1. Dear Editor,
Tuberculosis (TB) is one of the most important infectious diseases and considered among the top 10 causes of death in the world. According to World Health Organization (WHO) report in 2020, about 10 million infected cases of TB and also 1.5 million death occurred form this disease in 2019 [1], [2]. Moreover, about one fourth of infected patients by Mycobacterium tuberculosis (Mtb) are involved with more severe complications such as infection by drug-resistant TB (DR-TB) strains, co-infection with HIV virus, as well as latent TB (LTBI) infection. Although individuals affected by LTBI are asymptomatic and cannot infect others, but in general, some of them may develop to active-TB form that it is accounted as a concern for health services [3], [4], [5]. In addition, the standard treatment of TB is also a time consuming process and in some cases such as co-infection with HIV, DR-TB, and cases with side effects, therapeutic protocols may face with serious limitations. Therefore, it seems we will require to develop the novel therapeutic agents which reduce the course of treatment, and can cover the other problems in treating TB [5], [6], [7]. Vitamin D was first used by Williams in the beginning of 1849; he noticed that the fish liver oil improves the appetite and strength of the TB patients [8]. Rook et al. (1986) showed that calcitriol, the active biological form of Vitamin D, has in vitro anti-TB effects [9]. Based on the evidence, vitamin D influences the host immune system responses. It can activate the monocytes and lymphocytes of human through their vitamin D receptors (VDRs). For instance, this vitamin stimulates the physiological processes such as monocytes activities, restraining the proliferation of lymphocytes, stimulation of the immunoglobulin and cytokine production, stimulation of the reactive nitrogen and oxygen stress responses, restraining the matrix metalloproteinase activity, autophagy stimulation, and production of human antimicrobial peptides (AMPs) [10], [11]. In recent studies, it has been demonstrated that the serum level of vitamin D has a significant relationship with susceptibility rate to TB [10], [11], [12]. Furthermore, it has been shown that the polymorphisms such as Taql in the VDR gene also affect the development of TB [10]. Thus, it seems that vitamin D by improving the immune system responses causes the shorten course of TB treatment, increasing the intercellular elimination of Mtb, reducing transmissibility, and also improvement of the intensity and strength of infection by DR-TB strains [13].
Several clinical trials have so far been done about the effects of vitamin D on the treatment of TB, but some of which have shown controversial results. Moreover, the role of vitamin D supplement in the treatment of TB has not properly determined in the meta-analysis studies. The aim of this study was to evaluate the role of vitamin D on reducing the sputum conversion time in the patients with drug-susceptible TB and multi-drug resistant TB (MDR-TB). The polymorphism of Taql can also modify the effect of vitamin D supplement on time conversion.
Using the key words “Tuberculosis” and “Vitamin D”, the clinical trial studies related to our study were searched from the clinical trial website (https://clinicaltrials.gov/). Then, the free access documents were retrieved for the clinical trial studies. The required information from these studies were extracted and listed in Table 1.
Table 1.
Characteristics of included studies.
| Author | Publish year | Setting | Clinical trials register No. | Intervention vitamin D3 | Dosage | Female/MaleAge | Statistics of studies |
Refs. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| case | control | p-value | ||||||||
| Martineau | 2011 | UK | NCT00419068 | vitamin D3 2.5 mg |
2, 4 and 6 weeks | NA 30.6 |
36 (95% CI 31.8–40.2) | 43.5 (95% CI 36.5–50.5) |
HR: 1.39; 95% CI 0.90–2.16, P = 0.41 | [10] |
| Daley | 2015 | India | NCT00366470 | vitamin D3 2.5 mg |
0, 2, 4, and 6 weeks | 58/189 42.65 |
43·0 (33·3–52·8) | 42·0 (33·9–50·1) | 0·952 | [12] |
| Mily | 2015 | Bangladesh | NCT01580007 | vitamin D3 500 mg |
Once daily for 2 months | 23/19 27.4 ± 12 |
61.3% (38/62) | 42.2% (27/64) |
0.032 | [14] |
| Salahuddin | 2013 | Saudi Arabia | NCT01130311 | vitamin D3 600,000 IU |
2 doses | 118/141 28.05 ± 61 |
6.68 ± 2.04, 6.3–7.03 | 6.85 ± 2.50, 6.4–7.29 | 0.16 | [15] |
| Tukvadze | 2015 | Georgia | NCT00918086 | vitamin D3 1.25 mg |
Thrice weekly for 8 week | 72/127 33.25 |
29; 95% CI: 24, 36 |
27; 95% CI: 23, 36 | HR: 0.86; 95% CI: 0.63, 1.18; P = 0.33 | [16] |
| Bekele | 2018 | Ethiopia | NCT01698476 | vitamin D3 5000 IU |
FOR 16 weeks | 147/201 30.47 |
1.07 0.43–2.65 P = 0.879 |
1.05 0.42–2.63 P = 0.904 |
NA | [17] |
| Ganmaa | 2017 | Mongolia | NCT01657656 | vitamin D3 3.5 mg |
four oral doses | 134/256 33 |
NA | NA | Adjusted hazard ratio, 1.09; 95% confidence interval, 0.86–1.36; P = 0.48. |
[18] |
| Ralph | 2013 | Indonesia | NCT00677339 | vitamin D3 50,000 IU |
Once daily for 4 weeks | 69/131 27.5 |
59% (44/75) |
65% (52/80) |
Risk difference 7%, 95% CI 29 to 22%. | [19] |
The Odds Ratio (OR) and Hazard Ratio (HR) indices at 95% confidence intervals were used to evaluate the relationship between the vitamin D supplementation as adjunctive therapy and the sputum conversion. Using OR the effect of polymorphism of Taql gene on the time of sputum conversion was also measured. Pooling the data was done in the present study by use of the Comprehensive Meta-Analysis (CMA) software, version 2.2 (Biostat, Englewood, NJ). Based on Dersimonian and Laird method, the random effect models was used for the cases with high levels of heterogeneity (I2 index >25% and Cochran’s Q-test p-value <0.05) [3].
Overall, eight studies that had been fulfilled between 2011 and 2018 were matched with our criteria studies. We evaluated the information about 1811 people, so that 34.29% of them were women and the rest were men. Also, the mean age of the patients was measured 31.6 ± 15. In the study by Ralph et al. (2013), they used of both L-arginine and vitamin D for some groups, but we omitted the information of L-arginine groups in order to merely to evaluate the pure effect of vitamin D on TB.
Based on the primary statistical analysis, vitamin D supplementation increases the sputum smear and culture conversion (OR: 3.197; 2.39–4.26 with 95% CIs; I2: 64.44; Q value: 11.24 and Egger’s intercept: 1.81). However, the consumption of that had no impact on the time of sputum smear and culture conversion (HR: 1.05; 0.88–1.25 with 95% CIs; I2: 38.11; Q value: 3.23; Eggers’ intercept: 1.29). According to the secondary analysis, vitamin D had no impacts on the sputum conversion in the MDR-TB patients (OR: 0.91; 0.58–1.41 with 95% CIs; p-value: 0.69). In addition, we did not find a significant relationship between the effect of Taql gene polymorphism in adjunctive therapy of vitamin D and shortening the duration of sputum conversion (tt allele with OR: 1.12 and p value: 0.61; Tt allele with OR: 1.03 and p value: 0.86; TT allele with OR: 1.23 and p value: 0.31). Moreover, Taql polymorphism had no impacts on shortening the duration of sputum smear and culture conversion (HR: 1.43; 0.90–2.26 with 95% CIs; p value: 0.12). Regarding the statistical analysis, we also performed a supplementary analysis about the impact of vitamin D supplementation on the size of the lung cavity, total white blood cells (WBC) as well as deaths, although there was not found the significant relationship in this respect. For the three recent items, the statistical analysis results was OR: 0.92; p value: 0.49, OR: 1.18; p value: 0.145, and OR: 1.1; p value: 0.76 respectively. Vitamin D is one of the most well-known bioactive compounds that has an important effect on the reduction of pathogenesis of infectious diseases by modulating the immune system responses (Fig. 1).
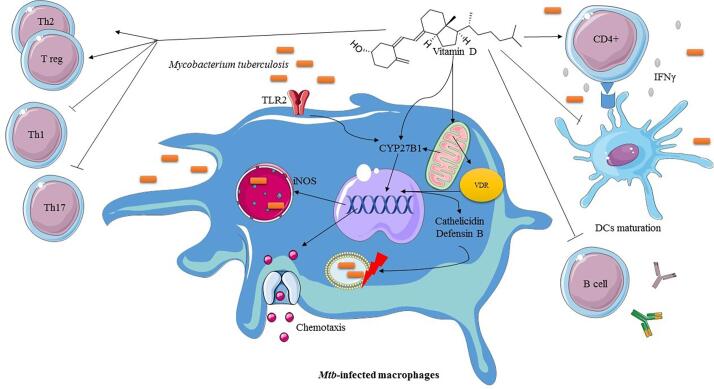
Fig. 1.
The crucial role of vitamin D in the immunopathogenesis of TB. Vitamin D has immunomodulatory effects on the immune-system, so that it suppresses Th1, Th17, B cell as well as DCs maturation. However, it also stimulates the proliferation of T regulatory (T reg) cells, which in turn inhibit the excessive T cell-mediated immunity and tissue damage. In the other hand, it also has a specific receptor on the macrophages, vitamin D receptor (VDR). After the binding to the VDR, vitamin D triggers the processes such as the production of natural human antimicrobial peptides (AMPs), nitric oxide synthesis and chemotaxis to provoke immune response for clearance of M. tuberculosis (Mtb).
By restraining the CD4+ T cell proliferation, inducing the IFN-γ, IL-2, and formation of VDR-RXR complex, vitamin D can shift the immune system response towards Th2/T regulatory (T reg) cells, and indeed effects on the final outcome of the infection by Mtb [20]. In the present study we demonstrated that vitamin D as adjunctive substance increases the sputum smear and culture conversion (OR: 3.19; p value: 0.01). However, based on our analysis, the consumption of vitamin D had no impact on the time of the sputum smear and culture conversion (HR: 1.05; p-value: 0.54). Furthermore, we observed no significant relationship between the vitamin D supplementation and sputum conversion in MDR-TB cases, total white blood cell counts, reduction of lung cavity size, death, and Taql polymorphism; previous studies had also controversial results. Jolliffe et al. (2019) showed that vitamin D supplementation can accelerate sputum smear conversion in the MDR-TB patients (aHR 1.15), but our findings were in contradiction with their results [21]. Wu et al. (2018) showed that vitamin D can increase the sputum smear and culture conversion (OR 1.21, p value: 0.007) and our results was also consistent with their results. However, Wu et al. stated that consuming vitamin D had a significant relationship with increasing the lymphocyte counts in the TB patients, but our findings was contradictory with the previous studies [22]. Wu et al. (2018) showed that vitamin D supplementation had no impact on the increasing of sputum conversion, but their results was not consistent with our results [22]. Nonetheless, the difference in the results could be due to the low sample size and limited studies. Although in the present study the existing clinical trials was evaluated up to April 2020, but we require more studies and more volunteers to evaluate the validity of the present study results.
In summary, we demonstrated that vitamin D supplementation increases the sputum smear and culture conversion in the TB patients. However, vitamin D has no impact on shortening the duration of sputum conversion in the TB patients. Regarding the present studies, it seems that vitamin D supplementation can increase the sputum conversion by improving the disinfecting activity of the macrophages. Hence, it can be concluded that vitamin D can be recommended for the adjunctive therapy in treating and prevention of TB, in combination with the anti-tuberculosis drugs and for the prophylactic aims.
Ethical Statement
Ethical Statement is not applicable for this manuscript.
References
- 1.World Health Organization. Global tuberculosis report 2019. World Health Organization; 2020.
- 2.Keikha M., Eslami M., Yousefi B., Karbalaei M. Overview of multistage subunit tuberculosis vaccines: advantages and challenges. Rev Med Microbiol. 2020 [Google Scholar]
- 3.Keikha M. There is significant relationship between Beijing genotype family strains and resistance to the first-line anti-tuberculosis drugs in the Iranian population. J Clin Tuberculosis Other Mycobacterial Diseases. 2020;19 doi: 10.1016/j.jctube.2020.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najafi A., Mahdian N., Yousefi B., Karbalaei M., Ezatpour B., Eslami M. New vaccine candidates as a scientific solution against the dream of tuberculosis vaccine. Rev Med Microbiol. 2020;31(3):126–134. [Google Scholar]
- 5.Dheda K., Gumbo T., Maartens G., Dooley K.E., McNerney R., Murray M. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;5(4):291–360. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 6.Centis R., Sotgiu G., Migliori G.B. Management of extensively drug-resistant tuberculosis. Lancet Respir Med. 2017;5(4):237–239. doi: 10.1016/S2213-2600(16)30437-4. [DOI] [PubMed] [Google Scholar]
- 7.Eslami M., Shafiei M., Ghasemian A., Valizadeh S., Al-Marzoqi A.H., Shokouhi Mostafavi S.K. Mycobacterium avium paratuberculosis and Mycobacterium avium complex and related subspecies as causative agents of zoonotic and occupational diseases. J Cell Physiol. 2019;234(8):12415–12421. doi: 10.1002/jcp.28076. [DOI] [PubMed] [Google Scholar]
- 8.Williams C.J. Cod-liver oil in phthisis. Lond J Medi. 1849;1(1):1. [PMC free article] [PubMed] [Google Scholar]
- 9.Rook G.A., Steele J., Fraher L., Barker S., Karmali R., O'riordan J, Stanford J Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57(1):159. [PMC free article] [PubMed] [Google Scholar]
- 10.Martineau A.R., Timms P.M., Bothamley G.H., Hanifa Y., Islam K., Claxton A.P. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377(9761):242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammadi A., Khanbabaei H., Nasiri-Kalmarzi R., Khademi F., Jafari M., Tajik N. Vitamin D receptor ApaI (rs7975232), BsmI (rs1544410), Fok1 (rs2228570), and TaqI (rs731236) gene polymorphisms and susceptibility to pulmonary tuberculosis in an Iranian population: a systematic review and meta-analysis. J Microbiol Immunol Infect. 2019 doi: 10.1016/j.jmii.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Daley P., Jagannathan V., John K.R., Sarojini J., Latha A., Vieth R. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15(5):528–534. doi: 10.1016/S1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]
- 13.Nunes-Alves C., Booty M.G., Carpenter S.M., Jayaraman P., Rothchild A.C., Behar S.M. In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol. 2014;12(4):289–299. doi: 10.1038/nrmicro3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mily A., Rekha R.S., Kamal S.M., Arifuzzaman A.S., Rahim Z., Khan L. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PLoS ONE. 2015;10(9) doi: 10.1371/journal.pone.0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salahuddin N., Ali F., Hasan Z., Rao N., Aqeel M., Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis. 2013;13(1):22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tukvadze N., Sanikidze E., Kipiani M., Hebbar G., Easley K.A., Shenvi N. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;102(5):1059–1069. doi: 10.3945/ajcn.115.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekele A., Gebreselassie N., Ashenafi S., Kassa E., Aseffa G., Amogne W. Daily adjunctive therapy with vitamin D 3 and phenylbutyrate supports clinical recovery from pulmonary tuberculosis: a randomized controlled trial in Ethiopia. J Intern Med. 2018;284(3):292–306. doi: 10.1111/joim.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganmaa D., Munkhzul B., Fawzi W., Spiegelman D., Willett W.C., Bayasgalan P. High-dose vitamin D3 during tuberculosis treatment in Mongolia. A randomized controlled trial. Am J Respir Crit Care Med. 2017;196(5):628–637. doi: 10.1164/rccm.201705-0936OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ralph A.P., Waramori G., Pontororing G.J., Kenangalem E., Wiguna A., Tjitra E. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sloka S., Silva C., Wang J., Yong V.W. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflamm. 2011;8(1):56. doi: 10.1186/1742-2094-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolliffe D.A., Ganmaa D., Wejse C., Raqib R., Haq M.A., Salahuddin N. Adjunctive vitamin D in tuberculosis treatment: meta-analysis of individual participant data. Eur Respir J. 2019;53(3):1802003. doi: 10.1183/13993003.02003-2018. [DOI] [PubMed] [Google Scholar]
- 22.Wu H.X., Xiong X.F., Zhu M., Wei J., Zhuo K.Q., Cheng D.Y. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med. 2018;18(1):108. doi: 10.1186/s12890-018-0677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]