Abstract
Background:
Heart chymase rather than angiotensin converting enzyme has higher specificity for angiotensin (Ang) I conversion into Ang II in humans. A new pathway for direct cardiac Ang II generation has been revealed through the demonstration that Ang-(1-12) is cleaved by chymase to generate Ang II directly. Herein, we address whether Ang-(1-12), chymase mRNA and activity levels can be differentiated in human atrial tissue from normal and diseased hearts, and if these measures associate with various pathological heart conditions.
Materials and Methods:
Atrial appendages were collected from 11 non-failing donor hearts and 111 patients undergoing heart surgery for the correction of valvular heart disease, resistant atrial fibrillation or ischemic heart disease. Chymase mRNA was analyzed by real-time PCR and enzymatic activity by high-performance liquid chromatography using Ang-(1-12) as the substrate. Ang-(1-12) levels were determined by immunohistochemical staining.
Results:
Chymase gene transcripts, chymase activity, and immunoreactive-Ang-(1-12) expression levels were higher in left compared to right atrial tissue, irrespective of cardiac disease. Also, left atrial chymase mRNA expression was significantly higher in stroke vs. non-stroke patients, and in cardiac surgery patients who had a history of postoperative atrial fibrillation vs. non-atrial fibrillation. Correlation analysis showed that left atrial chymase mRNA was positively related to left atrial enlargement, as determined by echocardiography.
Conclusions:
As Ang-(1-12) expression and chymase gene transcripts and enzymatic activity levels were positively linked to left atrial size in patients with left ventricular heart disease an important alternate Ang II forming pathway, via Ang-(1-12) and chymase, in maladaptive atrial and ventricular remodeling in humans is uncovered.
Keywords: chymase, angiotensin-(1-12), angiotensin II, human, heart disease, atria
Introduction
The renin-angiotensin system (RAS) plays a crucial role in the regulation of blood pressure and fluid balance while in the heart increased angiotensin II (Ang II), the main effector hormone of the RAS, contributes to adverse myocardial remodeling, cardiac fibrosis, and arrhythmias. Although increased cardiac RAS components are linked to the pathogenesis of heart disease, the outcomes of trials evaluating the efficacy of angiotensin converting enzyme (ACE) inhibitors, Ang II receptor blockers (ARBs) or a direct renin inhibitor have not fully achieved the benefits that would be expected from a robust experimental literature documenting Ang II as a cause of or a major contributor to the pathogenesis of heart disease.1–5 To reconcile the gap between treatment effects in animal experiments and clinical trial results, proposed explanations include species differences in Ang II processing pathways in humans and animals,6,7 compensatory ACE inhibition escape,8,9 failure of drugs to access the intracellular sites at which Ang II acts,10–13 or these factors in combination. A new perspective to this problem emanates from the identification of an alternate tissue Ang II forming mechanism in which an endogenously produced extended form of angiotensin I (Ang I) liberates Ang II directly through a non-ACE pathway.14,15
Angiotensin-(1-12) [Ang-(1-12)], a newly identified peptide from rat small intestine14 is expressed in the heart and kidneys of rats at concentrations much higher than those of Ang I and Ang II.14 Through a series of publications we showed: 1) increased expression and content of Ang-(1-12) in the hypertrophied heart of spontaneously hypertensive rats (SHR);16 2) the production of Ang II from Ang-(1-12) to be independent of ACE in the isolated heart of two rat hypertensive strains and three normotensive control rats,15 the heart of anephric Wistar Kyoto (WKY) rats,17 and rats fed a low salt diet;18 and 3) a contribution of chymase in Ang-(1-12) metabolism in the heart of SHR19 while ACE accounts for Ang-(1-12) metabolism in the circulation of both WKY and SHR.20 In extending these experimental findings to the human heart we identified Ang-(1-12) and chymase in left atrial specimens from patients undergoing a heart procedure for primary control of atrial fibrillation21 and left ventricular tissue from healthy subjects.22.
The significant differential roles of ACE and chymase in Ang-(1-12) metabolism between rats and human heart tissue coupled with our demonstration of co-expression of Ang-(1-12) with chymase21 suggested that this alternate substrate for Ang II production, being cleaved by chymase rather than ACE, may contribute to the pathogenesis of arrhythmias and the structural cardiac remodeling associated with diseases of the left heart. Since activation of autonomic nervous system mechanisms contribute to heart disease pathogenesis and particularly atrial fibrillation (AF),23 we also explored relations among atrial Ang-(1-12), chymase activity, brain and atrial natriuretic (BNP, ANP) peptides in atrial appendage specimens from patients undergoing heart surgery. In addition, since several studies reported molecular and biochemical differences between the two atria from humans and rodents,24 we also examined the potential differences in chymase activity and gene expression between left (LA) and right atrial (RA) tissues from both diseased and non-failing donor hearts, which had been rejected for use in transplantation surgery.
Methods
Ethic statement
The study was approved by the Wake Forest University Health Sciences (IRB 22619). Atrial appendages were obtained from 111 patients undergoing cardiac surgery at the Wake Forest Baptist Medical Center (Winston-Salem, NC, USA). Left and right segments of the atrial appendages were resected during cardiopulmonary bypass for correction of left cardiac valve replacement [aortic valve replacement (AVR), mitral valve regurgitation (MVR)], or coronary artery bypass grafting (CABG) (Table 1). Medical history, medications and specific surgical indication are documented in Table 1. Preoperative transthoracic echocardiograms25 and consent forms were obtained prior to cardiac surgery. Control right and left atrial tissues were obtained from Duke Human Heart repository’s (IRB#00079656) non-failing donor heart bank (n=11), that had been declined for transplantation (Table 1).
Table 1.
Sources of Atrial Tissue and Primary Subjects Characteristics
| VARIABLE |
Left Atria |
Right Atria |
|---|---|---|
| Control Subjects | ||
| Number of specimens | 11 | 11 |
| Gender (male/female) | 7/4 | 7/4 |
| Median age, yrs | 58 | 58 |
| Cardiac Surgery Patients | ||
| Number of specimens | 30 | 99 |
| Gender (male/female) | 19/11 | 76/23 |
| Median age, yrs. | 65 | 64.5 |
| Surgical Procedures: | ||
| Aortic valve disease; n (%) | 6 (20%) | 23 (23%) |
| Aortic valve repair/replacement | (2) | (18) |
| Aortic valve repair/replacement + MAZE | (3) | (4) |
| AVR + MVR | (1) | |
| AVR + MVR + CABG (triple procedure) | (1) | |
| Mitral valve disease; n (%) | 18 (60%) | 10 (10%) |
| Mitral valve repair/replacement | (10) | (7) |
| Mitral valve repair/replacement + MAZE | (7) | (2) |
| MVR + CABG + MAZE (triple procedure) | (1) | (1) |
| Ischemic Heart Disease; n (%) | 6 (20%) | 66 (67%) |
| CABG only | (54) | |
| CABG + Aortic valve repair/replacement | (7) | |
| CABG + Mitral valve repair/replacement | (4) | (3) |
| CABG +MAZE | (2) | (2) |
| Past Medical History | ||
| Hypertension; n (%) | 14 (46%) | 61 (61%) |
| Primary Atrial Fibrillation; n (%) | 16 (53%) | 15 (15%) |
| Diabetes mellitus; n (%) | 4 (13%) | 34 (34%) |
| Prior Medications | ||
| ACEnzyme Inhibitors | 12 (40%) | 33 (30%) |
| Angiotensin II Receptor Blockers | 8 (27%) | 22 (22%) |
| Calcium Channel Blockers | 9 (30%) | 25 (25%) |
| Beta blockers | 15 (50%) | 60 (61) |
| Statins | 13 (43%) | 61 (62%) |
| Diuretics | 15 (50%) | 40 (40%) |
| Loop Diuretics (furosemide) | (11) | (15) |
| Thiazide Diuretics (hydrochlorothiazide) | (4) | (16) |
| Loop & Thiazide Diuretics | (1) | |
| Aldosterone Blocking (spironolactone) | (3) | |
| Thiazide & Potassium Sparing Diuretics | (3) | |
| Loop & Aldosterone Blocking Diuretics | (1) | |
| Loop & Thiazide & Potassium sparing | (1) |
Ang-(1-12) immunohistochemistry
Human angiotensin-(1-12) was synthetized for us by AnaSpec Inc. (San Jose, CA). Immunohistochemistry was performed using an affinity purified polyclonal antibody directed to the COOH-terminus of the full length of the sequence of human Ang-(1-12) [Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Val11-Ile12]. Excised segments of the left and right atrial appendages were immediately immersed in a solution of 4% paraformaldehyde for 24 h and then transferred into 70% ethanol. After dehydration, the tissues were embedded in paraffin and cut into 5 pm thick sections. Slides were warmed for 1 h (55°C), deparaffinized in xylene, and, after being subsequently dipped in serial solutions of ethanol (100%, 95%, 85% and 70%), were rinsed in phosphate buffered saline (PBS). The slides were incubated in an antigen retrieval buffer (Antigen Unmasking Solution H-3300; Vector Laboratories Inc., Burlington, CA) and washed with double distilled water. Slides were then incubated for 5 min in 3% hydrogen peroxide to block the endogenous peroxidase. The sections were blocked with 1% bovine serum in phosphate buffered saline (PBS) with 5% normal goat serum for 1 h at room temperature and then incubated with the affinity-purified human Ang-(1-12) primary antibody (1:1000 dilution in 1% bovine serum in PBS with 5% normal goat serum) overnight at 4°C. Sections independently treated with 5% normal goat serum in the absence of the primary antibody served as negative controls. Additional controls included sections treated with the primary antibody preincubated with a 20-fold excess of human Ang-(1-12) peptide. In prior studies, we documented that this human Ang-(1-12) antibody does not cross-react with either Ang I or Ang II;21,22 however, we observed cross reactivity with human angiotensinogen (AGT). Although literature data described low, if any, expression of angiotensinogen in human heart,26 additional sections were incubated with human angiotensinogen antibody (IBL, MN, 1:50). After incubating with the primary antibody, each section was washed three times in PBS. The sections were blocked with 1% bovine serum in PBS with 5% normal goat serum for 1 h at room temperature and then incubated with biotinylated goat anti-rabbit secondary antibody (1:400 dilutions in 1% bovine serum in PBS with 5% normal goat serum; Vector Laboratories Inc., Burlington, CA) for 3 h. After washing the secondary antibody with PBS, sections were stained with 3,3’-diaminobenzidine (DAB, Sigma-Aldrich Chemical Co. St. Louis, MO) in Tris-buffered saline (0.05 mol/L, pH 7.65), and counterstained with hematoxylin before being dehydrated and mounted. The Ang-(1-12) staining-positive rates were calculated using Image J software (http://imagej.nih.gov/ij/), a public domain Java image processing program developed by the National Institutes of Health.
Chymase activity assay
Native plasma membranes (PMs) were prepared as described previously.21,22 Frozen atrial tissue (30-60 mg) was homogenized at 4°C in 1 mL reaction buffer (25 mM HEPES, 125 mM NaCl, and 10 mM ZnCl2, pH 7.4) using a Tissue Lyzer (Qiagen, Inc., Valencia, CA) for 90 seconds at 20 Hz. The homogenate was centrifuged at low spin (200 g) for 1 minute at 4°C to remove the connective tissue and cell debris. The supernatant was transferred into a new tube and centrifuged at 28,000 g for 20 minutes at 4°C. The pellet (native membranes) was resuspended in the reaction buffer and stored at −80°C until assayed for chymase activity.
125I-Ang-(1-12) metabolism by human atrial tissue PMs were studied in the absence or presence of the chymase inhibitor chymostatin. Highly purified human 125I-Ang-(1-12) substrate [1 nmol/L of 125I-Ang-(1-12)] was added to native PMs in the presence of a mixture of enzyme and peptidase inhibitors (chymostatin, lisinopril, MLN-4760, SCH39370, amastatin, bestatin, benzyl succinate and p-chloromercuribenzoate; each 50 pM) or in the absence of chymostatin only for 30 minutes at 37°C. At the end of the incubation time the reaction was stopped by adding equal volume of 1% phosphoric acid, mixed well, centrifuged (28,000 g for 20 minutes to remove the native PMs) and stored at 4°C. On the day of analysis, the samples were filtered before separation by reverse-phase high-performance liquid chromatography (RP-HPLC). We used a linear gradient from 10% to 50% mobile phase B at a flow rate of 0.35 mL/minute at 32°C. The solvent system consisted of 0.1% phosphoric acid (mobile phase A) and 80% acetonitrile/0.1% phosphoric acid (mobile phase B). Eluted 125I products, monitored by an in-line flow-through gamma detector (BioScan Inc., Washington, DC), were identified by comparing them with the retention time of synthetic standard 125I peptides. Data were analyzed using Shimadzu LC Solution acquisition software (Kyoto, Japan). The metabolic products are documented as the percent of Ang peptides fraction generated from the parent substrate by chymase. Chymase activity was calculated as the amount of parent 125I-Ang-(1-12) hydrolyzed into specific 125I-Ang II in the absence or presence of chymostatin and reported as fmoles of Ang II generated from the parent 125I-Ang-(1-12) in fmol/mg/min.
Analysis of gene expression by quantitative real-time PCR
Quantitative real-time PCR was used to detect gene mRNA levels in human atrial tissue. Total RNA was extracted from frozen, pulverized atria using TRIzol Reagent and processed according to the manufacturer’s recommendations. The quality and quantity of RNA samples were determined by spectrometry and agarose gel electrophoresis. Complementary first strand DNA was synthesized from oligo (dT)-primed total RNA, using the Omniscript RT kit (Qiagen Inc, CA). Relative quantification of mRNA levels by real-time PCR was performed using a SYBR Green PCR kit (Qiagen Inc. CA). Amplification and detection were performed with the QuantStudio 3 Real-Time PCR Systems (Thermo Fisher Scientific Inc.). Only one peak from the dissociation curve was found from each pair of oligonucleotide primers tested. Real-time PCR was carried out in duplicate; a no-template control was included in each run to check for contamination. It was also confirmed that no amplification occurred when samples were not subjected to reverse transcription. Sequence-specific oligonucleotide primers were designed according to published GenBank sequences (www.ncbi.nlm.nih.gov/Genbank) and confirmed with Oligo-Analyzer 3.0. The relative target mRNA levels in each sample were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Echocardiography
Preoperative transthoracic echocardiograms were performed by highly trained sonographers using a 1-5 MHz phased array transducer (Philips S5-1) and Philips iE33 sector scanner (Philips Medical Systems, Andover, MA). Digitally-stored images were reviewed and final reports were completed off-line (Xcelera 3.1; Koninklijke Philips Electronics, Amsterdam, The Netherlands) by cardiologists board certified in adult echocardiography. The preoperative transthoracic echocardiograms reported here were the studies most proximate to the patient’s surgery. An experienced investigator trained in perioperative echocardiography (LG), who was masked to biochemical and histological findings, manually reviewed all stored images in conjunction with the archived echocardiographic report. Transthoracic echocardiograms were performed and analyzed according to American Society of Echocardiography recommendations.27 Left ventricular end-diastolic and end-systolic internal diameters (LVID and LVIS, respectively), and end diastolic LV posterior wall (LVPW) and interventricular septal diameters (IVS) and left ventricular end-systolic left atrial diameter measurements were acquired and measured from the parasternal long-axis view using two-dimensional guided M-mode echocardiography by the leading edge-to-leading edge technique. Left ventricular end diastolic and end systolic volumes (EDV and ESV, respectively) and left atrial (LA) volume at end ventricular systole were measured by the biplane method of disks (modified Simpson’s rule) using apical 4-chamber and apical 2-chamber. Left ventricular ejection fraction was calculated as LVEF (%) = [(EDV-ESV/EDV)] × 100%. Mitral inflow measurements of early and late filling velocities were obtained using pulsed Doppler, with the sample volume placed at the tips of mitral leaflets from an apical four-chamber orientation. The early-to-late filing velocity ratio (E/A) was calculated in those patients who were in sinus rhythm at the time of the examination.
Statistics
Most of the groups did not pass the D’Agostino and Pearson omnibus normality test and therefore nonparametric evaluation was performed for these comparisons. Statistical differences were tested by Mann-Whitney test for two-group analysis. Differences between immunoreactive Ang-(1-12) staining in patients’ LA and RA, as well as the difference in chymase activities in normal LA and RA were evaluated by the Student’s t-test for unpaired analysis. In evaluating differences among echocardiographic variables, Kruskal-Wallis test followed by Dunn’s Multiple Comparison Test were employed and the results are presented as mean ± SE. Spearmen’s correlation coefficient was used for analyzing associations among molecular and biochemical parameters. Values of p < 0.05 were considered significant. Statistical analysis was carried out using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA).
Results
Healthy subject RA and LA tissues
Demographic characteristics of non-failing control heart donors are presented in Table 1. LA and RA were obtained from 11 subjects (7 males and 4 females, mean age of 57 years) whose hearts were rejected for transplantation. The absence of pathological heart function had been documented in these subjects by echocardiographic examination or by a negative finding in the patient’s medical record.
Analysis of chymase activity and chymase mRNA expression in control RA and LA are presented in Figure 1. Comparing to RA tissue, chymase activity and mRNA expression were 4- and 15-fold higher in LA, respectively. Similarly, ANF and BNP mRNA expression were higher in LA than in RA while comparable levels of AGT and ACE transcripts were found in both atria from control hearts (Figure 1). In addition, chymase mRNA correlated positively with chymase activity only in LA (r=0.76; p=0.0086) not RA (r=0.30; p=0.3743), whereas no significant correlations were observed between chymase mRNA level or activity and the gene expression levels of ANF, BNP, ACE, and AGT (data not shown).
Figure 1.
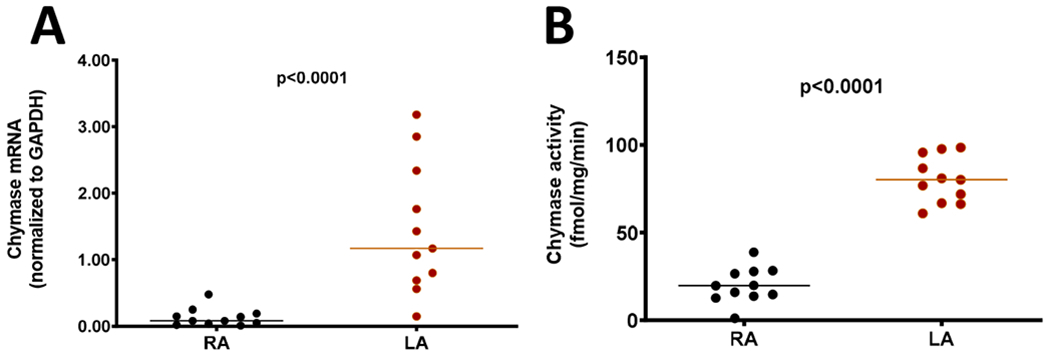
Chymase activity and mRNA levels of chymase, AGT, ACE, ANF, and BNP in right (RA) versus left atria tissues (LA) obtained from non-failing human control hearts.
Diseased RA and LA tissues
Table 1 also includes sources of atrial appendage tissue and cardiac surgery patient characteristics. A total of 129 right and left atrial appendage specimens were obtained from 111 subjects, who were predominately male and older than 60 years of age (Table 1). Among LA tissues collected, most patients had undergone mitral valve replacement (60%), while the majority of RA tissue came from patients with ischemic heart disease (67%). Among the examined tissue, 13 LA (43%) and 9 RA (9%) specimens were obtained from patients in whom the surgical intervention was associated with placement of incisions within the atria to block the conduction of errant electrical impulses for correction of atrial fibrillation.28 Hypertension (61%) and diabetes (34%) were more prevalent in patients from whom RA was obtained, while primary atrial fibrillation was found in half of the LA donors (53%) as opposed to only 15% of RA tissue donors.
The state of cardiac function prior to surgical intervention are presented in Data In Brief.25 Mean left ventricular ejection fraction (LVEF) was preserved across all groups of patients except in those patients who underwent coronary artery bypass graft (CABG) surgery, where it was slightly reduced. When compared to the CABG only group, peak velocities of early mitral blood flow were higher in mitral valve repair groups with and without concomitant CABG. There were no other differences in echocardiographic parameters of LV function or morphology between patients undergoing the different cardiac surgery procedures.
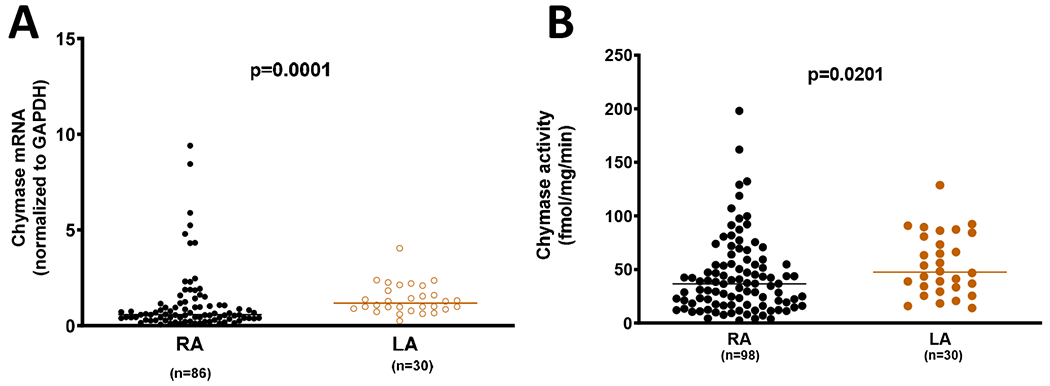
Compared to control subjects, chymase mRNA significantly increased in diseased RA (0.38 ± 0.07 vs. 0.18 ± 0.05, p=0.03) while chymase activity did not change (36.7 ± 8.5 vs. 26.6 ± 6.0, p=0.36). Surprisingly, chymase activity in the LA was dramatically decreased in diseased tissues compared to controls (48.5 ± 7.0 vs. 82.6 ± 3.7, p=0.0004), while there were no differences in chymase mRNA levels (1.45±0.22 vs. 1.71 ± 0.37, p=0.54). In both RA and LA, the mRNA levels of ANF, BNP, and ACE were increased in diseased vs. control tissue, while AGT mRNA was higher in RA, but not in LA, of diseased tissues when compared to controls (data not shown).
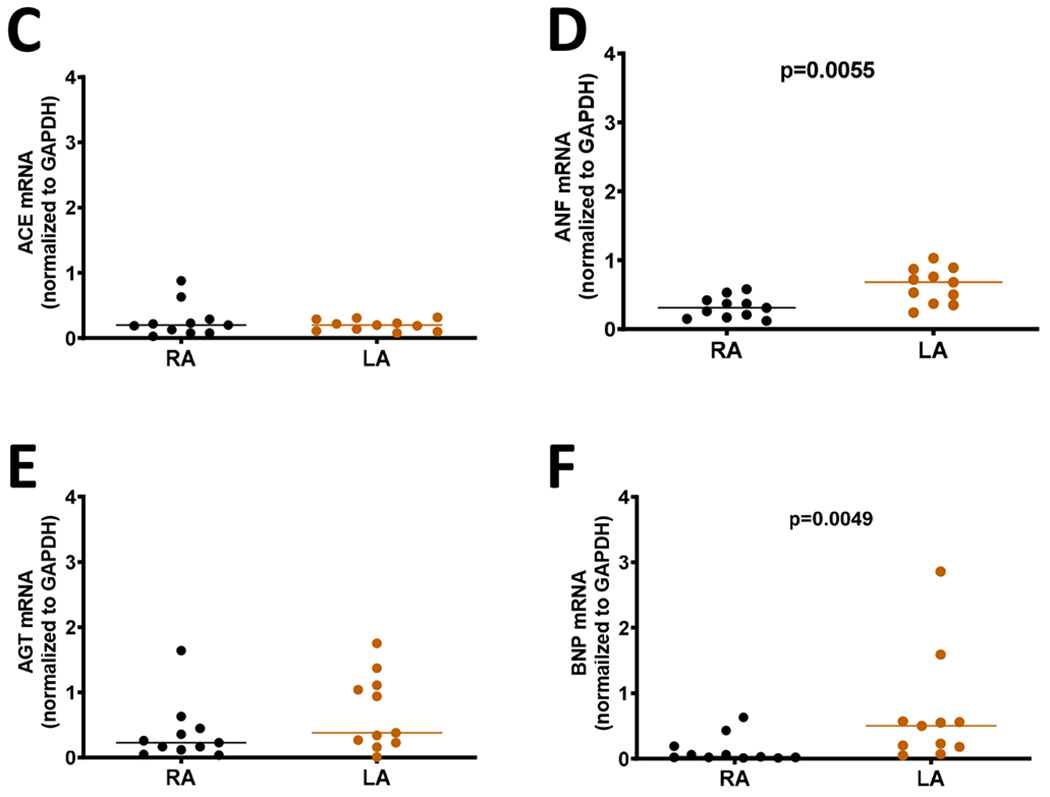
Ang-(1-12) staining was visualized within 11 LA and 17 RA tissues. The immunoreactive staining was primarily concentrated in the cytoplasm of cardiac myocytes (Figure 2A). There were striking differences between the Ang-(1-12) staining patterns between the left (Figure 2A, top) and RA (Figure 2A, bottom) with a consistently higher expression of the peptide in the LA. No immunostaining was detected in a control section treated with the primary antibody preincubated with 10x molar concentration (0.115 μM) of the Ang-(1-12) peptide to which the antibody was directed. In addition, specificity in detecting immunoreactive Ang-(1-12) was confirmed in sections in which an antibody against human AGT (1:50; IBL, MN) showed faint or no staining in atrial tissue. Quantitative analysis revealed 54% higher Ang-(1-12) intensity expression in LA compared to the RA (Figure 2B). The differences in staining between the two cardiac chambers were further validated in five subjects in whom tissue from both their right and left atrial appendages were obtained, although the difference did not reach statistical significance (p=0.0723).
Figure 2.
(A) Representative images of angiotensin-(1-12) [Ang-(1-12)] and angiotensinogen immunoreactive staining in human left (LA) and right atria (RA) obtained from patients undergoing cardiac surgery. i, no primary antibody/negative control; ii, Ang-(1-12) antibody; iii, Ang-(1-12) antibody blocked with human Ang-(1-12) peptide before staining; and iv, angiotensinogen antibody. Scale bar indicates 100 μm. (B) Quantitative analysis of Ang-(1-12) expression in the right (n = 17) and left atria (n = 11) obtained from patients undergoing cardiac surgery. Data are means ± SE.
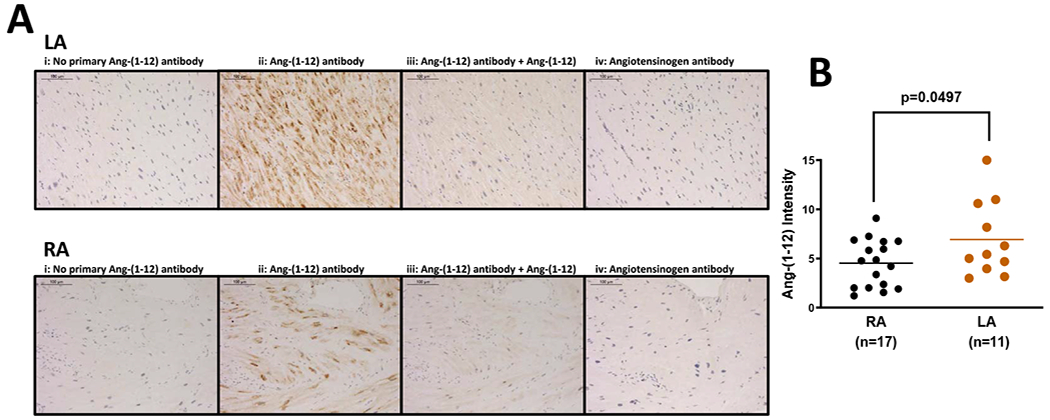
The increased Ang-(1-12) expression in LA was associated with higher values of chymase activity than those measured in RA tissue (Figure 3). Similarly, tissue chymase gene expression was significantly augmented in the left compared to the right atrium appendages (Figure 3). Regarding the other RAS components, tissue expression of AGT and ACE gene transcripts were not different in LA and RA (Figure 3). In contrast, expression of BNP and ANP were higher in LA when compared to the RA (Figure 3).
Figure 3.
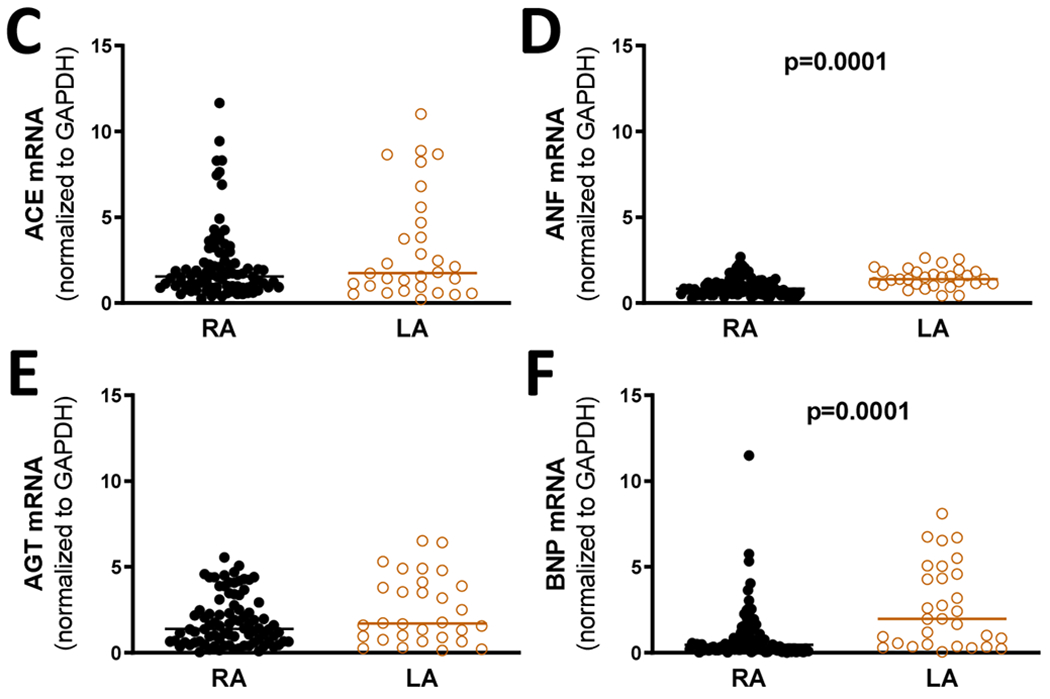
Chymase activity and mRNA levels of chymase, AGT, ACE, ANF, and BNP in right (RA) versus left atria tissues (LA) obtained from patients with heart diseases.
Ang-(1-12)/chymase axis in various pathological heart conditions
Chymase activities (Table 2) 25
Table 2.
Chymase activity in left and right atria
| Total | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SEM | P value | n | Mean ± SEM | P value | n | Mean ± SEM | P value | |
| LA chymase activity | |||||||||
| CHF | 3 | 84.3±5.7 | 0.054 | 3 | 84.3±5.7 | 0.018 | 0 | N/A | |
| Non-CHF | 27 | 51.0±5.4 | 16 | 46.1±6.1 | 11 | 58.0±9.9 | |||
| MR | 18 | 58.5±6.8 | 0.328 | 10 | 49.4±8.1 | 0.645 | 8 | 70.0±10.5 | 0.039 |
| Non-MR | 12 | 47.9±8.3 | 9 | 55.2±9.7 | 3 | 25.9±7.8 | |||
| ARB | 8 | 39.4±8.6 | 0.085 | 6 | 34.9±5.5 | 0.052 | 2 | 52.7±36.8 | 0.82 |
| Non-ARB | 22 | 59.7±6.1 | 13 | 60.1±7.7 | 9 | 59.2±10.6 | |||
| RA chymase activity | |||||||||
| HT | 61 | 48.6±5.0 | 0.052 | 48 | 46.7±5.5 | 0.023 | 13 | 55.3±12.4 | 0.74 |
| Non-HT | 37 | 34.3±4.3 | 27 | 28.6±3.7 | 10 | 49.4±11.6 | |||
| MVD | 5 | 70.0±25.3 | 0.080 | 1 | 59.09± | N/A | 4 | 72.8±32.5 | 0.290 |
| Non-MVD | 92 | 41.5±3.5 | 73 | 39.63±3.94 | 19 | 48.6±7.9 | |||
CHF: congestive heart failure; MR: mitral regurgitation; ARB: Angiotensin II receptor blocker; HT: hypertension; MVD: mitral valve disease.
LA chymase activity was significantly lower in male patients without congestive heart failure (CHF) compared to male CHF patients (p=0.018). Unfortunately, the female patient number was insufficient for statistical analysis. After combining male and female patients, the LA chymase activity tended to be lower in non-CHF vs. CHF patients (p=0.054). In female mitral regurgitation (MR) patients, LA chymase activity was dramatically higher in MR vs. non-MR patients (p=0.039). In right atria, chymase activity was higher only in male, but not in female, hypertensive (HT) patients compared with non-HT patients.
Chymase mRNA (Table 3) 25
Table 3.
Chymase mRNA levels in left and right atria
| Total | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SEM | P value | n | Mean ± SEM | P value | n | Mean ± SEM | P value | |
| LA chymase mRNA | |||||||||
| Stroke | 3 | 2.44±0.86 | 0.0092 | 1 | 1.08 | N/A | 2 | 3.12±0.92 | 0.0017 |
| Non-Stroke | 27 | 1.26±0.11 | 18 | 1.37±0.15 | 9 | 1.05±0.15 | |||
| PO-AF | 14 | 1.76±0.25 | 0.009 | 10 | 1.64±0.23 | 0.028 | 4 | 2.05±0.73 | 0.125 |
| Non-PO-AF | 16 | 1.05±0.09 | 9 | 1.04±0.08 | 7 | 1.06±0.18 | |||
| MVD | 5 | 1.91±0.58 | 0.0779 | 1 | 0.99 | N/A | 4 | 2.14±0.69 | 0.076 |
| Non-MVD | 23 | 1.23±0.12 | 16 | 1.33±0.15 | 7 | 1.02±0.19 | |||
| ARB | 8 | 1.06±0.23 | 0.1729 | 6 | 1.16±0.26 | 0.3570 | 2 | 0.77±0.52 | 0.337 |
| Non-ARB | 22 | 1.50±0.17 | 13 | 1.45±0.17 | 9 | 1.57±0.35 | |||
| RA chymase mRNA | |||||||||
| HT | 52 | 1.34±0.25 | 0.1886 | 41 | 1.26±0.29 | 0.4989 | 11 | 1.62±0.50 | 0.075 |
| Non-HT | 34 | 0.85±0.25 | 26 | 0.96±0.32 | 8 | 0.50±0.08 | |||
| CAD | 52 | 0.93±0.15 | 0.0698 | 43 | 0.87±0.15 | 0.0381 | 9 | 1.20±0.50 | 0.990 |
| Non-CAD | 24 | 1.71±0.54 | 15 | 2.01±0.82 | 9 | 1.19±0.44 | |||
| MVD | 4 | 2.45±1.22 | 0.1127 | 0 | N/A | 4 | 2.45±1.22 | 0.026 | |
| Non-MVD | 81 | 1.09±0.18 | 66 | 1.16±0.22 | 15 | 0.80±0.18 | |||
| AVD | 25 | 1.72±0.49 | 0.0389 | 21 | 1.75±0.57 | 0.0638 | 4 | 1.59±0.93 | 0.419 |
| Non-AVD | 59 | 0.90±0.15 | 45 | 0.89±0.17 | 14 | 0.94±0.33 | |||
| BB | 50 | 0.95±0.13 | 0.1659 | 39 | 0.86±0.13 | 0.1020 | 11 | 1.23±0.42 | 0.752 |
| Non-BB | 35 | 1.46±0.39 | 27 | 1.58±0.49 | 8 | 1.02±0.48 | |||
PO-AF: postoperative atrial fibrillation; ARB: Angiotensin II receptor blocker; HT: hypertension; CAD: coronary artery disease; MVD: mitral valve disease; AVD: aortic valve disease; BB: Beta blocker.
The stroke patient number in males was insufficient for statistical analysis. However, with both sexes together and females only, LA chymase mRNA were significantly higher in stroke versus non-stroke patients. In both sexes together and males alone with a history of postoperative atrial fibrillation (PO-AF), LA chymase mRNA was higher than in non-PO-AF patients. In RA, chymase mRNA was lower in male patients with coronary artery disease (CAD) vs. non-CAD (p=0.038), while it was higher in the combination of males and females with aortic valve disease (AVD) vs. non-AVD patients (p=0.039).
Ang-(1-12) (Table 4) 25
Table 4.
Ang-(1-12) levels in left and right atria
| Total | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SEM | P value | n | Mean ± SEM | P value | n | Mean ± SEM | P value | |
| LA Ang-(1-12) | |||||||||
| PO-AF | 8 | 8.27±1.29 | 0.0517 | 7 | 7.31±1.00 | 0.0676 | 1 | 15.00 | N/A |
| Non-PO-AF | 3 | 3.36±0.30 | 2 | 3.07±0.08 | 1 | 3.95 | |||
| ARB | 3 | 3.93±0.44 | 0.1143 | 2 | 3.92±0.77 | 0.2024 | 1 | 3.95 | N/A |
| Non-ARB | 8 | 8.06±1.39 | 7 | 7.07±1.13 | 1 | 15.00 | |||
| RA Ang-(1-12) | |||||||||
| AF | 4 | 2.48±0.78 | 0.0501 | 3 | 2.67±1.08 | 0.2370 | 1 | 1.91 | N/A |
| Non-AF | 13 | 5.14±0.64 | 9 | 4.74±0.87 | 4 | 6.03±0.67 | |||
PO-AF: postoperative atrial fibrilation; AF: atrial fibrillation; ARB: Angiotensin II receptor blocker.
Ang-(1-12) staining and quantification was performed in 23 patients including 11 LAs and 17 RAs. Five patients provided both LA and RA. In LA, there were tendencies, in both total (males and females combine) and male only patients, for Ang-(1-12) to be higher in those with a history of PO-AF vs. non-PO-AF (8.27 ± 1.29 vs. 3.36 ± 0.30, p=0.052 and 7.31 ± 1.00 vs. 3.07 ± 0.08, p=0.068 for total and male patients, respectively). However, in RA, Ang-(1-12) levels observed in those patients with a history of pre-existing AF (prior to the operation) tended to be lower than that observed in tissue from non-AF patient (2.48±0.78 vs. 5.14±0.64, p=0.050).
In cardiac patients, the chymase mRNA levels in LA were positively correlated with LA diameter and LA area (LAA) (r=0.571, p=0.011 and r=663, p=0.037, respectively). There was also a trend for of a positive relationship between LA chymase mRNA and LA Ang-(1-12) levels (p=0.089) (Figure 4).
Figure 4.
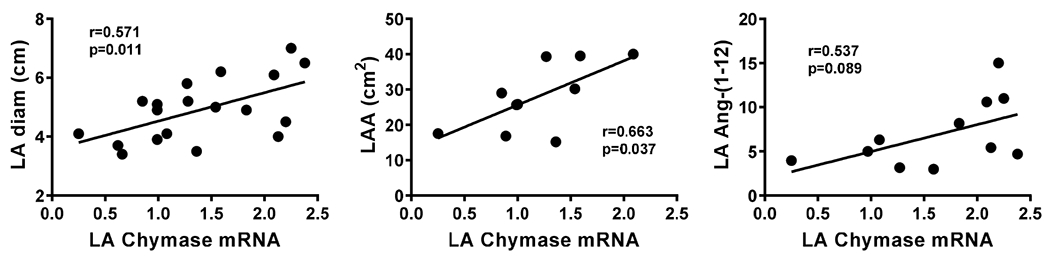
Correlation assays between left atrial (LA) chymase mRNA and LA diameter, area and Ang-(1-12) content.
Discussion
To our knowledge, this is the first study that systemically evaluated and compared the chymase/Ang-(1-12) in LA versus RA from both diseased and control subjects. In patients with left heart disease pathology, LA Ang-(1-12) expression and chymase gene transcripts and enzymatic activity levels positively associated with left atrial chamber size, suggesting a role for an Ang-(1-12)/chymase producing pathway to Ang II formation in the pathophysiology that characterizes enlargement of left atrial .
Little is known about the expression of angiotensin peptides in human heart disease, and no agreement exists as to whether cardiac intracrine Ang II production in humans follows biotransformation pathways identical to those described in rodents. In the pursuit of this objective, we show robust differences in the expression of Ang-(1-12) and chymase in the right and left atrial appendages from patients with heart disease. The high Ang-(1-12) expression in LA was associated with similar augmentation of the chymase gene and the enzymatic activity of its protein. The importance of these findings is that the increased expression and activity of this Ang II-forming pathway within atrial myocytes may not be amenable to blockade with ACE inhibitors or AT1 receptor blockers, as these drugs do not reach intracellular compartments.13
A differential Ang-(1-12) expression between the RA and LA was demonstrated in this study. A differential expression of Ang-(1-12) and chymase mRNA and activity between the two atria underscores a heretofore previously unrecognized selectivity of the biochemical components that upstream from Ang I lead to cardiac Ang II production. Stretch is a potent stimulus for cardiac Ang II production and release.29 The higher chymase/Ang-(1-12) found in the LA of patients with various heart diseases is in keeping with the observation that stretch is associated with activation of local Ang II activity.30 In addition, increased local Ang-(1-12) synthesis or myocyte uptake may be modulated by the characteristically heightened sympathetic activity found in left heart disease.31–34 Since Ang II stimulates presynaptic norepinephrine release, we suggest that stretch-induced activation of the Ang-(1-12)/chymase axis may be a linking mechanism by which Ang II facilitates increased net cardiac neurogenic drive. Moreover, increased cardiac sympathetic drives associated with volume overload causes mast cell degranulation and the attending release of chymase.35–37 Migration of mast cell-derived chymase from the cardiac interstitium into myocytes has been documented by us in patients with mitral valve insufficiency.38 Patients with MVR show increased norepinephrine release rates into the extravascular compartment33,39 while sympathetic overactivity contributes to the pathogenesis of AF.23 Therefore, the higher values of LA Ang-(1-12) expression, chymase mRNA, and activity may be related to the combined effects of the increased load imposed on the left atrium by either mitral valve incompetency or aortic valve restriction and increased cardiac sympathetic stimulation.
Increased expression of the Ang-(1-12)/chymase axis in human left heart disease is in keeping with experimental findings showing that chymase mediates the majority of Ang II production in the human heart6,40 while chymase inhibition attenuated Ang-(1-12)-induced experimental cardiac damage following ischemia-perfusion.41 Chymase is also an important Ang II forming mechanism within cardiomyocytes and fibroblasts.42,43 Moreover, the present findings extend our previous demonstration of chymase within LA myocytes of humans with enlarged left atria undergoing the Cox-Maze procedure for AF and the presence of chymase in left atrial myocytes of subjects with mitral valve regurgitation.38 As described elsewhere by us for the assessment of ACE 244 and chymase activities, 19,21,22 the chymase assay employed in these experiments obviated the use of synthetic substrates by evaluating the hydrolytic activity of the PM from the atrial tissue on 125I-Ang-(1-12).
The inconsistencies between chymase mRNA level and its enzymatic activity found in this study might be due to the post-transcriptional modifications that are involved in turning mRNA into protein, and the in vivo half-life of protein which also affects the analysis of protein level. Since chymase activity was measured by its ability to convert Ang-(1-12) to Ang II, this assay provides a better indication of the net ability of the enzyme to convert the substrate. Besides Ang II-production, chymase has other functions, such as mediating extracellular matrix (ECM) breakdown, which is also critical in cardiac fibrosis and remodeling45 and has not been determined in this study. Moreover, chymase is mainly produced and secreted from mast cells distributed in various tissues. Its activity has been found in blood which is affected by pathological conditions.46,47 This evidence suggests that it is also possible that chymase secreted from the mast cells in other tissues may migrate to the heart and affect the measured activity.
Compared to control subjects, chymase mRNA significantly increased in diseased RA while chymase activity did not change. Surprisingly, in LA, chymase activity was dramatically decreased in diseased tissues compared to controls, while there is no difference of chymase mRNA levels. Indeed, a limitation of this study is that the control tissue included in this study might not be representative of a “normal”, healthy heart. Although echocardiographic or medical records revealed no evidence of pathology, we cannot exclude the possibility that death from an acute injury might have affected the samples that were collected. Also, the manner of, and length of time for sample collection could have impacted chymase mRNA and activity levels in these tissue.45,48–50
In most cardiovascular diseases (CVDs), differences in pathophysiology, clinical presentation, and management have been observed between men and women. Estrogen status is believed to contribute to sex differences among CVDs. However, the exact roles and mechanisms of estrogen in female heart disease is not fully understood. The present study shows that in both RA and LA, chymase changes in diseased vs. control samples were different between male and female subjects (Table 2–4). In preclinical studies, using ovariectomized (OVX) rats, and cultured RBL-2H3 mast cells our group demonstrate that estrogen regulates mast cell proliferation and chymase expression through its receptor GPER45,48–54 The findings from these studies, as well as the present clinical data, strongly suggest a new mechanism that may underlie sex differences in heart disease; specifically, estrogens modulation of the Ang-(1-12)/chymase axis that leads to cardiac Ang II production and alterations in heart performance and structure. However, although current evidence shows that increased chymase is the “cause,” but not “consequence” of CVDs, we cannot exclude the possibility that differences in left atrial pressure, and cardiomyocyte stretch between male and female patients may differentially regulate chymase gene expression and activity.
The significant correlations between LA chymase gene transcript and LA diameter and area is clinically relevant as AF is mainly a disease of the left atrium, a concept that agrees with the effectiveness of ablation techniques to interrupt aberrant electrical pathways in the left atria55 and the association of AF with rare somatic mutations in left atrial myocytes.56 Correspondingly, in the patients with post-operation AF, LA chymase mRNA and LA Ang-(1-12) were higher when compared to patients without post-operation AF, strongly suggesting a role for LA Ang-(1-12)/chymase axis as an important mediator in the pathogenesis of post-operative AF.
Limitations and future studies
This clinical data shows the associations between chymase/Ang-(1-12) and pathological conditions of patients with heart disease. Our lab and others have previously shown that inhibition of chymase in rodent models protects the heart against various hemodynamic-related stresses.57–59 An orally active chymase inhibitor been recently evaluated in heart failure patients, underscores the importance of chymase in cardiovascular disease.60 All evidence suggests that chymase/Ang-(1-12), rather than the ACE/Ang II mediating pathway is the more important factor in human heart disease. Indeed, additional preclinical research, using more advanced molecular approaches such as gene knockout and overexpression models and cell systems, are needed to understand the exact roles of chymase/Ang-(1-12) in the pathogenesis of cardiovascular disease. We also realize that besides mediating Ang II formation, chymase might be involved in heart disease through Ang II-independent pathways, including inflammatory mechanisms,61,62 which need future investigations as well.
In summary, increased expression of the chymase/Ang-(1-12) pathway in the LA of subjects with heart disease, especially patients with heart failure and postoperative atrial fibrillation, implicates alternate mechanisms for tissue Ang II production in the processes accompanying adverse atrial and ventricular remodeling. The differential chymase and Ang-(1-12) expression levels in right and left atrial specimens provides an alternate rationale for the development of new therapeutic approaches to cardiac diseases. Our work has highly significant clinical implications because intracellular chymase-mediated Ang II formation is unaffected by ACE inhibitors or AT1 receptor antagonists, which act on the cell surface.63,64
Highlights:
Angiotensin (Ang)-(1-12)/chymase were higher in left versus right human atria
Left atrial (LA) Ang-(1-12)/chymase were positively associated with LA chamber size
LA Ang-(1-12)/chymase were associated with stroke and atrial fibrillation
Acknowledgment
This research was supported by grant HL-051952 from the National Heart Lung and Blood Institute (NHLBI) of the National Institutes of Health (CMF) and grants AG042758 and AG033727 (LG) from the National Institute on Aging (NIA), National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Authors have no conflict of interest to report.
References
- 1.Blood Pressure Lowering Treatment Trialists C, Turnbull F, Neal B, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008;336(7653):1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blood Pressure Lowering Treatment Trialists C, Turnbull F, Neal B, et al. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25(5):951–958. [DOI] [PubMed] [Google Scholar]
- 3.Disertori M, Barlera S, Staszewsky L, Latini R, Quintarelli S, Franzosi MG. Systematic review and meta-analysis: renin-Angiotensin system inhibitors in the prevention of atrial fibrillation recurrences: an unfulfilled hope. Cardiovasc Drugs Ther. 2012;26(1):47–54. [DOI] [PubMed] [Google Scholar]
- 4.Laight DW. Therapeutic inhibition of the renin angiotensin aldosterone system. Expert Opin Ther Pat. 2009;19(6):753–759. [DOI] [PubMed] [Google Scholar]
- 5.Turnbull F, Neal B, Algert C, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165(12):1410–1419. [DOI] [PubMed] [Google Scholar]
- 6.Balcells E, Meng QC, Johnson WH Jr., Oparil S, Dell’Italia LJ. Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol. 1997;273(4):H1769–1774. [DOI] [PubMed] [Google Scholar]
- 7.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289(6):H2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennezat PV, Berlowitz M, Sonnenblick EH, Le Jemtel TH. Therapeutic implications of escape from angiotensin-converting enzyme inhibition in patients with chronic heart failure. Curr Cardiol Rep. 2000;2(3):258–262. [DOI] [PubMed] [Google Scholar]
- 9.van den Meiracker AH, Man in ‘t Veld AJ, Admiraal PJ, et al. Partial escape of angiotensin converting enzyme (ACE) inhibition during prolonged ACE inhibitor treatment: does it exist and does it affect the antihypertensive response? J Hypertens. 1992;10(8):803–812. [PubMed] [Google Scholar]
- 10.Baker KM, Booz GW, Dostal DE. Cardiac actions of angiotensin II: Role of an intracardiac renin-angiotensin system. Annu Rev Physiol. 1992;54:227–241. [DOI] [PubMed] [Google Scholar]
- 11.De Mello WC, Danser AH. Angiotensin II and the heart : on the intracrine renin-angiotensin system. Hypertension. 2000;35(6):1183–1188. [DOI] [PubMed] [Google Scholar]
- 12.Dostal DE, Baker KM. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res. 1999;85(7):643–650. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Thomas CM, Yong QC, Chen W, Baker KM. The intracrine renin-angiotensin system. Clin Sci (Lond). 2012;123(5):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350(4):1026–1031. [DOI] [PubMed] [Google Scholar]
- 15.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008;294(5):H2242–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jessup JA, Trask AJ, Chappell MC, et al. Localization of the novel angiotensin peptide, angiotensin-(1-12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2008;294(6):H2614–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrario CM, Varagic J, Habibi J, et al. Differential regulation of angiotensin-(1-12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol. 2009;296(4):H1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata S, Kato J, Kuwasako K, Kitamura K. Plasma and tissue levels of proangiotensin-12 and components of the renin-angiotensin system (RAS) following low- or high-salt feeding in rats. Peptides. 2010;31(5):889–892. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1-12) by neonatal cardiac myocytes. PLoS One. 2011;6(1):e15759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moniwa N, Varagic J, Simington SW, et al. Primacy of angiotensin converting enzyme in angiotensin-(1-12) metabolism. Am J Physiol Heart Circ Physiol. 2013;305(5):H644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue. PLoS One. 2011;6(12):e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad S, Wei CC, Tallaj J, et al. Chymase mediates angiotensin-(1-12) metabolism in normal human hearts. J Am Soc Hypertens. 2013;7(2):128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linz D, Ukena C, Mahfoud F, Neuberger HR, Bohm M. Atrial autonomic innervation: a target for interventional antiarrhythmic therapy? J Am Coll Cardiol. 2014;63(3):215–224. [DOI] [PubMed] [Google Scholar]
- 24.Onuoha GN, Nicholls DP, Alpar EK, Ritchie A, Shaw C, Buchanan K. Regulatory peptides in the heart and major vessels of man and mammals. Neuropeptides. 1999;33(2):165–172. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Varagic J, Nagata S, Kon ND, Groban L, Ahmad S, VonCannon JL, Wright KN, Sun X, Deal D, Ferrario CM. Atrial angiotensin-(1-12)/chymase expression data in patient of heart diseases. Data In Brief (Submitted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urata H, Hoffmann S, Ganten D. Tissue angiotensin II system in the human heart. Eur Heart J. 1994;15 Suppl D:68–78. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. [DOI] [PubMed] [Google Scholar]
- 28.Cox JL, Boineau JP, Schuessler RB, Kater KM, Lappas DG. Five-year experience with the maze procedure for atrial fibrillation. Ann Thorac Surg. 1993;56(4):814–823; discussion 823-814. [DOI] [PubMed] [Google Scholar]
- 29.Yang BC, Phillips MI, Ambuehl PE, Shen LP, Mehta P, Mehta JL. Increase in angiotensin II type 1 receptor expression immediately after ischemia-reperfusion in isolated rat hearts. Circulation. 1997;96(3):922–926. [DOI] [PubMed] [Google Scholar]
- 30.Cingolani HE, Perez NG, Cingolani OH, Ennis IL. The Anrep effect: 100 years later. Am J Physiol Heart Circ Physiol. 2013;304(2):H175–182. [DOI] [PubMed] [Google Scholar]
- 31.Kienzle MG, Ferguson DW, Birkett CL, Myers GA, Berg WJ, Mariano DJ. Clinical, hemodynamic and sympathetic neural correlates of heart rate variability in congestive heart failure. Am J Cardiol. 1992;69(8):761–767. [DOI] [PubMed] [Google Scholar]
- 32.Malliani A, Montano N. Sympathetic overactivity in ischaemic heart disease. Clin Sci (Lond). 2004;106(6): 567–568. [DOI] [PubMed] [Google Scholar]
- 33.Mehta RH, Supiano MA, Oral H, et al. Compared with control subjects, the systemic sympathetic nervous system is activated in patients with mitral regurgitation. Am Heart J. 2003;145(6):1078–1085. [DOI] [PubMed] [Google Scholar]
- 34.Schomig A, Haass M, Richardt G. Catecholamine release and arrhythmias in acute myocardial ischaemia. Eur Heart J. 1991;12 Suppl F:38–47. [DOI] [PubMed] [Google Scholar]
- 35.Arizono N, Matsuda S, Hattori T, Kojima Y, Maeda T, Galli SJ. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab Invest. 1990;62(5):626–634. [PubMed] [Google Scholar]
- 36.Melendez GC, Li J, Law BA, Janicki JS, Supowit SC, Levick SP. Substance P induces adverse myocardial remodelling via a mechanism involving cardiac mast cells. Cardiovasc Res. 2011. ;92(3):420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ries P, Fuder H. Differential effects on sympathetic neurotransmission of mast cell degranulation by compound 48/80 or antigen in the rat isolated perfused heart. Methods Find Exp Clin Pharmacol. 1994;16(6):419–435. [PubMed] [Google Scholar]
- 38.Ferrario CM, Ahmad S, Nagata S, et al. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond). 2014;126(7):461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta RH, Supiano MA, Oral H, et al. Relation of systemic sympathetic nervous system activation to echocardiographic left ventricular size and performance and its implications in patients with mitral regurgitation. Am J Cardiol. 2000;86(11):1193–1197. [DOI] [PubMed] [Google Scholar]
- 40.Dell’Italia LJ, Husain A. Dissecting the role of chymase in angiotensin II formation and heart and blood vessel diseases. Curr Opin Cardiol. 2002;17(4):374–379. [DOI] [PubMed] [Google Scholar]
- 41.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82(1):40–50. [DOI] [PubMed] [Google Scholar]
- 42.Re RN, Cook JL. Noncanonical intracrine action. JAm SocHypertens. 2011;5(6):435–448. [DOI] [PubMed] [Google Scholar]
- 43.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol. 2008;294(4):H1675–1684. [DOI] [PubMed] [Google Scholar]
- 44.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. [DOI] [PubMed] [Google Scholar]
- 45.Dell’Italia LJ, Collawn JF, Ferrario CM. Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling. Circ Res. 2018;122(2):319–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Gu Y, Lewis DF, Alexander JS, Granger DN. Elevated plasma chymotrypsin-like protease (chymase) activity in women with preeclampsia. Hypertens Pregnancy. 2010;29(3):253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishio H, Takai S, Miyazaki M, Horiuchi H, Osawa M, Uemura K, Yoshida K, Mukaida M, Ueno Y, Suzuki K. Usefulness of serum mast cell-specific chymase levels for postmortem diagnosis of anaphylaxis. Int J Legal Med. 2005. November;119(6):331–4. [DOI] [PubMed] [Google Scholar]
- 48.Hendrix S, Kramer P, Pehl D, et al. Mast cells protect from post-traumatic brain inflammation by the mast cell-specific chymase mouse mast cell protease-4. FASEB J. 2013;27(3):920–929. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh WY, Chang TH, Chang HF, et al. Renal chymase-dependent pathway for angiotensin II formation mediated acute kidney injury in a mouse model of aristolochic acid I-induced acute nephropathy. PLoS One. 2019;14(1):e0210656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kempuraj D, Selvakumar GP, Thangavel R, et al. Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer’s Disease Pathogenesis. Front Neurosci. 2017;11:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, da Silva J, Alencar A, et al. Mast Cell Inhibition Attenuates Cardiac Remodeling and Diastolic Dysfunction in Middle-aged, Ovariectomized Fischer 344 x Brown Norway Rats. J Cardiovasc Pharmacol. 2016;68(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Jessup JA, Zhao Z, et al. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2.Lewis rats. PLoS One. 2013;8(10):e76992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Sun X, Ahmad S, Su J, Ferrario CM, Groban L. Estrogen modulates the differential expression of cardiac myocyte chymase isoforms and diastolic function. Mol Cell Biochem. 2019;456(1-2):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Z, Wang H, Lin M, Groban L. GPR30 decreases cardiac chymase/angiotensin II by inhibiting local mast cell number. Biochem Biophys Res Commun. 2015;459(1):131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666. [DOI] [PubMed] [Google Scholar]
- 56.Gollob MH, Jones DL, Krahn AD, et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354(25):2677–2688. [DOI] [PubMed] [Google Scholar]
- 57.Takai S, Jin D, Chen H, Li W, Yamamoto H, Yamanishi K, Miyazaki M, Higashino H, Yamanishi H, Okamura H. Chymase inhibition improves vascular dysfunction and survival in stroke-prone spontaneously hypertensive rats. JHypertens. 2014;32(8):1637–48. [DOI] [PubMed] [Google Scholar]
- 58.Maeda Y, Inoguchi T, Takei R, Hendarto H, Ide M, Inoue T, Kobayashi K, Urata H, Nishiyama A, Takayanagi R. Chymase inhibition prevents myocardial fibrosis through the attenuation of NOX4-associated oxidative stress in diabetic hamsters. J Diabetes Investig. 2012;3(4):354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, da Silva J, Alencar A, Zapata-Sudo G, Lin MR, Sun X, Ahmad S, Ferrario CM, Groban L. Mast Cell Inhibition Attenuates Cardiac Remodeling and Diastolic Dysfunction in Middle-aged, Ovariectomized Fischer 344 x Brown Norway Rats. J Cardiovasc Pharmacol. 2016;68(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanefendt F, ThuB U, Becka M, Boxnick S, Berse M, Schultz A, Otto C. Pharmacokinetics, Safety, and Tolerability of the Novel Chymase Inhibitor BAY 1142524 in Healthy Male Volunteers. Clin Pharmacol Drug Dev. 2019;8(4):467–79. [DOI] [PubMed] [Google Scholar]
- 61.Gurwitz D Mast-cell chymase: a new target for anti-inflammatory drugs. Trends Mol Med. 2000;6(3):99. [Google Scholar]
- 62.Palaniyandi SS, Nagai Y, Watanabe K, Ma M, Veeraveedu PT, Prakash P, Kamal FA, Abe Y, Yamaguchi K, Tachikawa H, Kodama M, Aizawa Y. Chymase inhibition reduces the progression to heart failure after autoimmune myocarditis in rats. Exp Biol Med (Maywood). 2007. October;232(9):1213–21. [DOI] [PubMed] [Google Scholar]
- 63.Baker KM, Chernin MI, Schreiber T, et al. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. RegulPept. 2004;120(1-3):5–13. [DOI] [PubMed] [Google Scholar]
- 64.Baker KM, Kumar R. Intracellular angiotensin II induces cell proliferation independentof AT1 receptor. Am J Physiol Cell Physiol. 2006;291(5):C995–1001. [DOI] [PubMed] [Google Scholar]


