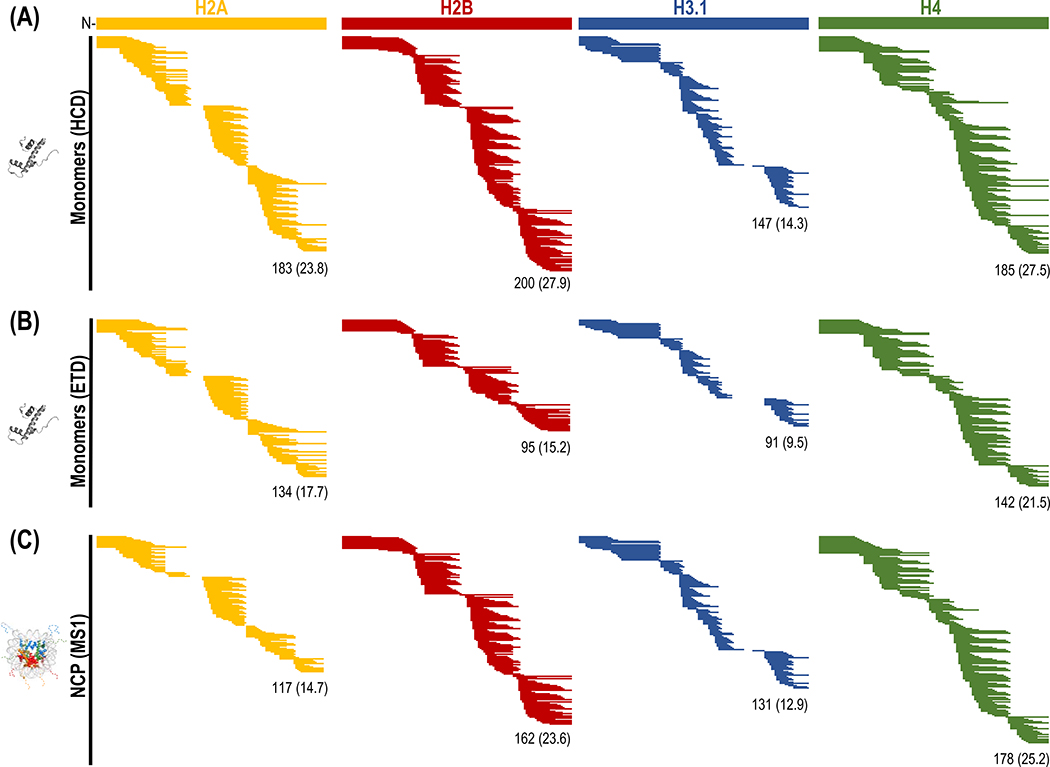
We then tested the overall protein coverage and reproducibility of the column using the four core histones and performed digestions in triplicates. To generate a comprehensive map of the peptides produced upon digestion with the dual protease column, we applied two different fragmentation methods, HCD that is available in Orbitrap systems and is more effective for small, low mass species, and ETD that is better suited for the analysis of high charge state cationic molecules [18] and is anticipated to provide better resolution of tail peptides. The number of peptides identified ranged from ~90 to ~200 with a 42% (H2B) to 63% (H4) overlap between the two methods (Fig. 1B). HCD resulted in a greater sequence depth compared to ETD, due to the slower scan rate of the latter (~⅔ the rate of HCD) that results in less frequent peptide sampling [22]. Both methods however resulted in complete coverage of H2B and H4 and >94% for H2A and H3.1 (Fig. 2A, 2B). Despite the larger pool of peptides identified by HCD and the higher redundancy values, most tail peptides were identified by both HCD and ETD, rendering either method suitable for HX measurements of histone tails at the peptide level (MS1). Pearson correlation coefficients of peptide intensities obtained in at least two replicates were >0.90. Standard deviations using HCD were inferior compared to ETD, presumably due to the higher number of small peptides identified (Fig. S1A, Table S1). These belonged mainly to the histone folds (starting at AA ~30 for H2A, H2B, H4 and AA 46 for H3 and extending to the C-terminus) and could still be qualified for HX measurements, however caution should be exercised during their selection due to their reduced reproducibility. Profiling the specificity of the column using these peptide identifications revealed cleavage sites characteristic to pepsin (Leu, Met) and protease type XIII (Arg) activities [14] (Fig. S1B).
Figure 2. Peptide maps generated upon digestion with the dual protease column.
Coverage maps of histone monomers using (A) HCD and (B) ETD. Each line corresponds to a unique peptide identified in at least two replicates (n=3). (C) Peptides identified in mononucleosomes at the MS1 level. The total number of peptides identified is given at the bottom of each figure along with the average AA overlap value (redundancy) in parenthesis. A detailed list of peptides identified is given in Table S1.
As histones form the stable core of nucleosomes, we next attempted to determine peptides generated for NCPs by a standard in-line digestion using the dual protease column. Peptides generated upon digestion with the dual protease column were profiled at the MS1 level, as would typically occur for HX-MS measurements. Using the peptide libraries generated above, we extracted the precursor ions and inspected them in HDExaminer. For all histones, the number of peptides validated ranged from ~117 (H2A) to ~178 (H4) (Fig. 2C). Compared to peptides identified from monomeric histones, identifications from the nucleosome matched with >85% of peptides from H3.1 and H4 monomers, ~78% from H2B, and ~60% from H2A. While the total number of peptide identification decreased, sequence coverage was unaffected, remaining similar between NCPs and monomers. Interestingly, the number of peptides corresponding to the tails was only marginally reduced relative to the monomers, yielding similar coverage. Taken together, these results indicate that despite the increase in sample complexity (histone octamer ~110 kDa), the presence of DNA and the lower amount of protein injected (0.5 μg per histone in NCPs vs. 1 μg in monomers), the developed method generated high resolution peptide maps for both the tails and the histone cores.
Given the importance of the tails and their PTMs in regulating transcriptional processes, we next focused our analysis on the improvements the dual protease column offers towards for deuterium measurements at the MS1 level that is the most commonly used approach in deuterium exchange studies. We also calculated redundancy values at the residue level, corresponding to the number of times a given AA has been identified in detected overlapping peptide fragments (Fig. S2A); higher values are desirable since they provide highly resolved information on protein structure, dynamics and interactions, and further validate deuterium measurements [17]. For all histones, a large number of overlapping tail (first 30 AAs) peptides with overhanging residues at their C-termini (e.g. H4) or both termini (e.g. H3) were detected upon HCD fragmentation (Fig. 2A, Table S1), well outperforming peptide identifications reported to date for this class of proteins using other proteases. For example, pepsin generates only one large peptide (1–50) for H3.1, whereas cathepsin-L generates two shorter peptides (1–21, 1–22) in addition to 1–50, improving resolution in this region [7]). In contrast, the dual protease column generated up to 16 overlapping peptides for H3.1 in residues 1–30 and resulted in almost complete residue resolution for AAs 5–8 and 15–26, covering 70% of the tail (Fig. S2B). Similarly, near-single residue resolution was obtained for AAs 14–28 (50% of the tail) in H2A and 15–26 (43% of the tail) in H4. These are areas that contain known target residues for PTMs and the ability to calculate exchange rates at the residue level using a traditional bottom-up approach opens new avenues for histone structural studies. An increase in the site-resolution may further be feasible through the use of advanced computational approaches, using overlapping peptides that do not share common termini [21]. Single residue resolution was achieved in mononucleosomes, with the exception of H2A that was slightly lower (AAs 17–28). Redundancy values calculated per residue were in the range of 6–35 with the majority exceeding a value of 10 (Fig. S2B). These correspond to ~2 to 4-fold increase compared to previous studies with pepsin or cathepsin-L/pepsin [7,23]. Similar redundancy values have been reported for other, non-histone proteins using the dual protease digestion scheme, even for proteins up to 900 residues long [17], indicating the unique advantages the dual protease digestion has to offer.
Next, we were interested to assess the method we developed on deuterated samples to evaluate how the presence of deuterium that results in isotopic expansion will impact protein coverage and redundancy. We employed the dual protease digestion configuration and performed deuterium exchange measurements of NCPs for three different time points (60 s, 600 s, 3600 s). In total, we were able to analyze 66 peptides in H2A (80% coverage), 73 in H2B (97% coverage), 51 in H3 (80% coverage) and 70 in H4 (96% coverage) for their D2O content. Between 40–60% of the undeuterated peptides previously identified under regular H2O buffer conditions (Fig. 2C) were able to be re-identified and analyzed in their deuterated states. This decrease is attributed to the limited incubation time with guanidine HCl (1 min) prior to digestion, in contrast to the undeuterated ones that were fully denatured. Despite the overall decrease in peptides generated, the tails were represented by a high number of peptides, offering unprecedented redundancy in this region compared to previously reported methods. We detected 19 peptides in H2A, 9 in H2B, 15 in H3 and 24 in H4; this corresponded to a marginal decrease in average redundancy values (8 to 12) compared to the denatured, undeuterated peptides (11 to 17) (Fig. S2C). This is anticipated, as flanking histone tails extend outside the boundaries of NCPs and are accessible for proteolysis. The deuterium uptake for all histones is depicted in the form of a heat map for individual time points (Fig. 3). It is shown that the tails are almost fully deuterated over the time course of the experiment in contrast to the histone folds that exhibit low deuterium uptake, even at the latest time point. For the investigation of histone fold protein dynamics, a higher labeling temperature or longer labeling time points that will allow for higher deuterium uptake to be measured in the course of the experiment is recommended.
Figure 3: Deuterium uptake of histones in NCPs by HX-MS.
Heat maps depicting H-to-D exchange for each histone in nucleosome core particles (NCPs) (A). Amino acid sequences for individual histones are shown on top. Color coding of heat maps is based on deuterium update (%) calculated based on normalizing D-uptake values to a fully deuterated NCP control sample as described in Mayne, 2016. Individual time points of exchange are 60, 600 and 3600 seconds. Individual D-uptake values are given in Table S1.
Extending resolution of histone tails to the residue level
Localization of the deuterium content at the residue level occurs either at the peptide level through the identification of many overlapping peptides with overhanging N- and C-terminal residues [21] or at the fragment ion level (MS2) using fragmentation methods that proceed with a low degree of intramolecular amide hydrogen migration and prevent hydrogen scrambling (i.e. electron capture dissociation and ETD) [20,24]. We took advantage of ETD capabilities available in the Orbitrap Fusion™ Lumos™ Tribrid™ MS to obtain residue resolution maps across the extensively modified tail regions (first 30 AAs). The majority of tail peptides identified (92%) were detected with charges +4 to +8 that correlated with length, rendering them excellent candidates for ETD fragmentation (Fig. S3). We calculated overlap per residue at the MS2 level, considering fragment ions from overlapping peptides and different charge states that result in separate MS2 spectra (Fig. S2A). Pairing complementary fragment ions from overlapping peptides is anticipated to yield much richer ion ladders and separate true N- and C-terminal ions from noise [22]. Summing all cleavage sites from overlapping peptides, and omitting residues N-terminal to proline at which ETD cleavage does not occur, full residue coverage of the tails was obtained, with the exception of H2B. Residue overlap was highest for H2A and H4 where >90% of the residues had values >10, followed by H3.1 and H2B where >60% had values >10 (Fig. 4). Despite the decreased coverage observed (~80%) for H2B compared to the other histones, high overlap values were obtained for most lysine residues that undergo N-terminal modifications that are important.
Figure 4. Resolution and redundancy of histone tails at the MS2 level.
Amino acid redundancy of histone tails (AAs 1–30) in monomers and NCPs using ETD fragmentation. These have been calculated as depicted in Fig. S2A.
We next analyzed whole NCPs using the same ETD workflow as for histone monomers. Pearson’s correlations of peptide intensities were >0.93 indicating excellent reproducibility (Fig. S1). The number of overlapping peptides identified within the tails was in the range of ~8–11 for individual histones, providing full coverage in this region. Similar to the monomers, overlap values at the MS2 level were highest for H2A and H4 (80% of residues had values >5), followed by H2B (73%) and H3.1 (64%) (Fig. 4). To our knowledge, this is the first time that overlap values of this magnitude are reported for NCP histones at residue resolution. In a recent, first of its kind study, where ETD was employed for the analysis of NCPs following pepsin digestion, the resolution obtained for the first 30 AAs by single peptides (~40–50 AA long), was ~13% for H2A and H2B and ~48% for H3.1 and H4 indicating many non-resolved segments [23]. Using the dual protease column and performing ETD on all peptides identified, we demonstrate here that >97% residue resolution is achieved in NCPs for H2A, H3.1 and H4, and >90% for H2B. Of note, these results are obtained using <25 msec ETD reaction time for charge states >+4; improvements in resolution and redundancy may be achieved by further method optimization i.e. higher intensity of fluoranthene ions or of the AGC target at MS2, however, this was beyond the scope of this study.
In conclusion, our study highlights protease type XIII as an exceptional enzyme for the analysis of histone tails by HX-MS that have thus far been represented poorly in such studies. In combination with pepsin, we demonstrate that high sequence resolution may be achieved for all histones by measuring the mass of intact peptides at the MS1 level. We further demonstrate that the tail peptides generated upon dual protease digestion are excellent candidates for ETD and showcase unprecedented redundancy of the tails at single residue resolution. The methods presented herein are not restricted by sample complexity or buffer composition and create numerous capabilities for chromatin research and drug discovery. For example, combinatorially modified histone peptides are employed extensively in peptide microarrays [25,26] and high-resolution structures (i.e. [27]) for interrogating binding motifs of effector proteins, antibodies and histone-modifying enzymes. Linear epitopes present in these peptides however are substantially different compared to the dynamic nucleosome structure; our method may be readily applied for probing these interactions in the context of whole nucleosomes, representative of physiological conditions. Furthermore, certain histone PTMs have been implicated in influencing interactions between the DNA, H3/H4 and H2A/H2B, leading to altered nucleosome structures [28,29]. Monitoring structural changes elicited by such modifications will provide direct insights into their role in nucleosome stability and will inform transcriptional processes and chromatin dynamics. Poorly characterized PTMs, i.e. crotonylation and butyrylation [30,31], or oncogenic histone mutations [32,33] are a few other examples amenable to the methodology reported herein.
Supplementary Material
Highlights.
Protease XIII is introduced as a novel protease for the analysis of histones by HX-MS
Optimized digestion conditions generates many short, overlapping N-terminal peptides
Improved resolution and redundancy is obtained for the typically underrepresented histone tails
The method is readily applicable to the HX-MS investigation of histone dynamics in mononucleosomes
Acknowledgements
This work was supported by Thermo Scientific, by a SPARC Award to M.P. from the Broad Institute of MIT & Harvard (#800332) and by a grant from the National Institute of Health (NIH) to J.D.J. at the Broad Institute of MIT & Harvard (U54-HG008097). The authors would like to acknowledge Susan Klaeger (Broad Institute) for useful discussions on ETD methods, Susan Bird (Thermo Scientific) and Jeff Morrow (Sierra Analytics) for support.
Footnotes
Data availability
The original mass spectra and sequence database have been deposited in the public proteomics repository MassIVE and are accessible at ftp://MSV000083879@massive.ucsd.edu when providing the dataset password: histone. If requested, also provide the username: MSV000083879. This dataset will be made public upon acceptance of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ, Crystal structure of the nucleosome core particle at 2.8 A resolution, Nature. 389 (1997) 251–260. [DOI] [PubMed] [Google Scholar]
- [2].Strahl BD, Allis CD, The language of covalent histone modifications, Nature. 403 (2000) 41–45. [DOI] [PubMed] [Google Scholar]
- [3].Luger K, Richmond TJ, The histone tails of the nucleosome, Curr. Opin. Genet. Dev. 8 (1998) 140–146. [DOI] [PubMed] [Google Scholar]
- [4].Arents G, Burlingame RW, Wang BC, Love WE, Moudrianakis EN, The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix, Proc. Natl. Acad. Sci. U. S. A. 88 (1991) 10148–10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE, The Protein Data Bank, Nucleic Acids Res. 28 (2000) 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Balasubramaniam D, Komives EA, Hydrogen-exchange mass spectrometry for the study of intrinsic disorder in proteins, Biochim. Biophys. Acta. 1834 (2013) 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Papanastasiou Malvina, Mullahoo James, DeRuff Katherine C, Bajrami Besnik, Johnston Stephen E, Peckner Ryan, Myers Samuel A, Carr Steven A, Jaffe Jacob D , Chasing tails: cathepsin-L improves structural analysis of histones by HX-MS, Mol. Cell. Proteomics. 18 (2019) 2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schräder CU, Lee L, Rey M, Sarpe V, Man P, Sharma S, Zabrouskov V, Larsen B, Schriemer DC, Neprosin, a Selective Prolyl Endoprotease for Bottom-up Proteomics and Histone Mapping, Mol. Cell. Proteomics. 16 (2017) 1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hamuro Y, Zhang T, High-Resolution HDX-MS of Cytochrome c Using Pepsin/Fungal Protease Type XIII Mixed Bed Column, J. Am. Soc. Mass Spectrom. 30 (2019) 227–234. [DOI] [PubMed] [Google Scholar]
- [10].Nirudodhi SN, Sperry JB, Rouse JC, Carroll JA, Application of Dual Protease Column for HDX-MS Analysis of Monoclonal Antibodies, J. Pharm. Sci. 106 (2017) 530–536. [DOI] [PubMed] [Google Scholar]
- [11].Cravello L, Lascoux D, Forest E, Use of different proteases working in acidic conditions to improve sequence coverage and resolution in hydrogen/deuterium exchange of large proteins, Rapid Commun. Mass Spectrom. 17 (2003) 2387–2393. [DOI] [PubMed] [Google Scholar]
- [12].Mazon H, Marcillat O, Forest E, Vial C, Local dynamics measured by hydrogen/deuterium exchange and mass spectrometry of creatine kinase digested by two proteases, Biochimie. 87 (2005) 1101–1110. [DOI] [PubMed] [Google Scholar]
- [13].Man P, Montagner C, Vernier G, Dublet B, Chenal A, Forest E, Forge V, Defining the interacting regions between apomyoglobin and lipid membrane by hydrogen/deuterium exchange coupled to mass spectrometry, J. Mol. Biol. 368 (2007) 464–472. [DOI] [PubMed] [Google Scholar]
- [14].Zhang H-M, Kazazic S, Schaub TM, Tipton JD, Emmett MR, Marshall AG, Enhanced digestion efficiency, peptide ionization efficiency, and sequence resolution for protein hydrogen/deuterium exchange monitored by Fourier transform ion cyclotron resonance mass spectrometry, Anal. Chem. 80 (2008) 9034–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang H-M, McLoughlin SM, Frausto SD, Tang H, Emmett MR, Marshall AG, Simultaneous reduction and digestion of proteins with disulfide bonds for hydrogen/deuterium exchange monitored by mass spectrometry, Anal. Chem. 82 (2010) 1450–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Englander JJ, Del Mar C, Li W, Englander SW, Kim JS, Stranz DD, Hamuro Y, Woods VL Jr, Protein structure change studied by hydrogen-deuterium exchange, functional labeling, and mass spectrometry, Proc. Natl. Acad. Sci. U. S. A. 100 (2003) 7057–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mayne L, Kan Z-Y, Chetty PS, Ricciuti A, Walters BT, Englander SW, Many overlapping peptides for protein hydrogen exchange experiments by the fragment separation-mass spectrometry method, J. Am. Soc. Mass Spectrom. 22 (2011) 1898–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF, Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry, Proc. Natl. Acad. Sci. U. S. A. 101 (2004) 9528–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Olsen JV, Macek B, Lange O, Makarov A, Horning S, Mann M, Higher-energy C-trap dissociation for peptide modification analysis, Nat. Methods. 4 (2007) 709–712. [DOI] [PubMed] [Google Scholar]
- [20].Rand KD, Adams CM, Zubarev RA, Jørgensen TJD, Electron capture dissociation proceeds with a low degree of intramolecular migration of peptide amide hydrogens, J. Am. Chem. Soc. 130 (2008) 1341–1349. [DOI] [PubMed] [Google Scholar]
- [21].Kan Z-Y, Walters BT, Mayne L, Englander SW, Protein hydrogen exchange at residue resolution by proteolytic fragmentation mass spectrometry analysis, Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 16438–16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guthals A, Clauser KR, Frank AM, Bandeira N, Sequencing-grade de novo analysis of MS/MS triplets (CID/HCD/ETD) from overlapping peptides, J. Proteome Res. 12 (2013) 2846–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Karch KR, Coradin M, Zandarashvili L, Kan Z-Y, Gerace M, Englander SW, Black BE, Garcia BA, Hydrogen-Deuterium Exchange Coupled to Top- and Middle-Down Mass Spectrometry Reveals Histone Tail Dynamics before and after Nucleosome Assembly, Structure. (2018). 10.1016/j.str.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rand KD, Zehl M, Jensen ON, Jørgensen TJD, Protein hydrogen exchange measured at singleresidue resolution by electron transfer dissociation mass spectrometry, Anal. Chem. 81 (2009) 5577–5584. [DOI] [PubMed] [Google Scholar]
- [25].Rothbart SB, Krajewski K, Strahl BD, Fuchs SM, Peptide microarrays to interrogate the “histone code,” Methods Enzymol. 512 (2012) 107–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shanle EK, Shinsky SA, Bridgers JB, Bae N, Sagum C, Krajewski K, Rothbart SB, Bedford MT, Strahl BD, Histone peptide microarray screen of chromo and Tudor domains defines new histone lysine methylation interactions, Epigenetics Chromatin. 10 (2017) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert J-P, Barsyte-Lovejoy D, Felletar I, Volkmer R, Müller S, Pawson T, Gingras A-C, Arrowsmith CH, Knapp S, Histone recognition and large-scale structural analysis of the human bromodomain family, Cell. 149 (2012) 214–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bowman GD, Poirier MG, Post-translational modifications of histones that influence nucleosome dynamics, Chem. Rev. 115 (2015) 2274–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Müller MM, Muir TW, Histones: at the crossroads of peptide and protein chemistry, Chem. Rev. 115 (2015) 2296–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, Lu Z, Ye Z, Zhu Q, Wysocka J, Ye Y, Khochbin S, Ren B, Zhao Y, Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification, Cell. 146 (2011) 1016–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li Y, Sabari BR, Panchenko T, Wen H, Zhao D, Guan H, Wan L, Huang H, Tang Z, Zhao Y, Roeder RG, Shi X, Allis CD, Li H, Molecular Coupling of Histone Crotonylation and Active Transcription by AF9 YEATS Domain, Mol. Cell. 62 (2016) 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang Y, Shan C-M, Wang J, Bao K, Tong L, Jia S, Molecular basis for the role of oncogenic histone mutations in modulating H3K36 methylation, Sci. Rep. 7 (2017) 43906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lowe BR, Maxham LA, Hamey JJ, Wilkins MR, Partridge JF, Histone H3 Mutations: An Updated View of Their Role in Chromatin Deregulation and Cancer, Cancers. 11 (2019). 10.3390/cancers11050660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.