Abstract
Despite respiratory motor neuron death, ventilation is preserved in SOD1G93A rats. Compensatory respiratory plasticity may counterbalance the loss of these neurons. Phrenic long-term facilitation (pLTF; a form of respiratory plasticity) in naïve rats is 5-HT2 and NADPH oxidase-dependent. Furthermore, 5-HT2A, not 5-HT2B, receptor-induced phrenic motor facilitation is NADPH oxidase-independent in naïve rats. pLTF is NADPH oxidase-dependent in pre-symptomatic, but not end-stage, SOD1G93A rats. Here, we hypothesized that in the putative phrenic motor nucleus (PMN) of SOD1G93A rats vs. wild-type littermates: 1) pre-symptomatic rats would have greater 5-HT2B receptor expression that decreases at end-stage; and 2) 5-HT2A receptor expression would increase from pre-symptomatic to end-stage. Putative PMN 5-HT2A receptor expression was reduced when comparing across (but not within) pre-symptomatic vs. end-stage groups (p<0.05). In contrast, putative PMN 5-HT2B receptor expression was increased when comparing across pre-symptomatic vs. end-stage groups, and within end-stage groups (p<0.05). These data suggest a potential role for 5-HT2 receptors in pLTF and breathing in SOD1G93A rats.
Keywords: Serotonin, plasticity, breathing, respiratory motor neuron, amyotrophic lateral sclerosis
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that affects both upper and lower motor neurons. Patients with ALS survive only 3-5 years after the disease has been diagnosed and death most commonly occurs due to respiratory failure (Lechtzin et al., 2002; Bourke et al., 2001; Lyall et al., 2001). However, despite the loss of respiratory motor neurons, patients are able to maintain breathing capacity. Once the disease becomes more severe, there is a turning point at which the patient rapidly succumbs to ventilatory failure leading to ventilator-dependence or death due to a lack of compensation to maintain breathing. The mechanisms by which patients are able to compensate for breathing remain unknown.
Although 90% of ALS cases are sporadic, the other roughly 10% of cases are familial and are a result of genetic mutations. Overexpression of superoxide dismutase 1 (SOD-1) is one cause of familial ALS, although the pathogenesis in transgenic rodent models overexpressing human SOD1 is still up for debate. Regardless of the mechanism of pathogenesis, rodent models including hSOD1G93A (Rosen et al., 1993; Gurney et al., 1994; Howland et al., 2002), hSOD1G37R (Wong et al., 1995), hSOD1G85R (Bruijn et al., 1997), hSOD1Quad (Wang et al., 2003), and hSOD1H46R (Nagai et al., 2001) develop clinical symptoms associated with both familial and sporadic human cases of ALS (Boillée et al., 2006). Interestingly, hSOD1G93A rats experience progressive motor neuron loss but do not develop breathing deficits until late in the disease (Nichols et al., 2013; Dale et al., 2006; Tankersley et al., 2006). This indicates respiratory compensation may be occurring to maintain ventilation despite dramatic phrenic motor neuron loss.
One way that has been speculated to induce respiratory compensation following motor neuron loss is through respiratory plasticity by neighboring surviving motor neurons. Acute intermittent hypoxia (AIH) is one mode to induce respiratory plasticity, which is called phrenic long-term facilitation (pLTF) (Dale-Nagle et al., 2010; Hayashi et al., 2003; Feldman et al., 2003; Bach and Mitchell, 1996). AIH-induced pLTF occurs predominately through the activation of Gq (5-HT2A/B) (Bach and Mitchell, 1996; McFarlane et al., 2011) coupled receptor-dependent pathways. Specifically, pLTF is induced through the activation of the serotonin receptor (5-HT2) in the phrenic motor nucleus at cervical spinal cord regions 3-5 of naïve rats (Bach and Mitchell, 1996). When 5-HT2A/B receptors become activated following AIH, protein kinase C theta (PKCΦ) is activated and leads to the new synthesis of brain-derived neurotrophic factor (BDNF) (McGuire and Ling 2004; Baker-Herman et al., 2004, Devinney, et al. 2015). BDNF then binds to the mTrkB receptor (Baker-Herman et al., 2004) and subsequently activates MEK and the phosphorylation of ERK (Hoffman et al., 2012), ultimately resulting in pLTF. pLTF also requires reactive oxygen species (ROS) formation via NADPH oxidase activity since ROS disinhibits phosphatase action on PKC (i.e., ROS formation allows pLTF to be evoked; MacFarlane and Mitchell, 2007; MacFarlane et al., 2011).
Pharmacological activation of 5-HT2A and 5-HT2B receptors also leads to respiratory plasticity called phrenic motor facilitation (pMF) in naïve adult rats (MacFarlane and Mitchell 2009; MacFarlane et al., 2011). Furthermore, pMF via 5-HT2A activation is NADPH oxidase-independent, while 5-HT2B-induced pMF is NADPH oxidase-dependent (MacFarlane et al., 2011). Interestingly, AIH-induced pLTF in pre-symptomatic SOD1G93A rats is NADPH oxidase-dependent, but end-stage SOD1G93A rats exhibit NADPH oxidase-independent pLTF (Nichols, et al. 2015). Thus, in this study, we hypothesized that there would be a shift in the 5-HT2 receptor balance in the putative phrenic motor nucleus of SOD1G93A rats vs. age-matched, wild-type littermates. Specifically, we hypothesized that pre-symptomatic SOD1G93A rats would have greater 5-HT2B receptor expression that decreases over time to end-stage. In contrast, we hypothesized that 5-HT2A receptor expression would increase from pre-symptomatic to end-stage. . However, we found that 5-HT2A receptor expression in the putative phrenic motor nucleus was not different between SOD1G93A rats and wild-type littermates regardless of disease state (i.e., neither pre-symptomatic nor end-stage), while 5-HT2B receptor expression was only increased in the putative phrenic motor nucleus of end-stage SOD1G93A rats compared to wild-type littermates. Interestingly, we also found that 5-HT2A receptor expression is reduced, while 5-HT2B receptor expression is upregulated, over time in the putative phrenic motor nucleus in both wild-type and SOD1G93A rats. This study provides knowledge into how 5-HT2A/B receptor expression changes both within and outside of the putative phrenic motor nucleus following motor neuron death induced by SOD1 overexpression. Furthermore, our results suggest changes in 5-HT2A/B receptor expression, and thus their downstream mechanisms, in response to motor neuron death may contribute to the maintenance of breathing throughout disease progression in SOD1G93A rats.
2. Methods
2.1. Animals and Tissue Samples
Male SOD1G93A Sprague Dawley rats were bred to wild-type female rats (Taconic Laboratories, Germantown, NY). Neurophysiological experiments were performed at the University of Wisconsin-Madison on adult male pre-symptomatic (3-4 months of age) or end-stage (5-6 months of age) SOD1G93A (MT) and age-matched, wild-type (WT) littermates (Nichols et al., 2015). End-stage was defined as a 20% reduction in peak body mass (Nichols et al., 2015). Immediately following neurophysiological protocols for another study (Nichols et al., 2015), rats were transcardially perfused with cold 4% paraformaldehyde in phosphate buffered saline (0.1 M PBS, pH 7.4; Nichols et al., 2015). Spinal cords were immediately removed following perfusion, post-fixed (4% paraformaldehyde in 0.1 M PBS) at 4°C overnight, and then cryoprotected in graded sucrose (20% sucrose for 3 days and 30% sucrose for an additional 3 days) at 4°C until sinking. The spinal cords were transversely sectioned to contain the largest portion of the phrenic motor nucleus (C4; 40 μm) using a freezing-sliding microtome (Leica SM 2000R, Germany), and stored at −20°C in an antifreeze solution (30% glycerol, 30% ethylene glycol, 40% PBS). All experimental procedures were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison. Tissue was then transferred to the University of Missouri-Columbia where the immunohistochemistry protocols, imaging, and analyses stated below were performed.
2.2. Immunohistochemistry
Six sections from the cervical region housing the phrenic motor nucleus (C4) were selected for each animal (pre-symptomatic WT: n=21 for 5-HT2A and n=25 for 5-HT2B; pre-symptomatic MT: n=19 for 5-HT2A and n=23 for 5-HT2B; end-stage WT: n=24 for 5-HT2A and n=17 for 5-HT2B; end-stage MT: n=25 for 5-HT2A and n=22 for 5-HT2B) and were washed with 1X PBS three times for five minutes on a shaker at room temperature. Antigen retrieval was performed by adding a 0.01 M citrate in DDI water solution to the tissue wells and shaken in a Labent Hybaid Maxi 14 Hybridization Oven Incubator (National Labnet Company) at 60°C for 30 minutes. Three, five minute washes were repeated in 1X PBS at room temperature on a shaker. Sections were then incubated at room temperature in a blocker solution (1X PBS + 0.2% Triton + 5% normal donkey serum) on a shaker for one hour. Sections were then incubated in primary antibody solution (1X PBS + 0.1% Triton + 5% normal donkey serum + antibodies against NeuN (mouse polyclonal, 1:500, Millipore) and either 5-HT2A (rabbit polyclonal, 1:200; Neuromics, Edina, MN) or 5-HT2B (rabbit polyclonal, 1:300; Neuromics, Edina, MN) overnight at 4°C on a shaker. The following day, tissue was washed three times for five minutes at room temperature on a shaker in 1X PBS. The tissue was then incubated for two hours at room temperature on a shaker in the dark in secondary antibody solution (1X PBS + 0.1% Triton + 5% normal donkey serum + donkey anti-mouse Alexa-Fluor 555 (1:1000; Molecular Probes, Eugene, OR) and donkey anti-rabbit Alexa-Fluor 488 (1:1000; Molecular Probes, Eugene, OR)). Tissue was then washed again while covered in 1X PBS three times for 5 minutes on a shaker at room temperature. The tissue was then mounted on positively charged glass slides (Thermo Fisher Scientific, Waltham, MA) and allowed to dry before ProLong™ Gold anti-fade reagent (Thermo Fisher Scientific, Waltham, MA) was applied and a coverslip was put onto the slides. Covered slides were stored at 4°C until quantification of staining was performed.
2.3. Imaging and Analysis
Photomicrographs were taken at the same settings for all images per antibody per group (i.e., gains: pre-symptomatic 5-HT2A=740.01 and NeuN=770.24, end-stage 5-HT2A=765.28 and NeuN=770.22; pre-symptomatic 5-HT2B = 802.25 and NeuN = 765.29, end-stage 5-HT2B=797.28 and NeuN=804.95). The photomicrographs encompassed the putative phrenic motor nucleus (Mantilla et al., 2009; Boulenguez et al., 2007; Watson et al., 2009) and the non-phrenic ventral horn, and were taken using a Leica DM4000 confocal microscope at 20x magnification with Leica Application Suite X (LAS X) software. Densitometry of the 5-HT2A/B receptors was performed on Z-stacked (5μm in z-plane per image) images by creating a region of interest that encompassed the putative phrenic motor nucleus (same region of interest used for all images). Images were taken and analyzed as 8-bit stacks at a resolution of 1024 × 1024 (366.67μm x 366.67μm). Thresholds in ImageJ for analysis of immunopositivity were: pre-symptomatic 5-HT2A: 17-24; pre-symptomatic 5-HT2B: 33-43; end-stage 5-HT2A: 17-34; and end-stage 5-HT2B: 45-55. Immunopositive pixels for 5-HT2A/B receptors were evaluated using ImageJ within the putative phrenic motor nucleus and in the non-phrenic ventral horn in which 5-HT2A/B optical density was expressed as an average density per section/animal. The fractional area occupied by 5-HT2A/B label in raw image files was also computed by Image J (Nichols et al., 2015). Within the putative phrenic motor nucleus, the 5-HT2A/B fractional area was interpreted as the percentage of the total field for the putative phrenic motor nucleus occupied by label positive pixels. However, the fractional area occupied by 5-HT2A/B label in raw image files was calculated manually for the non-phrenic ventral horn. Total label positive pixels were determined for the putative phrenic motor nucleus (fractional area multiplied by total pixels in the putative phrenic motor nucleus) and for the entire ventral horn (fractional area multiplied by total pixels in the ventral horn). Total label positive pixels for the non-phrenic ventral horn were determined by subtracting total label positive pixels in the putative phrenic motor nucleus from those in the area outside of the region of interest in the 20x magnification images. Total label positive pixels for the non-phrenic ventral horn were then divided by the total pixels of the non-phrenic ventral horn (total pixels of the putative phrenic motor nucleus were subtracted from the total pixels in the area outside of the region of interest in the 20x magnification images).
Photomicrographs were also taken at 4x magnification to provide a visual representation of the location of the putative phrenic motor nucleus (C4) within the ventral horn. In addition, photomicrographs were taken at 40x to enable the visualization of 5-HT2A/B receptor expression on and near neurons using an Olympus BX51 equipped with a three-axis motorized stage (Ludl Electronic Products Ltd., Hawthorne, NY, USA). Filter sets for Cy2 [ex. λ480nm; em. λ510nm] and Cy3 [ex. λ550 nm; λ570 nm] were used to identify positively labeled neurons and 5-HT2A/B receptors. Using the same focal plane, digital images were captured with each filter using a cooled monochrome digital camera (ORCA-AG, Hamamatsu, Bridgewater, NJ, USA) and the software package Neurolucida (version 9, MicroBrightField, Willston, VT, USA).
2.4. Statistical Analysis
A one-way ANOVA was used to compare 5-HT2A/B optical density and fractional area within groups in the putative phrenic motor nucleus and the non-phrenic ventral horn. A student’s t-test was used to compare 5-HT2A/B optical density and fractional area between groups in the putative phrenic motor nucleus and the non-phrenic ventral horn. A two-way ANOVA was used to compare 5-HT2A/B optical density and fractional area in the putative phrenic motor nucleus and the non-phrenic ventral horn between the different stages of disease progression (pre-symptomatic vs. end-stage) in WT and MT rats. The LSD post hoc test was used to detect significantly different individual comparisons, where differences between groups were considered significant if P < 0.05 and all values were expressed as means ± 1 SEM.
3. Results
3.1. 5-HT2A receptor expression recovers back towards wild-type levels at end-stage in the non-phrenic ventral horn, but is unaffected in the putative phrenic motor nucleus
Representative photomicrographs for 5-HT2A receptors from C4 transverse sections are shown in Figs. 1&2 A–J. 5-HT2A receptor positive staining was evaluated in pre-symptomatic (Fig. 1) and end-stage (Fig. 2) SOD1G93A (MT) and age-matched wild-type (WT) rats at 20x magnification (Figs. 1&2 B,D,G,I). The putative phrenic motor nucleus is predominately located at C4 (indicated by the yellow dotted circle in Figs. 1–4) in the ventral horn. When visualized using immunofluorescence for 5-HT2A receptors (indicated by green fluorescence in Figs. 1&2 A–J), the receptors were localized to the putative phrenic motor nucleus, but were also present in the non-phrenic ventral horn to a lesser degree (area outside of the putative phrenic motor nucleus in the 20x magnification images). Additionally, using NeuN (indicated by red fluorescence in Figs. 1&2 A–J), neurons were visualized both within and outside of the putative phrenic motor nucleus. Interestingly, it appears that 5-HT2A receptors do not solely exist on the membranes of neurons within the putative phrenic motor nucleus and the non-phrenic ventral horn (e.g., on the cell membranes of cells that are not motor neurons). In pre-symptomatic and end-stage animals, we observed no differences between MT and age-matched WT rats in optical density or fractional area of 5-HT2A receptor expression within the putative phrenic motor nucleus (Fig. 1K & 1L; p>0.05). However, 5-HT2A receptor optical density (p=0.005) and fractional area (p<0.001) were both significantly decreased in pre-symptomatic MT animals in the non-phrenic ventral horn when compared to WT animals. In contrast, this decrease in 5-HT2A optical density and fractional area returns to WT levels in the non-phrenic ventral horn of MT rats at end-stage (p>0.05; Figs. 2K & 2L).
Figure 1: 5-HT2A receptor expression in the putative phrenic motor nucleus and non-phrenic ventral horn in pre-symptomatic wild-type and SOD1G93A mutant in C4 spinal cord sections.
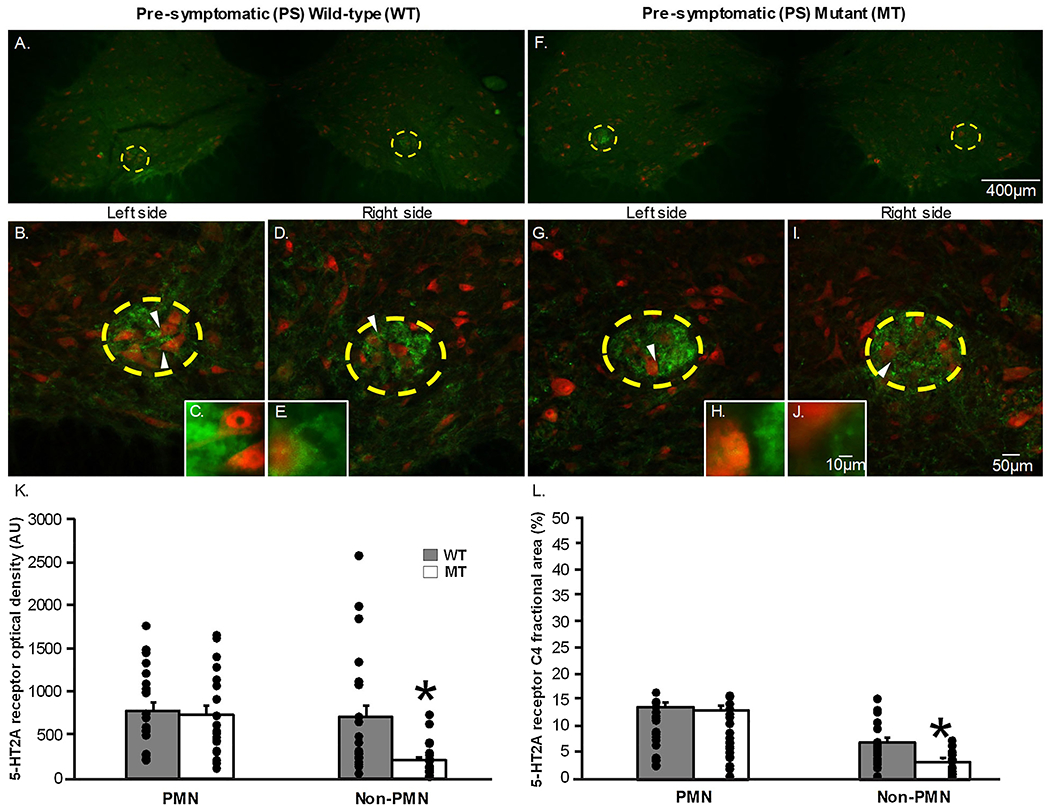
The representative photomicrographs display motor neurons (NeuN; red) and 5-HT2A (green) receptor expression within the putative phrenic motor nucleus (yellow dashed circle) and the non-phrenic ventral horn (area outside of the yellow dashed circle) from C4 spinal cord sections in pre-symptomatic (PS; 1A-J) wild-type (WT; 1A-E) and SOD1G93A (Mutant; MT; 1F-J) rats at 4x (1A&F), 20x (1B,D,G,I), and 40x (1C,E,H,J) magnification. The white arrows in the 20x magnification images indicate what neurons are displayed in the 40x magnification images. 5-HT2A receptor expression is represented as optical density (AU; 1K) and fractional area in C4 (1L) in the putative phrenic motor nucleus and non-phrenic ventral horn). Note, there were no significant differences in 5-HT2A optical density when comparing MT rats to age-matched WT littermates in the putative phrenic motor nucleus within PS and ES animals (p>0.05). However, there is a significant decrease in 5HT2A optical density and fractional area in the non-phrenic ventral horn of PS MT rats compared to PS WT rats.
Figure 2: 5-HT2A receptor expression in the putative phrenic motor nucleus and non-phrenic ventral horn in end-stage wild-type and SOD1G93A mutant in C4 spinal cord sections.
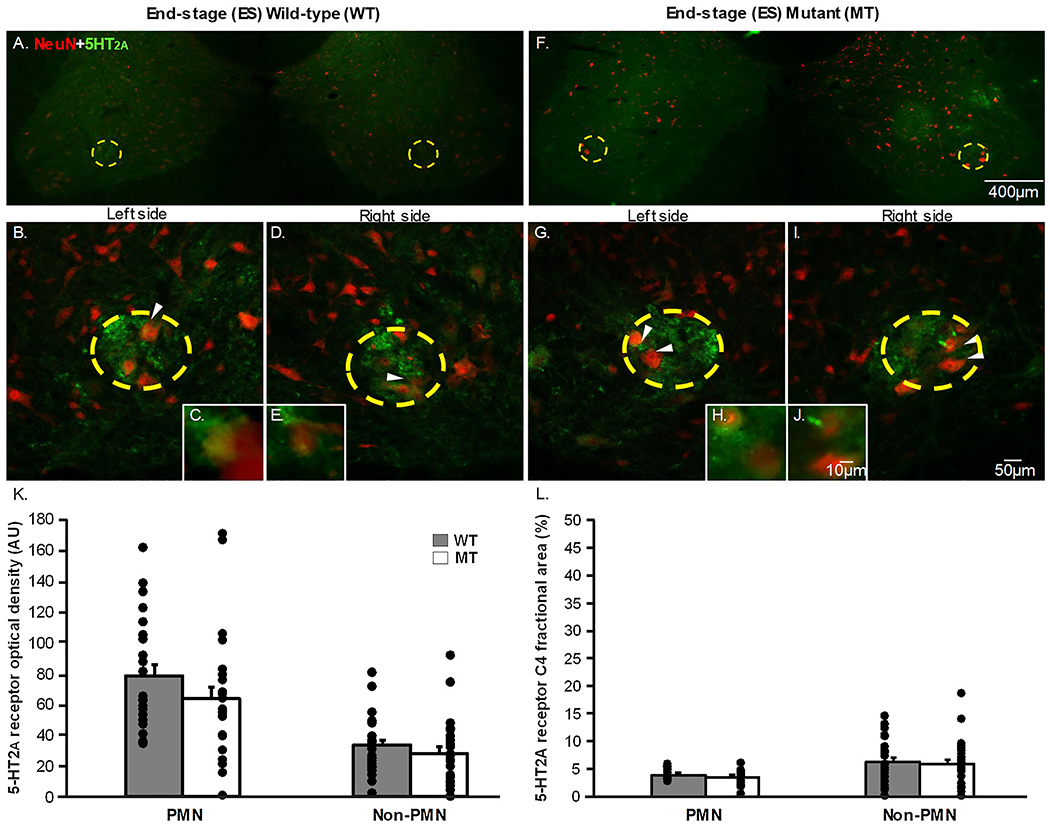
The representative photomicrographs display motor neurons (NeuN; red) and 5-HT2A (green) receptor expression within the putative phrenic motor nucleus (yellow dashed circle) and the non-phrenic ventral horn (area outside of the yellow dashed circle) from rat C4 spinal cord sections in end-stage (ES; 2A-J) wild-type (WT; 2A-E) and SOD1G93A (Mutant; MT; 2F-I) rats at 4x (2A&F), 20x (2B,D,G,I), and 40x (2C,E,H,J) magnification. The white arrows in the 20x magnification images indicate what neurons are displayed in the 40x magnification images. 5-HT2A receptor expression is represented as optical density (AU; 2K) and fractional area (2L) in the putative phrenic motor nucleus and the non-phrenic ventral horn. Note, there were no significant differences in 5-HT2A optical density when comparing MT rats to age-matched WT littermates neither in the putative phrenic motor nucleus nor the non-phrenic ventral horn at ES (p>0.05).
Figure 4: 5-HT2B receptor expression in the putative phrenic motor nucleus and non-phrenic ventral horn in end-stage wild-type and SOD1G93A mutant in C4 spinal cord slices.
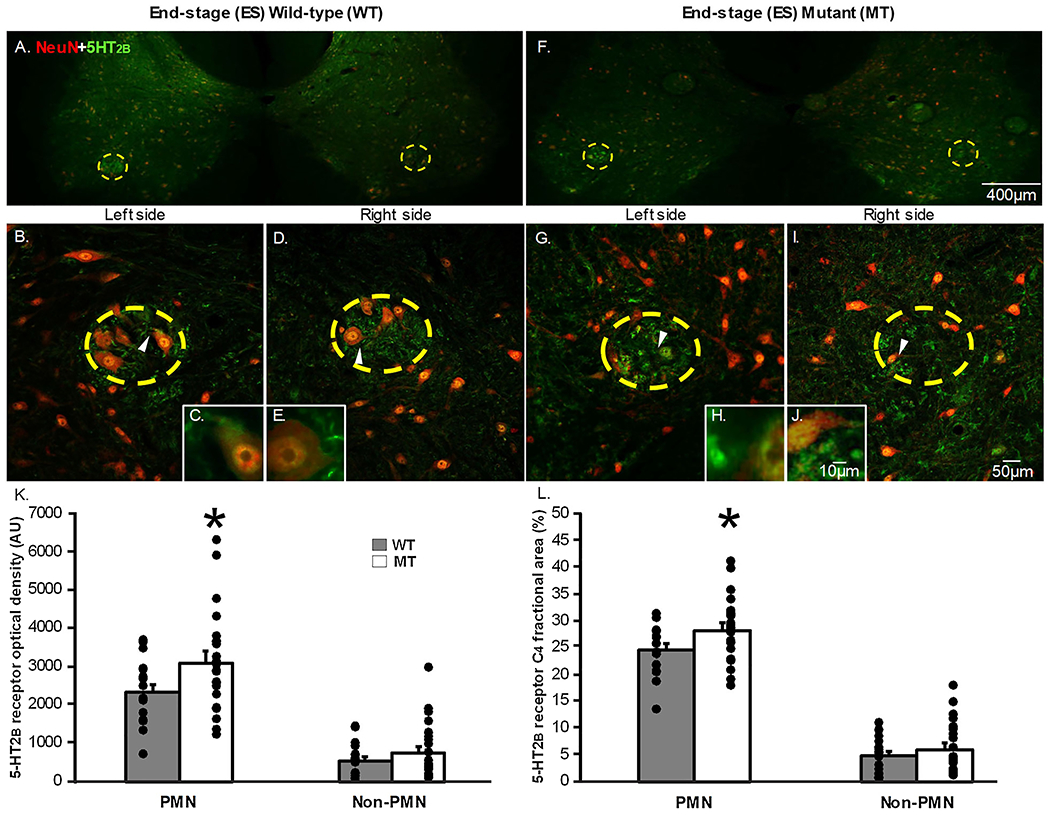
The representative photomicrographs display motor neurons (NeuN; red) and 5-HT2B (green) receptor expression within the putative phrenic motor nucleus (yellow dashed circle) and the non-phrenic ventral horn from rat C4 spinal cord sections (area outside of the yellow dashed circle) in end-stage (ES; 4A-J) wild-type (WT; 4A-E) and SOD1G93A (Mutant; MT; 4F-J) rats at 4x (4A&F), 20x (4B,D,G,I), and 40x (4C,E,H,J) magnification. The white arrows in the 20x magnification images indicate what neurons are displayed in the 40x magnification images. 5-HT2B receptor expression is represented as optical density (AU; 4K) and fractional area (4L) in the putative phrenic motor nucleus (PMN) and non-phrenic ventral horn. 5-HT2B optical density (p=0.032) and fractional area (p=0.04) was significantly increased in MT ES rats within the putative phrenic motor nucleus when comparing MT rats to age-matched WT littermates at ES.
3.2. 5-HT2B receptor expression is increased from wild-type levels at end-stage
Figures 3&4 A–J depict representative photomicrographs from C4 transverse sections for 5-HT2B receptor positive staining. Sections from the same rats as in the previous section were evaluated for 5-HT2B receptor positive staining (Fig. 3A–J, 4A–J). 5-HT2B receptor optical density was quantified at 20x magnification both within the putative phrenic motor nucleus (indicated by the yellow dashed circle) and in the non-phrenic ventral horn in pre-symptomatic (Fig. 3B,D,G,I) and end-stage (Fig. 4B,D,G,I) wild-type (WT) and SOD1G93A mutant (MT) rats. The fractional area of 5-HT2B receptor expression was also quantified within the putative phrenic motor nucleus and the non-phrenic ventral horn. Neurons were visualized both within and outside of the putative phrenic motor nucleus using NeuN (indicated by red fluorescence in Figs. 3&4 A–J). Once again, 5-HT2B receptors (indicated by green immunofluorescence; Fig. 3&4 A–J) were localized to the putative phrenic motor nucleus, but existed in the non-phrenic ventral horn to a much lesser degree (Figs. 3 & 4). Optical density (Fig. 3K) and fractional area (Fig. 3L) for 5-HT2B receptor expression within the putative phrenic motor nucleus and the non-phrenic ventral horn were not different when comparing pre-symptomatic MT and age-matched WT animals (p>0.05) However, we did observe that end-stage MT animals had a significant increase in both 5-HT2B optical density (Fig. 4K; p=0.042) and fractional area (Fig. 4L; p=0.045) within the putative phrenic motor nucleus. There was no difference in 5-HT2B receptor expression at end-stage in the non-phrenic ventral horn observed between MT and age-matched WT animals (Fig. 4K&L; p>0.05).
Figure 3: 5-HT2B receptor expression in the putative phrenic motor nucleus and non-phrenic ventral horn in pre-symptomatic wild-type and SOD1G93A mutant in C4 spinal cord sections.
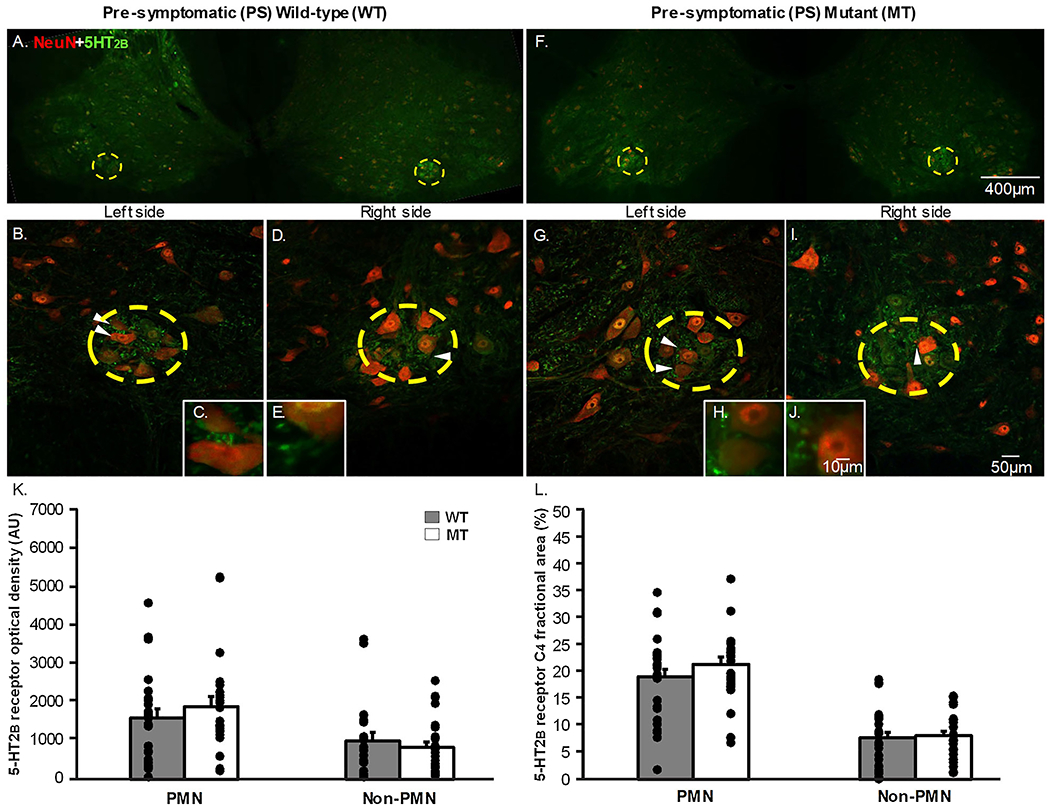
The representative photomicrographs display motor neurons (NeuN; red) and 5-HT2B (green) receptor expression within the putative phrenic motor nucleus (yellow dashed circle) and the non-phrenic ventral horn (area outside of the yellow dashed circle) from rat C4 spinal cord sections in pre-symptomatic (PS; 3A-J) wild-type and SOD1G93A (Mutant; MT; 3F-J) rats at 4x (3A&F), 20x (3B,D,G,I), and 40x (3C,E,H,J) magnification. The white arrows in the 20x magnification images indicate what neurons are displayed in the 40x magnification images. 5-HT2B receptor expression is represented as optical density (AU; 3K) and fractional area (3L) in the putative phrenic motor nucleus and non-phrenic ventral horn. 5-HT2B optical density and fractional area was not different in MT PS rats within the putative phrenic motor nucleus as well as within the non-phrenic ventral horn when comparing MT rats to age-matched WT littermates (p>0.05).
3.3. 5-HT2A/B receptor expression over disease progression
A two-way ANOVA was used to determine if 5-HT2A receptor expression within the putative phrenic motor nucleus and the non-phrenic ventral horn increases over time in MT rats. Surprisingly, we found that optical density and fractional area for 5-HT2A receptor expression within the putative phrenic motor nucleus was significantly decreased in end-stage vs. pre-symptomatic rats (both WT and MT; p<0.05). In the non-phrenic ventral horn, fractional area for 5-HT2A receptor expression was significantly increased in end-stage vs. pre-symptomatic MT rats (p<0.05). Lastly, optical density for 5-HT2A receptor expression in the non-phrenic ventral horn was significantly decreased at end-stage in wild-type rats (p<0.05 vs. pre-symptomatic rats).
A two-way ANOVA was also used to determine if 5-HT2B receptor expression within the putative phrenic motor nucleus and the non-phrenic ventral horn decreases over time in MT rats. However, we found that we found that optical density and fractional area for 5-HT2B receptor expression within the putative phrenic motor nucleus was significantly increased in end-stage vs. pre-symptomatic rats (both WT and MT; p<0.05). There were no significant differences in the non-phrenic ventral horn for 5-HT2B receptor expression between end-stage and pre-symptomatic WT or MT rats (p>0.05).
4. Discussion
The main findings of this study are that: 1) 5-HT2A receptor expression does not change within the putative phrenic motor nucleus when comparing WT and MT rats within pre-symptomatic and end-stage time-points; and 2) 5-HT2B receptor expression is upregulated at end-stage only within the putative phrenic motor nucleus of MT rats vs. pre-symptomatic WT littermates. To our surprise, 5-HT2A receptor expression does not change within the putative phrenic motor nucleus when comparing WT and MT rats within pre-symptomatic and end-stage time-points, but is decreased in pre-symptomatic MT animals in the non-phrenic ventral horn and returns towards WT levels by end-stage (Figs. 1&2). Thus, even though 5-HT2A receptor expression is unaffected in the putative phrenic motor nucleus of end-stage MT rats when compared to WT littermates (Fig. 2), this does not eliminate a role for 5-HT2A receptors for pLTF and breathing. Previous studies using models of spinal cord injury have found that intrathecal administration of a 5-HT2A receptor agonist induced respiratory recovery. Conversely, blocking 5-HT2A receptors resulted in a decrease in phrenic burst amplitude and respiratory rate (Zhou et al., 2001). This study highlights a critical role for the involvement of 5-HT2A receptors in respiration following phrenic motor loss after cervical spinal cord injury (Zhou et al., 2001), which may also be the case in SOD1G93A rats.
Interestingly, 5-HT2A receptor expression was reduced in WT littermates as well as MT rats over time within the putative phrenic motor nucleus, while 5-HT2B receptor expression was increased in WT littermates as well as MT rats over time within the putative phrenic motor nucleus. These receptor changes are observed when comparing end-stage to pre-symptomatic expression, regardless of SOD1 expression. Since the effects are observed in WT littermates, we suggest that 5-HT2B receptors may become more heavily utilized to maintain respiration later in life as 5-HT2A receptors decrease. Other groups have reported an age-related decline in 5-HT2 receptors (Wang et al., 1995) and serotonin 2A binding sites (Marcusson et al., 1984) in the frontal cortex of healthy human subjects. Thus, our observation of a decline in 5-HT2A receptors in the cervical spinal cord, and more specifically the putative phrenic motor nucleus, is consistent with previous studies in the CNS and to our knowledge the first to describe an age-related decline in putative phrenic 5-HT2A receptors. Additionally, Tadros et al. reported 5-HT2B receptor gene expression was up-regulated in older (~ 28 months of age) CBA/CaJ mice compared to younger (~ 12 months of age) CBA/CaJ mice in the inferior colliculus nucleus of the midbrain. Thus, our observation of an age-related increase in 5-HT2B receptor expression in the putative phrenic motor nucleus in the cervical spinal cord is consistent with previous studies in the CNS, and to our knowledge the first to describe an age-related increase in putative phrenic 5-HT2A receptors.
4.1. 5-HT2A/B receptor expression and pLTF
We found that 5-HT2B receptor expression is upregulated at end-stage only within the putative phrenic motor nucleus of MT vs. WT rats (Fig. 4K&L). Similar findings have been reported in other models of motor neuron loss. In spinal cord injury, patients experience spasticity that is thought to be caused by loss of serotonergic axons. Normally serotonin mediates motor neuron excitability through an increase in persistent calcium current, but with loss of serotonergic innervation to lower motor neurons there is a loss of serotonergic transmission and the motor neurons become hypoexcitable (Heckmen et al., 2009). To compensate for this loss of brainstem serotonergic input following spinal cord injury, motor neurons produced 5-HT2B and 5-HT2C receptors that were constitutively active (Murray et al., 2010a; Murray et al., 2010b; Fouad et al., 2010). Although this 5-HT2 receptor compensation was shown to contribute to the recovery of locomotor function, it also resulted in hyperexcitability of the motor neurons and lead to spasticity (Murray et al., 2010a; Murray et al., 2010b). ALS patients also experience a degeneration of serotonergic innervation and a depletion of serotonin, which has been recapitulated and studied in a mouse model of ALS (SOD1G86R) (Dentel et al., 2012). Interestingly, pre-symptomatic SOD1G86R mice had decreased serotonin in the brainstem, spinal cord, and cortex, while serotonin turnover remained unchanged (Dentel et al., 2012). SOD1G86R mice also developed spasticity that was alleviated by the 5-HT2B/C inverse agonist SB206553 (Dentel et al., 2012). These studies suggest that loss of central serotonin due to depletion, not the turnover of serotonin, contributes to early development of ALS and that compensation through constitutively active 5-HT2B and 5-HT2C receptors leads to motor neuron hyperexcitability and spasticity (Heckmen et al., 2009; Dentel et al., 2012; Murray et al., 2010a; Murray et al., 2010b; Fouad et al., 2010). Therefore, it is possible that the increase in 5-HT2B receptor expression we observed in MT rats at end-stage is in response to loss of serotonergic innervation and contributes to plasticity of the surviving motor neurons by making them hyperexcitable.
Previous studies conducted by Nichols et al. (2014) found that blockade of 5-HT receptors with methysergide did not impact breathing at end-stage in MT rats, but that blockade of downstream 5-HT2A and 5-HT2B (Gq) receptor pathway modulators (e.g., new BDNF synthesis and MEK) did impact pLTF (Nichols et al., 2014). Furthermore, ERK/MAPK signaling is required for the induction of AlH-induced pLTF in naïve rats, but not the maintenance of pLTF (Hoffman et al., 2012). Similarly, 5-HT2A receptors are required for the initiation of AlH-induced pLTF but not the maintenance (Fuller et al., 2001). It has also been shown that blocking either 5-HT2A or 5-HT2B prior to AIH attenuates pLTF, indicating the activation of both isoforms during AlH-induced pLTF (Tadjalli and Mitchell, 2019). Recent studies have evaluated pMF in naïve rats by examining MEK/ERK MAPK signaling downstream from 5-HT2A/B receptors and found that inhibition of MEK/ERK MAPK blocks 5-HT2A, but not 5-HT2B pMF (Tadjalli and Mitchell, 2019). Taken together with what was previously discussed, we believe that: 1) 5-HT2B receptor expression becomes upregulated in end-stage MT rats following loss of serotonergic input (whether those receptors are constitutively active or not has yet to be determined); 2) 5-HT2A receptor expression is unchanged in the putative phrenic motor nucleus at end-stage in MT rats because initiation of pLTF may have already begun during the pre-symptomatic stage; and 3) that both receptors are involved in pLTF and breathing because blocking 5-HT2 receptors or downstream signaling modulators resulted in compromised pLTF or breathing in MT rats at end-stage (Nichols et al., 2014; Nichols et al., 2017). Since 5-HT2A and 5-HT2B receptor expression in the putative phrenic motor nucleus is either not different or upregulated in end-stage MT rats vs. WT, age-matched littermates, we suggest both 5-HT2A and 5-HT2B receptors have a role in pLTF. We speculate that there may be compensation by one 5-HT2 isoform becoming constitutively active to preserve pLTF via the Q-pathway as the other 5-HT2 isoform becomes depleted or desensitized to serotonin throughout disease progression. Given that the overexpression of SOD1 does not initially downregulate reactive oxygen species in MT rats, there may be a switch from 5-HT2B receptor-NADPH oxidase-dependent to 5-HT2A receptor-NADPH oxidase-independent pLTF to preserve plasticity at end-stage through the formation of additional reactive oxygen and nitrogen species, as discussed in later sections. Since we suggest a role for 5-HT2 receptors in pLTF and breathing, future studies could focus on the intrathecal delivery of the isoform specific antagonists for 5-HT2A and 5-HT2B receptors both before and after AIH to delineate their role in pLTF and breathing in end-stage MT rats (i.e., if isoform antagonism abolishes pLTF or compromises breathing). If combined antagonism of these receptors does not impact pLTF or compromise breathing, then compensatory mechanisms may be occurring through activated Gs-coupled receptors (i.e., 5-HT7 or A2A receptors). Other possible explanations for the 5-HT2A/B receptor expression observed and compensatory mechanisms via 5-HT2A/B receptors contributing to pLTF and breathing are discussed in the following sections.
4.2. 5-HT2 receptor expression on other cell types
Upon closer investigation of the photomicrographs, it appears that the 5-HT2 receptors are not solely expressed on surviving motor neurons. Therefore, other cell types such as microglia and/or astrocytes, which both express 5-HT2 receptors, may express these receptors to maintain receptor expression at wild-type levels (MacFarlane, Vinit, & Mitchell, 2011; Hirst et al., 1998). In congruence with work from MacFarlane et al. 2011, we also report expression of 5-HT2A/B receptors on neighboring neuropil (MacFarlane, Vinit, & Mitchell, 2011). In vitro research has shown that serotonin and ATP facilitate microglial migration (Krabbe et al., 2012). Thus, it is possible that motor neuron death itself could lead to the release of ATP and signal microglial migration. In SOD1G93A mice, microglial 5-HT2B receptor expression was found to be upregulated in the lumbar (L3-5) spinal cord tissue and that blocking 5-HT2B receptors on microglia accelerated disease progression (El Oussini et al., 2016), which could also explain why we see an increase in 5-HT2B receptors in end-stage MT rats. Another study evaluating astroglial reactivity in the cervical spinal cord found that astroglial activation occurred in pre-symptomatic MT rats in the ventral horn of the spinal cord before motor neuron degeneration, which was further increased at end-stage when compared to non-transgenic rats (Howland et al., 2002). Therefore, neurons and other cell types, specifically astroglia, outside of the putative phrenic motor nucleus could be upregulating their expression of 5-HT2A receptors by end-stage in the non-phrenic ventral horn, resulting in the return towards WT levels of 5-HT2A expression as our data suggest (Fig. 2). To differentiate these roles, future immunohistochemical studies could be used to further investigate the expression of 5-HT2A and 5-HT2B receptors on microglia and astrocytes.
4.3. The interaction of ROS and 5-HT2A receptors
Lastly, we speculate that other RNS and/or ROS (e.g., nitric oxide or hydrogen peroxide) are formed following 5-HT2A activation independently of NADPH oxidase. This may subsequently lead to the inhibition of phosphatases and activation of PKC, resulting in pLTF at end-stage. When Macfarlane and Mitchell pretreated naïve rats with the nitric oxide synthase (NOS) inhibitor L-NAME, pLTF was attenuated, indicating the requirement for NOS and nitric oxide in pLTF. In vitro studies have also shown that serotonin can directly bind to and activate nNOS leading to not only NO production, but ROS production as well (Bréard & Grillon, 2009). Together, these studies may indicate 5-HT2A activation, independently of NADPH oxidase, that leads to the pLTF observed at end-stage in MT rats. Finally, isoform specific antagonism of 5-HT2A and 5-HT2B receptors could not only determine which isoform is required for pLTF in pre-symptomatic and end-stage MT rats, but the underlying mechanisms of pLTF that may include the production of RNS and ROS that are not scavenged by the overexpression of superoxide dismutase I. Future studies would have to utilize RNS and ROS inhibitors (against nitric oxide or hydrogen peroxide) to determine if they are necessary for pLTF. If either RNS and ROS (e.g., nitric oxide or hydrogen peroxide) are required for pLTF, then 5-HT2A or 5-HT2B receptor antagonists should be used to determine if the activation of these receptor isoforms lead to loss of downstream RNS and/or ROS production. These studies would provide insight as to which isoform is responsible for alternative RNS and/or ROS production that could contribute to pLTF despite the presence of superoxide dismutase overexpression and being independent of NADPH oxidase.
4.4. Significance
Overall, this study demonstrates that phrenic motor neuron death in MT rats leads to upregulation of 5-HT2B receptors in the putative phrenic motor nucleus at end-stage, while 5-HT2A remains unaffected in the putative phrenic motor nucleus throughout disease progression. We suspect that 5-HT2 receptor expression is changing on the surviving phrenic motor neurons as a compensatory measure to combat the loss of serotonergic input, as shown previously in other models of motor neuron loss. Because 5-HT2A/B receptors and their downstream modulators have been shown to be required for pMF and pLTF, their expression may be indicative of the mechanism by which respiratory plasticity is being elicited leading to increased output from the surviving motor neurons at end-stage in MT rats. The increase in 5-HT2B receptors may be due to the compensatory production of constitutively active 5-HT2B receptors. Although these receptors may contribute to pLTF and the maintenance of breathing through increased motor neuron excitability, spasticity, particularly of the diaphragm, can occur which may hinder breathing capacity late in disease. Additionally, we speculate that microglia and astrocytes are migrating to the putative phrenic motor nucleus and increasing their expression of 5-HT2 receptors in response to motor neuron death, although this has yet to be discerned. This study highlights a potential role for 5-HT2 receptor-dependent mechanisms for pLTF as well as the maintenance of breathing, especially following motor neuron loss. If the role of each of the 5-HT2 receptor isoforms in pLTF and breathing could be delineated throughout disease progression, then these receptors or their downstream modulators could be pharmacologically targeted to maintain elevated output of the surviving motor neurons and preserve breathing in those with ALS and similar motor neuron diseases.
Highlights.
Phrenic 5-HT2A receptor expression is reduced over time in SOD1G93A rats.
Phrenic 5-HT2A receptor expression is unchanged in SOD1G93A rats vs. wild-type rats.
Phrenic 5-HT2B receptor expression is increased over time in SOD1G93A rats.
Phrenic 5-HT2B receptor expression is unchanged in pre-symptomatic SOD1G93A rats.
Phrenic 5-HT2B receptor expression is upregulated in end-stage SOD1G93A rats.
Acknowledgements
Supported by NIH K99/R00 HL119606 (NLN), the Missouri Spinal Cord Injury/Disease Research Program (SCIDRP; NLN), and the University of Missouri College of Veterinary Medicine Committee on Research (COR; NLN). The authors thank the Dalton Imaging Core Facilities for the use of the confocal microscope, and Dr. David Kline at the Dalton Cardiovascular Research Center for the use of the Neurolucida microscopy system.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- SOD1
superoxide dismutase 1
- pLTF
phrenic long-term facilitation
- MT
mutant
- WT
wild-type
- pMF
phrenic motor facilitation
- NOS
nitric oxide synthase
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
All authors declare no conflicts of interest, financial or otherwise in relation to this work.
References
- Andersen PM, Sims KB, Xin WW, Kiely R, O’Neill G, Ravits J, Pioro E, Harati Y, Brower RD, Levine JS & Heinicke HU (2003). Sixteen novel mutations in the Cu/Zn superoxide dismutase gene in amyotrophic lateral sclerosis: a decade of discoveries, defects and disputes. Amyotrophic lateral sclerosis and other motor neuron disorders, 4(2), 62–73. [DOI] [PubMed] [Google Scholar]
- Bach KB, & Mitchell GS (1996). Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respiration physiology, 104(2–3), 251–260. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, & Mitchell GS (2004). BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nature neuroscience, 7(1), 48. [DOI] [PubMed] [Google Scholar]
- Boillée S, Velde CV, & Cleveland DW (2006). ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron, 52(1), 39–59. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Gestreau C, Vinit S, Stamegna JC, Kastner A, & Gauthier P (2007). Specific and artifactual labeling in the rat spinal cord and medulla after injection of monosynaptic retrograde tracers into the diaphragm. Neuroscience letters, 417(2), 206–211. [DOI] [PubMed] [Google Scholar]
- Bourke SC, Shaw PJ, & Gibson GJ (2001). Respiratory function vs sleep-disordered breathing as predictors of QOL in ALS. Neurology, 57(11), 2040–2044. [DOI] [PubMed] [Google Scholar]
- Bréard M, & Grillon C (2009). Serotonin binds to purified neuronal nitric oxide synthase: a possible explanation for ROS production induced by 5HT in the presence of nNOS. Free radical research, 43(3), 206–213. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, & Cleveland DW (1997). ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron, 18(2), 327–338. [DOI] [PubMed] [Google Scholar]
- Dale EA, Nashold LJ, Mahamed S, Svendsen CN, & Mitchell GS (2006). Sustained ventilatory capacity in a rat model of amyotrophic lateral sclerosis (ALS). A1213–A1213. [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, & Mitchell GS (2010). Multiple pathways to long-lasting phrenic motor facilitation New frontiers in respiratory control (pp. 225–230). Springer, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentel C, Palamiuc L, Henriques A, Lannes B, Spreux-Varoquaux O, Gutknecht L, René F, Echaniz-Laguna A, Gonzalez de Aguilar JL, Lesch KP and Meininger V (2012). Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: a link to spasticity. Brain, 136(2), 483–493. [DOI] [PubMed] [Google Scholar]
- Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, & Mitchell GS (2015). Phrenic long-term facilitation requires PKCθ activity within phrenic motor neurons. Journal of Neuroscience, 35(21), 8107–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oussini H, Bayer H, Scekic-Zahirovic J, Vercruysse P, Sinniger J, Dirrig-Grosch S, Dieterlé S, Echaniz-Laguna A, Larmet Y, Müller K & Weishaupt JH (2016). Serotonin 2B receptor slows disease progression and prevents degeneration of spinal cord mononuclear phagocytes in amyotrophic lateral sclerosis. Acta neuropathologica, 131(3), 465–480. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, & Nattie EE (2003). Breathing: rhythmicity, plasticity, chemosensitivity. Annual review of neuroscience, 26(1), 239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Rank MM, Vavrek R, Murray KC, Sanelli L, & Bennett DJ (2010). Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. Journal of neurophysiology, 104(6), 2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, & Mitchell GS (2001). Selected contribution: phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. Journal of applied physiology, 90(5), 2001–2006. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, & Mitchell GS (2008). Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. Journal of Neuroscience, 28(9), 2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW and Deng HX (1994). Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science, 264(5166), 1772–1775. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Hinrichsen CFL, & McCrimmon DR (2003). Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. Journal of applied physiology, 94(4), 1421–1430. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, Theiss R, & Schuster J (2009). Motoneuron excitability: the importance of neuromodulatory inputs. Clinical Neurophysiology, 120(12), 2040–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst WD, Cheung NY, Rattray M, Price GW, & Wilkin GP (1998). Cultured astrocytes express messenger RNA for multiple serotonin receptor subtypes, without functional coupling of 5-HT1 receptor subtypes to adenylyl cyclase. Molecular brain research, 61(1–2), 90–99. [DOI] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, MacFarlane PM, & Mitchell GS (2012). Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. Journal of applied physiology, 113(8), 1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, & DeGennaro LJ (2002). Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proceedings of the National Academy of Sciences, 99(3), 1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe G, Matyash V, Pannasch U, Mamer L, Boddeke HW, & Kettenmann H (2012). Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain, behavior, and immunity, 26(3), 419–428. [DOI] [PubMed] [Google Scholar]
- Lechtzin N, Rothstein J, Clawson L, Diette GB, & Wiener CM (2002). Amyotrophic lateral sclerosis: evaluation and treatment of respiratory impairment. Amyotrophic Lateral Sclerosis and other motor neuron disorders, 3(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Liu J, Lillo C, Jonsson PA, Velde CV, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, & Brännström T (2004). Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron, 43(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Lyall RA, Donaldson N, Polkey MI, Leigh PN, & Moxham J (2001). Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain, 124(10), 2000–2013. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, & Mitchell GS (2009). Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. The Journal of physiology, 587(22), 5469–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, & Mitchell GS (2009). Spinal nitric oxide synthase activity is necessary for phrenic long-term facilitation following intermittent hypoxia. [Google Scholar]
- MacFarlane PM, Vinit S, & Mitchell GS (2011). Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience, 178, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, & Sieck GC (2009). Retrograde labeling of phrenic motoneurons by intrapleural injection. Journal of neuroscience methods, 182(2), 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcusson JO, Morgan DG, Winblad B and Finch CE (1984). Serotonin-2 binding sites in human frontal cortex and hippocampus. Selective loss of S-2A sites with age. Brain Research, 311(1), pp.51–56. [DOI] [PubMed] [Google Scholar]
- McGuire M, & Ling L (2004). Activation of protein kinase C near/in phrenic motoneurons is required for phrenic long-term facilitation in rats. Am J Respir Crit Care Med, 169(7), A433. [Google Scholar]
- Murray KC, Stephens MJ, Ballou EW, Heckman CJ, & Bennett DJ (2010). Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. Journal of neurophysiology, 105(2), 731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D’amico J, Harvey PJ, Li X, Harris RLW, Ballou EW, Anelli R and Heckman CJ (2010). Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT 2C receptors. Nature medicine, 16(6), 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH, & Itoyama Y (2001). Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. Journal of Neuroscience, 21(23), 9246–9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, & Feldman JL (2007). Episodic stimulation of α1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. Journal of Neuroscience, 27(16), 4435–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Craig TA, & Tanner MA (2018). Phrenic long-term facilitation following intrapleural CTB-SAP-induced respiratory motor neuron death. Respiratory physiology & neurobiology, 256, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN and Mitchell GS (2013). Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. American journal of respiratory and critical care medicine, 187(5), 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Johnson RA, Satriotomo I, & Mitchell GS (2014). Neither serotonin nor adenosine-dependent mechanisms preserve ventilatory capacity in ALS rats. Respiratory physiology & neurobiology, 197, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Satriotomo I, Allen LL, Grebe AM & Mitchell GS (2017). Mechanisms of enhanced phrenic long-term facilitation in SOD1G93A rats. J. Neurosci. 37(24), 5834–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Satriotomo I, Harrigan DJ, & Mitchell GS (2015). Acute intermittent hypoxia induced phrenic long-term facilitation despite increased SOD1 expression in a rat model of ALS. Experimental neurology, 273, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Vinit S, Bauernschmidt L, & Mitchell GS (2015). Respiratory function after selective respiratory motor neuron death from intrapleural CTB–saporin injections. Experimental neurology, 267, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, & Rahmani Z (1993). Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature, 362(6415), 59. [DOI] [PubMed] [Google Scholar]
- Tadjalli A, & Mitchell GS (2019). Cervical Spinal 5-HT2A and 5-HT2B receptors are both necessary for moderate acute intermittent hypoxia-induced phrenic long-term facilitation. Journal of Applied Physiology. 127(2), 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zettel ML, Zhu X, Lynch-Erhardt M and Frisina RD, (2007). Serotonin 2B receptor: upregulated with age and hearing loss in mouse auditory system. Neurobiology of aging, 28(7), pp. 1112–1123. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Haenggeli C, & Rothstein JD (2007). Respiratory impairment in a mouse model of amyotrophic lateral sclerosis. Journal of applied physiology, 102(3), 926–932. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, & Goshgarian HG (2001). Serotonin 2 receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. Journal of applied physiology, 91(6), 2665–2673. [DOI] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, & Price DL (1995). An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron, 14(6), 1105–1116. [DOI] [PubMed] [Google Scholar]
- Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, & Borchelt DR (2003). Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Human molecular genetics, 12(21), 2753–2764. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Fowler JS, Schlyer D, MacGregor RR, Hitzemann RJ, Gur RC and Wolf AP (1995). Evaluation of age-related changes in serotonin 5-HT2 and dopamine D2 receptor availability in healthy human subjects. Life sciences, 56(14), pp.PL249–PL253. [DOI] [PubMed] [Google Scholar]
- Watson C, Paxinos G, Kayalioglu G (2009). The spinal cord: a Christopher and Dana Reeve Foundation text and atlas. London: Academic. [Google Scholar]


