Abstract
Telomeres are DNA repeats at the ends of linear chromosomes and are replicated by telomerase, a ribonucleoprotein reverse transcriptase. Telomere length regulation and chromosome end capping are essential for genome stability and are mediated primarily by the shelterin and CST complexes. POT1-TPP1, a subunit of shelterin, binds the telomeric overhang, suppresses ATR-dependent DNA damage response, and recruits telomerase to telomeres for DNA replication. POT1 localization to telomeres and chromosome end protection requires its interaction with TPP1. Therefore, the POT1-TPP1 complex is critical to telomere maintenance and full telomerase processivity. The aim of this mini-review is to summarize recent POT1-TPP1 structural studies and discuss how the complex contributes to telomere length regulation. In addition, we review how disruption of POT1-TPP1 function leads to human disease.
Abbreviations: POT1, Protection of telomere 1; TPP1 also known as ACD, Adrenocortical Dysplasia Protein Homolog; TRF1, Telomere Repeat binding Factor 1; TRF2, Telomere Repeat binding Factor 2; RAP1, Repressor/Activator Protein 1; TIN2, TRF1- and TRF2-Interacting Nuclear Protein 2; TERT, Telomerase Reverse Transcriptase; TERC, Telomerase RNA; CST, CTC1, Stn1 and Ten1; SMCHD1, Structural Maintenance Of Chromosomes Flexible Hinge Domain Containing 1; CTC1, Conserved Telomere Capping Protein 1; Stn1, Suppressor of Cdc Thirteen; Ten1, Telomere Length Regulation Protein; ATR, Ataxia Telangiectasia and Rad3-related Protein; ATM, Ataxia Telangiectasia Mutated protein; RPA, Replication Protein A; TSPYL5, Testis-specific Y-encoded-like protein 5; USP7, ubiquitin-specific-processing protease 7
Keywords: Telomeres, Telomerase, Shelterin, POT1, TPP1
1. Introduction
Telomeres are tandem nucleic acid repeats, (TTAGGG in vertebrates), located at the ends of eukaryotic chromosomes [1], [2]. Telomeres are replicated by telomerase, a ribonucleoprotein reverse transcriptase. Its catalytic core consists of TERT, a reverse transcriptase, and TERC, an RNA sequence containing the template for telomere replication [3]. While TERC is expressed ubiquitously, TERT expression is tightly regulated and becomes suppressed in the somatic cells of adults. Telomeres allow cells to overcome two key problems associated with linear chromosomes: chromosome end protection and end replication. Telomeres protect chromosome ends from erroneous exonuclease degradation and DNA damage response (DDR), which result in chromosome end fusions and genomic instability [4]. Telomeres also prevent gene erosion by providing a solution to the end replication problem [5], [6], [7], [8].
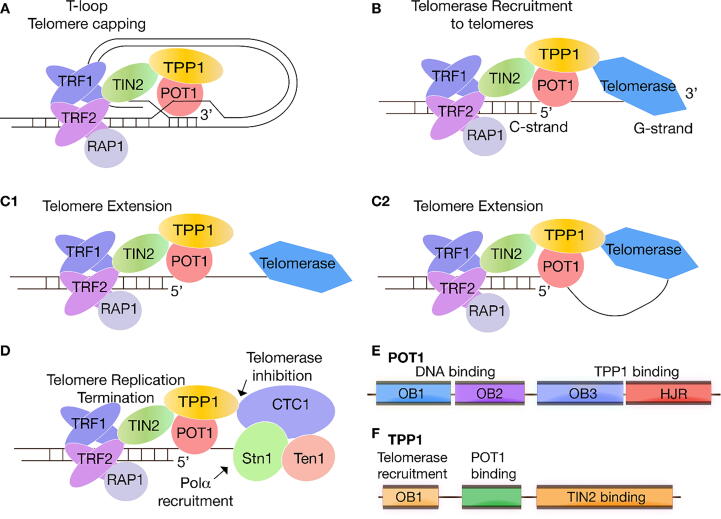
Telomere length regulation is extremely critical to organismal health because telomeres that are either too short or too long may result in genomic instability (and possibly oncogenesis). Apart from telomerase, telomere homeostasis is maintained via two separate multi-subunit nucleoprotein complexes, shelterin and CST [9], [10], [11], [12] (Fig. 1). Shelterin consists of six protein subunits: TRF1, TRF2, TIN2, TPP1 (ACD), POT1 and RAP1, which are held together in a single nucleoprotein complex by TIN2 [10], [13] (Fig. 1A-D). TIN2 makes direct contacts with TRF1, TRF2 and TPP1 [14], [15]. Current evidence suggests that TIN2 does not bind POT1 or RAP1 [13].
Fig. 1.
Schematic of shelterin and CST activities at telomeres. A. Shelterin facilitates T-loop formation at the ends of our chromosomes. B. Telomerase is recruited by the POT1-TPP1 processivity factor. C1 and C2. Two possible mechanisms of telomerase-dependent telomere extension: telomerase may have a transient interaction with TPP1 (C1) or remain tethered to TPP1 during telomere replication (C2). D. Inhibition of telomerase dependent telomere replication by the CST complex. E. POT1 contains three OB folds (OB1, OB2, OB3) and one Holliday Junction Resolvase (HJR) domain. While the N-terminal OB1 and OB2 bind ssDNA, OB3 and the HJR domain bind TPP1. F. TPP1 contains an N-terminal OB-fold involved in telomerase recruitment to telomeres, and a POT1- and TIN2-binding domains.
The shelterin complex has a plethora of functions associated with both telomere capping/end protection, and telomere length regulation. It has been demonstrated that shelterin “caps” telomeres to prevent the single-stranded telomeric overhangs from being recognized as DNA breaks. This process effectively suppresses ATM- and ATR-dependent DNA damage signaling pathways, thus preventing unwarranted activation of DNA repair mechanisms, at telomeres including classical non-homologous end joining (c-NHEJ) and homology-directed repair (HDR). Both of these processes are associated with deleterious chromosomal end-to-end fusions in shelterin knockdown studies [16], [17]. Shelterin achieves chromosome end protection from DDR in part by forming a lasso-like structure, known as the T-loop (Fig. 1A) [18], [19]. T-loops are formed through the invasion of the single-stranded G-overhang into upstream double-stranded DNA. If the G-overhang is missing or too short to form the T-loop, shelterin recruits Apollo/SNM1B to telomeres [20]. Apollo/SNM1B facilitates the formation of the G-overhang through its 5′-to-3′ DNA exonuclease activity, a process that, in turn, enables telomerase-dependent telomere replication and chromosome end capping [21], [22].
The subcomplex POT1-TPP1 is critical to telomere capping and telomere length regulation. Depletion of POT1 leads to catastrophic ATR-dependent DDR, significantly elongated telomeres, cell cycle arrest and is embryonically lethal [24], [25], [26]. TPP1 loss leads to reduced levels of POT1 at telomeres, diminished telomerase processivity, ATR-dependent DDR, and p53-dependent cell growth arrest [27], [28], [29] (Fig. 1C1, C2). Although much is known on how shelterin protects telomeres directly, recent studies have uncovered new players involved in shelterin-mediated chromosome end protection. It was recently found that SMCHD1, a protein implicated in a variety of cellular functions including DNA damage repair, promotes ATM-dependent DDR at uncapped telomeres. Current data indicates that SMCHD1 acts upstream of the ATM-dependent DDR by promoting ATM activation [23]. The recent discovery of novel shelterin partners suggests that our current understanding of telomere maintenance remains incomplete.
Telomere length is further regulated by the CST complex. CST is a heterotrimeric nucleoprotein complex composed of Stn1, Ten1 and CTC1 in higher eukaryotes [12], [30] (Fig. 1D). The main functions of CST include termination of the telomerase extension reaction [31] and recruitment of polα/primase to telomeres for C-strand fill-in [32], [33], [34], [35], [36] (Fig. 1D). CST inhibits telomerase activity potentially through sequestration of the single-stranded telomeric overhang and possibly by interfering with POT1-TPP1-dependent recruitment of telomerase to telomeres [12], [37].
Although both of these complexes function primarily at telomeres, shelterin and the CST proteins may have “moonlighting” functions. The shelterin component RAP1 has been found in extratelomeric sites and it’s been shown to protect against obesity in mice [38], [39]. This suggests it may act as a transcriptional regulator in metabolic signaling pathways. Another example, the mammalian CST complex rescues stalled DNA replication forks at non-telomeric sites. It also promotes origin firing during replication restart and some suggest it may be responsible for loading Polα/primase to these sites [40]. Nevertheless, the non-telomeric functions of the shelterin and CST complexes remain poorly understood.
Missense mutations that render shelterin and CST dysfunctional, lead to telomere length dysregulation and a host of diseases referred to as telomere syndromes or telomeropathies. These include bone marrow failure (BMF), dyskeratosis congenital (DKC), idiopathic pulmonary fibrosis (IPF), and Coats Plus (CP) [41], [42], [43], [44], [45], [46]. For example, specific mutations of POT1 or TPP1 have been identified in BMF and CP, and in a number of cancers including familial melanoma, glioma, and chronic lymphocytic leukemia as well as breast, stomach and parathyroid cancers [47], [48], [49], [50], [51], [52]. Structural data is now beginning to shed light on precisely how such mutations lead to disease [53], [54], [55].
This review aims to evaluate recent structural and functional studies of POT1-TPP1 and outline our current understanding of its role in telomere maintenance and disease.
2. POT1-TPP1 structure
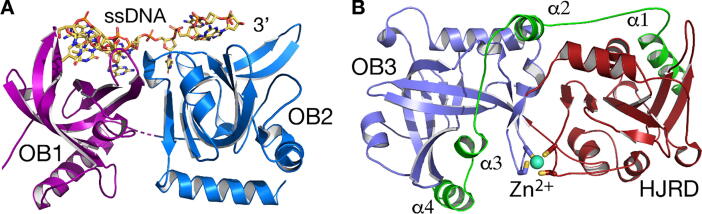
POT1 consists of four domains: three OB folds and a Holliday junction resolvase domain (HJRD) (Fig. 1E). It is the only shelterin component that binds single-stranded telomeric DNA (Fig. 1A). The two N-terminal OB folds bind the single-stranded telomeric overhang [56], [57] while the C-terminal portion binds TPP1 [54] (Figs. 1E, F and 2A, B). Interestingly, a 4-cysteine zinc binding cluster (C382, C385, C503, C506), located between the two C-terminal domains, appears to stabilize the extended conformation adopted by this portion of POT1 (Fig. 2B). It is worth noting that the POT1 HJRD, unlike other known holliday junction resolvase domains, does not bind double stranded DNA (Fig. 2B) [54], [58].
Fig. 2.
POT1 structures in complex with TPP1 or DNA. A. POT1 N-terminal 2 OB folds (OB1 and OB2) in complex with single-stranded telomeric DNA (PDB: 1XJV) B. POT1 C-terminal OB fold (OB3) and holliday junction resolvase domain (HJRD) in complex with TPP1 (green). The cysteine cluster is coordinated to a Zn2+ ion shown as blue sphere. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
POT1-TPP1 binding is mediated by the C-terminal OB-fold (OB3) and HJR domains of POT1 (PDB ID: 5UN7) (Fig. 2B). Both domains make extensive, primarily hydrophobic, interactions with the TPP1 central peptide, residues 266–332, which form an extended coil with four equally distributed α-helices (α1-4). The TPP1 peptide straddles the two POT1 domains, stretching across the length of the whole molecule. Contacts between TPP1 and the POT1 HJRD are mediated primarily by helix α1 of TPP1. Helix α2 binds at the interface of the POT1 OB3 and HJRD, while helices α3 and α4 bind the POT1 OB3 fold (Fig. 2B). Although most of the POT1-TPP1 contacts involve the four helices of TPP1, limited contacts do occur throughout the loop regions connecting these four helices.
TPP1 contains, in addition to POT1-binding domains, an N-terminal OB fold and a TIN2 binding domain at its C-terminus (Fig. 1F). The TPP1 N-terminal OB fold (PDB ID: 2I46), comprising residues 86–250, contains the ‘TEL-patch’ - a group of surface residues involved in the recruitment of telomerase to telomeres and stimulation of telomerase processivity [59]. The N-terminal tail of 86 residues is not conserved across organisms but is important for stimulating telomerase processivity in primates [29], [60], [61]. The C-terminal region of TPP1 contains a short sequence of residues (510–540) shown to be necessary and sufficient for TIN2 binding [62]. In fact, recent evidence shows that TIN2-TPP1 binding leads to increased telomerase activity, suggesting TIN2 cooperates with the POT1-TPP1 complex to stimulate telomerase processivity [15].
3. The POT1-TPP1 complex is highly specific for single-stranded telomeric DNA
POT1 binds the telomeric overhang with high affinity in a specific manner, a process mediated by its two N-terminal OB folds. The OB fold nearest the N-terminus binds six-nucleotides while OB2 binds 3 nucleotides. POT1 ssDNA binding, positions the 3′-end of the DNA within the canonical binding pocket of OB2, thus protecting it from exonuclease degradation as well as from access by telomerase for telomere replication. The affinity of human POT1 for telomeric DNA is in the range of 10–20 nM [54], [56], [57] and is enhanced by TPP1 binding. This could be attributed to the fact that TPP1 tethers POT1 to the shelterin complex and therefore places POT1 in proximity to the telomeric overhang.
The ability of POT1 to bind single-stranded telomeric DNA with high affinity and specificity also allows it to discriminate against TERRA, which would deplete POT1 binding to the telomeric overhang and risk telomere exposure. TERRA is the single-stranded RNA transcribed from telomeric DNA and therefore could contain multiple POT1 binding sites [63], [64], [65]. The specificity of POT1 for ssDNA over ssRNA is attributed to the substitution of the second deoxythymidine nucleotide in the 5′-TTAGGG-3′ repeat for ribouridine in TERRA [65]. Interestingly, TPP1 binding to POT1 enhances POT1′s specificity for DNA over RNA [65].
4. POT1 suppresses DNA damage response
The ends of eukaryotic chromosomes can be recognized as DNA strand breaks. DNA breaks induce cell cycle arrest and activate DNA repair via ATM- and ATR-dependent signaling pathways, which involve NHEJ and HDR mechanisms [1], [4]. NHEJ results in chromosome end-to-end fusions [1], [16], [66], [67], [68], [69] while HDR results in telomere sister chromatid exchanges, loss of telomeric DNA, and the formation of unstable telomere-free chromosome ends [70], [71]. Eukaryotic chromosome ends avoid recombination mechanisms through the shelterin mediated formation of the T-loop. T-loop formation is facilitated by the shelterin complex, and most recently it was shown that TRF2 alone is sufficient for this process [1], [16], [72].
T-loop formation involves the invasion of the single-stranded overhang into upstream double stranded telomeric DNA. Apollo, recruited to telomeres by TRF2 [21], uses its exonuclease activity to process telomeres immediately after replication, generating the 3′ single-stranded overhang. In doing so, it promotes T-loop formation as well as telomerase-dependent telomere replication. It also eliminates blunt-end telomeres (that activate DDR) and promotes POT1 binding [73] thus preventing ATR-dependent DDR [74]. ATR signaling is activated by the binding of the replication protein A (RPA) heterotrimer to the single-stranded DNA [75]. POT1 suppresses ATR signaling by directly outcompeting RPA for binding to the telomeric overhang [76]. TPP1 facilitates POT1 recruitment to telomeres and increases its affinity for ssDNA ten-fold [29], which could explain why TPP1 knockdown results in ATR-dependent DDR.
5. POT1-TPP1 is a telomerase processivity factor
The POT1-TPP1 complex plays a critical role in telomere length regulation. POT1 inhibits telomere extension by sequestering the telomeric overhang, thus preventing access of telomerase to telomeres. POT1-TPP1 together facilitate telomere extension by recruiting of telomerase to telomeres for DNA synthesis [29], [59], [77]. POT1-TPP1 therefore acts like a switch ether promoting or inhibiting telomerase activity. It is worth noting that short telomeres are preferentially extended by telomerase [78]. Long telomeres are coated with a large number of POT1-TPP1 complexes which render the telomeric overhang inaccessible to telomerase thus inhibiting telomerase-dependent telomere extension [79]. These results may suggest a model whereby POT1-TPP1 recruits and stimulates telomerase recruitment during late S-phase triggering telomere extension. After telomere elongation, a switch may occur leading to telomerase inhibition.
POT1-TPP1 dependent telomerase recruitment to telomeres involves contacts between the TEL-patch and the N-terminal domain of TERT (TEN) [77]. Interestingly, recent evidence shows that the ubiquitin-specific-processing protease 7 (USP7) also interacts with the same TPP1 N-terminal OB fold. The proximity of the USP7 and telomerase binding sites on TPP1 suggests a possible regulatory mechanism of telomerase access to telomeres [80]. The facts that POT1-TPP1 binding to ssDNA decreases the dissociation of the ssDNA from telomerase [81] and that the presence of POT1-TPP1 during telomere replication increases the rate of telomerase translocation provide additional evidence supporting this mechanism [81]. Another recent study shows that TIN2 cooperates with POT1-TPP1 to stimulate telomerase processivity [15]. This finding is not surprising since TIN2 interacts with TPP1 and TPP1 binds POT1, thus TIN2 assists with the localization of the POT1-TPP1 complex to telomeres [15]. It remains to be seen whether TIN2 also increases telomerase processivity by stabilizing the POT1-TPP1 interaction with TERT.
6. POT1-TPP1 and cancer
Telomere elongation is crucial to the continued division and survival of cancer cells [82], [83], [84]. In somatic cells, telomerase is inactive and telomeres get shorter with each round of cell division, eventually triggering replicative senescence [85], [86], [87]. Cells that have lost tumor suppressor pathways, such as p53, may bypass senescence and continue proliferating, further shortening telomeres to a critical length [88], [89]. At this ‘crisis’ stage, cells exhibit chromosome recombination and genomic instability, leading to extensive cell death. Rarely, a cell may escape crisis by reactivating telomerase or, in some cases, via homologous recombination based telomere replication, known as the ALT mechanism [90], [91].
Mutations in the shelterin complex that lead to telomere dysfunction and dysregulation are prevalent in cancer [92]. POT1-TPP1 mutations are implicated in multiple cancer types including melanoma, glioma, and chronic lymphocytic leukemia (CLL) [51], [52], [93], [94], [95], [96]. Recent studies have shown that POT1 mutations are also found in breast, stomach and parathyroid cancers [50], [97], [98]. Missense mutations at the N-terminal two OB folds of POT1, such as Y36N, Y89C, Q94G, Y223C, and R273L, render POT1 unable to bind telomeric ssDNA [49], [51], [52] (Fig. 3A). The crystal structure of POT1 with telomeric ssDNA shows Y89 and Y223 engaged in pi-stacking interactions with guanine nucleotides of the telomeric DNA that would be disrupted by mutation to a non-aromatic residue (PDB: 1XJV – Fig. 3A). Y36, Q94, and R273 make electrostatic interactions with the telomeric DNA. The much shorter, non-polar or hydrophobic side chains of the mutant Y36N, Q94G, and R273L lead to complete loss of interaction with the ssDNA (Fig. 3A). The biological consequences of CLL-associated Y36N and Y223C were observed in human fibrosarcoma cells expressing the POT1 variants. These cells exhibited chromosomal abnormalities associated with telomere uncapping, such as irregular telomere length, end-to-end fusions, and telomere fragility [49].
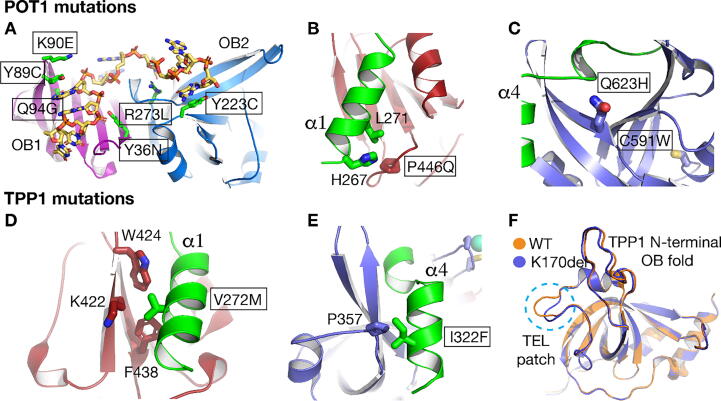
Fig. 3.
Structures of POT1-DNA and POT1-TPP1 showing selected mutations implicated in cancer and telomere syndromes. A. Structure of the POT1-N-terminal OB folds in complex with single-stranded telomeric DNA (PDB ID: 1XJV). The telomere syndrome mutation K90E is shown in stick. Mutations described in detail in the text are denoted by boxes. B. Holliday Junction resolvase domain (HJRD – red) of POT1 in complex with helix a1 of the TPP1 peptide (green). The cancer mutation P446Q and the TPP1 interacting residues H267 and L271 are shown in stick. C. POT1 (OB3) (blue) in complex with TPP1 (green) showing the cancer mutations C591W and Q623H. C591 is buried and contributes to the fold of the protein. In the C591W mutant, the much larger tryptophan side chain would perturb the fold of the OB fold. Q623 makes direct contact with the amide backbone of the TPP1 peptide. D. POT1 (HJRD) (red) in complex with TPP1 (green) showing TPP1 V272 and its interactions with POT1 K422, W424 and F438. E. I322 interacts with the P357 of the POT1 (OB3). F. Overlay of wild type (WT – PDB ID: 2I46) TPP1 and K170 deletion (K170del PDB ID: 5I2Y and 5I2X) of the N-terminal TPP1 OB fold. The region of the K170del in the TPP1 structure is highlighted with a blue dashed circle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To this end, research from our lab and others (PDB ID: 5UN7, 5H65 – Fig. 3B and C) shows how several of the reported cancer mutations that localize to the C-terminal portion of POT1 (POT1C) disrupt the POT1-TPP1 complex [54]. Several of these mutations directly alter the ability of POT1 to bind TPP1, while others are likely to disrupt the fold of the protein. In our study, we specifically interrogated the POT1C cancer mutations L343F, P446Q, P475L, R477T, A532P, I535F, C591W and Q623H found in patients with either familial glioma, melanoma, or CLL [49], [52]. Three of these mutations, P446Q, C591W, and Q623H (Fig. 3B and C), exhibited a reduction in POT1C-TPP1 binding, consistent with the structural data showing that these residues made direct contacts with TPP1. Biochemical studies show that these mutant proteins bind telomeric DNA with lower affinity than the wild type POT1 [79]. A decrease in DNA binding is expected to translate into defective telomere capping and an increase in DDR. In addition, defective DNA binding will most likely lead to an increase in telomere length due to persistent telomere replication by telomerase. Long telomeres are fragile and tend to collapse, generating telomere free ends. Cells with telomere free ends are prone to senescence, apoptosis and, in rare cases, genomic instability leading to cancer.
POT1 is also implicated in the ALT mechanism utilized by cancer cells (primarily osteosarcomas) to maintain their telomeres through homologous recombination [99], [100], [101], [102]. Promyelocytic leukemia (PML) nuclear bodies cluster around ALT telomeres and are directly involved in telomere recombination [103], [104]. In addition, shelterin and DNA repair proteins (TRF1, TRF2, TIN2, RAP1, MRE11, RAD50, NBS1) are required for the formation of ALT-associated PML bodies (APBs) at telomeres [105]. Testis-specific Y-encoded-like protein 5 (TSPYL5) was recently identified as another component of the PML body crucial to the viability of ALT+ cells [106]. TSPYL5 prevents POT1 proteasomal degradation in ALT cells by inhibiting USP7-dependet POT1 deubiquitylation [106]. The fact that TSPYL5 is essential to POT1 protection and the survival of ALT+ cells make it a promising target for ALT+ cancer therapies [106].
Regarding TPP1 mutations, a study on familial melanoma found that mutations V272M and I322F located in the POT1-binding domain (Fig. 3D and E) were also associated with the disease [93].
7. POT1-TPP1 and telomere syndromes
POT1 mutants implicated in telomere syndromes are defective in efficient chromosome end capping and telomerase recruitment to telomeres. Both of these defects lead to significantly short telomeres and, in some cases, telomere free ends. In cells with high turnover, such as bone marrow, short telomeres lead to premature senescence and are associated with telomeropathies.
There are four known mutations within the POT1-TPP1 complex implicated in the telomere syndromes BMF, IPF, and CP. These include the TPP1 A72E, K170E and K170Δ [107], [108], [109] and the POT1 K90E and S322L mutants [110]. A72E is located at the N-terminal 86 residues of TPP1. Structural data on this region of TPP1 is lacking, however we can speculate that A72E affects telomerase processivity in primates. K170 is located away from the canonical binding pocket of the N-terminal TPP1 OB fold and forms part of the TEL-patch (Fig. 3F). The K70 deletion (K70Δ) distorts the location and organization of adjacent amino acids (Fig. 3F) resulting in reduced telomerase processivity at telomeres [107].
The POT1 K90E mutation (Fig. 3A) localizes to the very N-terminal OB fold of POT1 and at the tip of the indentation comprising the canonical OB pocket. Although this pocket is involved in DNA binding, K90 is not (Fig. 3A). Although K90E does not appear to affect POT1-TPP1 or POT1-ssDNA binding, one study found that it did affect CST complex localization to telomeres [111]. This observation raises questions about a possible cooperation between POT1-TPP1 and the CST complex. In mice, the POT1b paralog recruits the CST complex to telomeres [24], [73], [112], [113] and, in humans, interaction between POT1 and the CST complex has been reported [31], [114]. However, direct binding between POT1 and CST has yet to be shown and the details of this mechanism remain unclear.
The POT1 mutant S322L is located at the intersection of the DNA and TPP1 binding domains of POT1 but its precise role remains unknown [115]. Identified in siblings with CP, the POT1 S322L mutant was expressed at normal levels, bound TPP1, and suppressed ATR-dependent DDR. However, the S322L POT1 mutant failed to regulate telomerase, resulting in extended telomeric overhangs, defective C-strand fill-in, and telomere truncations [115]. The current hypothesis is that mutations like S322L may interfere with possible cooperation between shelterin and CST [115], presumably needed to maintain a healthy length of telomeres. Structural and secondary structure prediction analysis suggest that S322L is located in the linker connecting the N- and C-terminal portions of POT1. It is common for unstructured loop regions of proteins to contain serine or threonine residues that are post-translationally modified. One, possibility is that S322 is a phosphorylation site and that the leucine mutant disrupts this process.
8. Summary and outlook
Apart from telomerase, telomere length regulation has been attributed to two major protein complexes, shelterin and CST. In depth understanding of the function of these complexes may hold the key to understanding and halting disease progression. A plethora of work from multiple labs has shed light into the functions of POT1-TPP1. Despite the wealth of data, there are still significant questions that remain to be addressed. For example, we still do not fully understand how the full length POT1-TPP1-DNA complex assembles at telomeres. Structural elucidation of the full length complex will be invaluable to our understanding of how these two proteins carry out their functions. Similarly, evidence now suggests that there is crosstalk between shelterin and CST. Undertesting how these two complexes cooperate to regulate telomerase and telomere homeostasis will be invaluable. Finally, understanding how the POT1-TPP1 mutations lead to cancer and telomere syndromes will be instrumental in our effort to identify and tailor therapies for these human diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The research was funded by the NIH grant R01CA201312 and the Wistar Cancer Center Support Grant P30 CA10815.
Author statement
The review was written by TA and ES with contributions from SP.
References
- 1.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326(5955):948–952. doi: 10.1126/science.1170633. Epub 2009/12/08. doi: 10.1126/science.1170633. PubMed PMID: 19965504; PMCID: PMC2819049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyne J., Ratliff R.L., Moyzis R.K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci USA. 1989;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. Epub 1989/09/01. doi: 10.1073/pnas.86.18.7049. PubMed PMID: 2780561; PMCID: PMC297991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shampay J., Blackburn E.H. Generation of telomere-length heterogeneity in Saccharomyces cerevisiae. Proc Natl Acad Sci. 1988;85(2):534–538. doi: 10.1073/pnas.85.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palm W., de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. Epub 2008/08/06. doi: 10.1146/annurev.genet.41.110306.130350. PubMed PMID: 18680434. [DOI] [PubMed] [Google Scholar]
- 5.Cech T.R., Lingner J. Telomerase and the chromosome end replication problem. Ciba Found Symp. 1997;211:20–28. doi: 10.1002/9780470515433.ch3. Epub 1997/01/01. doi: 10.1002/9780470515433.ch3. PubMed PMID: 9524749. [DOI] [PubMed] [Google Scholar]
- 6.Lingner J., Cooper J.P., Cech T.R. Telomerase and DNA end replication: no longer a lagging strand problem? Science. 1995;269(5230):1533–1534. doi: 10.1126/science.7545310. Epub 1995/09/15. doi: 10.1126/science.7545310. PubMed PMID: 7545310. [DOI] [PubMed] [Google Scholar]
- 7.Cech T.R. Beginning to understand the end of the chromosome. Cell. 2004;116(2):273–279. doi: 10.1016/s0092-8674(04)00038-8. Epub 2004/01/28. doi: 10.1016/s0092-8674(04)00038-8. PubMed PMID: 14744437. [DOI] [PubMed] [Google Scholar]
- 8.Shay J.W., Wright W.E. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72–76. doi: 10.1038/35036093. Epub 2001/06/20. doi: 10.1038/35036093. PubMed PMID: 11413492. [DOI] [PubMed] [Google Scholar]
- 9.Bandaria J.N., Qin P., Berk V., Chu S., Yildiz A. Shelterin protects chromosome ends by compacting telomeric chromatin. Cell. 2016;164(4):735–746. doi: 10.1016/j.cell.2016.01.036. PubMed PMID: 26871633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):2100–2110. doi: 10.1101/gad.1346005. PubMed PMID: 16166375. [DOI] [PubMed] [Google Scholar]
- 11.Giraud-Panis M.J., Teixeira M.T., Geli V., Gilson E. CST meets shelterin to keep telomeres in check. Mol Cell. 2010;39(5):665–676. doi: 10.1016/j.molcel.2010.08.024. Epub 2010/09/14. doi: 10.1016/j.molcel.2010.08.024. PubMed PMID: 20832719. [DOI] [PubMed] [Google Scholar]
- 12.Rice C., Skordalakes E. Structure and function of the telomeric CST complex. Comput Struct Biotechnol J. 2016;14:161–167. doi: 10.1016/j.csbj.2016.04.002. PubMed PMID: 27239262; PMCID: PMC4872678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor M.S., Safari A., Xin H., Liu D., Songyang Z. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc Natl Acad Sci U S A. 2006;103(32):11874–11879. doi: 10.1073/pnas.0605303103. Epub 2006/08/02. doi: 10.1073/pnas.0605303103. PubMed PMID: 16880378; PMCID: PMC1567669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank A.K., Tran D.C., Qu R.W., Stohr B.A., Segal D.J., Xu L. The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres. PLoS Genet. 2015;11(7) doi: 10.1371/journal.pgen.1005410. Epub 2015/08/01. doi: 10.1371/journal.pgen.1005410. PubMed PMID: 26230315; PMCID: PMC4521702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pike A.M., Strong M.A., Ouyang J.P.T., Greider C.W. TIN2 Functions with TPP1/POT1 To Stimulate Telomerase Processivity. Mol Cell Biol. 2019;39(21) doi: 10.1128/MCB.00593-18. Epub 2019/08/07. doi: 10.1128/MCB.00593-18. PubMed PMID: 31383750; PMCID: PMC6791651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denchi E.L., de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448(7157):1068–1071. doi: 10.1038/nature06065. Epub 2007/08/10. doi: nature06065 [pii] 10.1038/nature06065. PubMed PMID: 17687332. [DOI] [PubMed] [Google Scholar]
- 17.Tong A.S., Stern J.L., Sfeir A., Kartawinata M., de Lange T., Zhu X.D. ATM and ATR Signaling Regulate the Recruitment of Human Telomerase to Telomeres. Cell Rep. 2015;13(8):1633–1646. doi: 10.1016/j.celrep.2015.10.041. Epub 2015/11/21. doi: 10.1016/j.celrep.2015.10.041. PubMed PMID: 26586433; PMCID: PMC4662887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Lange T. T-loops and the origin of telomeres. Nat Rev Mol Cell Biol. 2004;5(4):323–329. doi: 10.1038/nrm1359. PubMed PMID: 15071557. [DOI] [PubMed] [Google Scholar]
- 19.Doksani Y., Wu J.Y., de Lange T., Zhuang X. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell. 2013;155(2):345–356. doi: 10.1016/j.cell.2013.09.048. PubMed PMID: 24120135; PMCID: PMC4062873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touzot F., Callebaut I., Soulier J., Gaillard L., Azerrad C., Durandy A. Function of Apollo (SNM1B) at telomere highlighted by a splice variant identified in a patient with Hoyeraal-Hreidarsson syndrome. Proc Natl Acad Sci U S A. 2010;107(22):10097–10102. doi: 10.1073/pnas.0914918107. Epub 2010/05/19. doi: 10.1073/pnas.0914918107. PubMed PMID: 20479256; PMCID: PMC2890423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Overbeek M., de Lange T. Apollo, an Artemis-related nuclease, interacts with TRF2 and protects human telomeres in S phase. Curr Biol. 2006;16(13):1295–1302. doi: 10.1016/j.cub.2006.05.022. Epub 2006/05/30. doi: 10.1016/j.cub.2006.05.022. PubMed PMID: 16730176. [DOI] [PubMed] [Google Scholar]
- 22.Wu P., van Overbeek M., Rooney S., de Lange T. Apollo contributes to G overhang maintenance and protects leading-end telomeres. Mol Cell. 2010;39(4):606–617. doi: 10.1016/j.molcel.2010.06.031. Epub 2010/07/14. doi: 10.1016/j.molcel.2010.06.031. PubMed PMID: 20619712; PMCID: PMC2929323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vancevska A., Ahmed W., Pfeiffer V., Feretzaki M., Boulton S.J., Lingner J. SMCHD1 promotes ATM-dependent DNA damage signaling and repair of uncapped telomeres. EMBO J. 2020:e102668. doi: 10.15252/embj.2019102668. Epub 2020/02/23. doi: 10.15252/embj.2019102668. PubMed PMID: 32080884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hockemeyer D., Daniels J.P., Takai H., de Lange T. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126(1):63–77. doi: 10.1016/j.cell.2006.04.044. Epub 2006/07/15. doi: 10.1016/j.cell.2006.04.044. PubMed PMID: 16839877. [DOI] [PubMed] [Google Scholar]
- 25.Wu L., Multani A.S., He H., Cosme-Blanco W., Deng Y., Deng J.M. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126(1):49–62. doi: 10.1016/j.cell.2006.05.037. Epub 2006/07/15. doi: 10.1016/j.cell.2006.05.037. PubMed PMID: 16839876. [DOI] [PubMed] [Google Scholar]
- 26.Guo X., Deng Y., Lin Y., Cosme-Blanco W., Chan S., He H. Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J. 2007;26(22):4709–4719. doi: 10.1038/sj.emboj.7601893. Epub 2007/10/20. doi: 10.1038/sj.emboj.7601893. PubMed PMID: 17948054; PMCID: PMC2080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Wang X., Flores E.R., Yu J., Chang S. Dysfunctional telomeres induce p53-dependent and independent apoptosis to compromise cellular proliferation and inhibit tumor formation. Aging Cell. 2016;15(4):646–660. doi: 10.1111/acel.12476. Epub 2016/04/27. doi: 10.1111/acel.12476. PubMed PMID: 27113195; PMCID: PMC4933665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kibe T., Zimmermann M., de Lange T. TPP1 Blocks an ATR-Mediated Resection Mechanism at Telomeres. Mol Cell. 2017;66(2):300. doi: 10.1016/j.molcel.2017.04.004. Epub 2017/04/22. doi: 10.1016/j.molcel.2017.04.004. PubMed PMID: 28431234. [DOI] [PubMed] [Google Scholar]
- 29.Wang F., Podell E.R., Zaug A.J., Yang Y., Baciu P., Cech T.R. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445(7127):506–510. doi: 10.1038/nature05454. Epub 2007/01/24. doi: 10.1038/nature05454. PubMed PMID: 17237768. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J., Chan H., Cash D.D., Miracco E.J., Ogorzalek Loo R.R., Upton H.E. Structure of Tetrahymena telomerase reveals previously unknown subunits, functions, and interactions. Science. 2015;350(6260):aab4070. doi: 10.1126/science.aab4070. Epub 2015/10/17. doi: 10.1126/science.aab4070. PubMed PMID: 26472759; PMCID: PMC4687456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L.Y., Redon S., Lingner J. The human CST complex is a terminator of telomerase activity. Nature. 2012 doi: 10.1038/nature11269. [DOI] [PubMed] [Google Scholar]
- 32.Feng X., Hsu S.J., Bhattacharjee A., Wang Y., Diao J., Price C.M. CTC1-STN1 terminates telomerase while STN1-TEN1 enables C-strand synthesis during telomere replication in colon cancer cells. Nat Commun. 2018;9(1):2827. doi: 10.1038/s41467-018-05154-z. Epub 2018/07/22. doi: 10.1038/s41467-018-05154-z. PubMed PMID: 30026550; PMCID: PMC6053418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng X., Hsu S.J., Kasbek C., Chaiken M., Price C.M. CTC1-mediated C-strand fill-in is an essential step in telomere length maintenance. Nucleic Acids Res. 2017 doi: 10.1093/nar/gkx125. PubMed PMID: 28334750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu P., Jia S., Takasugi T., Smith E., Nandakumar J., Hendrickson E. CTC1-STN1 coordinates G- and C-strand synthesis to regulate telomere length. Aging Cell. 2018;17(4) doi: 10.1111/acel.12783. Epub 2018/05/19. doi: 10.1111/acel.12783. PubMed PMID: 29774655; PMCID: PMC6052479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C., Dai X., Chai W. Human Stn1 protects telomere integrity by promoting efficient lagging-strand synthesis at telomeres and mediating C-strand fill-in. Cell Res. 2012;22(12):1681–1695. doi: 10.1038/cr.2012.132. PubMed PMID: 22964711; PMCID: PMC3515754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F., Stewart J.A., Kasbek C., Zhao Y., Wright W.E., Price C.M. Human CST has independent functions during telomere duplex replication and C-strand fill-in. Cell Rep. 2012;2(5):1096–1103. doi: 10.1016/j.celrep.2012.10.007. PubMed PMID: 23142664; PMCID: PMC3513692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L.Y., Redon S., Lingner J. The human CST complex is a terminator of telomerase activity. Nature. 2012;488(7412):540–544. doi: 10.1038/nature11269. Epub 2012/07/06. doi: 10.1038/nature11269. PubMed PMID: 22763445. [DOI] [PubMed] [Google Scholar]
- 38.Martinez P., Gomez-Lopez G., Garcia F., Mercken E., Mitchell S., Flores J.M. RAP1 protects from obesity through its extratelomeric role regulating gene expression. Cell Rep. 2013;3(6):2059–2074. doi: 10.1016/j.celrep.2013.05.030. Epub 2013/06/26. doi: 10.1016/j.celrep.2013.05.030. PubMed PMID: 23791526; PMCID: PMC5889507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeung F., Ramirez C.M., Mateos-Gomez P.A., Pinzaru A., Ceccarini G., Kabir S. Nontelomeric role for Rap1 in regulating metabolism and protecting against obesity. Cell Rep. 2013;3(6):1847–1856. doi: 10.1016/j.celrep.2013.05.032. Epub 2013/06/26. doi: 10.1016/j.celrep.2013.05.032. PubMed PMID: 23791522; PMCID: PMC5523811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart J.A., Wang F., Chaiken M.F., Kasbek C., Chastain P.D., 2nd, Wright W.E. Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J. 2012;31(17):3537–3549. doi: 10.1038/emboj.2012.215. Epub 2012/08/07. doi: 10.1038/emboj.2012.215. PubMed PMID: 22863775; PMCID: PMC3433780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aravinthan A.D., Alexander G.J. Telomere, telomerase and liver disease. Liver Int. 2018;38(1):33–34. doi: 10.1111/liv.13630. Epub 2017/12/23. doi: 10.1111/liv.13630. PubMed PMID: 29272567. [DOI] [PubMed] [Google Scholar]
- 42.Bernardes de Jesus B., Blasco M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013;29(9):513–520. doi: 10.1016/j.tig.2013.06.007. Epub 2013/07/24. doi: 10.1016/j.tig.2013.06.007. PubMed PMID: 23876621; PMCID: PMC3896987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blackburn E.H., Epel E.S., Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–1198. doi: 10.1126/science.aab3389. Epub 2016/01/20. doi: 10.1126/science.aab3389. PubMed PMID: 26785477. [DOI] [PubMed] [Google Scholar]
- 44.Carneiro M.C., de Castro I.P., Ferreira M.G. Telomeres in aging and disease: lessons from zebrafish. Dis Model Mech. 2016;9(7):737–748. doi: 10.1242/dmm.025130. Epub 2016/08/03. doi: 10.1242/dmm.025130. PubMed PMID: 27482813; PMCID: PMC4958310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiodi I., Mondello C. Telomere and telomerase stability in human diseases and cancer. Front Biosci (Landmark Ed). 2016;21:203–224. doi: 10.2741/4385. Epub 2015/12/29. doi: 10.2741/4385. PubMed PMID: 26709770. [DOI] [PubMed] [Google Scholar]
- 46.Heidenreich B., Kumar R. TERT promoter mutations in telomere biology. Mutat Res. 2017;771:15–31. doi: 10.1016/j.mrrev.2016.11.002. Epub 2017/03/28. doi: 10.1016/j.mrrev.2016.11.002. PubMed PMID: 28342451. [DOI] [PubMed] [Google Scholar]
- 47.Bagcchi S. POT1: a genetic link for familial glioma. Lancet Oncol. 2015;16(1) doi: 10.1016/S1470-2045(14)71178-7. Epub 2014/12/20. doi: 10.1016/S1470-2045(14)71178-7. PubMed PMID: 25524796. [DOI] [PubMed] [Google Scholar]
- 48.Calvete O., Martinez P., Garcia-Pavia P., Benitez-Buelga C., Paumard-Hernandez B., Fernandez V. A mutation in the POT1 gene is responsible for cardiac angiosarcoma in TP53-negative Li-Fraumeni-like families. Nat Commun. 2015;6:8383. doi: 10.1038/ncomms9383. Epub 2015/09/26. doi: 10.1038/ncomms9383. PubMed PMID: 26403419; PMCID: PMC4598567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramsay A.J., Quesada V., Foronda M., Conde L., Martinez-Trillos A., Villamor N. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat Genet. 2013;45(5):526–530. doi: 10.1038/ng.2584. PubMed PMID: 23502782. [DOI] [PubMed] [Google Scholar]
- 50.Richard M.A., Lupo P.J., Morton L.M., Yasui Y.A., Sapkota Y.A., Arnold M.A. Genetic variation in POT1 and risk of thyroid subsequent malignant neoplasm: a report from the Childhood Cancer Survivor Study. PLoS ONE. 2020;15(2) doi: 10.1371/journal.pone.0228887. Epub 2020/02/11. doi: 10.1371/journal.pone.0228887. PubMed PMID: 32040538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robles-Espinoza C.D., Harland M., Ramsay A.J., Aoude L.G., Quesada V., Ding Z. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet. 2014;46(5):478–481. doi: 10.1038/ng.2947. Epub 2014/04/02. doi: 10.1038/ng.2947. PubMed PMID: 24686849; PMCID: PMC4266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi J., Yang X.R., Ballew B., Rotunno M., Calista D., Fargnoli M.C. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat Genet. 2014;46(5):482–486. doi: 10.1038/ng.2941. PubMed PMID: 24686846; PMCID: PMC4056593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman H., Rice C., Skordalakes E. Structural analysis reveals the deleterious effects of telomerase mutations in bone marrow failure syndromes. J Biol Chem. 2017;292(11):4593–4601. doi: 10.1074/jbc.M116.771204. PubMed PMID: 28154186; PMCID: PMC5377775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rice C., Shastrula P.K., Kossenkov A.V., Hills R., Baird D.M., Showe L.C. Structural and functional analysis of the human POT1-TPP1 telomeric complex. Nat Commun. 2017;8:14928. doi: 10.1038/ncomms14928. PubMed PMID: 28393830; PMCID: PMC5394233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shastrula P.K., Rice C.T., Wang Z., Lieberman P.M., Skordalakes E. Structural and functional analysis of an OB-fold in human Ctc1 implicated in telomere maintenance and bone marrow syndromes. Nucleic Acids Res. 2018;46(2):972–984. doi: 10.1093/nar/gkx1213. Epub 2017/12/12. doi: 10.1093/nar/gkx1213. PubMed PMID: 29228254; PMCID: PMC5778599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loayza D., Parsons H., Donigian J., Hoke K., de Lange T. DNA binding features of human POT1: a nonamer 5'-TAGGGTTAG-3' minimal binding site, sequence specificity, and internal binding to multimeric sites. J Biol Chem. 2004;279(13):13241–13248. doi: 10.1074/jbc.M312309200. PubMed PMID: 14715659. [DOI] [PubMed] [Google Scholar]
- 57.Lei M., Podell E.R., Cech T.R. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol. 2004;11(12):1223–1229. doi: 10.1038/nsmb867. PubMed PMID: 15558049. [DOI] [PubMed] [Google Scholar]
- 58.Chen C., Gu P., Wu J., Chen X., Niu S., Sun H. Structural insights into POT1-TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat Commun. 2017;8:14929. doi: 10.1038/ncomms14929. PubMed PMID: 28393832; PMCID: PMC5394241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nandakumar J., Bell C.F., Weidenfeld I., Zaug A.J., Leinwand L.A., Cech T.R. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature. 2012;492(7428):285–289. doi: 10.1038/nature11648. PubMed PMID: 23103865; PMCID: PMC3521872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grill S., Bisht K., Tesmer V.M., Shami A.N., Hammoud S.S., Nandakumar J. Two Separation-of-Function Isoforms of Human TPP1 Dictate Telomerase Regulation in Somatic and Germ Cells. Cell Rep. 2019;27(12):3511–3521. doi: 10.1016/j.celrep.2019.05.073. e7. Epub 2019/06/20. doi: 10.1016/j.celrep.2019.05.073. PubMed PMID: 31216472; PMCID: PMC6599638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grill S., Tesmer V.M., Nandakumar J. The N Terminus of the OB Domain of Telomere Protein TPP1 Is Critical for Telomerase Action. Cell Rep. 2018;22(5):1132–1140. doi: 10.1016/j.celrep.2018.01.012. Epub 2018/02/02. doi: 10.1016/j.celrep.2018.01.012. PubMed PMID: 29386102; PMCID: PMC5815312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu C., Rai R., Huang C., Broton C., Long J., Xu Y. Structural and functional analyses of the mammalian TIN2-TPP1-TRF2 telomeric complex. Cell Res. 2017;27(12):1485–1502. doi: 10.1038/cr.2017.144. Epub 2017/11/22. doi: 10.1038/cr.2017.144. PubMed PMID: 29160297; PMCID: PMC5717407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azzalin C.M., Reichenbach P., Khoriauli L., Giulotto E., Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318(5851):798–801. doi: 10.1126/science.1147182. Epub 2007/10/06. doi: 10.1126/science.1147182. PubMed PMID: 17916692. [DOI] [PubMed] [Google Scholar]
- 64.Luke B., Lingner J. TERRA: telomeric repeat-containing RNA. EMBO J. 2009;28(17):2503–2510. doi: 10.1038/emboj.2009.166. Epub 2009/07/25. doi: 10.1038/emboj.2009.166. PubMed PMID: 19629047; PMCID: PMC2722245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nandakumar J., Podell E.R., Cech T.R. How telomeric protein POT1 avoids RNA to achieve specificity for single-stranded DNA. Proc Natl Acad Sci U S A. 2010;107(2):651–656. doi: 10.1073/pnas.0911099107. Epub 2010/01/19. doi: 10.1073/pnas.0911099107. PubMed PMID: 20080730; PMCID: PMC2796979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pardo B., Marcand S. Rap1 prevents telomere fusions by nonhomologous end joining. EMBO J. 2005;24(17):3117–3127. doi: 10.1038/sj.emboj.7600778. Epub 2005/08/13. doi: 10.1038/sj.emboj.7600778. PubMed PMID: 16096640; PMCID: PMC1201357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riha K., Heacock M.L., Shippen D.E. The role of the nonhomologous end-joining DNA double-strand break repair pathway in telomere biology. Annu Rev Genet. 2006;40:237–277. doi: 10.1146/annurev.genet.39.110304.095755. Epub 2006/07/11. doi: 10.1146/annurev.genet.39.110304.095755. PubMed PMID: 16822175. [DOI] [PubMed] [Google Scholar]
- 68.Chan S.W., Blackburn E.H. Telomerase and ATM/Tel1p protect telomeres from nonhomologous end joining. Mol Cell. 2003;11(5):1379–1387. doi: 10.1016/s1097-2765(03)00174-6. Epub 2003/05/29. doi: 10.1016/s1097-2765(03)00174-6. PubMed PMID: 12769860. [DOI] [PubMed] [Google Scholar]
- 69.Mieczkowski P.A., Mieczkowska J.O., Dominska M., Petes T.D. Genetic regulation of telomere-telomere fusions in the yeast Saccharomyces cerevisae. Proc Natl Acad Sci U S A. 2003;100(19):10854–10859. doi: 10.1073/pnas.1934561100. Epub 2003/09/10. doi: 10.1073/pnas.1934561100. PubMed PMID: 12963812; PMCID: PMC196892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rai R., Chen Y., Lei M., Chang S. TRF2-RAP1 is required to protect telomeres from engaging in homologous recombination-mediated deletions and fusions. Nat Commun. 2016;7:10881. doi: 10.1038/ncomms10881. Epub 2016/03/05. doi: 10.1038/ncomms10881. PubMed PMID: 26941064; PMCID: PMC4785230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bailey S.M., Cornforth M.N., Kurimasa A., Chen D.J., Goodwin E.H. Strand-specific postreplicative processing of mammalian telomeres. Science. 2001;293(5539):2462–2465. doi: 10.1126/science.1062560. Epub 2001/09/29. doi: 10.1126/science.1062560. PubMed PMID: 11577237. [DOI] [PubMed] [Google Scholar]
- 72.Timashev L.A., de Lange T. Characterization of t-loop formation by TRF2. Nucleus. 2020 doi: 10.1080/19491034.2020.1783782. Epub 2020/06/23. doi: 10.1080/19491034.2020.1783782. PubMed PMID: 32564646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu P., Takai H., de Lange T. Telomeric 3' overhangs derive from resection by Exo1 and Apollo and fill-in by POT1b-associated CST. Cell. 2012;150(1):39–52. doi: 10.1016/j.cell.2012.05.026. Epub 2012/07/04. doi: 10.1016/j.cell.2012.05.026. PubMed PMID: 22748632; PMCID: PMC3392515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barrientos K.S., Kendellen M.F., Freibaum B.D., Armbruster B.N., Etheridge K.T., Counter C.M. Distinct functions of POT1 at telomeres. Mol Cell Biol. 2008;28(17):5251–5264. doi: 10.1128/MCB.00048-08. Epub 2008/06/04. doi: 10.1128/MCB.00048-08. PubMed PMID: 18519588; PMCID: PMC2519730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vassin V.M., Anantha R.W., Sokolova E., Kanner S., Borowiec J.A. Human RPA phosphorylation by ATR stimulates DNA synthesis and prevents ssDNA accumulation during DNA-replication stress. J Cell Sci. 2009;122(Pt 22):4070–4080. doi: 10.1242/jcs.053702. Epub 2009/10/22. doi: 10.1242/jcs.053702. PubMed PMID: 19843584; PMCID: PMC2776501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kratz K., de Lange T. Protection of telomeres 1 proteins POT1a and POT1b can repress ATR signaling by RPA exclusion, but binding to CST limits ATR repression by POT1b. J Biol Chem. 2018;293(37):14384–14392. doi: 10.1074/jbc.RA118.004598. Epub 2018/08/08. doi: 10.1074/jbc.RA118.004598. PubMed PMID: 30082315; PMCID: PMC6139565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaug A.J., Podell E.R., Nandakumar J., Cech T.R. Functional interaction between telomere protein TPP1 and telomerase. Genes Dev. 2010;24(6):613–622. doi: 10.1101/gad.1881810. Epub 2010/03/17. doi: 24/6/613 [pii] 10.1101/gad.1881810. PubMed PMID: 20231318; PMCID: 2841338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ge Y., Wu S., Xue Y., Tao J., Li F., Chen Y. Preferential extension of short telomeres induced by low extracellular pH. Nucleic Acids Res. 2016;44(17):8086–8096. doi: 10.1093/nar/gkw464. Epub 2016/05/26. doi: 10.1093/nar/gkw464. PubMed PMID: 27220467; PMCID: PMC5041450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu M., Kiselar J., Whited T.L., Hernandez-Sanchez W., Taylor D.J. POT1-TPP1 differentially regulates telomerase via POT1 His266 and as a function of single-stranded telomere DNA length. Proc Natl Acad Sci U S A. 2019;116(47):23527–23533. doi: 10.1073/pnas.1905381116. Epub 2019/11/07. doi: 10.1073/pnas.1905381116. PubMed PMID: 31685617; PMCID: PMC6876245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zemp I., Lingner J. The shelterin component TPP1 is a binding partner and substrate for the deubiquitinating enzyme USP7. J Biol Chem. 2014;289(41):28595–28606. doi: 10.1074/jbc.M114.596056. Epub 2014/08/31. doi: 10.1074/jbc.M114.596056. PubMed PMID: 25172512; PMCID: PMC4192509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Latrick C.M., Cech T.R. POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 2010;29(5):924–933. doi: 10.1038/emboj.2009.409. Epub 2010/01/23. doi: 10.1038/emboj.2009.409. PubMed PMID: 20094033; PMCID: PMC2837173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reddel R.R. Telomere maintenance mechanisms in cancer: clinical implications. Curr Pharm Des. 2014;20(41):6361–6374. doi: 10.2174/1381612820666140630101047. Epub 2014/07/01. doi: 10.2174/1381612820666140630101047. PubMed PMID: 24975603; PMCID: PMC4262939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jafri M.A., Ansari S.A., Alqahtani M.H., Shay J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016;8(1):69. doi: 10.1186/s13073-016-0324-x. Epub 2016/06/22. doi: 10.1186/s13073-016-0324-x. PubMed PMID: 27323951; PMCID: PMC4915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klingelhutz A.J. The roles of telomeres and telomerase in cellular immortalization and the development of cancer. Anticancer Res. 1999;19(6A):4823–4830. Epub 2000/03/04. PubMed PMID: 10697595. [PubMed] [Google Scholar]
- 85.Cong Y.-S., Wright W.E., Shay J.W. Human Telomerase and Its Regulation. MMBR. 2002;66(3):407–425. doi: 10.1128/MMBR.66.3.407-425.2002. Epub 2002/09/05. doi: 10.1128/mmbr.66.3.407-425.2002. PubMed PMID: 12208997; PMCID: PMC120798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bernadotte A., Mikhelson V.M., Spivak I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016;8(1):3–11. doi: 10.18632/aging.100871. Epub 2016/01/26. doi: 10.18632/aging.100871. PubMed PMID: 26805432; PMCID: PMC4761709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Urquidi V., Tarin D., Goodison S. Role of telomerase in cell senescence and oncogenesis. Annu Rev Med. 2000;51:65–79. doi: 10.1146/annurev.med.51.1.65. Epub 2000/04/25. doi: 10.1146/annurev.med.51.1.65. PubMed PMID: 10774453. [DOI] [PubMed] [Google Scholar]
- 88.Artandi S.E., Attardi L.D. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun. 2005;331(3):881–890. doi: 10.1016/j.bbrc.2005.03.211. Epub 2005/05/04. doi: 10.1016/j.bbrc.2005.03.211. PubMed PMID: 15865944. [DOI] [PubMed] [Google Scholar]
- 89.Chin L., Artandi S.E., Shen Q., Tam A., Lee S.L., Gottlieb G.J. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97(4):527–538. doi: 10.1016/s0092-8674(00)80762-x. Epub 1999/05/25. doi: 10.1016/s0092-8674(00)80762-x. PubMed PMID: 10338216. [DOI] [PubMed] [Google Scholar]
- 90.Henson J.D., Neumann A.A., Yeager T.R., Reddel R.R. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21(4):598–610. doi: 10.1038/sj.onc.1205058. Epub 2002/02/19. doi: 10.1038/sj.onc.1205058. PubMed PMID: 11850785. [DOI] [PubMed] [Google Scholar]
- 91.Neumann A.A., Watson C.M., Noble J.R., Pickett H.A., Tam P.P., Reddel R.R. Alternative lengthening of telomeres in normal mammalian somatic cells. Genes Dev. 2013;27(1):18–23. doi: 10.1101/gad.205062.112. Epub 2013/01/12. doi: 10.1101/gad.205062.112. PubMed PMID: 23307865; PMCID: PMC3553280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel T.N., Vasan R., Gupta D., Patel J., Trivedi M. Shelterin proteins and cancer. Asian Pac J Cancer Prev. 2015;16(8):3085–3090. doi: 10.7314/apjcp.2015.16.8.3085. Epub 2015/04/30. doi: 10.7314/apjcp.2015.16.8.3085. PubMed PMID: 25921101. [DOI] [PubMed] [Google Scholar]
- 93.Aoude L.G., Pritchard A.L., Robles-Espinoza C.D., Wadt K., Harland M., Choi J. Nonsense mutations in the shelterin complex genes ACD and TERF2IP in familial melanoma. J Natl Cancer Inst. 2015;107(2) doi: 10.1093/jnci/dju408. Epub 2014/12/17. doi: 10.1093/jnci/dju408. PubMed PMID: 25505254; PMCID: PMC4334787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferrandon S., Saultier P., Carras J., Battiston-Montagne P., Alphonse G., Beuve M. Telomere profiling: toward glioblastoma personalized medicine. Mol Neurobiol. 2013;47(1):64–76. doi: 10.1007/s12035-012-8363-9. Epub 2012/10/16. doi: 10.1007/s12035-012-8363-9. PubMed PMID: 23065374. [DOI] [PubMed] [Google Scholar]
- 95.Lin T.T., Letsolo B.T., Jones R.E., Rowson J., Pratt G., Hewamana S. Telomere dysfunction and fusion during the progression of chronic lymphocytic leukemia: evidence for a telomere crisis. Blood. 2010;116(11):1899–1907. doi: 10.1182/blood-2010-02-272104. Epub 2010/06/12. doi: 10.1182/blood-2010-02-272104. PubMed PMID: 20538793. [DOI] [PubMed] [Google Scholar]
- 96.Bainbridge M.N., Armstrong G.N., Gramatges M.M., Bertuch A.A., Jhangiani S.N., Doddapaneni H. Germline mutations in shelterin complex genes are associated with familial glioma. J Natl Cancer Inst. 2015;107(1):384. doi: 10.1093/jnci/dju384. PubMed PMID: 25482530; PMCID: PMC4296199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao J., Zhang J., Long Y., Lu X. Expression of telomere binding proteins in gastric cancer and correlation with clinicopathological parameters. Asia Pac J Clin Oncol. 2011;7(4):339–345. doi: 10.1111/j.1743-7563.2011.01437.x. Epub 2011/12/14. doi: 10.1111/j.1743-7563.2011.01437.x. PubMed PMID: 22151982. [DOI] [PubMed] [Google Scholar]
- 98.Calvete O., Garcia-Pavia P., Dominguez F., Bougeard G., Kunze K., Braeuninger A. The wide spectrum of POT1 gene variants correlates with multiple cancer types. Eur J Hum Genet. 2017;25(11):1278–1281. doi: 10.1038/ejhg.2017.134. Epub 2017/08/31. doi: 10.1038/ejhg.2017.134. PubMed PMID: 28853721; PMCID: PMC5643968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cesare A.J., Reddel R.R. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11(5):319–330. doi: 10.1038/nrg2763. PubMed PMID: 20351727. [DOI] [PubMed] [Google Scholar]
- 100.De Vitis M., Berardinelli F., Sgura A. Telomere Length Maintenance in Cancer: At the Crossroad between Telomerase and Alternative Lengthening of Telomeres (ALT) Int J Mol Sci. 2018;19(2) doi: 10.3390/ijms19020606. Epub 2018/02/22. doi: 10.3390/ijms19020606. PubMed PMID: 29463031; PMCID: PMC5855828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dilley R.L., Greenberg R.A. ALTernative Telomere Maintenance and Cancer. Trends Cancer. 2015;1(2):145–156. doi: 10.1016/j.trecan.2015.07.007. Epub 2015/12/09. doi: 10.1016/j.trecan.2015.07.007. PubMed PMID: 26645051; PMCID: PMC4669901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heaphy C.M., Subhawong A.P., Hong S.M., Goggins M.G., Montgomery E.A., Gabrielson E. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179(4):1608–1615. doi: 10.1016/j.ajpath.2011.06.018. Epub 2011/09/06. doi: 10.1016/j.ajpath.2011.06.018. PubMed PMID: 21888887; PMCID: PMC3181356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Draskovic I., Arnoult N., Steiner V., Bacchetti S., Lomonte P., Londono-Vallejo A. Probing PML body function in ALT cells reveals spatiotemporal requirements for telomere recombination. Proc Natl Acad Sci U S A. 2009;106(37):15726–15731. doi: 10.1073/pnas.0907689106. Epub 2009/09/01. doi: 10.1073/pnas.0907689106. PubMed PMID: 19717459; PMCID: PMC2747187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Osterwald S., Deeg K.I., Chung I., Parisotto D., Worz S., Rohr K. PML induces compaction, TRF2 depletion and DNA damage signaling at telomeres and promotes their alternative lengthening. J Cell Sci. 2015;128(10):1887–1900. doi: 10.1242/jcs.148296. Epub 2015/04/25. doi: 10.1242/jcs.148296. PubMed PMID: 25908860. [DOI] [PubMed] [Google Scholar]
- 105.Jiang W.Q., Zhong Z.H., Henson J.D., Reddel R.R. Identification of candidate alternative lengthening of telomeres genes by methionine restriction and RNA interference. Oncogene. 2007;26(32):4635–4647. doi: 10.1038/sj.onc.1210260. Epub 2007/02/14. doi: 10.1038/sj.onc.1210260. PubMed PMID: 17297460. [DOI] [PubMed] [Google Scholar]
- 106.Episkopou H., Diman A., Claude E., Viceconte N., Decottignies A. TSPYL5 Depletion Induces Specific Death of ALT Cells through USP7-Dependent Proteasomal Degradation of POT1. Mol Cell. 2019;75(3):469–482. doi: 10.1016/j.molcel.2019.05.027. e6. Epub 2019/07/07. doi: 10.1016/j.molcel.2019.05.027. PubMed PMID: 31278054. [DOI] [PubMed] [Google Scholar]
- 107.Bisht K., Smith E.M., Tesmer V.M., Nandakumar J. Structural and functional consequences of a disease mutation in the telomere protein TPP1. Proc Natl Acad Sci U S A. 2016;113(46):13021–13026. doi: 10.1073/pnas.1605685113. Epub 2016/11/04. doi: 10.1073/pnas.1605685113. PubMed PMID: 27807141; PMCID: PMC5135350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo Y., Kartawinata M., Li J., Pickett H.A., Teo J., Kilo T. Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood. 2014;124(18):2767–2774. doi: 10.1182/blood-2014-08-596445. Epub 2014/09/11. doi: 10.1182/blood-2014-08-596445. PubMed PMID: 25205116; PMCID: PMC4215308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoffman T.W., van der Vis J.J., van der Smagt J.J., Massink M.P.G., Grutters J.C., van Moorsel C.H.M. Pulmonary fibrosis linked to variants in the ACD gene, encoding the telomere protein TPP1. Eur Respir J. 2019;54(6) doi: 10.1183/13993003.00809-2019. Epub 2019/09/14. doi: 10.1183/13993003.00809-2019. PubMed PMID: 31515401. [DOI] [PubMed] [Google Scholar]
- 110.Wilson T.L., Hattangady N., Lerario A.M., Williams C., Koeppe E., Quinonez S. A new POT1 germline mutation-expanding the spectrum of POT1-associated cancers. Fam Cancer. 2017;16(4):561–566. doi: 10.1007/s10689-017-9984-y. Epub 2017/04/09. doi: 10.1007/s10689-017-9984-y. PubMed PMID: 28389767. [DOI] [PubMed] [Google Scholar]
- 111.Pinzaru A.M., Hom R.A., Beal A., Phillips A.F., Ni E., Cardozo T. Telomere Replication Stress Induced by POT1 Inactivation Accelerates Tumorigenesis. Cell Rep. 2016;15(10):2170–2184. doi: 10.1016/j.celrep.2016.05.008. Epub 2016/05/31. doi: 10.1016/j.celrep.2016.05.008. PubMed PMID: 27239034; PMCID: PMC6145145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hockemeyer D., Palm W., Wang R.C., Couto S.S., de Lange T. Engineered telomere degradation models dyskeratosis congenita. Genes Dev. 2008;22(13):1773–1785. doi: 10.1101/gad.1679208. Epub 2008/06/14. doi: 10.1101/gad.1679208. PubMed PMID: 18550783; PMCID: PMC2492664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He H., Wang Y., Guo X., Ramchandani S., Ma J., Shen M.F. Pot1b deletion and telomerase haploinsufficiency in mice initiate an ATR-dependent DNA damage response and elicit phenotypes resembling dyskeratosis congenita. Mol Cell Biol. 2009;29(1):229–240. doi: 10.1128/MCB.01400-08. Epub 2008/10/22. doi: 10.1128/MCB.01400-08. PubMed PMID: 18936156; PMCID: PMC2612488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Diotti R., Kalan S., Matveyenko A., Loayza D. DNA-directed polymerase subunits play a vital role in human telomeric overhang processing. Mol Cancer Res. 2015;13(3):402–410. doi: 10.1158/1541-7786.MCR-14-0381. Epub 2014/12/19. doi: 10.1158/1541-7786.MCR-14-0381. PubMed PMID: 25519149; PMCID: PMC4369185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takai H., Jenkinson E., Kabir S., Babul-Hirji R., Najm-Tehrani N., Chitayat D.A. A POT1 mutation implicates defective telomere end fill-in and telomere truncations in Coats plus. Genes Dev. 2016;30(7):812–826. doi: 10.1101/gad.276873.115. Epub 2016/03/26. doi: 10.1101/gad.276873.115. PubMed PMID: 27013236; PMCID: PMC4826397. [DOI] [PMC free article] [PubMed] [Google Scholar]