Abstract
Purpose:
This study evaluates the pectoralis major (PM) tendon humeral insertion, utilizing imaging and histologic assessment in cadaveric specimens. Current descriptions of the pectoralis major tendon depict a bilaminar enthesis and clarification of the anatomy is important for diagnostic and surgical considerations.
Materials and Methods:
Fourteen fresh-frozen whole upper extremity specimens were used in this study. Magnetic resonance (MRI) and ultrasound (US) imaging of the PM muscles, tendons, and entheses were performed, followed by anatomic dissection and inspection. Morphology of the lateral tendon and entheses were evaluated, focused on the presence of layers. In eleven specimens, the lateral 3 cm of the PM tendon was carefully dissected from the footprint, while in three specimens, the tendon and humeral insertion were preserved and removed en bloc. Histology was performed in axial slabs along the medial-lateral length of the tendon and also evaluated for the presence of layers.
Results:
The superior-inferior and medial-lateral lengths of the PM footprint were 75 ± 9 mm and 7 ± 1 mm respectively. In all specimens, the clavicular and sternal head muscles and tendons were identified, with the clavicular head tendon generally being shorter. The medial-lateral length of the clavicular head tendon measured 19 ± 8 mm superiorly and 9 ± 3 mm inferiorly. The medial-lateral length of the sternal head tendon measured 38 ± 8 superiorly and 41 ± 18 mm inferiorly. All specimens demonstrated a unilaminar, not bilaminar, enthesis with abundant fibrocartilage on histology. Three specimens demonstrated interspersed entheseal fat and loose connective tissue at the enthesis on MRI and histology.
Conclusion:
The PM tendon humeral insertion consists of a unilaminar fibrocartilaginous enthesis. US, MRI, and histology failed to identify true tendon layers at the enthesis. Delaminating injuries reported in the literature may originate from a location other than the enthesis.
Level of Evidence: pectoralis major, magnetic resonance imaging, ultrasound, anatomy, histology, Anatomy Study, Cadaveric Dissection and Imaging
Classification of pectoralis major (PM) muscle and tendon injuries are a source of ongoing debate in the literature, mainly due to an evolving understanding of the complex anatomy. Classically, the muscular origin has been divided into two heads, a clavicular head superiorly, and a sternal head inferiorly. Several studies over the past century have sought to further elucidate the anatomy of the PM muscle. While these studies have consistently described a single clavicular head, the sternal head appears to be much more variable, having two to seven segments.2,13,18,21,28
There remains debate as to the anatomic configuration of the PM tendon, which can be divided into the medial tendon and the lateral insertion (or enthesis) at the lateral lip of the intertubercular sulcus or groove of the humerus. There is general consensus that the medial tendon is layered, with most authors describing a bilaminar appearance,2,12,13,18,21 although some have described a trilaminar appearance.14,28 However, the anatomy at the lateral insertion is less clear. Some authors have described coalescing of the lamina into a single tendon of insertion,10,16,18,28 while others have found a layered tendon insertion.12,13
In a previous investigation by Fung et al, the authors found variation in the lateral PM tendon insertion, with a bilaminar footprint in 4 out of 5 specimens (evident through blunt dissection).13 In all 5 specimens, there was an inferior continuity between the two laminae. Based on information obtained from these specimens, a classification of tendon failure has been proposed,12 and disseminated in the orthopedic surgery, sports medicine, and radiological literature. In addition, a specific sequence of tendon failure based on the “U” shaped enthesis has been proposed.9,11,15 These new patterns of PM tendon failure are based on the premise of the predominantly bilaminar footprint with a small region of unilaminar enthesis inferiorly. However, an entirely different failure scheme would be expected if another anatomic configuration were correct, such as an entirely unilaminar or bilaminar enthesis. In addition, the morphology of the lateral tendon and enthesis of the PM has other clinical implications. From the surgical perspective, insertional anatomy is important for repair procedures or transfer procedures where the tendon is harvested.22 From the diagnostic perspective, accurate anatomical knowledge is a pre-requisite for correct descriptions of injury, which clearly influences clinical decision-making and patient prognosis.
Notably, previous investigations on PM tendon anatomy have relied on anatomical dissection. However, it is well-known that it is possible to iatrogenically create non-anatomic planes during dissection.8 Preservation of the PM tendon with multi-modality imaging and histologic analysis would avoid this complication. The purpose of this study was to evaluate the morphology of the lateral tendon and enthesis of the pectoralis major using magnetic resonance (MR) and ultrasound (US) imaging with histologic correlation.
Materials and Methods
Cadavers and Specimen Preparation
Fourteen fresh-frozen whole upper extremity specimens (51 ± 14 years old at time of death, range 21–70 years, 9 males and 5 females, 6 left and 8 right shoulders) were obtained from a nonprofit whole-body donation company (United Tissue Network, Phoenix, AZ, USA) and used in accordance with institution guidelines. Upper extremities included intact scapulae and clavicles. None of the specimens had any evidence of surgery about the shoulder. The specimens were stored at −80°C and thawed for 24 hours in a water bath at room temperature prior to use.
Imaging
Magnetic resonance imaging of the PM, including the enthesis, was performed on a 3T clinical scanner (MR750, GE Healthcare, Milwaukee, WI, USA) using a 16-channel flexible coil. Shoulders were maximally adducted and oriented in the scanner in an equivalent manner to a patient being scanned in the head first, supine position. The free ends of the muscle were not specifically tensioned. Axial and sagittal oblique T1-weighted (TR/TE, 500–900 ms/9–11 ms) and T2-weighted (TR/TE, 4,000–7,000 ms/70 ms) fat-suppressed fast-spin echo sequences were used with field of view of 12 cm, matrix of 320 × 320, slice thickness of 3 mm, and 0 mm interslice gap. US imaging of the lateral tendon and enthesis was performed using a clinical scanner prior to dissection through the skin and directly on the tendon once exposed (Philips iU22, Philips, Bothell, WA, USA) and a linear transducer (L12–5).
Dissection and Anatomic Inspection
After imaging, the cadaveric specimens were dissected utilizing a standard deltopectoral interval approach until the PM was encountered. Both the anterior and posterior surfaces/laminae of the PM muscle and medial tendons were visually examined. A ruler was used to measure the superior-inferior dimensions of the footprint and the medial-lateral length between the myotendinous junction and insertion for each head at the superior and inferior portions. The presence or absence of adjoining tendinous or aponeurotic connections at the superior and inferior borders, such as a falciform ligament,25,26 aponeurotic expansion of the supraspinatus tendon23, or deltoid tendon connections2,20 were also noted. In all specimens, the lateral 3 cm of PM tendon was removed without dissection of the individual layers. In eleven specimens, the tendons were carefully removed from the footprint through a combination of sharp dissection and peeling. In three specimens, the lateral 3 cm of tendon and humeral insertion were preserved and removed en bloc.
Histologic Preparation
All samples were fixed in 10% zinc formalin and divided into three axial slabs (superior, middle, and inferior) corresponding to the cranial, middle, and caudal thirds of the tendon. The three specimens with tendon and bone were cut with a bandsaw (B16, Butcher Boy, Selmer TN, USA). Only three bony specimens were included due to initial concerns of potential tendon tissue damage related to bandsaw sectioning. Bony specimens were decalcified in 10% EDTA until complete as verified by radiography (Trident Specimen Radiography System, Hologic, Marlborough, MA, USA). All samples were paraffin embedded and 5 μm thick sections were cut and stained with hematoxylin and eosin (H&E). All slides were digitally scanned at 20x (Zeiss Axio Scan.Z1, Carl Zeiss Microscopy GmbH, Jena, Germany). In three select specimens, axial slabs were sub-sectioned in up to 10 different slabs due to a preponderance of interspersed loose connective tissue and fat at the enthesis, to evaluate for the potential presence or absence of histologically identifiable layers.
Imaging and Histologic Analysis
Magnetic resonance imaging and US images were examined in consensus by two musculoskeletal radiologists (B.K.H and E.Y.C., each with 8-years of experience in musculoskeletal radiology) for the presence of lateral tendon and enthesis layers. Distinct layers were defined as interposition of connective tissue between tendinous laminae or as signal intensity differences between laminae on images at the enthesis. In addition, medial-lateral widths of the PM footprint at the midportion were measured. Histologic slides were analyzed in consensus by a musculoskeletal pathologist (P.H., 50-years of experience in histopathology) and histotechnician (J.H.W., 17-years of experience in histopathology) for the presence of lateral tendon and enthesis layers. Layers were defined as loose connective tissue interposed between tendon extending from the bony attachment to the medial muscle. Histological presence of enthesis fibrocartilage, vascularity, and the presence of a tidemark were also noted.
Statistics
Statistical analyses were performed using SPSS (21.0, IBM Corporation, Armonk, NY, USA). The Shapiro–Wilk test was used to assess normality. The measurements obtained in specimens from females and males were compared using the Student’s t-test. Correlations were performed between footprint dimensions (superior-inferior lengths and medial-lateral widths) and donor age, heights, weights, and body mass indices. Paired Student’s t-tests were used to evaluate differences between medial-lateral tendon lengths at the superior and inferior portions of both heads. Histological measurements of medial-lateral length between the myotendinous junction and insertion were used when possible, but in cases where the myotendinous junction was not included in the histological block (e.g. most sternal head cases), gross ruler measurements were used instead. For statistical analysis, superior-inferior lengths grossly measured by a ruler were used and MRI measurements of medial-lateral widths of the footprint at the midportion were used. A p-value of < 0.05 was considered to indicate statistical significance.
Results
The superior-inferior length of the PM footprint measured 75 ± 9 mm (mean ± standard deviation) and the medial-lateral width of the footprint at the midportion measured 7.4 ± 1.3 mm. The mean footprint length (p=0.126) and width (p=0.178) were not statistically different between females and males. No significant correlations were found between superior-inferior footprint lengths and donor age (p=0.798), heights (p=0.432), weights (p=0.074), and body mass indices (p=0.656). No significant correlations were found between medial-lateral footprint widths and donor age (p=0.422), heights (p=0.201), weights (p=0.812), and body mass indices (p=0.961).
In all specimens, the clavicular and sternal head muscles were identified during the dissection. The medial-lateral length of the clavicular head tendon measured 19 ± 8 mm (range 7.8 – 35 mm) at the superior margin and 9 ± 3 mm (range 6 – 15 mm) at the inferior margin (p=0.001) (Figure 1A). On gross examination the clavicular head often had an extremely short tendon at its insertion onto the humerus, particularly at the inferior margin (Figures 2A and 3A). However, on imaging and histology, the clavicular head always had a tendon at the humeral insertion, although it measured as short as 6 mm. In all specimens the sternal head tendon demonstrated a greater medial-lateral length than the clavicular head tendon at all portions (Figures 2B and 3B). The medial-lateral length of the sternal head tendon measured 38 ± 8 (range 29 – 52 mm) at the superior margin and 41 ± 18 mm (range 11 – 75 mm) at the inferior margin (p=0.496) (Figure 1B).
Figure 1.

Schematic morphology of the PM tendon. (A) With the PM in-situ, the visible anterior tendon corresponds to the clavicular head, with labeled average measurements derived from this study. (B) With the PM muscle reflected laterally, the deep surface of the tendon is exposed which corresponds to the sternal head, also with labeled average.
Figure 2.
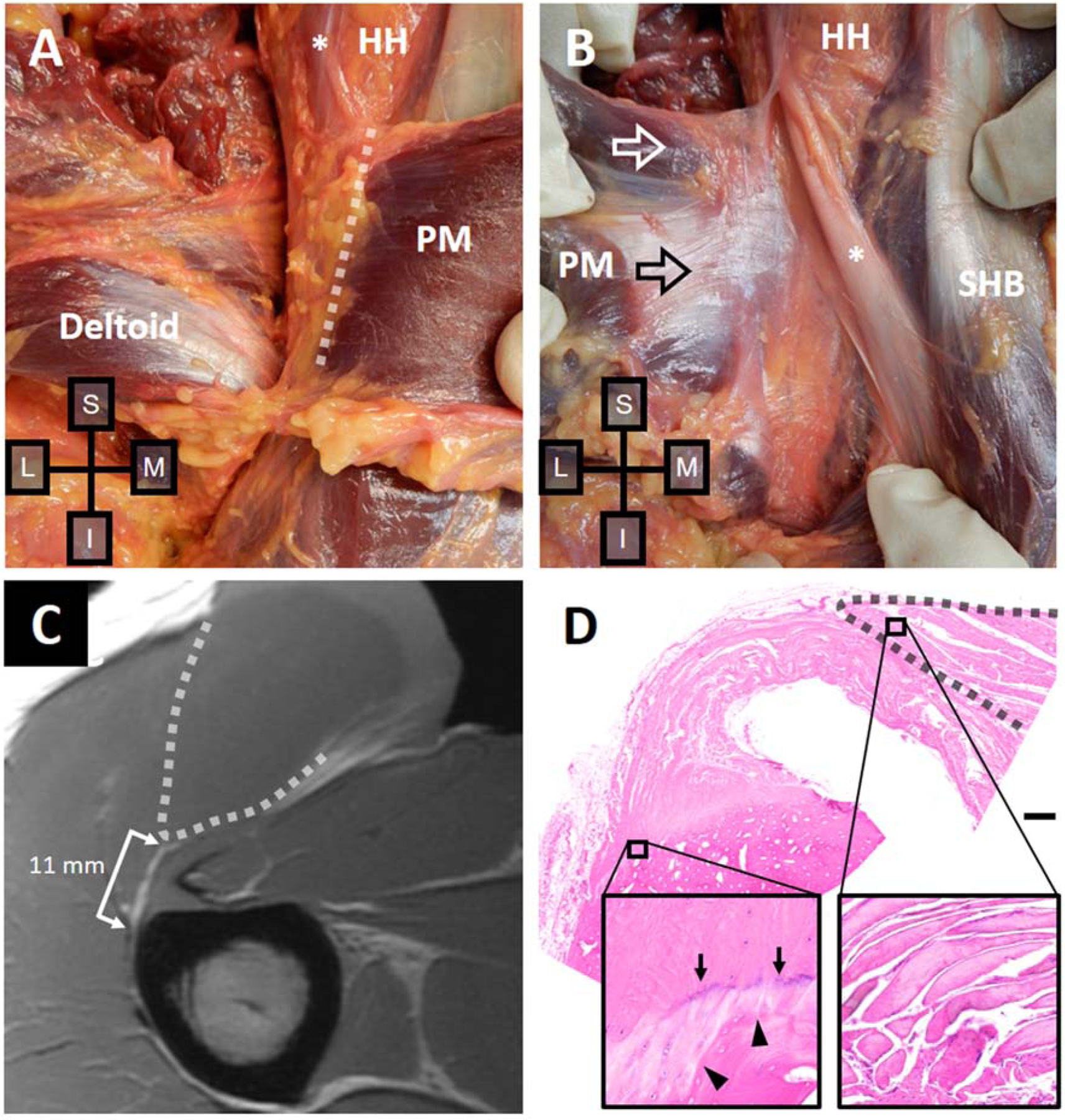
Right shoulder of a 21-year-old male donor. (A) With the deltoid reflected and PM in situ, the distal myotendinous junction of the clavicular head (dashed line) is located close to the humerus. (B) With the PM muscle reflected laterally, the posterior tendon of the sternal head is revealed (black arrow). The anterior muscle from the clavicular head (white arrow) is visible since it extends more proximally than the posterior tendon. (C) T1-weighted axial MRI at the midportion of the footprint shows the PM muscle (dashed line) and a PM tendon measuring 11 mm in width at the anterior surface. (D) H&E-stained slide shows a unilaminar tendon, which also measured 11 mm in width at the anterior surface. Fibrocartilage is present at the enthesis (left inset), sparsely populated by round cells in lacunae. An intermittent basophilic tidemark (arrows) proximal to the humerus edge (arrowheads) is present. Normal muscle appearance is seen more medially (right inset). Bar, 1 mm. PM, pectoralis major; HH, humeral head; *, long head biceps; SHB, short head biceps.
Figure 3.

Left shoulder of a 51-year-old male donor. (A) With the deltoid reflected and PM in situ, the distal myotendinous junction of the clavicular head (dashed line) is located adjacent to the humerus on gross examination. (B) With the PM muscle reflected laterally, the posterior tendon of the sternal head is revealed, which had a more cranial extension compared with the clavicular head. The distal myotendinous junction of the sternal head is shown (dashed line). (C) T1-weighted axial MRI at the midportion of the footprint shows the PM muscle (dashed line) and the PM tendon measuring 7 mm in width at the anterior surface. (D) H&E-stained slide shows a unilaminar tendon, which also measured 7 mm in width at the anterior surface. Fibrocartilage is present at the distal tendon margin (lower inset), sparsely populated by round cells in lacunae, often arranged in rows parallel to tendon fiber orientation (arrow). Normal muscle appearance is seen more medially (upper inset). Bar, 1 mm. PM, pectoralis major; HH, humeral head; *, long head biceps.
On gross examination of the superior portion of the footprint, the clavicular head appeared to extend more cranial compared with the sternal head in 5/14 specimens (Figure 2B), the sternal head appeared to extend more cranial compared with the clavicular head in 3/14 specimens (Figure 3B), and they appeared at the same level along their cranial margin in 6/14. In 9/14 specimens, dense connective tissue merged with the superior margin of the PM tendon, either representing the falciform ligament or aponeurotic expansion of the supraspinatus tendon. In 12/14 specimens, the pectoralis major and deltoid tendons were inseparable at the inferior margin on gross dissection.
On MRI evaluation, 3/14 specimens were initially interpreted as demonstrating a possible layered appearance at the enthesis (Figure 4 and 5), which was found on subsequent histology to represent entheseal fat and loose connective tissue, rather than true tendon layers. The remaining 11/14 specimens had no discernible lamina at the enthesis (Figures 2C, 3C, and 6A). None of the specimens demonstrated a layered enthesis on US imaging (Figure 6C), even with direct interrogation of the surgically exposed tendon.
Figure 4.

Left shoulder of a 69-year-old male donor. (A) T1-weighted axial MRI at the midportion of the footprint shows a unilaminar tendon on extending onto an irregular bony surface. (B) H&E-stained slide shows loosely packed tendon fascicles with artifactual separation merging onto a single, unified fibrocartilaginous enthesis. Note the relatively smooth tidemark (arrows) overlying the undulating and irregular bone surface. (C) T1-weighted axial MRI at the lower third of the footprint shows endotenon fat at the enthesis (arrow). (D) H&E-stained slide shows endotenon fat extending a limited distance medially into the distal tendon (arrows). Tendon fibers separated by fat become tightly juxtaposed (arrowheads) proximal to the fatty tissue. Bars, 500 μm.
Figure 5.

Right shoulder of a 36-year-old male donor. (A) T1-weighted axial MRI at the lower third of the footprint shows endotenon fat (arrow) and loose connective tissue in the lateral tendon, which was originally misinterpreted as a bilaminar enthesis. (B) H&E-stained slide shows several regions of loose connective tissue near the distal tendon margin (arrows) which did not extend into the medial tendon (upper inset 3 mm from lower) as would be expected for true layers from the clavicular and sternal heads. Bar, 500 μm.
Figure 6.

Right shoulder of a 48-year-old male donor. (A) T2-weighted fat-suppressed axial MRI at the midportion of the footprint shows a unilaminar tendon with mildly increased intra-tendinous signal at the enthesis (arrow). (B) H&E-stained slide shows clusters of vessels (*) invading a hypercellular region within the fibrocartilaginous enthesis. The hypercellular region gives way to normal fibrocartilaginous enthesis with round, clustered chondrocytes in lacunae (black arrowheads) and then tendon (not shown) more medially. (C) US image shows a unilaminar enthesis (arrow). Bar, 200 μm.
On histological evaluation, all samples had a unilaminar enthesis (Figures 2D and 3D). Abundant fibrocartilage was present at the lateral tendon/enthesis regions of all samples (Figures 2D, 3D, 4B, and 6B). Intra-tendinous blood vessels at the enthesis were present in 4/14 specimens (Figure 6B). As mentioned above, 3/14 specimens demonstrated isolated entheseal fat (Figure 4D) and loose connective tissue (Figure 5B) that did not extend more medially as would be expected for true layers.
Discussion
In this study, we investigated the lateral tendon and enthesis of the PM with the primary goal of determining whether a bilaminar tendon existed at the humeral insertion based on a histologic reference standard. The results from our histologic study demonstrate an entirely unilaminar enthesis. Previous studies that have assessed this region have relied on gross macroscopic findings, with some describing a unilaminar enthesis10,16,18,28 and others describing a bilaminar enthesis, with fusion of the inferior-most portion (forming a “U” shaped insertion).12,13 More recently, based on the concept of a “U” shaped, predominantly bilaminar insertion, authors have proposed a tendon injury classification.9,11,13,15 Specifically, differential laminar disruption was suggested to begin superiorly with the deep lamina, propagate inferiorly to then involve the superficial lamina, and then finally involve the superior fibers of the superficial lamina.15 Thus, in this injury classification, the tearing progresses from partial-thickness/incomplete-width, partial-thickness/complete width, full-thickness/incomplete-width, and finally to full-thickness, complete width. Our results do not support the use of this classification scheme.
In our specimens, the medial-lateral length of the PM tendon varied depending on the head (clavicular versus sternal) as well as the cranial-caudal location. In general, we found that the tendon of the clavicular head (mean 19 mm superiorly and 9 mm inferiorly) was shorter than the sternal head (mean 38 mm superiorly and 41 mm inferiorly). In the literature, various medial-lateral lengths have been reported. In six fresh-frozen specimens, Lee et al reported that the medial-lateral length of the superficial surface ranged from 5 – 15 mm.18 In two fixed cadavers, Wolfe et al reported a measurement of 5 mm, without specifying whether the measurement was of the clavicular or sternal head.28 Kretzler and Richardson reported that the anterior surface measured approximately 10 mm and the posterior surface measured approximately 25 mm, though the number of cadavers used for the experiment was unclear.17 The range of our measurements are in keeping with these previous studies.
In five fixed specimens, Fung et al reported much longer tendon lengths, including the clavicular head (mean 48 mm superiorly and 60 mm inferiorly) and sternal head (mean 54 mm superiorly and 58 mm inferiorly).13 In addition, Fung et al reported that tendon lengths increased in both layers from superior to inferior.13 In contrast, in our specimens the clavicular head typically had a much shorter tendon inferiorly compared with superiorly (Figure 1A). Differences in results may be related to small specimen numbers in all of our studies, or differences in technique since Fung et al used a unique digitizing system with serial dissections13 and we based our measurements on gross evaluation.
The true location of the myotendinous junction is important since this is an area that is classically more prone to injury, and depending on the injury location, different treatments may be prescribed.19 Our results confirm that the PM myotendinous junction is quite variable, being as close as 6 mm to the humerus for the clavicular head and as far as 75 mm from the humerus for the sternal head. In the cases where a short PM tendon exists, it is conceivable that a tear at the muscle or myotendinous junction may be misinterpreted as a distal tendon or enthesis tear. This wide anatomic variability calls into question previously reported frequencies of tear locations (e.g. myotendinous junction versus insertion)29 since it would be extremely difficult to determine anatomic location with certainty based solely on imaging.
The superior-inferior length of the PM footprint in our specimens measured 75 ± 9 mm, which is in range with Carey et al (72.3 ± 12.3 mm),7 de Figueiredo et al (80.8 ± 7.1 mm),10 and Fung et al (77 ± 14 mm).13 Jagiasi et al reported an average measurement of 46 ± 4.5 mm, but acknowledged that their smaller measurements may have been due to the origin of the cadavers, which were all from the Maharashtra region of India.16
At the superior margin of the PM footprint, we found a variety of appearances, with 5/14 demonstrating a more cranial clavicular head, 3/14 demonstrating a more cranial sternal head, and the rest at equal levels. This contrasts with the results from Fung et al where the posterior layers were always seen to extend more proximally than the anterior layers.13 We also found that in the majority of our specimens (9/14), dense connective tissue merged with the superior margin of the PM tendon. It was unclear whether this connective tissue represented the falciform ligament, which merges with the sternal head25 and has been reported to be present in up to 100% of cases26 or the aponeurotic expansion of the supraspinatus tendon, which has been reported to be present approximately half of the time.23 In fact, the falciform ligament and aponeurotic expansion may represent the same structure, but to the best of our knowledge this has not yet been fully elucidated in the literature. The close proximity and possible connection of the inferior PM and the deltoid tendons were noted in the majority of our specimens, similar to what has been previously described.2,24
Abundant fibrocartilage was present in the lateral tendon/enthesis regions of all of our samples, and in the three specimens harvested en bloc with bone, a classic tidemark was frequently observed separating the calcified from uncalcified fibrocartilage regions. Based on the structural classification of entheses popularized by Benjamin et al,6 our results suggest that the PM is a fibrocartilaginous enthesis at its insertion. Benjamin et al previously suggested that the PM insertion is a fibrous enthesis with small quantities of fibrocartilage, however also recognized the “mixed” nature of this attachment.3,4 In recent years there has been increasing interest in fibrocartilaginous entheses since they may be more frequently injured compared with fibrous entheses1 and may also provide insight into past physical activities from the bioarchaeological perspective, however there are clear pitfalls to this possible anatomic over-simplification.27 Also present in our samples was endotenon fat at the enthesis, which we demonstrate may be misinterpreted as layers on clinical imaging. Benjamin et al has highlighted the abundance of fat at various entheses about the body, which may play a role in proprioception, pain, and stress dissipation.5
Our study has several limitations. First, we had a limited number of samples and a formal power analysis was not performed since our primary objective was considered exploratory – namely to assess whether a unilaminar or multi-laminar enthesis existed. However, unlike many other previous studies, our specimens spanned a wide age range (21 – 70 years, with four donors <40 years of age). Second, only 3 out of 14 specimens were analyzed with the bony enthesis intact. However, as the primary goal was to determine whether a bilaminar tendon existed at the enthesis, our results were unlikely to be affected, since a bilaminar tendon, if present, would have also been shown at the cut margin. Third, our anatomical investigation did not include biomechanical analysis of the PM muscles and tendons and we are unable to comment on the biomechanical relevance of these anatomic findings. Fourth, histologic evaluation of the tendon is subject to some problems, including alterations of the intrinsic tension of the tendon due to separation from osseous and muscular attachments. Histologic sectioning can also create artifacts and artificial cleavage, though we note that the fibrocartilage at the enthesis tended to hold tissue planes together. Fifth, measurements in this study were made in consensus and reliability was not assessed. Finally, oblique sectioning of the tendon potentially creates challenges in analyzing structure. However, due to the complexity of the attachment, parallel sectioning relative to all fibers was not possible. Since the focus of this investigation was to assess the PM tendon insertion, we did not evaluate the more medial portions of the tendon histologically. Of note, our study was not designed to assess where the transition from the layered muscle to the unilaminar tendon occurred. It is certainly feasible that in PM tendon injuries, delamination events could occur within tendon layers that propagate laterally and result in partial-thickness and partial-width tear patterns described in the literature. However, it is unlikely that predicting insertional tear patterns based on a “U” shaped enthesis is possible based on our findings.
Conclusion
The distal PM tendon consists of a unilaminar fibrocartilaginous enthesis at the insertion of the lateral lip of the intertubercular groove of the humerus. This is the first investigation of the PM tendon insertion utilizing a combination of advanced imaging and histologic assessment. US and MRI failed to identify true tendon layers at the enthesis, and delaminating injuries reported in the literature may originate from a location other than the enthesis. Although injury classification patterns have been proposed based on the assumption of a bilaminar enthesis, we caution their continued use without further careful assessment of tear patterns and understanding of these anatomic considerations.
Acknowledgement of Funding:
The authors gratefully acknowledge grant support from the Veterans Affairs (Merit Awards I01CX001388 and I01RX002604) and NIH (R01AR075825, R01AR062581, R01AR068987, and R21AR073496).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
Institutional Review Board approval was not required for this cadaveric study.
References
- 1.Apostolakos J, Durant TJ, Dwyer CR, Russell RP, Weinreb JH, Alaee F et al. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J 2014;4:333–342. No doi [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley GT. The manner of insertion of the pectoralis major muscle in man. Anat Rec 1952. 113:301–307. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons--tendon “entheses”. Comp Biochem Physiol A Mol Integr Physiol 2002;133:931–945. 10.1016/s1095-6433(02)00138-1 [DOI] [PubMed] [Google Scholar]
- 5.Benjamin M, Redman S, Milz S, Buttner A, Amin A, Moriggl B et al. Adipose tissue at entheses: the rheumatological implications of its distribution. A potential site of pain and stress dissipation? Ann Rheum Dis 2004;63:1549–1555. 10.1136/ard.2003.019182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat 2006;208:471–490. 10.1111/j.1469-7580.2006.00540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey P, Owens BD. Insertional footprint anatomy of the pectoralis major tendon. Orthopedics 2010;33:23 10.3928/01477447-20091124-27 [DOI] [PubMed] [Google Scholar]
- 8.Chafik D, Galatz LM, Keener JD, Kim HM, Yamaguchi K. Teres minor muscle and related anatomy. J Shoulder Elbow Surg 2013;22:108–114. 10.1016/j.jse.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 9.Chiavaras MM, Jacobson JA, Smith J, Dahm DL. Pectoralis major tears: anatomy, classification, and diagnosis with ultrasound and MR imaging. Skeletal Radiol 2014;44:157–164. 10.1007/s00256-014-1990-7 [DOI] [PubMed] [Google Scholar]
- 10.de Figueiredo EA, Terra BB, Cohen C, Monteiro GC, de Castro Pochini A, Andreoli CV et al. The pectoralis major footprint: An anatomical study. Revista Brasileira de Ortopedia (English Edition) 2013;48:519–523. 10.1016/j.rboe.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ElMaraghy A, Devereaux M. Response to the Letter to the Editor regarding: a systematic review and comprehensive classification of pectoralis major tears. J Shoulder Elbow Surg 2013;22:e25–26. 10.1016/j.jse.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 12.ElMaraghy AW, Devereaux MW. A systematic review and comprehensive classification of pectoralis major tears. J Shoulder Elbow Surg 2012;21:412–422. 10.1016/j.jse.2011.04.035 [DOI] [PubMed] [Google Scholar]
- 13.Fung L, Wong B, Ravichandiran K, Agur A, Rindlisbacher T, Elmaraghy A. Three-dimensional study of pectoralis major muscle and tendon architecture. Clin Anat 2009;22:500–508. 10.1002/ca.20784 [DOI] [PubMed] [Google Scholar]
- 14.Grant JCB. A method of anatomy, descriptive and deductive. Baltimore,: Williams and Wilkins Co.; 1948. [Google Scholar]
- 15.Guiu R, Lefort H, Mihai I, Ernouf C, Domanski L. Regarding “A systematic review and comprehensive classification of pectoralis major tears”. J Shoulder Elbow Surg 2013. 22:e22–23. doi: 10.1016/j.jse.2012.10.030 [DOI] [PubMed] [Google Scholar]
- 16.Jagiasi JD, Valavi AS, Ubale TV, Sahu D. Insertion anatomy of the pectoralis major tendon. Journal of Clinical Orthopaedics & Trauma 2019;10:541–543. 10.1016/j.jcot.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kretzler HH Jr, Richardson AB. Rupture of the pectoralis major muscle. Am J Sports Med 1989. 17:453–458. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Brookenthal KR, Ramsey ML, Kneeland JB, Herzog R. MR imaging assessment of the pectoralis major myotendinous unit: an MR imaging-anatomic correlative study with surgical correlation. AJR Am J Roentgenol 2000. 174:1371–1375. [DOI] [PubMed] [Google Scholar]
- 19.Lee YK, Skalski MR, White EA, Tomasian A, Phan DD, Patel DB et al. US and MR Imaging of Pectoralis Major Injuries. Radiographics 2017;37:176–189. 10.1148/rg.2017160070 [DOI] [PubMed] [Google Scholar]
- 20.Leijnse JN, Han SH, Kwon YH. Morphology of deltoid origin and end tendons--a generic model. J Anat 2008;213:733–742. 10.1111/j.1469-7580.2008.01000.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis WH. Observations on the pectoralis major muscle in man. Bull Johns Hopkins Hosp 1901;12:172–177. [Google Scholar]
- 22.Moroder P, Schulz E, Mitterer M, Plachel F, Resch H, Lederer S. Long-Term Outcome After Pectoralis Major Transfer for Irreparable Anterosuperior Rotator Cuff Tears. J Bone Joint Surg Am 2017;99:239–245. 10.2106/JBJS.16.00485 [DOI] [PubMed] [Google Scholar]
- 23.Moser TP, Cardinal É, Bureau NJ, Guillin R, Lanneville P, Grabs D. The aponeurotic expansion of the supraspinatus tendon: anatomy and prevalence in a series of 150 shoulder MRIs. Skeletal Radiol 2015;44:223–231. 10.1007/s00256-014-1993-4 [DOI] [PubMed] [Google Scholar]
- 24.Perrin JB. Notes on some Variations of the Pectoralis Major, with its Associate Muscles. J Anat Physiol 1871;5:233–420 219. [PMC free article] [PubMed] [Google Scholar]
- 25.Shiu B, Jazini E, Robertson A, Henn RF, Hasan SA. Anatomical Relationship of the Axillary Nerve to the Pectoralis Major Tendon Insertion. Orthopedics 2017;40:e460–e464. 10.3928/01477447-20170208-04 [DOI] [PubMed] [Google Scholar]
- 26.Taylor SA, Fabricant PD, Bansal M, Khair MM, McLawhorn A, DiCarlo EF et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg 2015;24:511–519. 10.1016/j.jse.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 27.Villotte S, Knüsel CJ. Understanding Entheseal Changes: Definition and Life Course Changes. International Journal of Osteoarchaeology 2013;23:135–146. 10.1002/oa.2289 [DOI] [Google Scholar]
- 28.Wolfe SW, Wickiewicz TL, Cavanaugh JT. Ruptures of the pectoralis major muscle. An anatomic and clinical analysis. Am J Sports Med 1992;20:587–593. [DOI] [PubMed] [Google Scholar]
- 29.Zvijac JE, Schurhoff MR, Hechtman KS, Uribe JW. Pectoralis major tears: correlation of magnetic resonance imaging and treatment strategies. Am J Sports Med 2006;34:289–294. 10.1177/0363546505279573 [DOI] [PubMed] [Google Scholar]


