Abstract
Objective
Anticoagulation may be a challenge in coronavirus disease 2019 (COVID-19) extracorporeal membrane oxygenation due to endothelial injury and dysregulation of coagulation, which may increase the risk of thrombotic and bleeding complications. This report was created to describe the authors’ single institutional experience, with emphasis on the high rate of intracranial hemorrhage for the first 10 patients with COVID-19 placed on venovenous extracorporeal membrane oxygenation (VV ECMO).
Design
Case series, retrospective analysis.
Setting
Single institution.
Participants
Ten patients.
Interventions
None.
Measurements and Main Results
Patient characteristics, mortality, stroke rate, and length of stay data were collected in all patients. In addition, laboratory values of D-dimer and C-reactive protein and standard measurements of prothrombin and activated partial thromboplastin time were collected on all patients. Ten patients, each confirmed with COVID-19 via reverse transcription-polymerase chain reaction, were supported on VV ECMO for acute respiratory distress syndrome (ARDS) for a mean duration of 9.4 ± 7 days. Four of 10 patients had hemorrhagic strokes, 3 of which resulted in death. At 30 days after initiation of VV ECMO, a total of 7 survivors included 6 patients discharged from the hospital and 1 patient who remained in the intensive care unit.
Conclusions
In this small study of 10 patients, intracranial hemorrhage was a common complication, resulting in a high rate of death. The authors urge caution in the anticoagulation management of VV ECMO for patients with severe ARDS and COVID-19 patients. Close monitoring of all hematologic parameters is recommended during ECMO support while awaiting larger, multicenter studies to examine the best practice.
Key Words: extracorporeal membrane oxygenation, stroke, intraparenchymal hemorrhage, anticoagulation, COVID-19, SARS-CoV-2
Introduction
Infection with severe acute respiratory syndrome coronavirus 2 was designated a worldwide pandemic in March, 2020. Coronavirus disease 2019 (COVID-19), the illness caused by severe acute respiratory syndrome coronavirus 2, has rallied the world behind efforts to investigate and report the optimal clinical management and treatment for this disease. Despite maximal medical therapy, COVID-19 can progress to severe, refractory acute respiratory distress syndrome (ARDS), prompting clinicians to consider utilization of extracorporeal membrane oxygenation (ECMO) in appropriate cases, although early reports appeared to have high rates of mortality.1
In general, patients with severe ARDS supported with venovenous extracorporeal membrane oxygenation (VV ECMO) are anticoagulated to reduce the risk of circuit clot or associated venous thromboembolism. Patients with COVID-19 demonstrate complex pathophysiology, with multiorgan involvement, in particular changes in patients’ coagulation profiles stemming from the combination of inflammation and vascular endothelium activation.2 , 3 In addition, arterial and venous thromboeis appear to be a potential source of the organ dysfunction seen in COVID-19 patients.4 , 5 The risks and benefits of anticoagulation and the complex interplay among COVID-19 infection, inflammation, and hypercoagulability in this population remain unstudied in the setting of VV ECMO.
This case series describes the authors’ single institutional neurologic outcomes for the first 10 patients placed on VV ECMO for COVID-19, of whom 3 had severe intraparenchymal hemorrhagic strokes resulting in death, 1 patient had a small subarachnoid hemorrhage, and 1 patient had severe gastrointestinal bleeding. This case series describes a hemorrhagic stroke rate that far exceeds that expected for VV ECMO treatment in severe ARDS.
Methods
This study was approved by the institutional review board at the University of Pennsylvania. The authors’ ECMO team consists of a multidisciplinary group that has managed a robust VV ECMO lung rescue program including the ability to perform mobile ECMO.6 , 7 A decision was made to continue using VV ECMO during the COVID-19 pandemic with rigorous, multidisciplinary patient selection. Due to limited access to ECMO circuits and concern about an overwhelming number of consults for ECMO, the authors restricted the authors’ previously published criteria8 to the following:
-
•
Age <65
-
•
Absence of significant pre-existing comorbidities
-
•
Mild or no limitations in physical activity prior to COVID-19
-
•
No or mild evidence of other end-organ damage from current disease
-
•
Body mass index <45 (relative indication)
-
•
Smoking history <30 packs/yr
-
•
No cardiac arrest prior to cannulation
-
•
Ventilator duration < or = 7 days
All patients in this study were cannulated at the authors’ institution or at an outside hospital by the authors’ mobile ECMO team, and subsequently were admitted to specialized units staffed by critical care specialists and highly skilled intensive care unit (ICU) nurses trained in ECMO management.
The ECMO circuits were standardized per the authors’ institutional practice. Cardiohelp and Rotaflow (Maquet Getinge Group, Germany) devices were used for all patients, with standard cannulation using a femoral venous inflow cannula and a right internal jugular outflow cannula. Patients’ pump settings and lab values were obtained at close intervals for pre- and post-oxygenator monitoring. Standard safety checklists that included safety hand crank, wall, as well as tank oxygen supply were placed permanently at the bedside.
The authors’ standardized protocol for anticoagulation of patients on VV ECMO uses a heparin infusion, targeting an activated partial thromboplastin time (aPTT) of 40- to 50 seconds, and 50- to- 60 seconds if oxygenator failure or evidence of clotting occurs. The reference range for normal aPTT at the authors’ institution is 21.8- to- 32.5 seconds. One patient was anticoagulated with a bivalirudin infusion due to problems with recurrent clotting of their continuous renal replacement therapy circuit while on a heparin infusion prior to ECMO support. All patients at the time of cannulation received a standard 50- unit/kg intravenous unfractionated heparin bolus. Heparin infusion was started after cannulation and adjusted per the authors’ institutional provider–driven protocol, with the aPTT measured every 6 hours initially and every 12 hours once the target range was achieved. Additional standard laboratory values were collected at daily intervals including D-dimer, ferritin, fibrinogen, partial thromboplastin time, prothrombin time, international normalized ratio, and platelet counts.
Inclusion Criteria
Between March 21, 2020 and April 25, 2020, patients meeting inclusion criteria, with severe refractory ARDS due to COVID-19, who failed a trial of proning therapy with a muscle relaxant infusion, were placed on VV ECMO. Patients were considered if their PaO2/FIO2 was <80 on 100% oxygen, with appropriate positive end-expiratory pressure.9 , 10 Retrospective chart review was performed on all patients with COVID-19 requiring VV ECMO. All data were reviewed by 2 independent reviewers, A.A.U. and J.H. Data were placed in Excel 2019 (Microsoft). Data were summarized with means, standard deviations, and proportions within each cohort. Each cohort was analyzed and compared using an χ2 test or Fisher exact test for categorical variables and Kruskal-Wallis tests for continuous variables. All analyses were performed using Stata 14 (StataCorp, College Station, TX), and p < 0.05 was defined as statistically significant. Data for patient-specific averages are reported as mean ± 25th/75th quartile ranges. Laboratory data are reported as mean ± standard deviation.
The primary outcome was incidence of any type of stroke for the duration of VV ECMO. A diagnosis of stroke was suspected based on bedside findings of focal neurologic deficits, notably an abnormal pupillary examination in patients treated with heavy sedation and neuromuscular blockade agents. A stroke alert, with a formal emergency neurology consultation and a computed tomography scan, was obtained in all cases of suspected stroke. Intracranial bleeding was categorized as subarachnoid hemorrhage (SAH), intraparenchymal hemorrhage, or intraventricular extension of intraparenchymal hemorrhage. Ischemic stroke was defined by large- vessel occlusion and evidence of ischemia or infarction on computed tomography scan. Secondary outcomes evaluated included total days of ECMO support, time to decannulation, time to tracheostomy, and 30-day survival. The authors also evaluated patients for 30-day neurologic status after admission, number of circuit exchanges required for oxygenator clot, continuous venovenous hemofiltration circuit exchange events caused by clot, and evidence of pulmonary embolism. Laboratory values also were recorded and analyzed daily and at the time of stroke evaluation.
Results
Ten patients, each confirmed COVID-19 cases via reverse-transcription polymerase chain reaction, were cannulated for VV ECMO for ARDS for a mean duration between March 21, 2020 and April 25, 2020. The average duration for VV ECMO was 16.7 ± 5.3/22 days (mean ± 25th/75th quartile), with a range of 4- to- 51 days. All patients were cannulated using a 25F multistage femoral venous inflow. Regarding outflow, 1 patient had a 17F cannula, 6 had an 18F cannula, 1 had a 19F cannula, and 2 had a 20F cannula. There were no periprocedural complications and no evidence on physical examination of venous outflow obstruction from the right head and neck. The average initial VV ECMO settings were a flow of 4.5 ± 4.3/4.8 L/min, an average speed of 3,503 ± 3,331/3,637 RPM, and an average sweep of 3.8 ±3.3/4.0 L/min with 100% FIO2.
Patient characteristics, comorbidities, pertinent COVID-19 medications, and outcomes are listed in Table 1 . The average time from intubation to cannulation was 5.7 ± 3.25/7.8 days. At the time of cannulation, the mean PaO2/FIO2 ratio was 65.8 ± 57.0/71.0, mean compliance was 26.3 ±17.3/35 (100 mL/cmH2O), and mean plateau pressure was 28.8 ±27.0/29.75 (cmH2O). All 10 patients tested negative for other viral pathogens.
Table 1.
Baseline Data
| Baseline Data | All Patients (N = 10) | Non-Stroke (N = 6) | Stroke (N = 4) |
|---|---|---|---|
| Age, mean ± 25th/75th quartile | 50.7 ± 47.5/58.8 | 48.6 ± 36.5/58.25 | 53.7 ± 50.5/58.25 |
| Weight (kg), mean ± 25th/75th quartile | 107.5 ± 92.3/123.3 | 109.8 ± 98.0/166.0 | 104 ± 88.0/109.5 |
| Height (cm), mean ± 25th/75th quartile | 176.0 ± 171.3/177.8 | 174.0 ± 171.25/176.5 | 179.0 ± 173.8/181.75 |
| BMI, mean ± 25th/75th Quartile | 34.7 ± 29.9/39.5 | 36.2 ± 31.3/39.5 | 32.4 ± 26.9/34.1 |
| Sex (F=female) | 3 | 2 | 1 |
| Smoking history | 2 | 2 | 0 |
| Alcohol use history | 5 | 3 | 2 |
| Past medical Hx | |||
| Stroke | 1 | 0 | 1 |
| HTN | 5 | 3 | 2 |
| Asthma | 5 | 4 | 1 |
| COVID-19 treatments | |||
| Hydroxychloroquine | 9 | 5 | 4 |
| Remdesivir | 2 | 0 | 2 |
| Tocilizumab | 6 | 5 | 1 |
| Azithromycin | 10 | 6 | 4 |
| Steroids | 5 | 3 | 2 |
| Outcomes | |||
| ECMO decannulation | 6/10 | 6/6 | 1/4 |
| Terminal decannulation | 3/10 | 0/6 | 3/4 |
| Discharged | 6/10 | 6/6 | 0/4 |
| Tracheostomy | 7/10 | 6/6 | 1/4 |
| Ventilator weaned at 30 d | 6/10 | 6/6 | 0/4 |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; HTN, hypertension; Hx, history.
Six patients (60%) were decannulated successfully on reaching an appropriate clinical criterion. At 30 days after initiation of VV ECMO, 7 patients were alive; 6 patients were discharged by 30 days of hospital admission and 1 patient remained in the ICU. All 7 patients who survived to 30 days had tracheostomy placement, on average 25.0 ± 16.0/26.5 days after intubation. Four patients (40%) experienced hemorrhagic strokes on days 3, 4, 7, and 22 after VV ECMO start (Table 2 ). Three of these were intraparenchymal hemorrhage and 1 was mild SAH. The patient with a mild SAH had a prior distant history of an ischemic stroke and was anticoagulated with bivalirudin secondary to recurrent continuous dialysis circuit clotting. This patient with SAH was the only patient who remained hospitalized at 30 days after cannulation. All 3 patients with intraparenchymal stroke had clinical and radiographic evidence of herniation, and care was withdrawn within 24 hours after diagnosis (Fig 1 ). The patients without stroke demonstrated an average platelet count of 148 ± 93 (mean ± standard deviation [min = 36, max = 565]) during ECMO support versus stroke patient's average platelet count of 169 ± 56 (min = 102, max = 366) during ECMO support, which was not statistically different (p = 0.17). The aPTT on average for the duration of ECMO for the patients without stroke was 41.8 ± 10.1 seconds versus 52.8 ± 8.2 seconds for patients with stroke, which was not statistically different based on the small sample size (p = 0.09). In the patients without stroke, there were a total 54 of 329 (16.4%) aPTT results above the target range. In the stroke patients, there were a total of 63 of 346 (18.2%) aPTT results above the target range. None of the elevated aPTT results was related temporally to the 24-hour period preceding the stroke diagnosis. The fibrinogen level on average was higher in the stroke patients versus the patients without stroke at 513.7 ± 69.6 versus 344.4 ± 46.03 (p < 0.001). There was a total of 10 circuit exchanges during the total of 167 ECMO days in the 10 patients. Eight of 10 circuit exchanges occurred in the patients with stroke. All 8 circuit exchanges occurred due to rapidly declining oxygenator function due to clot, with a PaO2/FIO2 <200 on 100% oxygen. One circuit exchange occurred in the group without stroke due to an oxygenator clot and the last circuit exchange occurred to make a mobile transport console available for clinical use. All 6 patients without stroke were neurologically intact and participating in physical therapy at the time of discharge.
Table 2.
Characteristics of ICH Patients on VV ECMO and at the Time of Stroke
| Patient | A | B | C | D |
|---|---|---|---|---|
| CT Head Date | 4/12/20 | 4/11/20 | 4/27/20 | 4/18/20 |
| Imaging modality | Portable CTH | Unenhanced CTH with axial and coronal reformats | Unenhanced CTH, CT angio head/neck | Unenhanced CTH with axial and coronal reformats |
| Past medical history of stroke | No | No | No | Yes |
| Signs and symptoms prompting CTH | Polyuria, anisocoria Found upon unproning | Anisocoria | Anisocoria, gaze defect | AMS, agitation |
| Type of stroke | IPH, SAH | IPH | IPH with IVE, large vessel occlusion | SAH |
| Findings | 8.4 × 4.6 × 4.7 cm | 6.7 × 6.0 × 5.9 cm | 6.3 × 4.3 cm | Small Curvilinear |
| Location | Left frontal, Bilateral ACA | Left frontal, temporal | Left temporal | Left frontal Sulcus |
| Labs at time of CTH | ||||
| Platelet count (103/uL) | 139 | 197 | 134 | 335 |
| PTT (s) | 60.3 | 70.9 | 37.6 | 60.6 |
| BUN (mg/dL) | 35 | 46 | 152 | 35 |
| D-dimer (ug/mL) | 19.72 | 1.04 | 4.85 | 9.22 |
| Fibrinogen (mg/dL) | 250 | 756 | 555 | 388 |
| Ferritin (ng/mL) | None | 1596.3 | 983.8 | 1198.0 |
| SBP over 24 h prior to stroke (mmHg) | 117-140 | 89-139 | 103-132 | 102-131 |
| Presence of infection at the time of IPH | Pseudomonas VAP | None | MSSA VAP | None |
| Complication | Tonsillar, subfalcine herniation | Transtentorial, subfalcine herniation | Transtentorial | None, resolved |
| Anticoagulation | Heparin | Heparin | Heparin | Argatroban |
| Heparin dose at time of CTH | 14 units/kg/h | 12 units/kg/h | 11 unit/kg/h | 2.8 μg/kg/min |
| Antiplatelets at time of stroke | No | No | ASA 81 | No |
| Acute kidney injury | None | KDIGO 3 | KDIGO 3 | KIDGO 3 |
| CRRT | No | No | No | Yes |
| Management | Withdrawal of care | Withdrawal of care | Withdrawal of care | Supportive |
| Stroke on ECMO day | 22 | 7 | 3 | 4 |
| 30-day death | Yes | Yes | Yes | No |
Abbreviations: ACA, anterior cerebral artery; AMS, altered mental status; BUN, blood urea nitrogen; CT, computed tomography; CTH, clot time with heparinase; CRRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICH, intracranial hemorrhage; IPH, intraparenchymal hemorrhage; IVE, intraventricular extension; KDIGO, Kidney Disease Improving Global Outcomes; MSSA, methicillin-sensitive Staphylococcus aureus; PTT, partial thromboplastin time; SAH, subarachnoid hemorrhage; SBP, systolic blood pressure; VAP, ventilator-associated pneumonia.
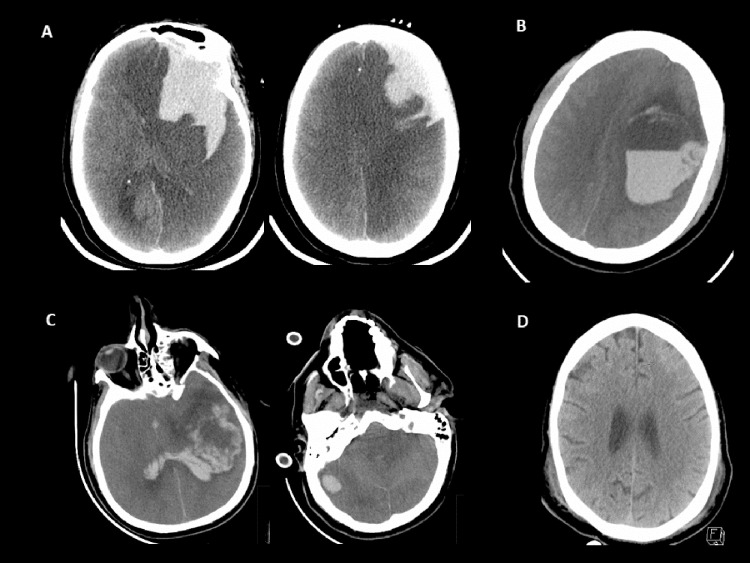
Fig 1.
Representative images of CTH. Patient A-D represented as columns in Table 2. (A) Multicompartment intracranial hemorrhage with intraparenchymal, subarachnoid, and subdural components with marked diffuse edema and secondary infarction of the left anterior and posterior cerebral artery territories owing to vascular compression. (B) Large left hemispheric intracerebral hemorrhage with fluid level consistent with coagulopathic hemorrhage. (C) Multifocal intracerebral hemorrhage with left hemispheric lobar hemorrhage and separate right cerebellar hemorrhage. (D) Small left frontal cortical subarachnoid hemorrhage.
Discussion
To the authors’ knowledge, this report was the first of its kind to focus on the rate of ICH for COVID-19 patients on VV ECMO. COVID-19 is a new disease, and it is important to report early institutional experiences, which may affect patient management at other ECMO hospitals. To better understand how unusual this rate of ICH is in VV ECMO, the authors reviewed the literature. Nasr et al analyzed data from the nationwide inpatient sample from 2001 to 2011, including 8,398 adults who received VV or venoarterial ECMO.11 The authors found that 10.9% had neurologic complications but only 3.6% had ICH. This sample, although large, included venoarterial and VV ECMO, which have different anticoagulation requirements and risk profiles. In addition, this study did not report if the ICH occurred while patients were on ECMO or after decannulation, but only noted that ICH occurred prior to hospital discharge. Lorusso et al. analyzed data from the Extracorporeal Life Support Organization to assess the incidence of neurologic outcomes in patients supported with VV ECMO. In an analysis of 4,988 patients supported with VV ECMO for non-COVID-19–related respiratory failure, ICH was diagnosed in 181 (3.6%) patients, with a mortality of 79.6%.12 The CESAR trial found neurologic injury was observed in 4% of patients; however, the type of neurologic injury was not differentiated into subtypes.13 In the EOLIA trial, of the 124 patients randomized to ECMO support, 3 patients experienced hemorrhagic stroke.14
The authors’ center has extensive experience offering ECMO to patients with severe ARDS, including a mobile program, which has been able to continue implementing ECMO cannulation in regional hospitals in a limited fashion during this pandemic. To date, at the authors’ institution, there has been less than 1% intraparenchymal hemorrhage during non-COVID lung rescue VV ECMO in 266 patients since 2015.
This unprecedented ICH rate in COVID-related ARDS requiring VV ECMO has prompted an evaluation of the anticoagulation practice by experts in hematology and neurology. In an abundance of caution, the authors now are using a venous thromboembolism (VTE) prophylaxis dose of 5,000- to- 7,500 units of subcutaneous heparin 3 times a day and 81 mg of aspirin daily for COVID-19 patients on VV ECMO. Using VTE prophylaxis alone has been done by others. Krueger et al, in 2017, described their experience with 61 patients with subcutaneous enoxaparin alone.15 The authors found thrombotic complications in 4 patients, 3 of them in the centrifugal pump after a run time of more than 5 days. No ICHs were reported in this single-center retrospective analysis. The authors opted for this approach after extensive discussion with hematology and neurology experts, keeping in mind the fatal nature of the IPH the authors experienced. Although the authors temporarily ceased using heparin infusions in COVID-19 ECMO patients, the authors have not yet experienced an increase in fatal thrombotic complications or reduced circuit durability.
The 3 initial reports from Wuhan, China reported the use of ECMO in 4 of 36, 6 of 52, and 5 of 173 critically ill COVID-19 patients.3 , 16 , 17 The neurologic outcomes for ECMO patients in these studies were not reported. Recently, Jacobs et al described the outcomes of 32 ECMO patients in 9 hospitals.18 Fifteen patients were decannulated and 10 of these patients died. One patient death was attributed to ICH and 2 to disseminated intravascular coagulopathy (DIC). The Extracorporeal Life Support Organization has created a live COVID ECMO dashboard and, as of May 23, 2020, the report recorded 591 completed extracorporeal life support runs, with a total of 1 stroke (<1%) and 35 ICHs (5%).19 The granular neurologic and hematologic outcomes data for COVID-19 from the currently published literature are limited, and it remains unclear if centers are experiencing similar rates of ICH or bleeding complications such as DIC.
Evidence is mounting that a subset of COVID-19 ICU patients can progress to DIC.20 , 21 Elevated D-dimer and fibrin/fibrinogen-degradation products have been identified as an early marker for disturbances in the coagulation pathway, whereas abnormalities in prothrombin time, partial thromboplastin time, and platelet counts are relatively uncommon in initial presentations.4 It is also possible that aPTT may measure anticoagulation levels inadequately in patients with COVID-19, but it is unclear why this only would manifest as increased bleeding in VV ECMO patients. For future patients, the authors will consider using heparin assay results in conjunction with aPTT to guide anticoagulation. Prior to 2015, the authors’ institution regularly used activated clotting time to guide anticoagulation with heparin infusion. Anecdotally, the authors’ patients had much higher rates of bleeding complications in that era. Venovenous ECMO bleeding typically is associated with platelet dysfunction.22 , 23 Based on the results, patients who had a stroke had more ECMO circuit exchanges. This simply may be a marker for a prothrombotic state or risk for microvascular thrombosis that resulted in parenchymal cerebral hemorrhage.
Coronavirus disease 2019 appears to be an independent risk factor for coagulopathy and thrombosis; however, the mechanism and pathophysiology are currently under active investigation. There have been reports of a high rate of thrombotic complications including VTE and stroke. However, the neurologic and hematologic outcomes of COVID-19 still are emerging, even with pathology report data appearing to indicate that thrombosis is the more common problem.24 It is essential to tease out the degree of contribution to coagulopathy from COVID-19 in multiorgan system illness. Acute respiratory distress syndrome, paralysis, and critical illness itself have been demonstrated to be risk factors for thrombosis. It is unclear why the rate of ICH in the authors’ COVID-19 population was so high, but it may be related to vascular inflammation associated with COVID-19.25 There is continued evidence that COVID-19 results in a cytokine storm and inflammatory cascade that may be exacerbated by extracorporeal circuitry and may be attenuated by anti-inflammatory agents (ie, corticosteroids).26 In addition, biomarkers associated with thrombosis, such as D-dimer, and actual thrombotic event rates have been elevated consistently in COVID-19.25 , 27 Further study, designed to test the balance of required anticoagulation in COVID-19 patients on ECMO, is warranted.
Limitations
There were several limitations to the study, which included the low number of patients studied. This report hoped to emphasize early reporting of sentinel unexpected events; however, results may be difficult to interpret due to low numbers. Additionally, this represented a single institutional outcome. The ECMO selection criteria vary from center to center; in addition, ECMO capabilities fluctuate based on the level of the COVID-19 surge capacity of a hospital system. Furthermore, anticoagulation policies may vary across institutions. Finally, conventional coagulation studies, such as aPT Student t testing, may have limited predictive value for actual coagulation status in COVID-19, and perhaps institutions should consider routine viscoelastic testing for this special patient population.
This article demonstrated the other hematologic spectrum of COVID-19, in particular in the setting of anticoagulation and extracorporeal devices. This article highlighted that this disease being grappled with is not just a prothrombotic disease but rather a disease that causes severe imbalance in bleeding and thrombosis risk, in particular with extracorporeal circulation. Based on the results, the authors urge close evaluation of anticoagulation strategies during the use of VV ECMO in COVID-19. Furthermore, the authors suggest all ECMO programs internally evaluate their anticoagulation protocols. Close neurologic monitoring is recommended based on this limited case series. Rapid reporting of complications remains essential as clinicians around the world apply various potentially lethal treatment modalities to this pandemic illness.
Conflicts of Interest
None.
References
- 1.Namendys-Silva S.A. ECMO for ARDS due to COVID-19. Heart Lung. 2020;49:348–349. doi: 10.1016/j.hrtlng.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald M.D., Laudanski K., Vernick W.J. Acute respiratory failure managed via inter-facility transport for extracorporeal life support: A 3-year experience. J Cardiothorac Vasc Anesth. 2019;33:1865–1870. doi: 10.1053/j.jvca.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Gutsche J.T., Miano T.A., Vernick W. Does a mobile ECLS program reduce mortality for patients transported for ECLS therapy for severe acute respiratory failure? J Cardiothorac Vasc Anesth. 2018;32:1137–1141. doi: 10.1053/j.jvca.2017.08.050. [DOI] [PubMed] [Google Scholar]
- 8.Gutsche J., Vernick W., Miano T.A. One-year experience with a mobile extracorporeal life support service. Ann Thorac Surg. 2017;104:1509–1515. doi: 10.1016/j.athoracsur.2017.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Guerin C., Reignier J., Richard J.C. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 11.Nasr D.M., Rabinstein A.A. Neurologic complications of extracorporeal membrane oxygenation. J Clin Neurol. 2015;11:383–389. doi: 10.3988/jcn.2015.11.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorusso R., Gelsomino S., Parise O. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: Findings from the Extracorporeal Life Support Organization Database. Crit Care Med. 2017;45:1389–1397. doi: 10.1097/CCM.0000000000002502. [DOI] [PubMed] [Google Scholar]
- 13.Peek G.J., Mugford M., Tiruvoipati R. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 14.Combes A., Hajage D., Capellier G. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 15.Krueger K., Schmutz A., Zieger B. Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: An observational study in more than 60 patients. Artif Organs. 2017;41:186–192. doi: 10.1111/aor.12737. [DOI] [PubMed] [Google Scholar]
- 16.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically illpatients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs J.P., Stammers A.H., St Louis J. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: Experience with 32 patients. ASAIO J. 2020;66:722–730. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Extracorporeal Life Support Organization. COVID-19 registry dashboard. Available at:https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx. Accessed May 23, 2020.
- 20.Lax S.F., Skok K., Zechner P. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: Results from a prospective, single-center, clinicopathologic case series [e-pub ahead of print] Ann Intern Med. 2020 May 14 doi: 10.7326/M20-2566. May 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wichmann D., Sperhake J.P., Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19 [e-pub ahead of print] Ann Intern Med. 2020 May 6 doi: 10.7326/M20-2003. May 23, 2020. [DOI] [PubMed] [Google Scholar]
- 22.Kalbhenn J., Schlagenhauf A., Rosenfelder S. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J Heart Lung Transplant. 2018;37:985–991. doi: 10.1016/j.healun.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Oliver W.C. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth. 2009;13:154–175. doi: 10.1177/1089253209347384. [DOI] [PubMed] [Google Scholar]
- 24.Rapkiewicz A.V., Mai X., Carsons S.E. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiezia L., Boscolo A., Poletto F. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horby P., Lim W.S., Emberson J. Effect of dexamethasone in hospitalized patients with COVID-19: Preliminary report. medRxiv. 2020 2020.06.22.20137273. [Google Scholar]
- 27.Cui S., Chen S., Li X. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]