Abstract
Background
Unenhanced chest computed tomography (CT) can assist in the diagnosis and classification of coronavirus disease 2019 (COVID-19), complementing to the reverse-transcription polymerase chain reaction (RT-PCR) tests; the performance of which has yet to be validated in emergency department (ED) setting. The study sought to evaluate the diagnostic performance of chest CT in the diagnosis and management of COVID-19 in ED.
Methods
This retrospective single-center study included 155 patients in ED who underwent both RT-PCR and chest CT for suspected COVID-19 from March 1st to April 1st, 2020. The clinical information, CT images and laboratory reports were reviewed and the performance of CT was assessed, using the RT-PCR as standard reference. Moreover, an adjudication committee retrospectively rated the probability of COVID-19 before and after the CT calculating the net reclassification improvement (NRI). Their final diagnosis was considered as reference. The proportion of patients with negative RT-PCR test that was directed to the referent hospital based on positive CT findings was also assessed.
Results
Among 155 patients, 42% had positive RT-PCR results, and 46% had positive CT findings. Chest CT showed a sensitivity of 84.6%, a specificity of 80.0% and a diagnostic accuracy of 81.9% in suggesting COVID-19 with RT-PCR as reference. Concurrently, corresponding values of 89.4%, 84.3% and 86.5% were retrieved with the adjudication committee diagnosis as reference. For the subgroup of patients with age > 65, specificity and sensitivity were 50% and 80.8%, respectively. In patients with negative RT-PCR results, 20% (18/90) had positive chest CT finding and 22% (4/18) of those were eventually considered as COVID-19 positive according to the adjudication committee. After CT, the estimated probability of COVID-19 changed in 10/104 (11%) patients with available data: 4 (4%) were downgraded, 6 (6%) upgraded. The NRI was 1.92% (NRI event −2.08% + NRI non-event 5.36%). No patient with negative RT-PCR but positive CT was eventually directed to hospital.
Conclusion
Chest CT showed promising sensitivity for diagnosing COVID-19 across all patients' subgroups. However, CT did not modify the estimated probability of COVID-19 infection in a substantial proportion of patients and its utility as an emergency department triage tool warrants further analyses.
Keywords: COVID-19, Coronavirus, CT, Computed tomography, RT-PCR, Screening, Pandemic, Triage
Abbreviations: ACR, American college of radiology; COVID-19, Coronavirus disease 2019; ED, Emergency department; FN, False Negative; FP, False Positive; LDCT, Low dose Computed Tomography; NEWS, National Early Warning Score; NPV, Negative Predictive Value; NRI, Net Reclassification Improvement; PPV, Positive Predictive Value; RT-PCR, Reverse transcription polymerase chain reaction; TP, True Positive; TN, True Negative
1. Introduction
During the current COVID-19 pandemic, triage is an essential process in most emergency departments (ED) owing to overcrowding and the impossibility of taking care of every patient immediately. Early diagnosis of COVID-19 is key to improving outcomes in the absence of specific therapeutic drugs or vaccines. Currently reverse-transcription polymerase chain reaction (RT-PCR) is the gold standard for diagnosing COVID-19.
The clinical utility of RT-PCR is limited however by global shortages of RT-PCR viral testing reagents, the limited number of laboratories that can meet rigorous quality standards, the length of time required for results to become available, and a clinically significant false negative rate. In parallel, there is a growing number of publications describing CT appearance in the setting of known or suspected COVID-19 infection [[1], [2], [3], [4]]. Some studies also suggest that chest computed tomography (CT) in particular may be positive in the setting of a negative RT-PCR test [[5], [6], [7], [8]]. As a result there is growing interest in the role and appropriateness of CT in the screening, diagnosis and management of patients with suspected or known COVID-19 infection [1,9].
CT based strategy for patients suspected for COVID-19 infection could improve sensitivity and specificity but has never been validated in ED setting. Data supporting such a strategy are lacking and current clinical merit further analysis. The use of a CT based strategy in the emergency department setting may allow determination of the level of priority of a given patient and facilitate direction into COVID positive cohorts from emergency departments to referral hospitals.
We therefore conducted this retrospective study to evaluate the diagnostic performance of CT in patients presenting to ED with COVID-19 suspicion compared with both RT-PCR, which is currently used as a gold standard, and a clinical adjudication committee decision.
2. Methods
2.1. Study Population and data source for RT-PCR results
From March 1st to April 1st 2020, patients with suspected COVID-19 infection who underwent both RT-PCR test and unenhanced CT screening at the emergency department (ED) of La Tour Hospital were included. This hospital is a secondary academically affiliated hospital and the 2nd largest ED in the canton of Geneva, Switzerland (23,343 visits in 2017). Patients who were included were all symptomatic (fever and/or dyspnea and/or cough) and if they required a hospital admission they were directed to the referent tertiary center, the Geneva University Hospital (HUG) (72,921 patients admitted in 2018) in accordance to the local policy recommendation to maintain and cohort patients with COVID-19 in a single referral center in each region. As in many centers, La Tour Hospital and HUG developed their own scale for hospital triage and admission decision, based mainly on clinical parameters. (Table 1 ). Pregnant women and patients under the age of 18 were excluded.
Table 1.
Hospital admission criteria for COVID-19.
| La Tour hospital | Geneva University Hospital |
|---|---|
|
|
Age, tachypnea, O2 saturation, temperature, blood pressure, pulse rate, consciousness.
Confusion, urea, respiratory rate > 20, blood pressure < 90 mmHg.
Following the experience in China, La Tour hospital integrated a modified version of the National Early Warning Score (NEWS) with age ≥ 65 years added as an independent risk factor based on recent reports [10]. Based on this score, patient with score > 4 must all be transferred to referent hospital for monitoring. Hospital admission could also be considered for patients with score 1–4. (Table 1) Final decision was left to the discretion of attending physicians.
In all patients, the delay between the two tests was less than 24 h (range 4 h–24 h). The RT-PCR results were extracted from the patients' electronic medical records in our hospital information system. Specimens' collection was performed by the same nurse study. Nasopharyngeal swab was used and send to the “Centre national de référence pour les infections virales émergentes” (CRIVE) at HUG. The RT-PCR assays were performed by using cobas® 6800 SARS-CoV-2 System from Roche, F. Hoffmann-La Roche Ltd.: Roche's cobas SARS-CoV-2 Test to detect novel coronavirus received FDA Emergency Use Authorization Ltd.
2.2. Chest CT protocol
CT examinations were performed using a 64 slices MDCT scanner (SOMATOM Definition; AS Siemens Healthineers, Forchheim, Germany). Patients were scanned in the supine position, during breath hold and with feet towards the gantry. The main scanning parameters were as follow: tube voltage = 100 kVp, automatic tube current modulation (Mean 50 mAs), pitch = 1.1 mm, matrix = 512 × 512, slice thickness = 10 mm, field of view = 350 mm × 350 mm. For each patient, two reconstructions were performed (utilizing the small 1 mm and large 3 mm thick images) and stored in the PACS system. No premedication with beta-blockers or nitrates was added before LDCT acquisition. All patients were examined with the same standardized examination protocol. CT were unenhanced with relatively low dose (2 to 2.5 mSV).
2.3. Image analysis
All images were reviewed independently by two experienced specialists with more than 10 years of experience in interpreting chest CT imaging (first by a radiologist and then by a pulmonologist). Both were blinded to RT-PCR results but were aware of the epidemiological characteristics and clinical symptoms of the patients. CT findings included ground glass opacity (GGO), consolidation, air bronchogram and nodular opacities. GGO was defined as hazy areas of increased opacity or attenuation with visible underlying vessels. Each specialist classified the abnormal CT according to GGO distribution of the affected lung parenchyma graded on a 3-point scale: 1 = light <30%, 2 = moderate 30–60%, 3 = severe >60%. Finally, the results of the classification were merged by consensus and the specialists classified the CT on positive or negative for COVID-19.
2.4. Adjudication committee
The adjudication committee was composed of 5 board-certified specialists in respiratory diseases and internal medicine. All were senior attending physicians with expertise in caring for patients with respiratory tract infections. After the completion of the study, the adjudication committee, blinded to the RT-PCR results, retrospectively reviewed the ED admission charts of all patients and graded the probability of COVID-19 by integrating data from several parts of the chart: the history section, the results of the clinical examination and the laboratory tests. They assess these probabilities independently, blinded to each other's assessments. First, each expert gave an individual opinion of the probability of the patient having COVID-19 on a three-point Likert scale (low, intermediate, high); second, each expert re-examined the cases where the committee had been in disagreement, in full knowledge of the other experts' first decisions. Finally, in plenary session, the adjudication committee made consensus decisions on cases that remained unresolved after the first two phases.
Adjudication committee was then informed of the CT interpretation and made a new evaluation of the probability of COVID-19 by incorporating the results of the CT and the radiologist's interpretation, repeating the 3-phases process. After integrating the results of RT-PCR (integrating serial analyses if available) and the clinical follow-up in the referral hospital, their final consensus decision was considered as the reference diagnosis for COVID-19 infection.
2.5. Outcomes
The study primary endpoints were the test diagnostic characteristics; specifically, sensitivity, specificity, positive and negative predictive values of CT first in comparison to RT-PCR as gold standard and, second, to the final adjudication committee decision. For patients with negative RT-PCR tests but positive CT results, follow-up RT-PCR analyses and/or CT images were tracked to further confirm the imaging diagnosis if available.
Secondary endpoints were the proportion of patients whose probability of COVID-19 changed (upgraded or downgraded) before and after CT and the proportion of modified diagnoses which matched the reference diagnosis, the proportion of patients for whom positive CT results but negative RT-PCR modified the patient's direction (hospital admission or outpatient follow-up); the proportion of patient for whom positive CT results modified the patient's direction according NEWS score 1–4 whilst awaiting the RT-PCR results.
2.6. Statistical analysis
Continuous variables were expressed as mean ± SD, and discrete variables were expressed as absolute numbers and percentages. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy of chest CT imaging were calculated, using RT-PCR as the gold standard. Similar tests were performed with final adjudication committee decision as standard of reference. A 95% confidence interval was provided by the Wilson score method. A p value <.05 was considered statistically significant. The performance of chest CT for identifying COVID-19 in different age groups (<60 years and ≥ 60 years) and by gender was compared by the Chi-square test.
The estimated probabilities of COVID-19 before and after the CT were compared, and the proportion of modified diagnoses (95% CI) was calculated. To assess whether CT helped clinicians to better reclassify patients in agreement with reference diagnoses, we calculated the net reclassification improvement (NRI). The absolute NRI calculates the absolute number of patients correctly reclassified: net reclassification of patients with CoVID-19 (NRI event) plus net reclassification of patients without CoVID-19 according to the adjudication committee (NRI non-event) on the total number of patients. Statistical analysis was performed with the SPSS (version 25.0; SPSS Inc., Chicago, Illinois, United States).
2.7. Sample size
Based on the findings of Ai et al. [5] who found a CT sensitivity of 97%, specificity of 25% and a prevalence of COVID-19 infection of 59% in a population with suspicion of infection (RT-PCR testing), the minimum sample size needed to evaluate the CT diagnostic performance with 80% power and confidence interval width of 14% is: 60 patients for sensitivity evaluation and 130 patients for specificity evaluation. In order to have sufficient cases to evaluate both sensitivity and specificity, our sample size needed would therefore be 130 patients.
Approval for the retrospective analysis of the patients with COVID-19 suspicion was obtained from the Swiss Ethics Commission (Commission cantonale d'éthique de la recherche CCER2020–00687) and written informed consent was waived.
3. Results
3.1. Study population characteristics
From March 1st to April 1st 2020, a total of 155 patients that underwent both RT-PCR and chest CT were included in our retrospective study analysis. The mean age was 60.0 ± 18.46 years, with 48% men.
Of 155 patients, 42% had positive and 58% had negative RT-PCR results. (Table 2 ) Of 65 patients with positive RT-PCR results, 85% had positive chest CT scans. Of 90 patients with negative RT-PCR result, 20% had positive chest CT scans and 22% of them (4/18) were eventually considered as CoVID-19 positive according to the adjudication committee. Abnormal CT were classified according to the extent of ground-glass opacities (<30% in 73%, 30–60% in 20%, >60% in 6%).
Table 2.
Sociodemographic data according RT-PCR and CT results and adjudication committee final decision
| Demographics (n = 155) | RT-PCR |
CT |
Diagnosis of Covid according to adjudication committee |
|||
|---|---|---|---|---|---|---|
| Positive (n = 65,42%) | Negative (n = 90, 58%) | Positive (n = 73, 47%) |
Negative (n = 82, 53%) |
Positive (n = 66,43%) |
Negative (n = 89, 57%) |
|
| Gender | ||||||
| Male (n) | 32 | 42 | 39 | 35 | 32 | 42 |
| Female (n) | 33 | 48 | 34 | 47 | 34 | 47 |
| Age | ||||||
| 18–40 (n) | 5 | 24 | 6 | 23 | 5 | 24 |
| 40–65 (n) | 32 | 31 | 38 | 25 | 34 | 36 |
| >65 (n) | 28 | 35 | 29 | 34 | 27 | 29 |
3.2. Diagnostic performance of CT
The primary outcome of the present study was the performance of chest CT in diagnosing COVID-19 with RT-PCR as reference [Table3]. The performance of CT to distinguish COVID-19 positive from negative was as follows: sensitivity of 84.6% (95% confidence interval [CI]: 73.52% to 92.37%), specificity of 80.0% (95% CI, 70.25% to 87.69%), and overall accuracy of 81.9%. The negative predictive value was 87.8% and positive predictive value was 75.3%.
For patients >65 years old (high-risk patients), CT sensitivity was 74.1% (95% CI: 53.72% to 88.89%), specificity was 78.4% (95% CI: 61.79% to 90.17%), and overall accuracy was 76.6%. In this subgroup population of high-risk patients, the negative predictive value was 80.6% and positive predictive value was 71.4%.
18/155 patients had positive chest CT scans but negative initial RT-PCR. Clinical follow-up and serial RT-PCR tracking by adjudication committee revealed a final diagnosis of COVID-19 in 4 patients (22%).
Diagnostic performance of CT with final adjudication committee considered as diagnosis reference was as follow [Table 3 ]: sensitivity of 89.4% (95% confidence interval [CI]: 79.36% to 95.63%), specificity of 84.3% (95% CI: 75.02% to 91.12%), and overall accuracy of 86.5%. The negative predictive value was 91.4% and positive predictive value was 75.0%.
Table 3.
Diagnostic Performance of CT with RT-PCR and with adjudication committee as references.
| RT-PCR | Adjudication committee | |
|---|---|---|
| All patients | ||
| Sensitivity | 84.6% | 89.4% |
| Specificity | 80.0% | 84.2% |
| Positive Predictive Value | 75.3% | 80.8% |
| Negative Predictive Value | 87.8% | 91.4% |
| Overall Accuracy | 81.9% | 86.5% |
| Patients >65 years old (high risk) | ||
| Sensitivity | 74.0% | 80.8% |
| Specificity | 78.4% | 81.6% |
| Positive Predictive Value | 71.4% | 75.0% |
| Negative Predictive Value | 80.6% | 86.1% |
| Overall Accuracy | 76.6% | 81.3% |
| Values are % or % (95% confidence interval) | ||
For patients >65 years old (high-risk patients), CT sensitivity was 80.78% (95% CI: 60.65% to 93.45%), specificity was 81.6% (95% CI: 65.67% to 92.26%), and overall accuracy was 81.3%. In this subgroup population of high-risk patients, the negative predictive value was 86.11% and positive predictive value was 75%.
3.3. Net reclassification index
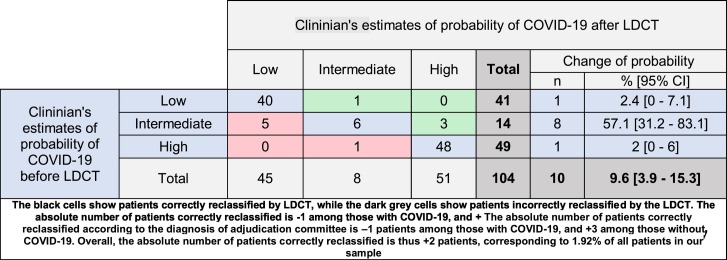
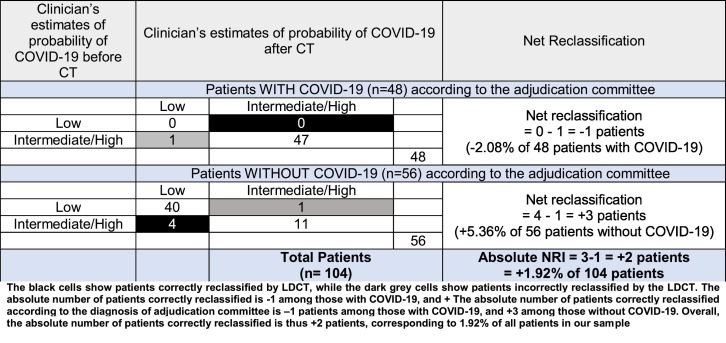
According to the adjudication committee, the initial probability of COVID-19 was high in 49 (47.1%), intermediate in 14 (13.5%) and low in 41 (39.4%) patients. After the CT, those probabilities became high in 51 (49%), intermediate in 8 (7.7%) and low in 45 (43.3%) patients. The CT results changed the probability of COVID-19 in 10 patients (9.6%). The probability was upgraded in 4 (3.8%) patients and downgraded in 6 (5.8%) [Table 4 ]. More than 57% of patients with intermediate pre-LDCT probability had their probability changed after CT. The changes in clinician's probability subsequently matched the reference diagnoses in 66.6% of modifications (4/6) and in 80.8% of all patients (84/104). The absolute number of patients correctly reclassified according to the diagnosis of adjudication committee is −1 patient among those with COVID-19, and +3 among those without COVID-19. Overall, the absolute number of patients correctly reclassified is thus +2 patients, corresponding to 1.92% of all patients in our sample. The NRI was 1.92% (NRI event −2.08% + NRI non-event 5.36%). [Table 5 ].
Table 4.
Clinician's estimates of probability of COVID-19 before and after CT.
Table 5.
Net reclassification index.
3.4. Direction of patients according the CT results
Follow-up information was available in 111 patients. 54/111 (49%) were directed to the referral hospital and 57/111 (51%) were followed in ambulatory setting as outpatients. 53 had NEWS score > 4, 25 had NEWS score between 1 and 4 and 33 had NEWS score = 0. CT results modified the direction of 16 patients awaiting results of RT-PCR (which could take up to 24 h). Ten patients with NEWS score between 1 and 4 were not referred because the CT revealed light GGO lesions (<30%). Six patients with NEWS score between 1 and 4 were admitted to the referral hospital because of moderate to severe GGO lesions on CT scan. Of note, 3 patients with NEWS score 1–4 and abnormal CT scan transferred to the referent hospital were denied admission because their hospital triage scale did not integrate the chest CT results. Among patients with positive chest CT but negative RT-PCR, none was eventually admitted to the referral hospital.
4. Discussion
This study assessed a CT based triage strategy in a Swiss ED setting during the COVID-19 pandemic. The strength of this study is to combine clinical probability and CT to determine whether the results would change likelihood of disease process. The diagnostic performance of CT for COVID-19 infection is promising, reflecting previous data from China [5]. With RT-PCR results as gold standard in 155 patients, the sensitivity, specificity, accuracy of chest CT in indicating COVID-19 infection were 84.6%, 80.0% and 75.3%, respectively. The positive predictive value and negative predictive value were 75.3% and 87.8%, respectively. In our study, the positive rate of RT-PCR assay for throat swab samples was 46% which similar than previous report (30–60%) [5].
One of the main concerns with the COVID-19 pandemic is the low to very low sensitivity of RT-PCR (50–83%) [5,11]. Patients with negative RT-PCR but CT scan suggestive of COVID-19 represents either a false negative RT-PCR or a false positive CT scan. To overcome this issue, those patients with discordant findings between negative RT-PCR and positive CT were tracked retrospectively in our study by an adjudication committee: based on the analysis of clinical symptoms, CT features and serial RT-PCR if available, 4/18 patients with discordant results were reclassified as clinically diagnosed cases of COVID-19. Therefore, with the adjudication committee decision as diagnostic reference, the diagnostic performance of CT scan was even better: sensitivity of 89.4%, specificity of 84.3%, and overall accuracy of 86.5%. The negative predictive value was 91.4% and positive predictive value was 75.0%.
Non-pharmaceutical interventions are widely implemented for pandemic mitigation (i.e. delaying and flattening the peak) [12]. Among these different measures massive testing in symptomatic patients and contact tracing followed by isolation are supported by WHO. According to current diagnostic criteria, viral nucleic acid test by RT-PCR assay plays a vital role in determining hospitalization and isolation for individual patients. However, its lack of sensitivity and relatively long time to obtaining test results were detrimental to the control of the disease epidemic. Furthermore, several countries, including Switzerland, faced frequent shortages of RT-PCR tests. Switzerland is among the countries with the highest number of CoVID-19 cases per capita in the world, but testing efforts are currently not detecting all infected people, including some with clinical disease compatible with CoVID-19 [13]. In this context, a strategy using CT-scanning alongside RT PCT for COVID-19 diagnosis may improve the effectiveness of screening in symptomatic patients. It may also improve timeliness of diagnosis and offer a solution when RT-PCR tests are unavailable.
Globally, specificity of CT scan was higher in our study than previously described [5] but remains less than optimal as other viruses and non -infectious pathologies can mimic GGO CT lesions [14]. In our study, 14 patients had positive CT but negative RT-PCR and were considered as potentially falsely positive by the adjudication committee. Furthermore, CT did not appear to add diagnostic value as positive results depend on pre-test probability and are more likely when the prior probability is low. Considering our CT specificity, the PPV in our cohort was 50% if pretest probability was low. To the best of our knowledge, our study is the first assessing the utility of CT scans that integrates the pre-test probability. CT results changed the probability of a diagnosis of COVID-19 in a small proportion of patients (9.6%), upgrading the probability in 3.8% and downgrading it in 5.8%. The absolute net reclassification index was 1.92% and NRI non-event was not superior to NRI event, meaning that CT doesn't help to exclude a diagnosis of CoVID-19.
Furthermore, the results of CT scan did not modify the direction of patients with suspected COVID-19 in the majority of the cases: only 16 patients of 155 had their direction changed after the results of CT scan. Ten patients (NEWS score 1–4) were maintained in ambulatory care because the CT revealed only light GGO lesions (<30%). Conversely, 6 patients without absolute admission criteria (NEWS score < 5) were admitted to referent hospital because of moderate to severe GGO lesion on CT scan. All of them had finally a positive RT-PCR. Of note, 3 of them were refused by the referent hospital and were re-transferred because of absence of clinical admission criteria. CT findings do not seem being associated with intensive care unit (ICU) admission [15] and their prognostic value for assessing mortality must be confirmed [16].
Overall, these results confirmed that when COVID-19 is suspected, patients should be isolated pending confirmation with (multiple) RT-PCR tests, or until quarantine has lapsed. If hospital admission criteria are present, patients should be transferred to the referral hospitals. The results of a CT scan did not change this in our study. Furthermore, using CT to diagnose COVID-19 patients is logistically challenging and can overwhelm available resources. The American College of Radiology (ACR) recently recommended against CT for screening tests: “..CT should be used sparingly and reserved for hospitalized, symptomatic patients with specific clinical indications for CT” [1].
5. Limitation of study
Our study presents the inherent limitations of any retrospective study: first, it included only individuals who received both RT-PCR and CT. In absence of specific guidelines or protocols dictating who must underwent imaging, CT prescription was left to the discretion of attending physician with a risk of selection bias that may skew the perceived performance of CT. It is reflected by the overall high positivity rate of RT-PCR in our population. Beyond this selection bias, Switzerland is among the countries with the highest number of CoVID-19 cases per capita, limiting the generalizability of the data in low incidence area. Second, even if data for performance analyses were complete, data on follow-up were missing in 44 patients and were excluded, exposing to biased estimates. Furthermore, the time of onset of symptoms was poorly identified limiting the assessment of the evolution of COVID-19 pneumonia on CT, and RT-PCR positivity [7]. CT abnormalities might predate RT-PCR positivity in symptomatic patients and in those without symptoms who subsequently test positive by RT-PCR. Chest X-ray performance was not assessed in this study as this test was not performed for safety reasons: even with proper cleaning protocols, as involved health-care workers technician could become vectors of infection to other vulnerable patients who require imaging. This was less an issue as a CT scan was dedicated to CoVID-19 and performed in closed-circuit with standardized cleaning delay. Finally, as the predominant pattern seen in COVID-19 pneumonia is GGO, detecting COVID-19 with use of chest X-Ray—where this type of abnormality is often undetectable, particularly in patients with few symptoms or low severity—will be limited.
6. Conclusion
CT utility remains uncertain for the diagnosis and management of COVID-19. Its diagnostic performance seems promising with good sensitivity across all patient subgroups. However, CT did not modify the estimated probability of COVID-19 infection in a substantial proportion of patients and its utility as a triage tool is debatable. Its prognostic value could be further enhanced if it was able to define early radiological abnormalities or patterns that predict a poor outcome such as ICU admission. More research is needed into the correlation of CT findings with clinical severity and progression, the predictive value of baseline CT or temporal changes for disease outcome, and the sequelae of acute lung injury induced by COVID-19. Meanwhile, we urge caution using systematically CT, keeping in mind that, now more than never, protecting limited resources is critical.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Necessity of informed consent for patient data utilizing was waived due to the current pandemic situation.
Disclosure
The authors have no disclosure.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declared no potential conflicts of interests associated with this study.
Acknowledgments
Dr. Antoine Rosset, Dre Jarosalva Toman, Dr. Philippe Hauser, Dr. Lise Luecker, Mme Dominique Ratheau, Dre Laurence Bouchardy.
References
- 1.https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
- 2.Li K., Wu J., Wu F., Guo D., Chen L., Fang Z., et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou S., Wang Y., Zhu T., Xia L., CT Features of Coronavirus Disease (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2019;2020:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 4.Song F., Shi N., Shan F., Zhang Z., Shen J., Lu H., et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;200642 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;200343 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;200432 doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chua F., Armstrong-James D., Desai S.R., Barnett J., Kouranos V., Kon O.M., et al. The role of CT in case ascertainment and management of COVID-19 pneumonia in the UK: insights from high-incidence regions. Lancet Respir Med. 2020;8(5):438–440. doi: 10.1016/S2213-2600(20)30132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao X., Wang B., Kang Y. Novel coronavirus infection during the 2019-2020 epidemic: preparing intensive care units-the experience in Sichuan Province. China Intensive Care Med. 2020;46(2):357–360. doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markel H., Lipman H.B., Navarro J.A., Sloan A., Michalsen J.R., Stern A.M., et al. Nonpharmaceutical interventions implemented by US cities during the 1918-1919 influenza pandemic. JAMA. 2007;298(6):644–654. doi: 10.1001/jama.298.6.644. [DOI] [PubMed] [Google Scholar]
- 13.Salathe M., Althaus C.L., Neher R., Stringhini S., Hodcroft E., Fellay J., et al. COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly. 2020;150:w20225. doi: 10.4414/smw.2020.20225. [DOI] [PubMed] [Google Scholar]
- 14.Hope M.D., Raptis C.A., Shah A., Hammer M.M., Henry T.S. six s. A role for CT in COVID-19? What data really tell us so far. Lancet. 2020;395(10231):1189–1190. doi: 10.1016/S0140-6736(20)30728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients With 2019 Novel coronavirus-infected pneumonia in Wuhan, China, JAMA 2020. [DOI] [PMC free article] [PubMed]
- 16.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan. China PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]