Summary
Acute psychological stress has long been known to decrease host fitness to inflammation in a wide variety of diseases, but how this occurs is incompletely understood. Using mouse models, we show that IL6 is the dominant cytokine inducible upon acute stress alone. Stress-inducible IL6 is produced from brown adipocytes in a beta-3-adrenergic-receptor-dependent fashion. During stress, endocrine IL6 is the required instructive signal for mediating hyperglycemia through hepatic gluconeogenesis, which is necessary for anticipating and fueling “fight or flight” responses. This adaptation came at the cost of enhancing mortality to a subsequent inflammatory challenge. These findings provide a mechanistic understanding of the ontogeny and adaptive purpose of IL6 as a bona fide stress hormone coordinating systemic immunometabolic reprogramming. This brain-brown fat-liver axis may provide new insights into brown adipose tissue as a stress-responsive endocrine organ and mechanistic insight into targeting this axis in the treatment of inflammatory and neuropsychiatric diseases.
Keywords: IL6, brown adipose tissue, acute stress, immunometabolism, gluconeogenesis, neuroimmunology, inflammation, beta-adrenergic receptors, tolerance
IN BRIEF
During acute psychological stress, brown adipocytes initiate a chain of events mediated by adrenergic signaling and IL6 release that metabolically fuels “fight or flight” adaptive responses but at the same time comes at an inflammatory cost.
Graphical Abstract
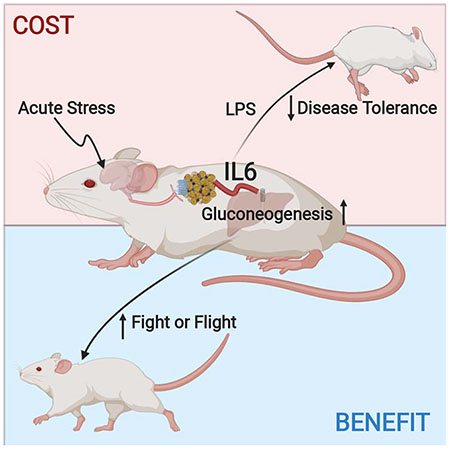
Introduction
Acute life stressors have been observed to decompensate a wide range of inflammatory diseases since antiquity (Hippocrates, 1849; Liu et al., 2017). Most chronic sterile inflammatory diseases are known to “flare” after acute stress, contributing significantly to morbidity and mortality. Indeed, psychosocial stress worsens most inflammatory diseases, including allergic diseases, autoimmune diseases, and cancers (Batty et al., 2017; Liu et al., 2002; Mohr et al., 2004; Roussou et al., 2013). In human studies, stress induces measurable changes in biology, such as in the magnitude of inflammatory cytokines and in functional changes within relevant end-organs, such as in pulmonary function in the case of allergic inflammation (Liu et al., 2002). Concordantly, several randomized controlled trials targeting stress management have largely lead to improvement in the morbidity of inflammatory diseases (Black and Slavich, 2016; Pbert et al., 2012; Simpson et al., 2014). However, the well-studied mediators of stress physiology, glucocorticoids and catecholamines, are primarily thought to be immunosuppressive (Russell and Lightman, 2019) and used therapeutically for this purpose, creating a paradox that many have tried to resolve for over 30 years (Frank et al., 2013; Munck et al., 1984). How does psychological stress, which leads to the production of immunosuppressive mediators such as cortisol and catecholamines, decrease host fitness to inflammation?
Studies dating back to 1990 have shown that psychological stress increase circulating levels of IL6 in humans and laboratory animals (Cheng et al., 2015; LeMay et al., 1990; Maes et al., 1998). The role that IL6 plays in the acute stress response, also referred to as the “fight or flight” response, is unclear. The idea that stress itself induces endocrine mediators like IL6, which is traditionally associated with inflammation, has since been supported by the detection of increased circulating cytokines in depression and anxiety (Felger and Lotrich, 2013; Khandaker et al., 2014), and by the association of IL6 polymorphisms in individuals with depression (Zhang et al., 2016). Moreover, there is a robust relationship between depression and anxiety and poor outcomes in inflammatory diseases (Eisner et al., 2005; Satin et al., 2009). This body of research has led to clinical trials assessing the efficacy of an antagonizing monoclonal antibody targeting IL6 receptor subunit alpha (IL6Ra), tocilizumab — used in rheumatoid arthritis and vasculitis — in depression (Kappelmann et al., 2018), despite little understanding of how and why stress induces IL6. The possibility that stress-inducible cytokines, as opposed to glucocorticoids or catecholamines, underlie how stress leads to poorer outcomes in inflammatory diseases has not been explored in depth.
Here we report that commonly utilized models of acute stress in mice induce endocrine IL6. Stress-induced IL6 requires consciousness and beta-3-adrenergic-receptor signaling in brown adipocytes. IL6 is required for stress hyperglycemia, a metabolic adaptation that enables “fight or flight” response, via hepatic gluconeogenesis. The cost of stress-induced IL6 is that it decreases host fitness to a subsequent inflammatory challenge. Our studies therefore mechanistically uncover the origin and adaptive function of IL6 in acute stress and its cost in the setting of inflammation in mice.
Results
Acute stress induces endocrine IL6
We found that standard laboratory models of acute stress—including tube restraint, cage switching, and social isolation—induced high levels of circulating IL6 (Figure 1A), consistent with previous studies demonstrating that stress alone induced IL6 (Cheng et al., 2015; LeMay et al., 1990; Maes et al., 1998). Unexpectedly, we found that a single conscious retro-orbital bleed induced IL6 (Figure 1A). To more comprehensively survey other stress-inducible immune mediators, we screened 32 inflammatory cytokines and chemokines in the circulation of stressed mice and identified a set of cytokines inducible by stress alone; IL6 was the most greatly induced cytokine and common to two different stress models (Figure 1B). We did not detect corresponding increases in the soluble IL6 receptor (Figure S1A) (Khokha et al., 2013). The absolute level of IL6 we detected fell in the middle range of reported levels (50 pg/mL to 200 ng/mL) in inflammatory contexts and above reported ranges post-exercise and diet-induced obesity (30-100 pg/mL) (Chowdhury et al.; Gao et al., 2014; Greenhill et al., 2011; Masuda et al., 2013; Osuchowski et al., 2006; Remick et al., 2005; Vida et al.; Wang et al., 2017; Yan et al., 2015). We also confirmed that acute stress-increased IL6 was independent of handlers (as indicated in the figure legend).
Figure 1. Acute stress induces endocrine IL6.
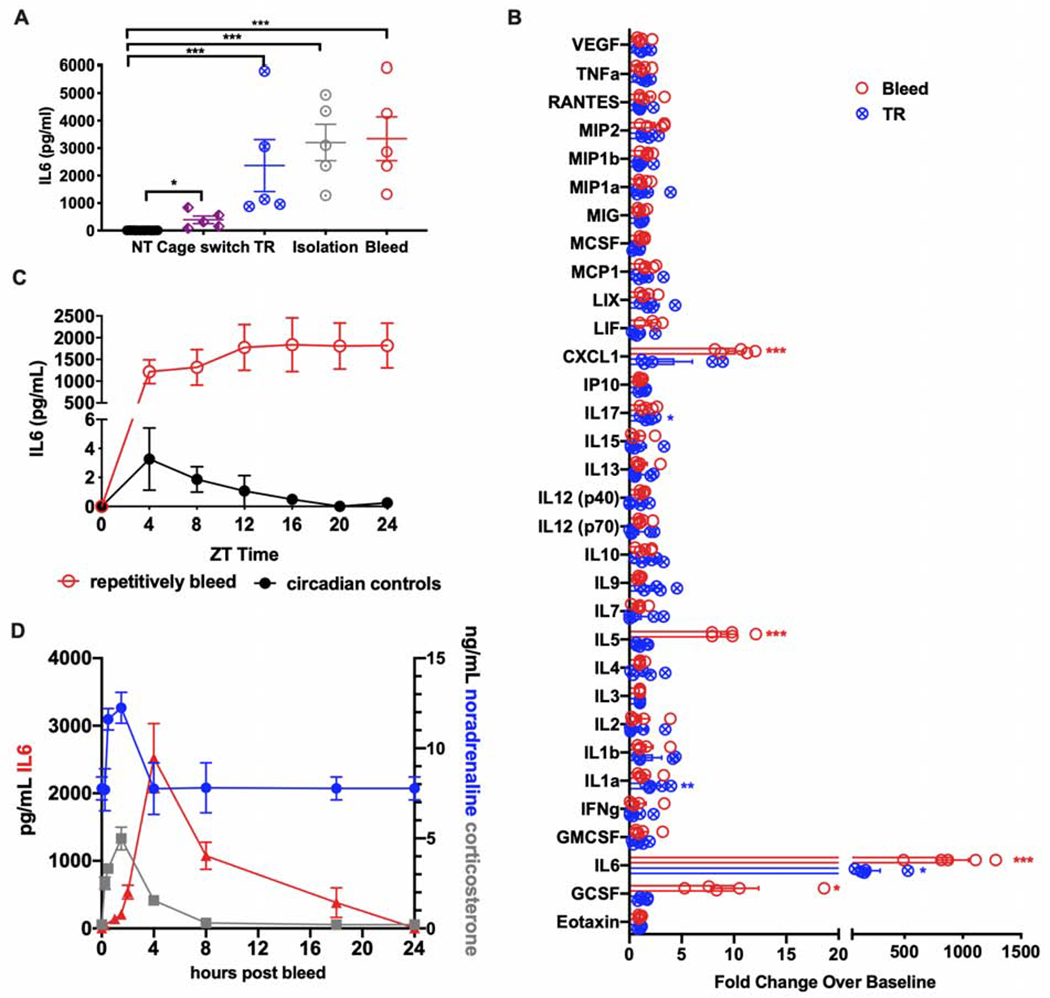
(A) Plasma IL6 levels in mice after exposure to one of the indicated stress challenges (n=5 per group, representative of 2 experiments). These experiments were performed by H.Q. NT, no treatment. TR, tube restraint.
(B) Fold change of the indicated inflammatory cytokines and chemokines four hours after a single retro-orbital bleed or tube restraint (TR). Results are presented as the ratio of cytokine or chemokine levels from stressed subjects compared with no stress controls (n=5 per group). Experiments were performed by R. D.
(C) Plasma IL6 levels following repetitive bleeding. Repetitive bleeding was applied to the same mice (n=5) every four hours, while circadian controls were non-stressed mice bled once at the indicated ZT times (n=5 for each time point). Experiments were performed by A.W.
(D) Plasma levels of noradrenaline, corticosterone, and IL6 post bleeding at indicated time point (n=5 per time point, representative of 2 experiments). * p<0.05, ** p<0.01, *** p<0.001, ****p<0.0001
To exclude circadian oscillations leading to fluctuations in IL6, we measured circulating IL6 over time by retro-orbital bleeding, and observed that repeated bleeding of the same animals sustained high IL6 levels. When individual cages of unmanipulated, entrained animals were bled at the corresponding Zeitgeber times (five mice per ZT time, bled only once at that ZT time), no such sustained increase in IL6 was noted, demonstrating that conscious bleeding itself increased circulating IL6 and that repeated bleeding sustained high IL6 levels (Figure 1C). Thus, in order to gain insight into the kinetics of IL6 after an acute stress, we subjected several groups of mice to a single retro-orbital bleed, and then sampled individual groups at different timepoints following the bleed. Circulatory cortisol and noradrenaline were increased within 15 minutes, peaked at two hours after acute stress and returned to baseline by four hours; however, IL6, which was significantly increased in blood by two hours, peaked at four hours, and was even detectable above baseline 18 hours after acute stress (Figure 1D). The unique kinetics of stress-induced endocrine IL6 suggested that it may be mediating more sustained aspects of stress physiology.
Since adrenal gland is thought to be the major mediator of the acute stress response and previous reports have described adrenally-derived IL6 (Path et al., 2000), we asked if the adrenal gland was required for stress-induced IL6. We found that adrenalectomized mice had significantly higher levels of IL6 following stress, suggesting that the adrenal gland negatively regulated IL6 (Figure S1B). To address previous reports of cross-talk between IL6 and adrenal hormones (Bethin et al., 2000; Path et al., 2000), we utilized an antagonistic anti-IL6Ra antibody and found that inhibition of IL6 signaling did not change circulating levels of corticosterone or noradrenaline after acute stress (Figure S1C and D). This model avoids the confounding developmental defects observed in constitutive IL6 knockout animals (Wallenius et al., 2002).
We then validated previous observations that circulating IL6 levels were increased in stressed humans (Felger and Lotrich, 2013; Khandaker et al., 2014). We acquired a community sample of individuals that were carefully assessed for high and low stress using a structured cumulative stress and adversity interview that assessed recent and past life events (Cumulative Adversity Interview, (Ansell et al., 2012; Seo et al., 2014; Turner and Lloyd, 1995)). The high and low groups were group matched on age, gender, education and body mass index (Figure S1E). We found significant overall increased IL6 levels in the high [74 pg/ml, SE: 35] versus low [3.9 pg/ml, SE: 2.78] stress groups (t=2.15, p<0.05) (Figure S1E). The absolute circulating level of stress-associated IL6 in humans was a hundred times lower in mice, reflecting inter-species variation and/or acuity, heterogeneity, and magnitude of stressors. Taken together, these data indicate that IL6 is an endocrine hormone inducible by acute stress alone, with different kinetics than the canonical stress hormones, corticosterone and noradrenaline.
Stress-inducible IL6 is produced by brown adipocytes
To understand the ontogeny of stress-induced IL6, we first ensured that IL6 was not induced by retro-orbital bleeding as a result of bacterial translocation from the skin or gastrointestinal tract (Kelly et al., 2015). Consistent with our observations that other acute-phase cytokines were not induced after stress, stress-induced IL6 was present in both gnotobiotic animals and animals deficient in key signaling pathways necessary for detecting bacteria (Figure 2A). Previous reports had suggested that hyperglycemia itself, a characteristic feature of the acute stress response (Esposito et al., 2002), was sufficient to induce IL6. To test this, we performed an oral glucose tolerance test four hours post retro-orbital bleeding and found that both glucose and water induced IL6, suggesting that the acute stress of gavaging and bleeding, but not hyperglycemia, was responsible for IL6 induction (Figure S1F and G). We also wanted to exclude the possibility that local damage to the retro-orbital plexus was inducing regional endothelial or immune release of IL6, and so sampled the contralateral orbital plexus and did not observe differences between traumatized and untraumatized eyes, suggesting that the contribution of systemic IL6 was not significantly affected by local damage (Figure S1H).
Figure 2. Stress-inducible IL6 is produced by brown adipocytes.
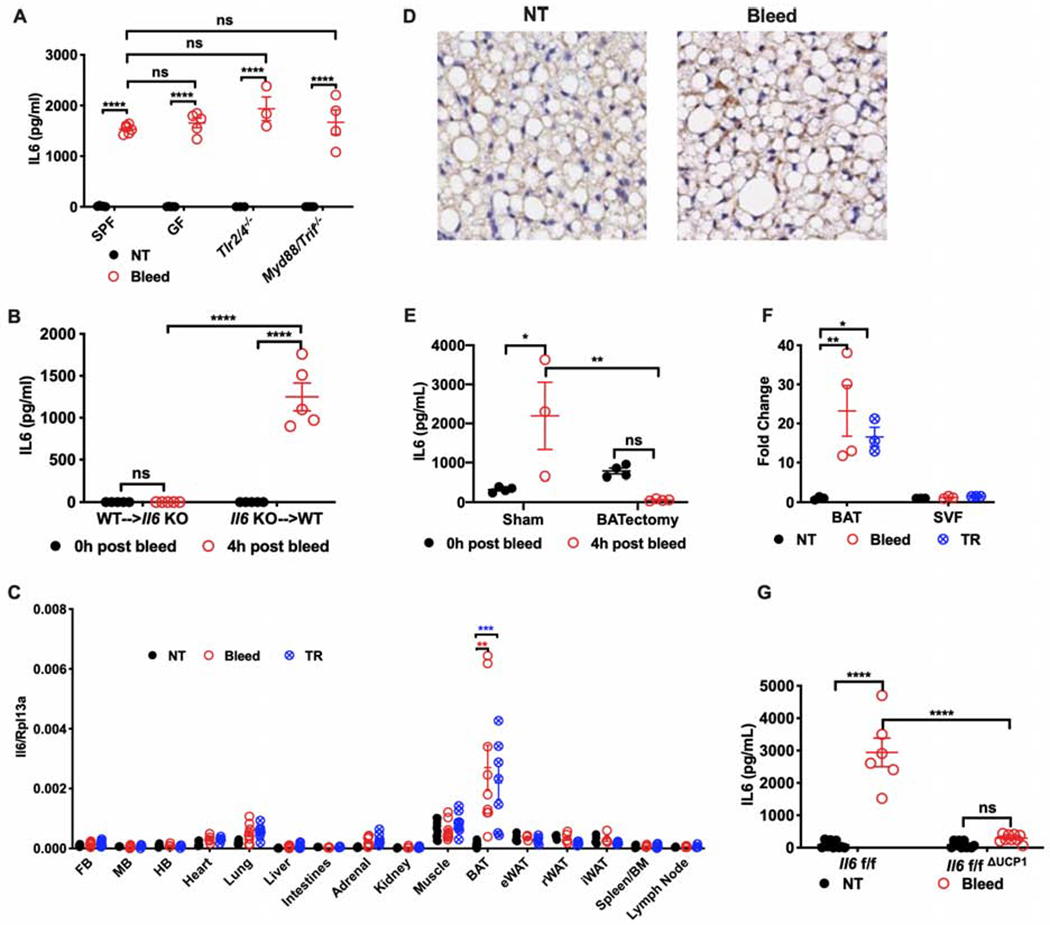
(A) Plasma levels of IL6 post retro-orbital bleeding from conventionally housed mice (SPF), germ free mice (GF), or mice deficient in key signaling pathways necessary for pathogen detection and response (n=8 for SPF, n=3 for GF, n=3 for Tlr2/4−/−, n=4 for Myd88/Trif−/−).
(B) Plasma level of IL6 from chimeric mice exposed to bleeding. WT→Il6 KO: Il6 knockout (KO) mice transplanted with bone marrow (BM) cells from wildtype (WT) mice; Il6 KO→WT: WT mice transplanted with BM cells from Il6 KO mice (n=5 per group).
(C) Transcriptional analysis of Il6 in tissues from stressed and control mice (n=5 per group, representative of 2 experiments). FB, forebrain. MB, midbrain. HB, hindbrain. BAT, brown adipose tissue. eWAT, epididymal white adipose tissue. rWAT, retroperitoneal white adipose tissue. iWAT, inguinal white adipose tissue. NT, no treatment. TR, tube restraint.
(D) Representative images of immunohistochemical staining for IL6 in brown adipose tissue harvested from bled mice or from controls (NT). IL6-positive staining is brown.
(E) Plasma level of IL6 post bleeding from mice with surgical removal of brown adipose tissue (BATectomy) or sham surgery controls (sham) (n=3 for sham, n=4 for BATectomy)
(F) Transcriptional analysis of Il6 in stromal vascular fraction (SVF) of brown adipose tissue (BAT) from stressed mice (n=3 for NT, n=4 for Bleed or TR group, representative of 2 experiments). Results are presented as fold increase relative to non-stressed controls (NT). TR, tube restraint.
(G) Plasma level of IL6 post bleeding from mice with Il6 genetically deleted in brown adipocytes (Il6f/fΔUCP1) compared with littermate controls (Il6f/f). NT, no treatment. * p<0.05, ** p<0.01, ***p<0.001, **** p<0.0001
See also Figure S1.
IL6 is produced by many cell types, including hematopoietic cells, myocytes, endothelial cells, adipocytes (Hunter and Jones, 2015). To identify the origin of IL6, we first performed mixed bone marrow chimera studies using IL6-deficient animals and found that stress-induced IL6 was not produced by radiosensitive cells (Figure 2B). Given the kinetics of plasma IL6 following acute stress, we reasoned that IL6 would be transcriptionally regulated. Thus, we screened multiple tissues for Il6 induction using both the bleeding and tube restraint models. We found that Il6 was robustly induced in the brown adipose tissue (BAT) (Figure 2C) and confirmed protein expression by immunohistochemistry (Figure 2D). We did not detect increased muscle Il6, which was consistent with the observation that mice did not significantly increase physical activity after an acute stressor (Figure S2B). To test if BAT was the sole source of stress-induced IL6, we surgically excised the BAT, which ablated the IL6 response to bleeding stress (Figure 2E).
Since BAT is a complex collection of cells including radioresistant immune cells (Wolf et al., 2017), we first asked if stress-induced Il6 would be present in the stromal vascular fraction (SVF), which includes all cells except adipocytes. The purity of our SVF isolation was verified by the absence of Ucp1 and beta-3 adrenergic receptor (Adrb3) expression (Figure S1I and J). Il6 transcriptional induction was not observed in the SVF fraction from either bled or restrained animals, implying that stress-induced IL6 was derived from brown adipocytes (Figure 2F). Thus, we generated an animal in which Il6 could be inducibly deleted in brown-adipocytes using Ucp1 promoter-driven Cre under the control of estrogen receptor (Il6f/fΔUCP1) and detected a significant attenuation of IL6 following acute stress in these animals (Figure 2G). Collectively, our data demonstrate that brown adipocytes are the source of stress-induced IL6.
ADRB3 mediates brown-adipocyte-derived IL6 in response to acute stress independently of thermoregulation
Since BAT is critical for non-shivering thermogenesis in cold and psychological stress (Kataoka et al., 2014), we assessed the effect of ambient temperature on stress-induced IL6. We subjected animals placed in standard cold acclimation (4°C), standard (22°C), and thermoneutral (32°C) conditions to bleeding stress, and did not note any effect on IL6 induction (Figure S1K). To test whether the key mediator of non-shivering thermogenesis, UCP1, was required for IL6, we tested the IL6 response in Ucp1-deficient animals. Consistent with a function independent from thermoregulation, we did not note differences in either endocrine IL6 levels or BAT transcriptional upregulation of Il6 (Figure 3A and B). We also did not note any temperature differences after bleeding stress in the presence of IL6Ra-blocking antibody compared to isotype control (Figure S1L). Transcriptional analyses of BAT after acute stress did not demonstrate induction of Ucp1 or other classic cold-responsive genes (Figure S1M). Interestingly, mRNA transcript for Il5, which was detected as a stress-induced cytokine after bleeding (Figure 1B), was also produced in the BAT. This finding is reminiscent of the reported role of IL5 in BAT adaptation to prolonged cold exposure (Lee et al., 2015; Qiu et al., 2014).
Figure 3. ADRB3 mediates brown adipocyte-derived IL6 in response to acute stress.
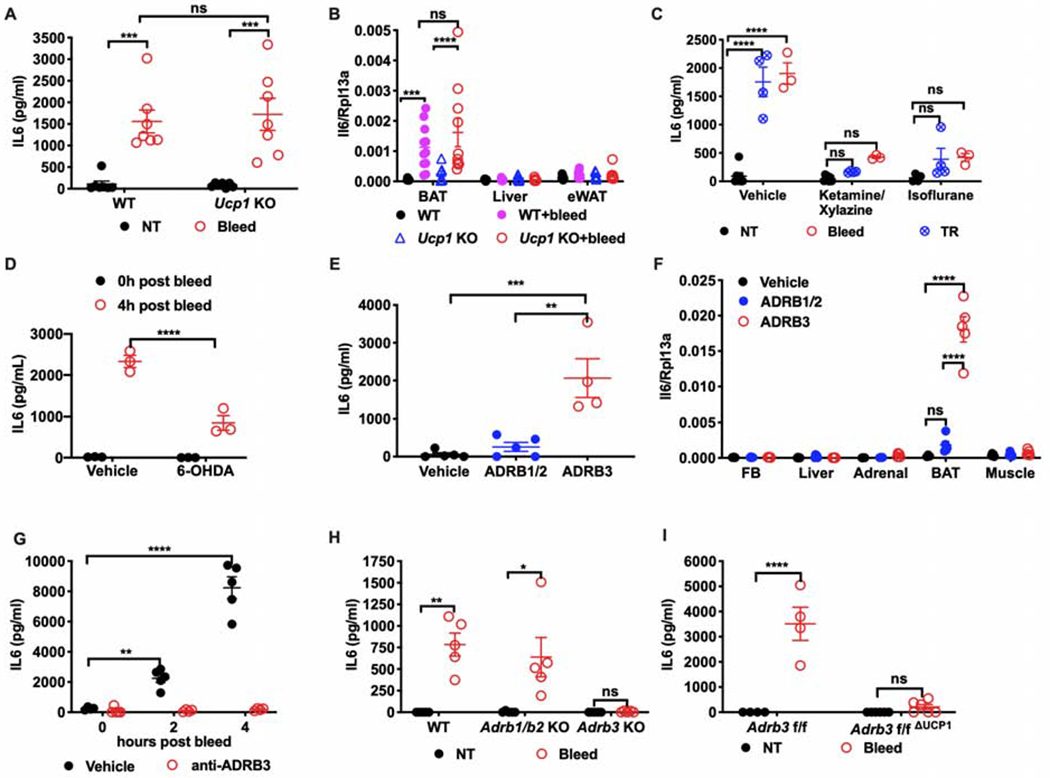
(A) Circulating IL6 levels (n=7 per group) and (B) mRNA expression of Il6 post bleeding in Ucp1 KO or WT mice (n=7 for WT, n=11 for WT + bleed, n=7 for Ucp1 KO, n=10 for Ucp1 KO + bleed). NT, no treatment. BAT, brown adipose tissue. eWAT, epididymal white adipose tissue.
(C) Plasma IL6 levels in mice bled or restrained after anesthesia induced by ketamine/xylazine or isoflurane (n=5 per group, representative of 2 experiments). NT, no treatment. TR, tube restraint.
(D) Circulating IL6 post bleeding from mice with chemical denervation of brown adipose tissue via 6-hydroxydopamine (6-OHDA) administration (n=3 per group).
(E) Circulating IL6 levels and (F) mRNA expression of Il6 two hours post injection of beta-adrenergic receptor agonists (n=5 per group). ADRB1/2: isoproterenol, ADRB1 and ADRB2 agonist. ADRB3: CL316,243, ADRB3 agonist. FB, forebrain. Adrenal, Adrenal gland. BAT, brown adipose tissue.
(G) Plasma level of IL6 post bleeding at indicated time points from mice pre-treated with the ADRB3 antagonist SR59203A (n=5 per group).
(H) Plasma level of IL6 post bleeding in Adrb1/2 KO or Adrb3 KO mice (n=5 per group). NT, no treatment.
(I) Plasma level of IL6 post bleeding from mice with conditional UCP1-mediated Adrb3 deletion (Adrb3f/fΔUCP1) or littermate controls (Adrb3f/f) (n=5 per group). NT, no treatment. * p<0.05, **p<0.01, *** p<0.001, **** p<0.0001
We reasoned that consciousness would be required for IL6 response to acute stress. Thus, we anesthetized animals with either ketamine/xylazine or isoflurane, after which we subjected them to tube restraint or retro-orbital bleeding, and found that anesthesia abrogated stress-induced IL6 (Figure 3C). Since sympathetic outflow to BAT is well-described in settings of acute exposure to cold (Kawate et al., 1994; Nguyen et al., 2017) and psychological stress (Kataoka et al., 2014), where projections originate from the rostral medullary raphe region and dorsomedial hypothalamus, we hypothesized that IL6 was induced via beta-adrenergic signaling. To test if sympathetic neurons were required for stress-induced IL6, we utilized 6-hydroxydopamine (6-OHDA) at doses which achieve significant BAT sympathectomy without significant effects on the central nervous system (Depocas et al., 1984). After 6-OHDA treatment, we found that stress-inducible IL6 was significantly attenuated, confirming that sympathetic outflow was required (Figure 3D). Since all three beta-adrenergic receptors are present in BAT (Figure S1J, N, and O), we asked which of these played a role in mediating stress-induced IL6. We thus challenged animals with ADRB agonists and found that the ADRB3 agonist CL316,243, but not the ADRB1/2 agonist isoproterenol, was sufficient to induce IL6 (Figure 3E and F), consistent with previous reports (Kosteli et al., 2010; Zhang et al., 2014). Concordantly, pre-treatment with the pharmacologic inhibitor of ADRB3, but not ADRB1/2 (Figure 3G and Figure S1P) or genetic deletion of Adrb3 (Figure 3H), abrogated stress-induced IL6. These experiments demonstrate that ADRB3 is necessary and sufficient for acute-stress-induced IL6. Since many cell types express Adrb3, we generated animals in which Adrb3 could be inducibly deleted from brown adipocytes (Adrb3f/fΔUCP1) and verified that tamoxifen induction efficiently deleted Adrb3 (Figure S1Q). As expected and consistent with Il6f/fΔUCP1 animals (Figure 2G), stress-inducible IL6 was significantly attenuated in Adrb3f/fΔUCP1 mice (Figure 3I). These data are consistent with a UCP1-independent ADRB3-dependent endocrine function of brown adipocytes and suggest that BAT may function as an endocrine organ sensitive to adrenergic outflow triggered by acute stress.
IL6 is necessary for maintaining hyperglycemia following acute stress
Given the large induction of IL6 by stress alone, we hypothesized that IL6 was coordinating stress physiology. Classical “fight or flight” physiology includes autonomic outflow and metabolic reprogramming towards catabolic metabolism, which is thought to fuel the increased energy demand anticipated in threatening situations (Russell and Lightman, 2019). We did not detect significant changes in the quantity of canonical stress hormones (Figures S1C–D) or stress-induced heart rate or hypertension (Figure S2A) after acute stress in the absence of IL6 function, suggesting that autonomic output was not significantly impacted by IL6. In contrast, using indirect calorimetry, we did detect significant differences in the overall energy expenditure of stressed animals in which IL6 signaling was antagonized (Figure 4A) despite no significant changes in total activity of animals in either group (Figure S2B). We thus hypothesized that organismal metabolic re-programming was likely a key function of stress-induced IL6, consistent with the numerous studies that have reported a role for IL6 in affecting organismal metabolism in various contexts (Covarrubias and Horng, 2014; Mauer et al., 2014; Pedersen and Febbraio, 2007; Scheller et al., 2011; Timper et al., 2017).
Figure 4. IL6 is necessary for promoting stress-hyperglycemia.
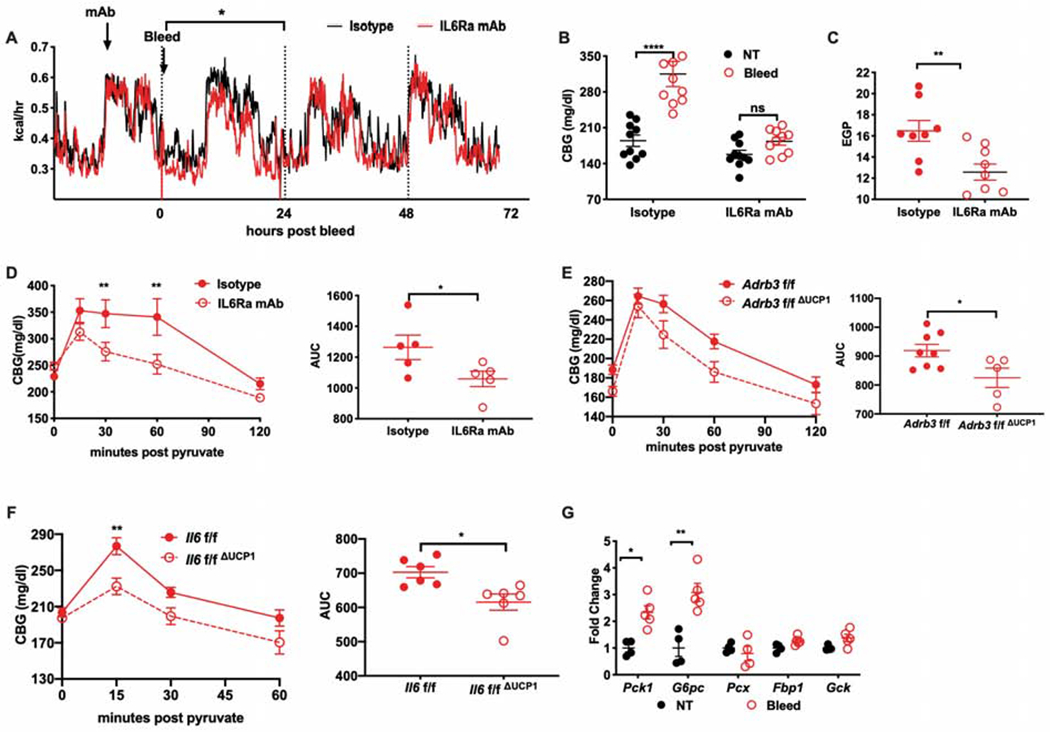
(A) Energy expenditure post bleeding from mice pretreated with IL6Ra antibody or isotype control (n=4 per group, representative of 2 experiments). Statistic represents the area under the curve between groups.
(B) Blood glucose levels post bleeding from mice pretreated with IL6Ra inhibitor or isotype control (n=10 per group). CBG: Capillary blood glucose
(C) Endogenous glucose production (EGP) post bleeding in mice pretreated with IL6Ra inhibitor or isotype control (n=8 for isotype, n=9 for IL6Ra mAb).
(D) Pyruvate tolerance test (PTT) performed four hours post bleeding in mice pretreated with IL6Ra antibody or isotype control (n=5 per group, representative of 2 experiments). AUC, area under curve.
(E) PTT performed four hours post bleeding in mice with conditional UCP1-mediated deletion of Adrb3 (Adrb3f/fΔUCP1) or (F) Il6 (Il6f/fΔUCP1) compared respectively with their littermate controls. (n=8 for Adrb3f/f, n=5 for Adrb3f/fΔUCP1, n=6 for Il6f/f or Il6f/fΔUCP1). AUC, area under curve.
(G) Gluconeogenesis-associated gene expression in the liver harvested three hours post bleeding (n=4 for NT, n=5 for Bleed). Pck1: phosphoenolpyruvate carboxykinase 1. G6pc: glucose-6-phosphatase catalytic subunit. Pcx: pyruvate carboxylase. Fbp1: fructose-1,6-bisphosphatase 1. Gck: glucokinase. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
We thus examined metabolic changes induced by acute stress at the peak of endocrine IL6 levels. We found that stress-induced hyperglycemia was durable at four hours after acute stress in an IL6Ra-dependent fashion (Figure 4B). A single bolus of stress-dosed IL6 was also sufficient to recapitulate the effects of acute stress (Figure S2C), indicating that IL6 was both necessary and sufficient to induce stress-hyperglycemia at this time point. Hyperglycemia is caused by impaired clearance (insulin resistance) and/or excess glucose production from glycogenolysis or gluconeogenesis. To determine which of these processes was causing hyperglycemia, we tested organismal insulin resistance with a glucose tolerance test (GTT) and did not detect significant differences between stressed and unstressed animals (Figure S2D). Likewise, we did not detect differences in GTT in mice challenged with a single bolus of stress-dosed IL6 (Figure S2E) nor did we detect changes in plasma insulin at peak glycemia after IL6 challenge (Figure S2F), suggesting the absence of an effect of IL6 on insulin-dependent glucose uptake. However, when we performed insulin tolerance tests (ITT), we observed that acute stress alleviated insulin-induced hypoglycemia at later time points (Figure S2G), suggesting that stress-induced IL6 may potentiate endogenous glucose production. Consistent with this idea, a single dose of recombinant IL6 was also sufficient to recapitulate the effects of acute stress on maintaining higher levels of glucose at late timepoints during ITT (Figure S2H), and IL6Ra blockade in stressed animals attenuated the ability of mice to maintain normoglycemia after insulin challenge (Figure S2I). We tested the contribution of glycogenolysis to stress hyperglycemia by examining glycogen content in liver, kidney and skeletal muscle after stress but did not detect significant differences (Figure S2J). We thus hypothesized that IL6 was inducing gluconeogenesis during stress.
Gluconeogenesis is typically engaged in hypoglycemic or net negative energy balance states, with the exception of forced intensive exercise, where muscle-derived IL6 has been shown to induce gluconeogenesis (Banzet et al., 2009; Febbraio et al., 2004). To test if gluconeogenesis was impaired in an IL6-dependent fashion, we measured endogenous glucose production after acute stress and found that it was significantly decreased in the absence of IL6 signaling (Figure 4C). This finding was supported by pyruvate tolerance tests (PTT) in stressed animals, where pyruvate conversion to glucose was impaired in the absence of IL6Ra signaling (Figure 4D). Consistent with our previous findings, we found that the effects of IL6Ra antagonism could be fully recapitulated using Il6f/fΔUCP1 and Adrb3f/fΔUCP1 animals, which lack stress-inducible IL6 (Figure 4E–F). Gluconeogenic capacity is mediated by key rate-limiting enzymes, many of which have been shown to be sensitive to IL6 signaling via STAT3 regulatory elements (Banzet et al., 2009). We thus assessed the hepatic transcriptional induction of Pck1, G6pc and other gluconeogenic genes and found that Pck1 and G6pc were significantly increased following acute stress (Figure 4G).
Given the close crosstalk between glucose and lipid metabolism (Weickert and Pfeiffer, 2006), observations of hyperlipidemia in patients receiving anti-IL6Ra antibodies, as well as the role of free fatty acids on hepatic glucose production (Boden et al., 1994), we studied lipid metabolism in response to acute stress. The circulating level of free fatty acids and glycerol were not significantly altered in response to retro-orbital bleeding (Figure S3A and B). Acute stress decreased circulating triglyceride (TG) levels (Figure S3C), which was likely a result of both enhanced TG clearance (Figure S3D) and suppressed hepatic TG production (Figure S3E). Consistent with clinical observations from patients treated with tocilizumab, anti-IL6Ra did increase TG in the non-stressed condition (Figure S3F). However, manipulation of IL6 signaling did not significantly impact triglyceride metabolism in response to acute stress, although stress-induced hypertriglyceridemia did trend lower in IL6Ra antagonized animals (Figure S3F). We did not detect differences in free fatty acids, glycerol, or β-hydroxybutyrate following intravenous injection of IL6 (Figure S3H–K) or IL6Ra blockade (Figure S3G), nor did we detect changes in lipolytic capacity in mice with conditional IL6Ra deletion in adipose tissue (Figure S3L). Finally, we were unable to detect differences in in vivo fatty acid turnover following anti-IL6Ra treatment (Figure S3M). Thus, we found that the dominant effect of IL6 during acute stress is in inducing gluconeogenesis in the absence of a negative energy state to support stress-induced hyperglycemia.
IL6Ra in the liver controls stress hyperglycemia through hepatocyte reprogramming
Since the liver and kidney are the major glucose-producing organs (Stumvoll et al., 1998), we surveyed the transcriptional induction of gluconeogenic genes in both organs and found that they were induced by bleeding stress only in the liver (Figure 5A and Figure S4A). We observed that Il6ra was significantly induced in liver but not in kidney (Figure 5B), suggesting that the liver may be the primary target for IL6 signaling in response to acute stress. Concordantly, we found that the IL6/STAT3 target genes, Saa3 and Socs3, were also induced in the liver in response to acute stress (Figure S4B and C). To directly assess gluconeogenesis in the liver and kidney, we developed a method to assess the contribution of pyruvate to gluconeogenesis in a tissue-specific manner during stress, and found that IL6Ra inhibitor suppressed gluconeogenesis from pyruvate in liver but not in kidney (Figure 5C and D). We did not detect differences in circulating gluconeogenic amino acids (Figure S4D). Finally, to directly assess the role of hepatocyte IL6Ra, we generated mice with hepatocyte-specific deletion of Il6ra (Figure S4E) and performed PTT after acute stress. We found that hepatocyte-specific deletion was sufficient to recapitulate the inhibitory effects of systemic IL6Ra blockade on gluconeogenesis after bleeding (Figure 5E) and restraint stress (Figure 5F). These data indicate that stress-inducible IL6 acts on the liver to induce hepatic gluconeogenesis.
Figure 5. IL6 mediates stress hyperglycemia through hepatocyte reprogramming.
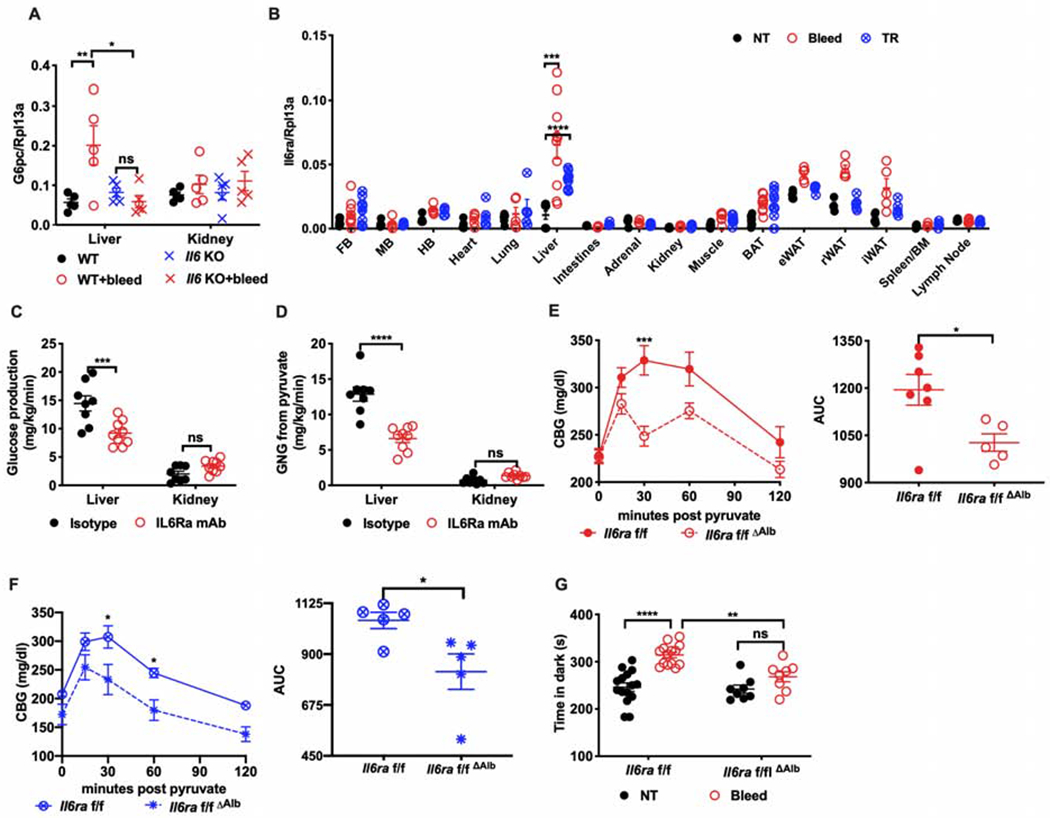
(A) G6pc mRNA in the liver and kidney of Il6 KO or WT mice four hours post bleeding (n=5 per group).
(B) Il6ra mRNA in the indicated tissues from stressed mice (n=5 per group, representative of 2 experiments)). FB, forebrain. MB, midbrain. HB, hindbrain. BAT, brown adipose tissue. eWAT, epididymal white adipose tissue. rWAT, retroperitoneal white adipose tissue. iWAT, inguinal white adipose tissue. NT, no treatment. TR, tube restraint.
(C) Rates of glucose production and (D) of gluconeogenesis from pyruvate in the liver or kidney of mice treated with IL6Ra inhibitor or isotype control (n=8 for isotype, n=9 for IL6Ra mAb).
(E) PTT four hours post bleeding or (F) tube restraint in mice with hepatocyte-specific deletion of Il6ra (Il6raf/fΔAlb) compared with littermate controls (Il6raf/f) (n=5 per group). AUC, area under the curve.
(G) Time in the dark using the light/dark box paradigm analyzed four hours post bleeding (bleed) from mice with hepatic deletion of Il6ra (Il6raf/fΔAlb) compared with littermate controls (Il6raf/f) (n=15 for Il6raf/f, n=9 for Il6raf/fΔAlb, representative of 3 experiments). * p<0.05, ** p<0.01, ***p<0.001, **** p<0.0001
See also Figure S4.
We reasoned that the purpose of activating gluconeogenesis during acute stress, when animals are neither hypoglycemic nor in net-negative energy balance states, is anticipatory of impending increased demand (“fight or flight” response). Consequently, impairment of hepatic gluconeogenesis should be sufficient to affect adaptive behavioral responses to acute stress. To test this, we utilized the light-dark box paradigm. The light-dark box paradigm is a common tool for studying stress response behaviors where animals are placed into a novel environment in which part of the apparatus is exposed under bright light and connected to another enclosed and opaque space by a small opening (Bourin and Hascoet, 2003). In this paradigm, animals must balance the need to explore the novel space with the fear of avoiding possible predation in the exposed area. A normal adaptive response is to spend more time in the dark enclosure. We thus established baseline responses of animals with conditional hepatic deletion of Il6ra and then compared the responses at the peak of endocrine IL6 after a single conscious retro-orbital bleeding. We found that hepatic IL6Ra was required for the normal behavioral response (Figure 5G and S4F). Taken in aggregate, we demonstrate that stress-induced IL6 mediates stress-hyperglycemia through hepatic IL6Ra signaling in positive energy balance states and that hepatic IL6Ra is necessary for a normal behavioral response to acute stress.
ADRB3-dependent IL6 from BAT potentiates lethal endotoxemia secondary to acute stress
Given the many reported roles of IL6 in affecting inflammatory responses, we reasoned that stress-induced IL6 may change the outcome of inflammation. We decided to use the lipopolysaccharide (LPS) model of inflammation, since in this model, mortality is due solely to the inflammatory response without any confounding contribution by pathogens. Animals were subjected to various stress models that induce IL6 followed by a subsequent LPS challenge. We found that priming animals with stress robustly enhanced mortality to LPS (Figure 6A). For the LPS studies, we opted to use the tube-restraint model to avoid confounders associated with the hemodynamic consequences of bleeding. Since we found that ADRB3 activation was sufficient and required for stress-induced IL6, we pre-treated animals with ADRB agonists and found that pre-treatment with ADRB3 agonist alone was sufficient to enhance LPS mortality (Figure 6B). Concordantly, a single injection of stress-dosed IL6 was sufficient to potentiate LPS-induced mortality (Figure 6C). Since stress-induced IL6 required consciousness, we tested whether or not animals anesthetized prior to tube restraint were still more susceptible to LPS-induced mortality, and found that consciousness was required for the stress-priming effect, an effect that could be bypassed with endogenous administration of IL6 (Figure 6D). To test if ADRB3-dependent IL6 was necessary, ADRB3 antagonist was applied alongside the restraint challenge, which negated the effects of stress-priming (Figure 6E). Finally, we asked if Il6f/fΔUCP1 animals, which lack stress-inducible IL6, would be resistant to stress-priming (Figure 6F). Consistent with our hypotheses, Il6f/fΔUCP1, which did not display altered susceptibility to LPS in the absence of stress-priming (Figure S5A), were resistant to the potentiating effects of tube-restraint on LPS mortality. We also tested the effects of stress-priming using our Il6raf/fΔAlb model. Here, regardless of stress-priming, animals lacking hepatic IL6 signaling were significantly more sensitive to endotoxemia, suggesting that the hepatic acute phase response was a required adaptation to endotoxemia (Figure S5B), consistent with previous reports (Castell et al., 1989; Wunderlich et al., 2010). We also tested if hyperglycemia induced by IL6 during stress was itself sufficient to prime LPS responses, and thus challenged animals to exogenous glucose to achieve stress hyperglycemia or an isocaloric isovolumetric dose of lipid, and found that glucose, but not lipid, was sufficient to prime the LPS response (Figure S5C).
Figure 6. ADRB3-dependent IL6 from BAT potentiates lethal endotoxemia secondary to acute stress.
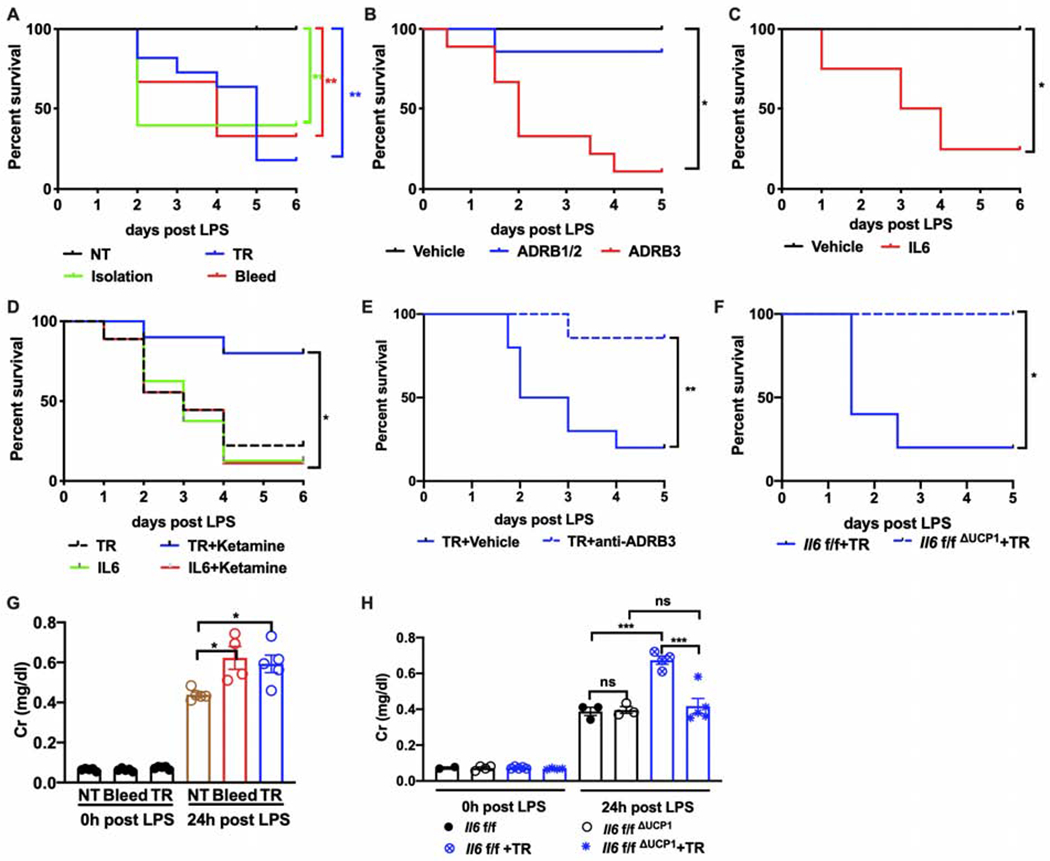
(A) Survival rate of LPS-induced endotoxemia in mice pre-exposed to the indicated stressors (n=10 per group). NT, no treatment. TR, tube restraint.
(B) Survival of endotoxemic animals pretreated with vehicle (n=8), ADRB1 and ADRB2 agonist (ADRB1/2, n=7) or ADRB3 agonist (ADRB3, n=9).
(C) Survival of endotoxemic animals pretreated with stress dose of IL6 (n=8 per group).
(D) Survival of endotoxemic animals pretreated with the indicated interventions. n=9 for tube restraint (TR), n=10 for TR with ketamine, n=8 for IL6, n=9 for IL6 with ketamine.
(E) Survival rate of endotoxemic mice pre-exposed to restraint with ADRB3 antagonist or vehicle injection. TR, tube restraint. (n=10 per group).
(F) Lethality in endotoxemia from mice pre-exposed to restraint with brown adipose tissue specific deletion of Il6 (Il6f/fΔUCP1) compared with littermate controls (Il6f/f) (n=10 per group).
(G) Circulating creatinine (Cr) levels 0 or 24 hours post LPS injection from mice pre-exposed to bleeding or restraint stress (n=5 per group). NT, no treatment. TR, tube restraint.
(H) Circulating creatinine (Cr) levels 0 or 24 hours post LPS injection from mice pre-exposed to restraint stress with brown adipose tissue specific deletion of Il6 (Il6f/fΔUCP1) compared with littermate controls (Il6f/f) (n=5 per group). TR, tube restraint.
* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
To determine how stress-priming enhanced inflammation-mediated mortality, we first measured cytokine levels and tissue inflammatory gene induction. We did not detect significant changes in circulating or tissue inflammatory cytokines post-LPS in a variety of experimental settings, nor did we detect any changes in tissue inflammatory transcripts or body temperature (Figure S5D–R, S6D). Thus, we hypothesized that stress-induced IL6 may be affecting host tolerance (Ferreira et al., 2011; Wang et al., 2016; Weis et al., 2017). Since our previous work isolated key brainstem functions (like maintaining heart and respiratory rate) as a target of host tolerance, we also assessed these parameters using our stress paradigm, but did not find significant differences (Figure S6A–F). We also did not find large differences in maintaining glycemia, fatty acid, or ketone body levels (Figure S6I–K), which we had previously shown to be important in host tolerance to LPS (Wang et al., 2016). Since end-organ dysfunction is a hallmark of inflammatory damage, we assessed biomarkers of vital organ function in stress-primed versus control animals, and found that stress-primed animals displayed significantly more renal and a trend towards more cardiac damage while hepatic damage appeared to be equivalent across conditions (Figure 6G and S6G,H). These markers of end-organ damage were absent in stress-primed Il6f/fΔUCP1 animals (Figure 6H), demonstrating that BAT-derived IL6 from stress was required for decreasing tolerance to inflammatory damage. Together, these findings suggest that stress decreased host tolerance to inflammation in a BAT-derived IL6-dependent fashion. The precise mechanism by which stress decreases host fitness to inflammation remains to be understood. Taken together, our studies suggest that stress-induced IL6, while adaptive for supporting fight-or-flight physiology, comes at the cost of decreasing host fitness to endotoxemia-induced inflammation.
Discussion
Psychological stress has been known to induce endocrine IL6 for nearly 30 years and has been shown in multiple species, including rats, mice, and humans (Cheng et al., 2015; LeMay et al., 1990; Maes et al., 1998). However, mechanistic understanding for this phenomenon has not been addressed. The evolutionary basis for IL6 induction during acute stress was unknown, and how this may connect to the long-observed connection between stress, metabolism, and inflammation was also unclear. Our study demonstrates that stress-induced IL6 is produced from brown adipocytes in an ADRB3-dependent fashion in mice. Thermogenic programs were not engaged in this context and this response was independent of ambient temperature. One key role of stress-induced endocrine IL6 is in reprogramming organismal metabolism by instructing hepatic gluconeogenesis in the absence of a net negative energy balance or hypoglycemic state, likely in anticipation of increased glucose demand. Hepatic IL6 signaling was also necessary for mediating normal behavioral responses in the light-dark box paradigm suggesting that hepatic organismal reprogramming is required for an adaptive “fight or flight” response. Finally, we found that stress-induced BAT-derived endocrine IL6 was necessary and sufficient for decreasing host tolerance to a subsequent inflammatory response using the endotoxemia model.
Gluconeogenesis is normally not engaged in positive energy balance states, with the exception of forced exercise (Banzet et al., 2009). In this setting, IL6 is derived from myocytes and induces hepatic gluconeogenesis. In our study, we did not observe any IL6 induction in muscle, nor did we observe increased activity after stress, and instead found that brown adipocytes were indispensable in the setting of acute stress. It is interesting to speculate why IL6, which can be derived from many different cell types, is produced by brown adipocytes in this context. The BAT has a number of features that make it an ideal endocrine organ responsive to acute psychologic stress. It is highly innervated, and thus capable of immediate responsiveness after detection of stress, a feature that has been clearly demonstrated in acute cold exposure for the purpose of defending body temperature. In addition, blood flow through BAT can be quickly increased (hyperemia) (Abreu-Vieira et al., 2015), and, in the setting of acute cold exposure, is optimal for quickly circulating warmed blood, or, in this case, a stress hormone. Interestingly, BAT hyperemia and thermogenesis can be decoupled, suggesting that there could be scenarios were uncoupled respiration is not necessary for hyperemia (Abreu-Vieira et al., 2015). In our study, we did not find that ambient temperature played a role in stress-inducible IL6; observe transcriptional induction of the thermogenic program; find differences in body temperature as a function of IL6 signaling; or demonstrate a requirement for UCP1. We speculate that perhaps other contextual inputs, such as through the cold-sensor TRPM8 (Ma et al., 2012; Moraes et al., 2017), may be required to activate the full thermogenic program. From this perspective, BAT can thus be considered a stress-responsive endocrine organ that may have several responses depending on other contextual inputs. Finally, BAT is highly enriched in ADRB3. Unlike ADRB1 or ADRB2, ADRB3 has been shown to be less easily desensitized (Rouget et al., 2004), thus providing more durable responsiveness to adrenergic outflow. Consistent with this idea, we found that repeated stress-exposure over the course of 24 hours maintained elevated plasma IL6 levels. The role of BAT in adult humans is controversial since its detection is dependent upon the approach employed (Leitner et al., 2017; Porter et al., 2016; van Marken Lichtenbelt et al., 2009). It is principally confined to clavicular and para-aortic areas, which may be why less stress-associated IL6 is recovered in humans compared to mice if it is even made in human BAT at all (Maes et al., 1998). On the other hand, recent work suggest that BAT of “humanized” mice is notably similar to human BAT (de Jong et al., 2019). It remains unclear how the mechanistic insights from our studies in rodents will translate to humans.
Our work further highlights the complexity of adrenergic signaling in physiology. While signaling on ADRB2 in immune cells has largely been shown to be anti-inflammatory (Agac et al., 2018; Nance and Sanders, 2007), our study suggests that ADRB3 activation after acute stress exposure may be detrimental for adaptation to a subsequent inflammatory challenge. The clinical observation that sympathetic mimetics enhance survival in septic shock may be resultant from the effects of supraphysiological dosing on supporting blood pressure that may be the dominant mechanism of protection regardless of its other effects.
Like adrenergic biology, the role of IL6 in inflammation and metabolism is similarly complex. IL6 is generally considered a pro-inflammatory cytokine, which is supported by the efficacy of IL6 blockade in inflammatory diseases (Kotch et al.). On the other hand, several studies demonstrate an anti-inflammatory role for IL6 (Mauer et al., 2014; Nandi et al.). In our study, we demonstrate that BAT-derived IL6 is required for stress to enhance end-organ damage and mortality caused by the LPS model. Likewise, we found that pre-exposure to peak plasma levels of stress-induced IL6 was sufficient to increase end-organ damage and potentiate mortality. In the LPS model, which may not be generalizable to other models of inflammation, we found that while stress did not meaningfully increase inflammatory magnitude, hemodynamic or cardiopulmonary parameters, or gross metabolic parameters, it nonetheless led to measurable worsening of end-organ function, and death. The mechanism underlying this phenomenon is unclear, but likely is a result of stress-induced IL6-dependent metabolic re-programming similar to that which has been previously published by our group and others (Ganeshan et al., 2019; Luan et al., 2019; Wang et al., 2016; Wang A, 2019; Weis et al., 2017). Whether other types of inflammatory challenges would also be affected by the models of acute stress used here, and, more generally, how different types, degrees, and durations of stress affect tissue tolerance in different inflammatory settings is an open question, as is the precise mechanism for how IL6 primes LPS-mediated mortality. Moreover, it remains unclear if the elevated levels of circulating IL6 seen in aging and obesity or after exercise would also drive differences in host tolerance to inflammation. Similarly, from a metabolic perspective, IL6 has been shown to be both insulin sensitizing and insulin desensitizing depending on the context. In contexts of chronically increased IL6 such as obesity or rheumatoid arthritis, IL6 has been shown to induce insulin resistance (Castaneda et al., 2019; Kim et al., 2004; Perry et al., 2015). On the other hand, in these same contexts, others have shown that IL6 induces insulin sensitivity (Carey et al., 2006; Findeisen et al., 2019; Matthews et al., 2010; Mauer et al., 2014; Steensberg et al., 2003; Timper et al., 2017), while IL6 has been shown to be insulin sensitizing in exercise (Benrick et al., 2012). Our study did not find that IL6 was playing a role in insulin sensitivity after stress. Our animals were all lean, chow-fed animals, and thus in a net energy positive state. One main finding of our study is that IL6 is a distinct inductive signal to instruct hepatic gluconeogenesis during net energy positive states. Unlike during fasting, ketogenic diets, or other hypoglycemic states where gluconeogenesis programs are mediated primarily by CREB/FoxO1 programs (Oh et al., 2013), which are responsive to low energy states, stress induces gluconeogenesis even in net positive energy balance states. Our data suggests that IL6 signaling in hepatocytes is required for gluconeogenesis in non-net negative energy balance states. While IL6 does not appear to increase gluconeogenesis at rest (Steensberg et al., 2003), our studies resonate well with other studies demonstrating STAT3 regulation of the same gluconeogenic genes such as Pck1 and G6pc and by studies demonstrating a role for IL6 in exercise-mediated hepatic gluconeogenesis (Banzet et al., 2009). Here, it is interesting to consider the similarities between psychological stress and forced exercise. Thus, we propose a model wherein IL6 is a necessary signal that instructs gluconeogenesis in net energy positive states as an anticipatory, (impending increase in glucose demand), as opposed to responsive adaptation (Figure 7).
Figure 7. Model.
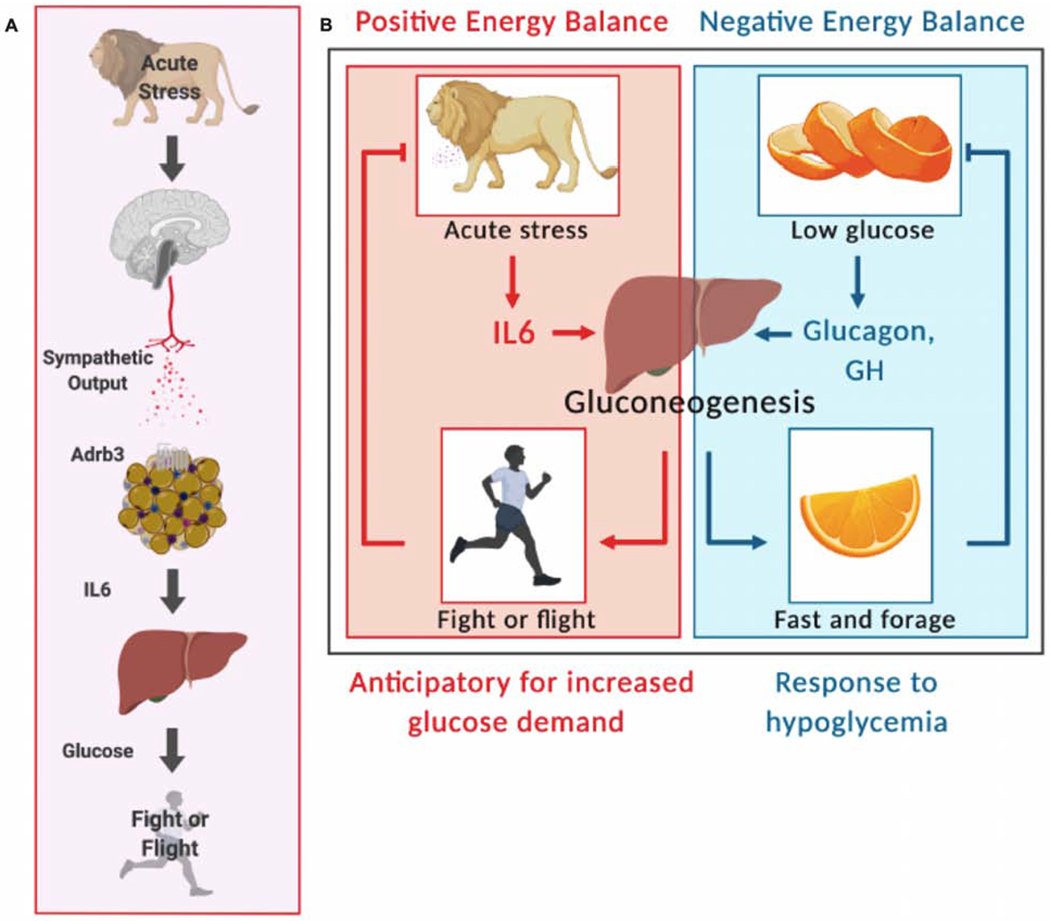
(A) Acute stresses engage a brain-BAT-liver axis to promote metabolic adaptation in order to support fight or flight physiology
(B) Acute stress, unlike fasting or other negative energy states, requires IL6, in contrast to hormones like glucagon or growth hormone (GH), to induce gluconeogenesis for anticipatory, (impending increase in glucose demand), as opposed to responsive adaptation.
We found that hepatic IL6 signaling was required for a normal behavioral adaptation to stress. Numerous studies have reported that intracranial IL6 and IL6 signaling using non-canonical trans-signaling modalities impact behavior (Rothaug et al., 2016). Our findings that hepatocyte expression of Il6ra is required for normal behavioral response to acute stress are in line with recent studies using chronic models of stress (Hodes et al., 2014; Niraula et al., 2019; Zhang et al., 2017), where the use of blocking antibodies to IL6Ra, which do not cross the blood brain barrier (Nellan et al., 2018), promoted resilience to social stress. These studies suggest a necessary peripheral role for IL6 in mediating behavioral changes, consistent with our findings. It is possible that hepatocyte IL6Ra would be required in these models of chronic stress as well. Indeed, the light-dark box paradigm we utilized in this study is commonly used in depression and anxiety studies, and IL6 has been implicated in depression for many years (Kappelmann et al., 2018). On the other hand, aerobic exercise, which also induces IL6 (Benrick et al., 2012; Pedersen and Febbraio, 2008; Reza et al., 2017), is highly associated with improved outcomes in depression and anxiety. Thus, it remains to be seen in on-going clinical trials (i.e. NCT02660528) if elevated IL6 observed in depressed patients is pathogenic.
Our studies suggest that the degree of stress experienced by an animal at the time of inflammatory challenge may be one important factor for determining its disease trajectory. Understanding which environmental factors – such as timing to the last meal bolus or other determinants that are proxies for the “stressfulness” of the environment – may contribute to the stochasticity observed in genetically identical organisms subjected to identical challenges is an area of active study that may shed insight into mechanistic determinants of disease trajectories.
In summary, this study identifies a brain-BAT-liver axis in mice whereby IL6 modulates glucose metabolism under conditions of acute stress and suggests that there is an adaptive purpose for inducing IL6 in acute stress. We speculate that maladaptive states may arise in chronic stress where IL6 becomes persistently elevated. Whether or not ADRB3 or IL6Ra receptor biology changes as a function of chronic IL6 states, as has been shown for the glucocorticoid and insulin receptors (Boucher et al., 2014; Cohen et al., 2012), remains to be seen. Should this brain-BAT-liver axis also be relevant in humans, our findings have implications for the pathogenesis of psychiatric diseases such as seasonal affective disorder, where depression occurs primarily as a function of cold and dark seasons, and also implicate ADRB3 and IL6 as potential therapeutic targets for preventing disease flares in conditions of pathologic inflammation.
Limitations, Caveats and Open Questions
These experiments were performed on mice in a single facility and largely on the C57BL/6J genetic background. The impact of the microbiota, genetic background, and facility-specific factors are unknown, and the unnatural settings in animal facilities likely affect the results and interpretation of physiology studies, including this one. It is likely that stress-induced IL6 plays additional roles besides those described in our studies. There are a number of open questions raised by our study. Does psychological stress affect all types of inflammation the same? How do different stressors and degrees of stress affect inflammation? Are our observations informative for placebo and nocebo biology? Finally, the translatability of this study to humans is to be determined.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for reagents may be directed to, and will be fulfilled by, the lead contact Andrew Wang (andrew.wang@yale.edu).
Materials Availability
This study did not generate new reagents. Mouse lines for this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
This study did not generate any datasets/code amenable for depositing into public repositories.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Male mice with 6-8 weeks of age were used. C57BL/6J, Adrb1/b2 KO, Ucp1 KO, Il6ra f/f, AlbCre, AdipoCre mice were purchased from Jackson Laboratories and bred at Yale University. Adrb3 KO mice were a kind gift from Dr. Natasa Petrovic (Stockholm University), Adrb3 f/f mice were a kind gift from Dr. Jean-Luc Balligand (UC Louvain), the UcpICreER animal was a kind gift from Dr. Christian Wolfrum (ETH Zurich), the Il6 f/f was a kind gift from Dr. Juan Hidalgo (Universitat Autonoma Barecelona) and the Tlr2/4 KO, and Myd88/Trif KO animals were kindly provided by Dr. Ruslan Medzhitov. Adrenalectomy and sham surgery mice were purchased from Jackson Laboratories. All animal experiments were performed according to institutional regulations upon review and approval of Yale University’s Institutional Animal Care and Use Committee.
For stress models, all experiments were done during the light period. Acute restraint stress was initiated by retaining a mouse in a ventilated 50ml Falcon tube for four hours. Social isolation was induced by individually housing mouse for three days. Retro-orbital bleeding (50 μL of blood drawn via a glass cuvette VWR #53432-921) was performed four hours before experiments, then blood was collected from the other eye for analysis. In the cage switch model, mice were removed from their home cage and placed in new cage of the same size with dirty bedding of other non-littermate males. Non-disturbed socially-housed littermates were applied as the controls. For anesthesia studies, ketamine/xylazine was injected intraperitoneally or animals were placed in isoflurane chambers. Verification of anesthesia was confirmed by lack of activity after toe pinching. Chemically sympathectomized with 6-hydroxydopamine (6-OHDA, sigma) was applied through intraperitoneal injection 24 hours before acute stress at a dosage of 10 mg/kg. For manipulation of ambient temperature, mice were either housed at thermoneutral (32°C) cabinets for two weeks, or placed in the cold (4°C) for three hours, or housed at standard housing (22°C).
For flux and metabolite measurements, mice underwent surgery under isoflurane anesthesia to place a catheter in the left jugular vein. Animals were individually housed with unrestricted access to food and water and were treated again with IL6Ra antibody or isotype control the night before a 120 min tracer infusion, performed after two hours of acclimation to a 1.25 inch diameter plastic restrainer (IBI Scientific) in which the mouse’s tail was drawn through the restraint and tethered with tape, while an adjustable nose cone permitted the mouse several centimeters of forward movement. At the conclusion of the tracer infusion, mice were euthanized via IV pentobarbital and liver and kidney were obtained and freeze-clamped in liquid N2 within 30 sec of euthanasia.
For LPS endotoxemia, mice were injected intraperitoneally with LPS derived from Escherichia coli 055:B5 (Sigma-Aldrich) diluted in PBS. Dosing varies dramatically from lot to lot. New lots are tested for LD50. In these studies, lethal doses are between 15 and 20 mg/kg. For experiments with antibody blockade, anti-IL6Ra or the isotype antibody (BE0047 and BE0090 respectively, Bio X Cell, 8 mg/kg) diluted in PBS was injected intravenously one night before experiment through retro-orbital injection. For experiments utilizing recombinant IL6, recombinant mouse IL6 (406-ML-025/CF, R&D) or PBS control was intravenously injected one hour before experiment at a dose of 5ng/100μl/mouse through the retro-orbital plexus. For experiments utilizing adrenergic agonists, ADRB1/2 agonist (Isoproterenol, 1351005, Sigma-Aldrich, 1mg/kg), ADRB3 agonist (CL316243, Sigma-Aldrich, 1mg/kg) were injected intraperitoneally two hours prior to blood and tissue harvesting. For adrenergic antagonists, propranolol (5 mg/kg, P0884, Sigma-Aldrich) and ADRB3 antagonist (SR59203A, 21407, Cayman Chemical, 5mg/kg) were applied once every two hours during four-hour-exposure of acute stresses, blood were collected at indicated time point, and lethal dose of LPS was administered two hours after last injection of ADRB3 antagonist.
Vital signs, including blood oxygen saturation, breath rate, and heart rate post LPS injection were monitored via pulse oximetry using the MouseOx Plus (Starr Life Sciences Corp.). Core body temperature was measured by rectal probe thermometry (Physitemp TH-5 Thermalert).
Human Subjects
A community sample of individuals signed written informed consent for research approved by the Yale Institutional Review Board and was carefully assessed for high and low stress using a structured cumulative stress and adversity interview that assessed recent and past life events (Cumulative Adversity Interview (CAI), (Ansell et al., 2012; Seo et al., 2014; Turner and Lloyd, 1995)). The high and low groups were group matched on age, gender, education and body mass index (BMI). All subjects participated in two standardized experiment sessions on consecutive days conducted between 2:00 and 4:00 pm in the afternoon. Baseline blood draws were drawn at 2 pm after a one-hour habituation period and two additional draws were conducted within 15 minutes. Change from baseline values were computed to assess baseline adjusted values and reduce variability across subjects.
METHOD DETAILS
Quantification of Plasma Cytokines
Undiluted plasma from stressed mice were screened by Eve Technologies using mouse cytokine arrays, the raw data including standard results were presented in Figure S7A. Except for the results in figure 1B and figure S1E from samples analyzed by Eve Technologies, other analyses were conducted in our laboratory. Plasma concentrations of IL6 were assayed by sandwich ELISA (Luan et al., 2019; Wang et al., 2016). Anti-mouse IL6 capture antibody (14-7061-85, eBioscience) was diluted (1:1000) in coating buffer (NaHPO4 PH 9) then incubated in enhanced protein-binding ELISA-grade plate (490012-252, VWR) overnight at 4°C. On the next day plates were blocked using PBS buffer with 10% FBS for one hour at room temperature. Afterwards, the standards and plasma from stressed mice were incubated in the plate overnight at 4°C. The highest concentration of recombinant IL6 standard (406-ML, R&D) was 10ng/ml followed by serial two-fold dilutions to create a standard curve, and plasma were diluted 1:5 (20ul plasma in 80ul blocking buffer per well). On the third day, biotin-conjugated anti-IL6 detection antibodies (554402, BD Pharmingen) was diluted (1:500) and incubated in the plate for one hour at room temperature, followed with another incubation of diluted (1:1000) HRP-conjugated streptavidin (554066, BD Biosciences) for half an hour at room temperature. Then plates were incubated in the dark at room temperature with TMB substrate reagent (555214, BD Biosciences) and the color was checked every five minutes. Then plates were read at 450nm instantly after stop solution (3M H2SO4) was added. Between each step, five to seven times of washing were normally applied. The raw data, results of standard curve and representative plate with color developed with TMB were displayed in Figure S7B–E. Plasma concentrations of TNFα and IL-1β were assayed by the same sandwich ELISA method. Recombinant TNFα (410-MY, R&D) and IL-1β (401-ML-010, R&D) were utilized as standards with the highest concentration at 10ng/ml and 1ng/ml respectively. Capture antibodies were anti-TNFα (14-742385, eBioscience) and anti-IL-1β (14-7012-81, Invitrogen). Detection antibodies conjugated with biotin were anti-mouse TNFα (13-7349-81, Invitrogen) and anti-mouse IL-1b (13-7112-85, eBioscience). HRP-conjugated streptavidin, TMB substrate reagent, and stop solution were the same with those in ELISA assay for IL6 measurement.
Quantification of Plasma Metabolites, Hormones, and Organ Injury Markers
Glycemia was measured by whole blood collection via the retro-orbital plexus and assessed using a glucometer (OneTouch), or, during the flux studies in IL6Ra treated mice, in blood collected from the tail vein using the YSI Glucose Analyzer. Plasma was separated using lithium heparin-coated microcentrifuge tubes (BD Diagnostics). Plasma L-type triglycerides (TG) and nonesterified fatty acids were measured using the kits according to manufacturer’s instructions (Wako Diagnostics). Plasma Glycerol and β-hydroxybutyrate were measured using the kits per manufacturer’s instructions (Sigma Aldrich and Cayman Chemical, respectively). Plasma amino acid concentrations were measured by gas chromatography-mass spectrometry (GC-MS) as described previously (Perry et al., 2018). Plasma levels of corticosterone (Enzo Life Sciences), insulin (Crystal Chem), and noradrenaline (Eagle Biosciences) were measured using kits according to the manufacturer’s protocols. Cardiac Troponin-I (CTNI) concentration and Alanine Aminotransferase (ALT) activity in the blood were measured by kits per manufacturers’ instructions (Life Diagnostics and Cayman Chemical, respectively). Plasma creatinine were assayed using HPLC by the George M. O’Brien Kidney Center at Yale.
Surgical Removal of Brown Adipose Tissue
Surgical removal of brown adipose tissue (BAT) was applied to seven weeks old male mice. A 1.5 cm incision was made to expose the intrascapular fat pads following intraperitoneal injection of ketamine/xylazine. Two lobes of darkly colored BAT were completely removed with little bleeding. Sham-operated mice were anesthetized and incisions were made into the muscles without tissue excision. Heat mats were applied to keep all animals warm during and after surgery until consciousness was fully recovered. Then mice were kept in room temperature, and IL6 secretion post retro-orbital bleeding was analyzed in these mice two days after surgery.
Metabolic Tolerance Tests
For oral GTT, D-Glucose (G8270, Sigma-Aldrich) or water gavage was performed; blood was collected afterwards through retro-orbital plexus at indicated time point for glucose and IL6 analysis. For intraperitoneal GTT, D-glucose was given through intraperitoneal injection at the dose of 2g/kg. CBG was analyzed using a glucometer (OneTouch) at indicated time points. For insulin tolerance tests, insulin (Novolin R) was administrated at 2 lU/kg through intraperitoneal injection. For pyruvate tolerance tests, pyruvate (Sigma-Aldrich) was applied at 2g/kg by intraperitoneal injection. CBG was analyzed at indicated time points. For lipid tolerance tests, intralipid 20% (1141, Sigma-Aldrich) was intraperitoneally injected at a volume of 200μl Per mouse. Plasma level of TG was analyzed at 0, 1,2, 3, and 4 hours after intralipid administration. For hepatic triglyceride production, poloxamer 407 (16758, Sigma-Aldrich) was dissolved in PBS and then intraperitoneally injected at 1g/kg. Plasma level of TG was analyzed at 0, 1, 2, 3, and 4 hours after poloxamer injection. Mice were fasted overnight before the hepatic TG production assay and assessment of glucose and palmitate turnover; for all other tolerance tests, stressed or unstressed mice were fasted for four hours before testing. Capillary blood glucose (CBG) was analyzed using a glucometer (OneTouch) at indicated time points.
Endogenous Glucose Production and Palmitate Turnover
After anti-IL6Ra treatment, endogenous glucose production and palmitate turnover from C57BL/6J mice were measured as precious described (Perry et al., 2017a; Perry et al., 2017b). In brief, IL6Ra inhibitor or vehicle control was injected through orbital venous plexus respectively before catheter placement and tracer infusions. Mice were infused with [3-3H] glucose (10 μCi/min, PerkinElmer), [U-13C16] potassium palmitate (2.5 μmol/kg/min, Cambridge Isotopes), and [3-13C] sodium lactate (40 μmol/kg/min, Sigma) continuously for a total of two hours following a 5 min 3X prime. Palmitate turnover was determined by gas chromatography-mass spectrometry (GC-MS) (Perry et al., 2017a). Plasma specific activity was measured using a scintillation counter and compared to tracer specific activity to measure whole-body endogenous glucose production, which can be attributed entirely to gluconeogenesis in a 16 hr fasted, glycogen-depleted mouse. Based on equations previously described (Perry et al Nat Comm 2017), and after verifying minimal (atom percent enrichment <1%, as compared to glucose 15-20%) renal and hepatic bicarbonate enrichment, we measured the whole-body ratio of pyruvate carboxylase flux (i.e. gluconeogenesis from pyruvate) to total gluconeogenesis by mass isotopomer distribution analysis (MIDA):
| (1) |
where XFE represents the fractional triose enrichment and is calculated as
| (2) |
In these calculations, we corrected for any [13C2] glucose synthesized from [13C2] trioses – as opposed to the condensation of two [13C1] trioses – by GC-MS measurement of the enrichment in the glucose C4C5C6 fragment, according to the equation
| (3) |
This ratio was measured in plasma (representing whole-body gluconeogenesis from pyruvate), liver, and renal cortex. By comparing the whole-body gluconeogenesis from pyruvate (GNG from pyruvateT) to that measured in liver (GNG from pyruvateL) and kidney (GNG from pyruvateK), we were able to measure the fractional contribution of the kidney to whole-body gluconeogenesis :
| (4) |
Absolute rates of gluconeogenesis from kidney could then be calculated by multiplying the measured endogenous glucose production by (equation 4), and gluconeogenesis from liver was calculated as the difference between total endogenous glucose production and gluconeogenesis from kidney. The rate of gluconeogenesis from liver and kidney was then multiplied by the fractional contribution of pyruvate to gluconeogenesis in those tissues to calculate the contribution of pyruvate to gluconeogenesis in each tissue.
qRT-PCR
Tissues were homogenized in 1ml RNA-Bee (Tel-Test, Inc) using a FastPrep-24 5G homogenizer (MP Biomedicals). RNA was purified using QIAGEN RNeasy columns according to the manufacturer’s instructions. cDNA was generated with reverse transcriptase (Clontech) using oligo-dT6 primers (Sigma-Aldrich). qRT-PCR was performed on a CFX96 Real-Time System (Bio-Rad) using PerfeCTa SYBR Green SuperMix (Quanta Biosciences). Relative expression units were calculated as transcript levels of target genes relative to Rpl13a. Primers used for qRT-PCR are listed in Table S1.
Stromal Vascular Fraction Isolation
Stromal vascular fraction (SVF) were isolated by density separation as previously reported (Aune et al., 2013). Briefly, interscapular BAT depots were minced and digested in collagenase at 37°C for 1 hour with constant agitation. Then after filtering through 70μM cell strainer, the cell suspensions were centrifuged at 500g for 5 min. The pellet was the SVF fraction.
Metabolic Cage
Energy expenditure after acute stress was measured by indirect calorimetry using metabolic cages (Promethion, Sable Systems International). A 2-day period of acclimation was followed by 2 days of steady-state recording prior to experimentation. Afterwards, C57BL/6J mice were given IL6Ra antibody or isotype control through intravenous injection; the next morning, both groups were subjected to retro-orbital bleeding stress.
Ambulatory Blood Pressure Measurements
A blood pressure transducer (TA11-PA-C10, commercially available through Data Sciences International) was surgically implanted under isoflurane anesthesia (1-3% in oxygen) into the carotid artery of mice using sterile surgical technique. Meloxicam was provided for 48 hours for post-operative analgesia and the skin was closed with surgical staples. Mice were allowed to recover for 7 days. Afterwards, surgical staples were removed, and the mice were transferred to a fresh cage and singly housed for data collection. There they were allowed to acclimate for several days prior to initiation of study. A 10 second segment was collected every minute for the duration of the experiment. Every dot in the figure reflects the averaged value over one hour reading period.
Behavioral Tests
The spontaneous exploratory behavior of mice following acute stress was analyzed three hours post retro-orbital bleeding using the light-dark paradigm. All experiments were performed between 11AM and 12 noon in male mice 6-8 weeks of age. Non-stressed mice were habituated to the environment three hours prior to being placed in the light/dark box; three days later, the same mice were transferred to the testing room one night before the experiment for acclimatization, then retro-orbital bleeding or tube restraint was performed three hours before light/dark box test. The light/dark box consisted of two of the same-sized chambers (18x10x13 cm), a dark chamber and an equal size light chamber connected by a small central aperture (3.8x3.8x3.8cm). Urine and feces were removed and the box was cleaned after each trial. Mice were initially placed in the corner of the light chamber facing away from the opening and monitored for 6 minutes after the first entry into the dark section. The latency time for the first passage from the light section to the dark one, transitions between the two compartments, and the amount of time spent in the dark were recorded (Mineur et al., 2007).
Immunohistochemistry
Mice were euthanized and perfused with PBS or fixative. Brown adipose tissues were immersion-fixed in 10% neutral buffered formalin. Then tissues were trimmed, processed, embedded, and sectioned and stained for IL6.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed using Prism 8.0 (GraphPad Software, Inc.). For parameters obtained from metabolic cage, the area under a curve was calculated followed by student’s t test. Student’s t test was used for two groups comparison. More than two groups were compared using one-way analysis of variance (ANOVA) followed by Tukey test. Samples at different time points from multiple groups were analyzed using two-way ANOVA followed by Tukey test. The log-rank Mantel-Cox test was used to compare Kaplan Meier curves. A p value less than 0.05 was considered statistically significant. Data are presented as the mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Supplementary Material
Figure S1. ADRB3 mediates brown adipocyte-derived IL6 in response to acute stress, Related to Figure 1, Figure 2, and Figure 3.
(A) Plasma concentrations of soluble IL6 receptor (s-IL6Ra) from bled mice (n=5 per group).
(B) Plasma IL6 levels post-bleeding in mice with bilateral adrenalectomy or sham (n=5 per group).
(C) Plasma noradrenaline and (D) corticosterone in mice pretreated with IL6Ra antibody or isotype control (n=5 per time point per group).
(E) Relative level of circulating IL6 from human with high or low stress history (n=12 for high stress history, n=13 for low stress history).
(F) Capillary blood glucose (CBG) and (G) plasma IL6 from WT mice after glucose or water gavage. Mice were bled then fasted for four hours before gavage (n=5 per group).
(H) Plasma IL6 levels in blood collected from the indicated site of mouse eyes four hours post bleeding (n=5 per group).
(I) qPCR analyses of Ucp1 and (J) Adrb3 in brown adipocyte tissue (BAT) or the stromal vascular fraction (SVF) from mice stressed with the indicated conditions (n=5 per group).
(K) Plasma concentration of IL6 four hours post bleeding from mice subjected to the indicated ambient temperature (n=3 per group, representative of 2 experiments).
(L) Body temperature after bleeding stress in the presence of IL6Ra antibody or isotype control (n=5 per group).
(M) Transcriptional analyses of cold-sensitive genes and Il5 in BAT (n=3-4 per group). Results are expressed as fold change relative to non-stressed control (NT).
(N-O) Transcriptional expression of beta-adrenergic receptors Adrb1 and Adrb2 in BAT or SVF from BAT of stressed mice (n=3-4 per group).
(P) Plasma IL6 levels at indicated time points post bleeding from mice pre-treated with an ADRB1/2 antagonist propranolol (anti-ADRB1/2) or PBS (n=5 per group).
(Q) Expression of Adrb3 in the indicated tissues from mice with conditional UCP1-medidated deletion of Adrb3 (n=4 per group). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S2. IL6 is necessary for promoting stress-hyperglycemia, Related to Figure 4.
(A) Continuous heart (HR) and blood pressure (BP) tracings in mice treated with IL6Ra mAb or isotype control followed by tube restraint (TR) (n=4 per group).
(B) Activity of mice treated with IL6Ra antibody of isotype control followed by retro-orbital bleeding (n=4 per group, representative of 2 experiments).
(C) Capillary blood glucose (CBG) one hour after injection of stress-dosed IL6 (n=10 per group).
(D) Glucose tolerance test (GTT) performed four hours post bleeding from mice pretreated with IL6Ra antibody or isotype control (n=5 per group, representative of 3 experiments).
(E) GTT performed one hour after injection with IL6 or vehicle control (n=5 per group).
(F) Plasma insulin levels one hour after injection with IL6 or vehicle control (n=6 for vehicle, n=8 for IL6).
(G) Insulin tolerance test (ITT) performed in animals four hours after a single retro-orbital bleeding (n=5 per group, representative of 2 experiments). NT, no treatment.
(H) Insulin tolerance test performed one hour after a stress-dose IL6 injection (n=6 per group).
(I) Insulin tolerance test performed in mice pretreated with IL6Ra antibody or isotype control (n=5 per group)
(J) Glycogen content in the indicated tissues of mice 3 hours post bleeding (muscle is gastrocnemius, n=5 per group). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S3. Lipid metabolism in response to acute stress or external IL6, Related to Figure 4.
(A-C) (A) Plasma non-esterified fatty acid (NEFA), (B) glycerol, and (C) triglycerides (TG) four hours post bleeding (n=28 for NT, n=29 for Bleed. Pooled from 3 experiments).
(D) Intralipid tolerance test performed four hours post bleeding (n=5 per group).
(E) Hepatic triglyceride secretion at indicted time points post poloxamer injection from bled mice or controls (NT) (n=5 per group).
(F-G) (F) Plasma TG levels or (G) NEFA assessed four hours post bleeding in mice pretreated with IL6Ra antibody or isotype control (n=5 per group).
(H-K) (H) Plasma level of TG, (I) NEFA, (J) Glycerol, (K) β-Hydroxybutyrate (BHB) one hour after IL6 injection (n=5 per group).
(L) In vivo lipolysis assay performed using ADRB3 agonist CL316,243 in mice with conditional IL6Ra deletion in the adipose tissue (Il6raf/fΔAdipo) or littermate controls (Il6raf/f) (n=6 per group).
(M) Palmitate turnover assessed in mice treated with IL6Ra antibody or isotype control (n=8 per group). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S4. IL6 mediates stress hyperglycemia through hepatocyte reprogramming, Related to Figure 5.
(A) Pck1 mRNA measured at four hours post bleeding from the liver and kidney of Il6 KO or WT mice (n=5 per group)
(B) Expression of Saa3 and (C) Socs3 in the indicated tissues from stressed mice (n=5 per group).
(D) Plasma level of amino acids four hours post bleeding in mice pretreated with IL6Ra antibody or isotype control (n=5 per group).
(E) Il6ra expression in the indicated tissue from mice with liver-specific deletion of Il6ra (Il6raf/fΔAlb) or littermate controls (Il6raf/f) (n=6 per group).
(F) Latency to dark in mice with liver-specific Il6ra deletion (n=15 for Il6raf/f, n=9 for Il6raf/fΔAlb, representative of 3 experiments). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S5. Inflammatory responses to a lethal endotoxemia are not affected by the pre-exposure of acute stress, Related to Figure 6.
(A) Survival rate of mice with brown adipose tissue specific deletion of Il6 (Il6f/fΔUCP1) comparing with littermate controls (Il6f/f) (n=6 per group, representative of 2 experiments).
(B) Survival rate of mice with liver specific deletion of Il6ra (Il6raf/fΔAlb) comparing with littermate controls (Il6raf/f) (n=6 per group, representative of 3 experiments).
(C) Survival of animals pre-treated with glucose or an isocaloric isovolumetric dose of intralipid followed by LPS injection (n=5 per group).
(D) Plasma IL6, (E) TNFα, and (F) IL-1β levels sampled 0, 2, 6, and 20 hours after LPS challenge in bled mice or non-bled controls (n=5 per group, representative of 2 experiments).
(G) Plasma IL6, (H) TNFα, and (I) IL-1β levels sampled 0, 2, 6, and 20 hours after LPS challenge in mice pre-challenged with ADRB3 agonist or vehicle control (n=5 per group).
(J) The peak levels of circulating IL6 and (K) TNFα during LPS-mediated endotoxemia from mice pre-exposed to tube restraint (TR) or controls (NT) (n=5 per group).
(L-R) Expression of inflammatory transcripts in the indicated tissues from LPS-treated mice pre-exposed to bleeding stress. Tissues were harvested three hours after LPS challenge (n=5 per group). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S6. BAT-derived IL6 from acute stress decreases host tolerance to inflammatory damage, Related to Figure 6.
(A-D) Vital signs before and 24 hours post LPS challenge from mice pre-exposed to acute stress. NT, no treatment. TR, tube restraint. HR, heart rate.
(E) Continuous systolic blood pressure (BP) and (F) heart rate (HR) tracings in mice with tube restraint and the subsequent LPS challenge. NT, no treatment. TR, tube restraint.
(G) Circulating alanine aminotransferase (ALT) and (H) cardiac troponin I (CTNI) levels 0 or 24 hours post LPS injection from mice pre-exposed to bleeding or restraint stress (n=5 per group). NT, no treatment. TR, tube restraint.
(I) Capillary blood glucose (CBG) levels post LPS injection at indicated time points from mice pre-exposed to restraint stress (n=5 per group, representative of 2 experiments). NT, no treatment. TR, tube restraint.
(J) Plasma non-esterified fatty acid (NEFA) levels post LPS injection at indicated time points from mice pre-exposed to restraint stress (n=5 per group, representative of 2 experiments).
(K) Plasma β-hydroxy-butyrate (BHB) levels post LPS injection at indicated time points from mice pre-exposed to restraint stress (n=5 per group, representative of 2 experiments).
Supplementary Figure 7. Raw data from ELISA analysis of IL6 , Related to Figure 1B, Figure 3D and Figure 3G.
(A) The raw data of IL6 from stressed mice including the standards concentration from Eve Technologies.
(B) The raw OD values from the plate presented below in (D).
(C) The layout of samples from the plate presented below in (D). Row A, C, E, and F were samples collected before bleeding. Row B,D, G, and H were samples at four hours post bleeding. NA, no samples in the well. Row A and B were related to figure 3D from mice treated with 6-OHDA 0 and 4 hours post bleeding. Row C and D were from mice with surgical denervation of BAT or sham controls (data not show). Row E, F, G, and H were related to figure 3G from mice with BAT-specific deletion of Il6 (Il6f/fΔUCP1) or littermate controls (Il6f/f) 0 and 4 hours post bleeding.
(D) The representative plate with color developed with TMB.
(E) The standard curve generated based on the OD value in (A) and concentrations in (B) from column 1 and 2.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse IL6 | eBioscience | Cat. # 14-7061-85 |
| Biotin-conjugated anti-IL6 | BD Pharmingen | Cat. # 554402 |
| HRP-conjugated streptavidin | BD Bioseiences | Cat. # 554066 |
| Anti-mouse TNFa | eBioscience | Cat. # 14-7423-85 |
| Anti-mouse IL-1β | Invitrogen | Cat. # 14-7012-81 |
| Biotin-conjugated anti-TNFa | Invitrogen | Cat. # 13-7349-81 |
| Biotin-conjugated anti-IL-1β | eBioscience | Cat. # 13-7112-85 |
| InVivoMAb anti-mouse IL-6R | Bio X Cell | Cat. # BE0047 |
| InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin | Bio X Cell | Cat. # BE0090 |
| Bacterial and Virus Strains | ||
| N/A | ||
| Biological Samples | ||
| Human plasma | Yale Stress Center | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Recombinant mouse IL6 | R & D | Cat. # 406-ML |
| Recombinant mouse TNFa | R & D | Cat. # 410-MY |
| Recombinant mouse IL-1β | R & D | Cat. # 401-ML-010 |
| NEFA standard solution | Wako Diagnostics | Cat. # 276-76491 |
| HR series NEFA-HR(2) color reagent A | Wako Diagnostics | Cat. # 999-34691 |
| HR series NEFA-HR(2) solvent A | Wako Diagnostics | Cat. # 995-34791 |
| HR series NEFA-HR(2) color reagent B | Wako Diagnostics | Cat. # 991-34891 |
| HR series NEFA-HR(2) solvent B | Wako Diagnostics | Cat. # 993-35191 |
| Multi-calibrator lipid | Wako Diagnostics | Cat. # 464-01601 |
| L-type triglyceride M enzyme color A | Wako Diagnostics | Cat. # 994-02891 |
| L-type triglyceride M enzyme color B | Wako Diagnostics | Cat. # 990-02991 |
| Intralipid 20% | Sigma-Aldrich | Cat. # 1141 |
| Poloxamer 407 | Sigma-Aldrich | Cat. # 16758 |
| Glucose, D-[3-3H] | PerkinElmer | Cat. # NET331C250UC |
| Potassium palmitate (U-13C16) | Cambridge Isotope Laboratories | Cat. # CLM-3943-PK |
| Sodium L-lactate-3-13C solution | Sigma-Aldrich | Cat. # 490040 |
| 6-hydroxydopamine | Sigma-Aldrich | Cat. # H4381 |
| Lipopolysaccharides from Escherichia coli O55:B5 | Sigma-Aldrich | Cat. # L2880 Lot #: 039M4004V |
| Isoproterenol | Sigma-Aldrich | Cat. # 1351005 |
| CL316243 | Sigma-Aldrich | Cat. # C5976 |
| Propranolol | Sigma-Aldrich | Cat. # P0884 |
| SR59203A | Cayman Chemical | Cat. # 21407 |
| Critical Commercial Assays | ||
| Mouse cytokine array/chemokine array 44-plex (MD44) | Eve Technologies | N/A |
| Human cytokine array/chemokine array 42-plex with IL-18(HD42) | Eve Technologies | N/A |
| Glycerol assay kit | Sigma-Aldrich | Cat. # MAK117-1KT |
| β-hydroxybutyrate colorimetric assay kit | Cayman Chemical | Cat. # 700190 |
| Corticosterone ELISA kit | Enzo Life Sciences | Cat. # ADI-900-097 |
| Mouse insulin ELISA kit | Crystal Chem | Cat. # 90080 |
| Noradrenaline ELISA kit | Eagle Biosciences | Cat. # NOR31-K01 |
| Mouse CTnI | Life Diagnostics | Cat. # CTNI-1-US |
| Alanine transaminase colorimetric activity assay kit | Cayman Chemical | Cat. # 700260 |
| Deposited Data | ||
| N/A | ||
| Experimental Models: Cell Lines | ||
| N/A | ||
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | 00064 |
| Mouse: Adrb1tm1Bkk Adrb2tm1Bkk | The Jackson Laboratory | 003810 |
| Mouse: B6.129-Ucp1 tm1KZ | The Jackson Laboratory | 003124 |
| Mouse: B6;SJL-Il6ratm11Drew / J | The Jackson Laboratory | 12944 |
| Mouse: B6. FVB(129)-Tg(Alb-cre)1Dlr / J | The Jackson Laboratory | 016832 |
| Mouse: B6. 129-Tg(Adipo q-cre/Esr1*)1Evdr / J | The Jackson Laboratory | 024671 |
| Mouse: Adrenalectomy and sham surgery | The Jackson Laboratory | https://www.jax.org/jax-mice-and-services/find-and-order-jax-mice/surgical-and-preconditioning-services/surgical-service-for-jax-mice |
| Oligonucleotides | ||
| Primers for mouse Il6 Forward | This paper | TGAACAACGATGATGCACTTG |
| Primers for mouse Il6 Reverse | This paper | CTGAAGGACTCTGGCTTTGTC |
| Primers for mouse Il6ra Forward | This paper | AGACCTGGGACCCGAGTTAC |
| Primers for mouse Il6ra Reverse | This paper | AAGGTCAAGCTCCTCCTTCC |
| Primers for mouse Fbp1 Forward | This paper | TGGTTCCGATGGACACAAGG |
| Primers for mouse Fbp1 Reverse | This paper | CCAATGTGACTGGGGATCAAG |
| Primers for mouse Gck Forward | This paper | TTACACTGGCCTCCTGATGG |
| Primers for mouse Gck Reverse | This paper | TTTGCAACACTCAGCCAGAC |
| Primers for mouse Ucp1 Forward | This paper | GTGAACCCGACAACTTCCGAA |
| Primers for mouse Ucp1 Reverse | This paper | TGCCAGGCAAGCTGAAACTC |
| Primers for Pck1, G6p, Pcx, Adrb3, Adrb1, Adrb2, Rpl13a, Ikkb, Tnfa, Cxcl1, Mx1, Il12b, Saa3, Prdm16, Il5, Ssa3, Socs3, See Table S1 | This paper | N/A |
| Recombinant DNA | ||
| N/A | ||
| Software and Algorithms | ||
| Prism 8.0 | GraphPad Software, Inc. | N/A |
| Other | ||
| N/A | ||
HIGHLIGHTS.
IL6 is the dominant endocrine cytokine induced by acute stress in mice.
Stress-inducible IL6 is produced in brown adipocytes via ADRB3 signaling.
IL6 is required for stress hyperglycemia and adaptive “fight or flight” responses.
Stress-induced IL6 decreases tolerance to a subsequent inflammatory challenge.
ACKNOWLEDGMENTS
We thank members of the Wang, Picciotto, Sinha, and Perry labs for helpful discussions. We are indebted to Dr. Ruslan Medzhitov for his guidance and support. We thank Ali Nasiri and Wanling Zhu for the mouse metabolic flux phenotyping, Dr. Mamula’s lab for sharing equipment, and Dr. Akiko Iwasaki for use of thermoneutral cabinets. We thank Mrs. Krista Wang, Drs. Ruth Franklin and Harding Luan for review of the manuscript. Invasive hemodynamic monitoring was performed through the George M. O’Brien Kidney Center at Yale (NIH Grant P30-DK079310). A.W. was supported by the NIH Clinical Investigator Award K08AI128745 (A.W.). K.I.W was supported by a Gruber Fellowship. Metabolic flux analysis performed in the Perry lab was supported by an NIH Pathway to Independence Award (K99/R00 CA215315, R.J.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Abreu-Vieira G, Hagberg CE, Spalding KL, Cannon B, and Nedergaard J (2015). Adrenergically stimulated blood flow in brown adipose tissue is not dependent on thermogenesis. American journal of physiology Endocrinology and metabolism 308, E822–829. [DOI] [PubMed] [Google Scholar]
- Agac D, Estrada LD, Maples R, Hooper LV, and Farrar JD (2018). The beta2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav Immun 74, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, and Sinha R (2012). Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry 72, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune UL, Ruiz L, and Kajimura S (2013). Isolation and differentiation of stromal vascular cells to beige/brite cells. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banzet S, Koulmann N, Simler N, Sanchez H, Chapot R, Serrurier B, Peinnequin A, and Bigard X (2009). Control of gluconeogenic genes during intense/prolonged exercise: hormone-independent effect of muscle-derived IL-6 on hepatic tissue and PEPCK mRNA. J Appl Physiol (1985) 107, 1830–1839. [DOI] [PubMed] [Google Scholar]
- Batty GD, Russ TC, Stamatakis E, and Kivimaki M (2017). Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ 356, j108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benrick A, Wallenius V, and Asterholm IW (2012). Interleukin-6 mediates exercise-induced increase in insulin sensitivity in mice. Exp Physiol 97, 1224–1235. [DOI] [PubMed] [Google Scholar]
- Bethin KE, Vogt SK, and Muglia LJ (2000). Interleukin-6 is an essential, corticotropinreleasing hormone-independent stimulator of the adrenal axis during immune system activation. Proceedings of the National Academy of Sciences of the United States of America 97, 9317–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, and Slavich GM (2016). Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann N Y Acad Sci 1373, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Chen X, Ruiz J, White JV, and Rossetti L (1994). Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 93, 2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Kleinridders A, and Kahn CR (2014). Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, and Hascoet M (2003). The mouse light/dark box test. Eur J Pharmacol 463, 55–65. [DOI] [PubMed] [Google Scholar]
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, et al. (2006). Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55, 2688–2697. [DOI] [PubMed] [Google Scholar]
- Castaneda S, Remuzgo-Martinez S, Lopez-Mejias R, Genre F, Calvo-Alen J, Llorente I, Aurrecoechea E, Ortiz AM, Triguero A, Blanco R, et al. (2019). Rapid beneficial effect of the IL-6 receptor blockade on insulin resistance and insulin sensitivity in non-diabetic patients with rheumatoid arthritis. Clin Exp Rheumatol 37, 465–473. [PubMed] [Google Scholar]
- Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R, Fabra R, and Heinrich PC (1989). Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett 242, 237–239. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Jope RS, and Beurel E (2015). A pre-conditioning stress accelerates increases in mouse plasma inflammatory cytokines induced by stress. BMC Neurosci 16, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, and Turner RB (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America 109, 5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias AJ, and Horng T (2014). IL-6 strikes a balance in metabolic inflammation. Cell Metab 19, 898–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JMA, Sun W, Pires ND, Frontini A, Balaz M, Jespersen NZ, Feizi A, Petrovic K, Fischer AW, Bokhari MH, et al. (2019). Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Nature Metabolism 1, 830–843. [DOI] [PubMed] [Google Scholar]
- Depocas F, Foster DO, Zaror-Behrens G, Lacelle S, and Nadeau B (1984). Recovery of function in sympathetic nerves of interscapular brown adipose tissue of rats treated with 6-hydroxydopamine. Can J Physiol Pharmacol 62, 1327–1332. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Katz PP, Lactao G, and Iribarren C (2005). Impact of depressive symptoms on adult asthma outcomes. Ann Allergy Asthma Immunol 94, 566–574. [DOI] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, and Giugliano D (2002). Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106, 2067–2072. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, and Pedersen BK (2004). Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53, 1643–1648. [DOI] [PubMed] [Google Scholar]
- Felger JC, and Lotrich FE (2013). Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246, 199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Marguti I, Bechmann I, Jeney V, Chora A, Palha NR, Rebelo S, Henri A, Beuzard Y, and Soares MP (2011). Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 145, 398–409. [DOI] [PubMed] [Google Scholar]
- Findeisen M, Allen TL, Henstridge DC, Kammoun H, Brandon AE, Baggio LL, Watt KI, Pal M, Cron L, Estevez E et al. (2019). Treatment of type 2 diabetes with the designer cytokine IC7Fc. Nature 574, 63–68. [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, and Maier SF (2013). Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun 33, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan K, Nikkanen J, Man K, Leong YA, Sogawa Y, Maschek JA, Van Ry T, Chagwedera DN, Cox JE, and Chawla A (2019). Energetic Trade-Offs and Hypometabolic States Promote Disease Tolerance. Cell 177, 399–413 e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Liu F, Fang L, Cai R, Zong C, and Qi Y (2014). Genkwanin inhibits proinflammatory mediators mainly through the regulation of miR-101/MKP-1/MAPK pathway in LPS-activated macrophages. PLoS One 9, e96741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, Mansell A, and Jenkins BJ (2011). IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol 186, 1199–1208. [DOI] [PubMed] [Google Scholar]
- Hippocrates (1849). The genuine works of Hippocrates/translated from the Greek, with a preliminary discourse and annotations by Francis Adams, in two volumes (London: The Sydenham Society; ). [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, et al. (2014). Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America 111, 16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, and Jones SA (2015). IL-6 as a keystone cytokine in health and disease. Nat Immunol 16, 448–457. [DOI] [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, Jones PB, and Khandaker GM (2018). Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 23, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N, Hioki H, Kaneko T, and Nakamura K (2014). Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab 20, 346–358. [DOI] [PubMed] [Google Scholar]
- Kawate R, Talan MI, and Engel BT (1994). Sympathetic nervous activity to brown adipose tissue increases in cold-tolerant mice. Physiol Behav 55, 921–925. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, and Hyland NP (2015). Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, and Jones PB (2014). Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 71, 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokha R, Murthy A, and Weiss A (2013). Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 13, 649–665. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, et al. (2004). Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53, 1060–1067. [DOI] [PubMed] [Google Scholar]
- Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, and Ferrante AW Jr. (2010). Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 120, 3466–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotch CA-OX, Barrett D, and Teachey DT Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, and Chawla A (2015). Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, Tal I, Dieckmann W, Gupta G, Kolodny GM, et al. (2017). Mapping of human brown adipose tissue in lean and obese young men. Proceedings of the National Academy of Sciences of the United States of America 114, 8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay LG, Vander AJ, and Kluger MJ (1990). The effects of psychological stress on plasma interleukin-6 activity in rats. Physiol Behav 47, 957–961. [DOI] [PubMed] [Google Scholar]
- Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, and Busse WW (2002). School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med 165, 1062–1067. [DOI] [PubMed] [Google Scholar]
- Liu YZ, Wang YX, and Jiang CL (2017). Inflammation: The Common Pathway of Stress-Related Diseases. Front Hum Neurosci 11, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, Herzog EL, Kang I, Pisani MA, Yu S, et al. (2019). GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y, Jin R, Ma L, Wang P, Zhu Z, et al. (2012). Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol 4, 88–96. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, et al. (1998). The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 10, 313–318. [DOI] [PubMed] [Google Scholar]
- Masuda K, Ripley B, Nishimura R, Mino T, Takeuchi O, Shioi G, Kiyonari H, and Kishimoto T (2013). Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proceedings of the National Academy of Sciences of the United States of America 110, 9409–9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews VB, Allen TL, Risis S, Chan MH, Henstridge DC, Watson N, Zaffino LA, Babb JR, Boon J, Meikle PJ, et al. (2010). Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53, 2431–2441. [DOI] [PubMed] [Google Scholar]
- Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, et al. (2014). Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 15, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, and Picciotto MR (2007). Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology 52, 1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Hart SL, Julian L, Cox D, and Pelletier D (2004). Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ 328, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes MN, de Assis LVM, Henriques FDS, Batista ML Jr., Guler AD, and Castrucci AML (2017). Cold-sensing TRPM8 channel participates in circadian control of the brown adipose tissue. Biochim Biophys Acta Mol Cell Res 1864, 2415–2427. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, and Holbrook NJ (1984). Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5, 25–44. [DOI] [PubMed] [Google Scholar]
- Nance DM, and Sanders VM (2007). Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21, 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi D, Mishra Mk Fau - Basu A, Basu A Fau - Bishayi B, and Bishayi B Protective effects of interleukin-6 in lipopolysaccharide (LPS)-induced experimental endotoxemia are linked to alteration in hepatic anti-oxidant enzymes and endogenous cytokines. [DOI] [PubMed] [Google Scholar]
- Nellan A, McCully CML, Cruz Garcia R, Jayaprakash N, Widemann BC, Lee DW, and Warren KE (2018). Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood 132, 662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NL, Barr CL, Ryu V, Cao Q, Xue B, and Bartness TJ (2017). Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol 312, R132–R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niraula A, Witcher KG, Sheridan JF, and Godbout JP (2019). Interleukin-6 Induced by Social Stress Promotes a Unique Transcriptional Signature in the Monocytes That Facilitate Anxiety. Biol Psychiatry 85, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Han HS, Kim MJ, and Koo SH (2013). CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep 46, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuchowski MF, Welch K, Siddiqui J, and Remick DG (2006). Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol 177, 1967–1974. [DOI] [PubMed] [Google Scholar]
- Path G, Scherbaum WA, and Bornstein SR (2000). The role of interleukin-6 in the human adrenal gland. Eur J Clin Invest 30 Suppl 3, 91–95. [DOI] [PubMed] [Google Scholar]
- Pbert L, Madison JM, Druker S, Olendzki N, Magner R, Reed G, Allison J, and Carmody J (2012). Effect of mindfulness training on asthma quality of life and lung function: a randomised controlled trial. Thorax 67, 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, and Febbraio MA (2007). Point: Interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol (1985) 102, 814–816. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, and Febbraio MA (2008). Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88, 1379–1406. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Camporez JG, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, et al. (2015). Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Peng L, Abulizi A, Kennedy L, Cline GW, and Shulman GI (2017a). Mechanism for leptin’s acute insulin-independent effect to reverse diabetic ketoacidosis. J Clin Invest 127, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Peng L, Cline GW, Butrico GM, Wang Y, Zhang XM, Rothman DL, Petersen KF, and Shulman GI (2017b). Non-invasive assessment of hepatic mitochondrial metabolism by positional isotopomer NMR tracer analysis (PINTA). Nat Commun 8, 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Wang Y, Cline GW, Rabin-Court A, Song JD, Dufour S, Zhang XM, Petersen KF, and Shulman GI (2018). Leptin Mediates a Glucose-Fatty Acid Cycle to Maintain Glucose Homeostasis in Starvation. Cell 172, 234–248 e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C, Herndon DN, Chondronikola M, Chao T, Annamalai P, Bhattarai N, Saraf MK, Capek KD, Reidy PT, Daquinag AC, et al. (2016). Human and Mouse Brown Adipose Tissue Mitochondria Have Comparable UCP1 Function. Cell Metab 24, 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, and Chawla A (2014). Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick DG, Bolgos G, Copeland S, and Siddiqui J (2005). Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun 73, 2751–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, Sharma M, and Kambadur R (2017). Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun 8, 1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothaug M, Becker-Pauly C, and Rose-John S (2016). The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta 1863, 1218–1227. [DOI] [PubMed] [Google Scholar]
- Rouget C, Breuiller-Fouche M, Mercier FJ, Leroy MJ, Loustalot C, Naline E, Frydman R, Croci T, Morcillo EJ, Advenier C, et al. (2004). The human near-term myometrial beta 3-adrenoceptor but not the beta 2-adrenoceptor is resistant to desensitisation after sustained agonist stimulation. British journal of pharmacology 141, 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussou E, Iacovou C, Weerakoon A, and Ahmed K (2013). Stress as a trigger of disease flares in SLE. Rheumatology international 33, 1367–1370. [DOI] [PubMed] [Google Scholar]
- Russell G, and Lightman S (2019). The human stress response. Nat Rev Endocrinol 15, 525–534. [DOI] [PubMed] [Google Scholar]
- Satin JR, Linden W, and Phillips MJ (2009). Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer 115, 5349–5361. [DOI] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, and Rose-John S (2011). The pro- and anti inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813, 878–888. [DOI] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, and Sinha R (2014). Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology 39, 670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R, Booth J, Lawrence M, Byrne S, Mair F, and Mercer S (2014). Mindfulness based interventions in multiple sclerosis--a systematic review. BMC Neurol 14, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Fischer CP, Sacchetti M, Keller C, Osada T, Schjerling P, van Hall G, Febbraio MA, and Pedersen BK (2003). Acute interleukin-6 administration does not impair muscle glucose uptake or whole-body glucose disposal in healthy humans. The Journal of physiology 548, 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, Brouwer E, Cid MC, Dasgupta B, Rech J, et al. Trial of Tocilizumab in Giant-Cell Arteritis. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Meyer C, Perriello G, Kreider M, Welle S, and Gerich J (1998). Human kidney and liver gluconeogenesis: evidence for organ substrate selectivity. The American journal of physiology 274, E817–826. [DOI] [PubMed] [Google Scholar]
- Timper K, Denson JL, Steculorum SM, Heilinger C, Engstrom-Ruud L, Wunderlich CM, Rose-John S, Wunderlich FT, and Bruning JC (2017). IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 trans-Signaling. Cell Rep 19, 267–280. [DOI] [PubMed] [Google Scholar]
- Turner RJ, and Lloyd DA (1995). Lifetime traumas and mental health: the significance of cumulative adversity. J Health Soc Behav 36, 360–376. [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, and Teule GJ (2009). Cold-activated brown adipose tissue in healthy men. The New England journal of medicine 360, 1500–1508. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, and Jansson JO (2002). Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8, 75–79. [DOI] [PubMed] [Google Scholar]
- Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, and Medzhitov R (2016). Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell 166, 1512–1525 e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, L. H, Hilliard B, Carvalho F, Rosen C, Ahasic A, Herzog E, Kang I, Pisani M, Yu S, Zhang C, Ring A, Young L, Medzhitov R (2019). GDF15 is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Yao HW, Chang CF, Wang SW, Wang SM, and Chen SH (2017). Suppression of interleukin-6 increases enterovirus A71 lethality in mice. J Biomed Sci 24, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert MO, and Pfeiffer AF (2006). Signalling mechanisms linking hepatic glucose and lipid metabolism. Diabetologia 49, 1732–1741. [DOI] [PubMed] [Google Scholar]
- Weis S, Carlos AR, Moita MR, Singh S, Blankenhaus B, Cardoso S, Larsen R, Rebelo S, Schauble S, Del Barrio L, et al. (2017). Metabolic Adaptation Establishes Disease Tolerance to Sepsis. Cell 169, 1263–1275 e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y, Boura-Halfon S, Cortese N, Haimon Z, Sar Shalom H, Kuperman Y, Kalchenko V, Brandis A, David E, Segal-Hayoun Y, et al. (2017). Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat Immunol 18, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich FT, Strohle P, Konner AC, Gruber S, Tovar S, Bronneke HS, Juntti-Berggren L, Li LS, van Rooijen N, Libert C, et al. (2010). Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab 12, 237–249. [DOI] [PubMed] [Google Scholar]
- Yan C, Zhang BB, Hua H, Li B, Zhang B, Yu Q, Li XY, Liu Y, Pan W, Liu XY, et al. (2015). The Dynamics of Treg/Th17 and the Imbalance of Treg/Th17 in Clonorchis sinensis-Infected Mice. PLoS One 10, e0143217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, Dai Z, White PJ, McGarrah RW, Ilkayeva OR, et al. (2019). BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu Z, Zhao G, Wang F, and Fang Y (2016). Identification of IL6 as a susceptibility gene for major depressive disorder. Sci Rep 6, 31264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, and Hashimoto K (2017). Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry 7, e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Mottillo EP, Zhao J, Gartung A, VanHecke GC, Lee JF, Maddipati KR, Xu H, Ahn YH, Proia RL, et al. (2014). Adipocyte lipolysis-stimulated interleukin-6 production requires sphingosine kinase 1 activity. The Journal of biological chemistry 289, 32178–32185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ADRB3 mediates brown adipocyte-derived IL6 in response to acute stress, Related to Figure 1, Figure 2, and Figure 3.
(A) Plasma concentrations of soluble IL6 receptor (s-IL6Ra) from bled mice (n=5 per group).
(B) Plasma IL6 levels post-bleeding in mice with bilateral adrenalectomy or sham (n=5 per group).
(C) Plasma noradrenaline and (D) corticosterone in mice pretreated with IL6Ra antibody or isotype control (n=5 per time point per group).
(E) Relative level of circulating IL6 from human with high or low stress history (n=12 for high stress history, n=13 for low stress history).
(F) Capillary blood glucose (CBG) and (G) plasma IL6 from WT mice after glucose or water gavage. Mice were bled then fasted for four hours before gavage (n=5 per group).
(H) Plasma IL6 levels in blood collected from the indicated site of mouse eyes four hours post bleeding (n=5 per group).
(I) qPCR analyses of Ucp1 and (J) Adrb3 in brown adipocyte tissue (BAT) or the stromal vascular fraction (SVF) from mice stressed with the indicated conditions (n=5 per group).
(K) Plasma concentration of IL6 four hours post bleeding from mice subjected to the indicated ambient temperature (n=3 per group, representative of 2 experiments).
(L) Body temperature after bleeding stress in the presence of IL6Ra antibody or isotype control (n=5 per group).
(M) Transcriptional analyses of cold-sensitive genes and Il5 in BAT (n=3-4 per group). Results are expressed as fold change relative to non-stressed control (NT).
(N-O) Transcriptional expression of beta-adrenergic receptors Adrb1 and Adrb2 in BAT or SVF from BAT of stressed mice (n=3-4 per group).
(P) Plasma IL6 levels at indicated time points post bleeding from mice pre-treated with an ADRB1/2 antagonist propranolol (anti-ADRB1/2) or PBS (n=5 per group).
(Q) Expression of Adrb3 in the indicated tissues from mice with conditional UCP1-medidated deletion of Adrb3 (n=4 per group). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S2. IL6 is necessary for promoting stress-hyperglycemia, Related to Figure 4.
(A) Continuous heart (HR) and blood pressure (BP) tracings in mice treated with IL6Ra mAb or isotype control followed by tube restraint (TR) (n=4 per group).
(B) Activity of mice treated with IL6Ra antibody of isotype control followed by retro-orbital bleeding (n=4 per group, representative of 2 experiments).
(C) Capillary blood glucose (CBG) one hour after injection of stress-dosed IL6 (n=10 per group).
(D) Glucose tolerance test (GTT) performed four hours post bleeding from mice pretreated with IL6Ra antibody or isotype control (n=5 per group, representative of 3 experiments).
(E) GTT performed one hour after injection with IL6 or vehicle control (n=5 per group).
(F) Plasma insulin levels one hour after injection with IL6 or vehicle control (n=6 for vehicle, n=8 for IL6).
(G) Insulin tolerance test (ITT) performed in animals four hours after a single retro-orbital bleeding (n=5 per group, representative of 2 experiments). NT, no treatment.
(H) Insulin tolerance test performed one hour after a stress-dose IL6 injection (n=6 per group).
(I) Insulin tolerance test performed in mice pretreated with IL6Ra antibody or isotype control (n=5 per group)
(J) Glycogen content in the indicated tissues of mice 3 hours post bleeding (muscle is gastrocnemius, n=5 per group). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S3. Lipid metabolism in response to acute stress or external IL6, Related to Figure 4.
(A-C) (A) Plasma non-esterified fatty acid (NEFA), (B) glycerol, and (C) triglycerides (TG) four hours post bleeding (n=28 for NT, n=29 for Bleed. Pooled from 3 experiments).
(D) Intralipid tolerance test performed four hours post bleeding (n=5 per group).
(E) Hepatic triglyceride secretion at indicted time points post poloxamer injection from bled mice or controls (NT) (n=5 per group).
(F-G) (F) Plasma TG levels or (G) NEFA assessed four hours post bleeding in mice pretreated with IL6Ra antibody or isotype control (n=5 per group).
(H-K) (H) Plasma level of TG, (I) NEFA, (J) Glycerol, (K) β-Hydroxybutyrate (BHB) one hour after IL6 injection (n=5 per group).
(L) In vivo lipolysis assay performed using ADRB3 agonist CL316,243 in mice with conditional IL6Ra deletion in the adipose tissue (Il6raf/fΔAdipo) or littermate controls (Il6raf/f) (n=6 per group).
(M) Palmitate turnover assessed in mice treated with IL6Ra antibody or isotype control (n=8 per group). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S4. IL6 mediates stress hyperglycemia through hepatocyte reprogramming, Related to Figure 5.
(A) Pck1 mRNA measured at four hours post bleeding from the liver and kidney of Il6 KO or WT mice (n=5 per group)
(B) Expression of Saa3 and (C) Socs3 in the indicated tissues from stressed mice (n=5 per group).
(D) Plasma level of amino acids four hours post bleeding in mice pretreated with IL6Ra antibody or isotype control (n=5 per group).
(E) Il6ra expression in the indicated tissue from mice with liver-specific deletion of Il6ra (Il6raf/fΔAlb) or littermate controls (Il6raf/f) (n=6 per group).
(F) Latency to dark in mice with liver-specific Il6ra deletion (n=15 for Il6raf/f, n=9 for Il6raf/fΔAlb, representative of 3 experiments). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S5. Inflammatory responses to a lethal endotoxemia are not affected by the pre-exposure of acute stress, Related to Figure 6.
(A) Survival rate of mice with brown adipose tissue specific deletion of Il6 (Il6f/fΔUCP1) comparing with littermate controls (Il6f/f) (n=6 per group, representative of 2 experiments).
(B) Survival rate of mice with liver specific deletion of Il6ra (Il6raf/fΔAlb) comparing with littermate controls (Il6raf/f) (n=6 per group, representative of 3 experiments).
(C) Survival of animals pre-treated with glucose or an isocaloric isovolumetric dose of intralipid followed by LPS injection (n=5 per group).
(D) Plasma IL6, (E) TNFα, and (F) IL-1β levels sampled 0, 2, 6, and 20 hours after LPS challenge in bled mice or non-bled controls (n=5 per group, representative of 2 experiments).
(G) Plasma IL6, (H) TNFα, and (I) IL-1β levels sampled 0, 2, 6, and 20 hours after LPS challenge in mice pre-challenged with ADRB3 agonist or vehicle control (n=5 per group).
(J) The peak levels of circulating IL6 and (K) TNFα during LPS-mediated endotoxemia from mice pre-exposed to tube restraint (TR) or controls (NT) (n=5 per group).
(L-R) Expression of inflammatory transcripts in the indicated tissues from LPS-treated mice pre-exposed to bleeding stress. Tissues were harvested three hours after LPS challenge (n=5 per group). * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
Figure S6. BAT-derived IL6 from acute stress decreases host tolerance to inflammatory damage, Related to Figure 6.
(A-D) Vital signs before and 24 hours post LPS challenge from mice pre-exposed to acute stress. NT, no treatment. TR, tube restraint. HR, heart rate.
(E) Continuous systolic blood pressure (BP) and (F) heart rate (HR) tracings in mice with tube restraint and the subsequent LPS challenge. NT, no treatment. TR, tube restraint.
(G) Circulating alanine aminotransferase (ALT) and (H) cardiac troponin I (CTNI) levels 0 or 24 hours post LPS injection from mice pre-exposed to bleeding or restraint stress (n=5 per group). NT, no treatment. TR, tube restraint.
(I) Capillary blood glucose (CBG) levels post LPS injection at indicated time points from mice pre-exposed to restraint stress (n=5 per group, representative of 2 experiments). NT, no treatment. TR, tube restraint.
(J) Plasma non-esterified fatty acid (NEFA) levels post LPS injection at indicated time points from mice pre-exposed to restraint stress (n=5 per group, representative of 2 experiments).
(K) Plasma β-hydroxy-butyrate (BHB) levels post LPS injection at indicated time points from mice pre-exposed to restraint stress (n=5 per group, representative of 2 experiments).
Supplementary Figure 7. Raw data from ELISA analysis of IL6 , Related to Figure 1B, Figure 3D and Figure 3G.
(A) The raw data of IL6 from stressed mice including the standards concentration from Eve Technologies.
(B) The raw OD values from the plate presented below in (D).
(C) The layout of samples from the plate presented below in (D). Row A, C, E, and F were samples collected before bleeding. Row B,D, G, and H were samples at four hours post bleeding. NA, no samples in the well. Row A and B were related to figure 3D from mice treated with 6-OHDA 0 and 4 hours post bleeding. Row C and D were from mice with surgical denervation of BAT or sham controls (data not show). Row E, F, G, and H were related to figure 3G from mice with BAT-specific deletion of Il6 (Il6f/fΔUCP1) or littermate controls (Il6f/f) 0 and 4 hours post bleeding.
(D) The representative plate with color developed with TMB.
(E) The standard curve generated based on the OD value in (A) and concentrations in (B) from column 1 and 2.
Data Availability Statement
This study did not generate any datasets/code amenable for depositing into public repositories.


