Abstract
Rationale & Objective
During the coronavirus disease 2019 (COVID-19) pandemic, New York encountered shortages in continuous kidney replacement therapy (CKRT) capacity for critically ill patients with acute kidney injury stage 3 requiring dialysis. To inform planning for current and future crises, we estimated CKRT demand and capacity during the initial wave of the US COVID-19 pandemic.
Study Design
We developed mathematical models to project nationwide and statewide CKRT demand and capacity. Data sources included the Institute for Health Metrics and Evaluation model, the Harvard Global Health Institute model, and published literature.
Setting & Population
US patients hospitalized during the initial wave of the COVID-19 pandemic (February 6, 2020, to August 4, 2020).
Intervention
CKRT.
Outcomes
CKRT demand and capacity at peak resource use; number of states projected to encounter CKRT shortages.
Model, Perspective, & Timeframe
Health sector perspective with a 6-month time horizon.
Results
Under base-case model assumptions, there was a nationwide CKRT capacity of 7,032 machines, an estimated shortage of 1,088 (95% uncertainty interval, 910-1,568) machines, and shortages in 6 states at peak resource use. In sensitivity analyses, varying assumptions around: (1) the number of pre–COVID-19 surplus CKRT machines available and (2) the incidence of acute kidney injury stage 3 requiring dialysis requiring CKRT among hospitalized patients with COVID-19 resulted in projected shortages in 3 to 8 states (933-1,282 machines) and 4 to 8 states (945-1,723 machines), respectively. In the best- and worst-case scenarios, there were shortages in 3 and 26 states (614 and 4,540 machines).
Limitations
Parameter estimates are influenced by assumptions made in the absence of published data for CKRT capacity and by the Institute for Health Metrics and Evaluation model’s limitations.
Conclusions
Several US states are projected to encounter CKRT shortages during the COVID-19 pandemic. These findings, although based on limited data for CKRT demand and capacity, suggest there being value during health care crises such as the COVID-19 pandemic in establishing an inpatient kidney replacement therapy national registry and maintaining a national stockpile of CKRT equipment.
Index Words: Continuous renal replacement therapy (CRRT), continuous kidney replacement therapy (CKRT), coronavirus disease 2019 (COVID-19), acute kidney injury (AKI), acute kidney injury stage 3 requiring dialysis (AKI 3D), shortages, mathematical model, pandemic, resource shortage, acute care, resource allocation, acute renal failure (ARF)
Graphical abstract
Plain-Language Summary.
Despite strategic planning, New York encountered continuous kidney replacement therapy (CKRT) shortages during the initial wave of the coronavirus disease 2019 (COVID-19) pandemic. To improve future planning, we developed mathematical models to project CKRT demand and capacity in the United States by state. Shortages were projected in 6 states during the initial wave of the COVID-19 pandemic, with possible shortages in 8 additional states. Currently, these models are based on limited data for CKRT demand and capacity across the United States. This limitation highlights the potential value of collecting national data for dialysis machines, supplies, and personnel using an inpatient kidney replacement therapy national registry and the creation of a national stockpile of CKRT equipment.
The coronavirus disease 2019 (COVID-19) pandemic, with more than 2,800,000 confirmed cases in the United States as of July 6, 2020, has created a surge in patients requiring intensive care.1 , 2 Among critically ill patients with COVID-19, 4.8% to 6.9% develop acute kidney injury stage 3 requiring dialysis (AKI 3D), a condition routinely managed with continuous kidney replacement therapy (CKRT) in the intensive care unit (ICU).3, 4, 5, 6, 7, 8, 9
Anticipating this surge, health care systems underwent crisis capacity activation for inpatient kidney replacement therapy (KRT), constituting a substantial adjustment to standards of care.4 , 7 , 10 , 11 Nephrologists used various strategies to improve KRT capacity, including procuring additional CKRT machines from manufacturers, decreasing the dose and duration of CKRT, and expanding the use of intermittent dialysis modalities such as hemodialysis (HD) and peritoneal dialysis (PD).10, 11, 12 Despite these efforts, New York hospital systems encountered CKRT shortages during the initial wave of the COVID-19 pandemic.11 , 13 , 14
In this time of uncertainty, mathematical models have informed capacity planning for ICU beds and ventilators, enabling increased ventilator production and distribution across the United States to mitigate shortages.15, 16, 17, 18 Similarly, mathematical models could improve CKRT capacity planning. The objective of this study was to develop mathematical models of CKRT demand and capacity to inform emergency planning, identify areas in which more data are needed, and mitigate CKRT shortages during the current COVID-19 pandemic and future health care crises.2 , 17, 18, 19
Methods
We developed mathematical models to project CKRT demand due to COVID-19, non–COVID-19 CKRT demand, and CKRT capacity during the initial wave of the COVID-19 pandemic. Model results were used to estimate nationwide and statewide CKRT shortages. Given the uncertainty in many of the model parameters, we first applied base-case parameter estimates and then varied them in sensitivity analysis.
CKRT Demand Due to COVID-19
Model Structure
The model simulated a US cohort of patients hospitalized due to COVID-19 between February 6, 2020, and August 4, 2020, reflecting the initial wave of the COVID-19 pandemic. We estimated new daily cases of AKI 3D from COVID-19 requiring CKRT and daily CKRT demand as follows:
-
(1)Daily CKRT demand due to COVID-19 = (new daily cases of AKI 3D from COVID-19 requiring CKRT) + (existing cases of AKI 3D from COVID-19 requiring CKRT), where
-
(i)New daily cases of AKI 3D from COVID-19 requiring CKRT = (daily number of hospitalizations for COVID-19) × (incidence of AKI 3D requiring CKRT among hospitalized patients with COVID-19), and
-
(ii)Existing cases of AKI 3D from COVID-19 requiring CKRT = (cases of AKI 3D from COVID-19 requiring CKRT on the previous day) − (cases of AKI 3D from COVID-19 no longer requiring CKRT on the current day)
-
(i)
Input Parameters
We obtained estimates of the daily number of hospitalized patients with COVID-19 from the Institute for Health Metrics and Evaluation (IHME) model (version 06/10/2020), a multistage hybrid model that uses COVID-19 death rates, viral transmission characteristics, and the impact of social interventions to provide daily estimates of hospitalizations and deaths due to COVID-19.16 The IHME model accounts for uncertainty in the number of hospitalizations in each state from fixed and random-effect estimations influenced by state characteristics.16 This range of uncertainty is used to produce several iterations of model-generated results, which are aggregated to create 95% uncertainty intervals (UIs). When appropriate, we present estimates derived from the IHME model as mean with 95% UI.
For this simulated cohort, we determined the incidence of AKI 3D requiring CKRT, the time from hospitalization to the development of AKI 3D requiring CKRT, and the duration of CKRT from the published literature (Table 1 ).3, 4, 5 , 7 , 8 , 20 , 21 We estimated an incidence of AKI 3D requiring CKRT from the largest New York study of patients with COVID-19.8 Within this study, 5.2% of hospitalized patients with COVID-19 developed AKI 3D in the ICU. Although only 46% of these patients received CKRT, this was likely due to the expanded use of HD in the ICU from CKRT capacity constraints.4 , 7 , 10 , 11 Because most of these critically ill patients would have preferentially received CKRT instead of HD in the pre–COVID-19 era, we assumed an incidence of AKI 3D requiring CKRT among hospitalized patients with COVID-19 of 5.2%.8
Table 1.
Input Parameters for Base-Case Model Simulations of CKRT Demand and Capacity During the Initial Wave of the COVID-19 Pandemic in the United States
| Parameter | Base-Case Value | Range in Sensitivity Analysis | References |
|---|---|---|---|
| CKRT demand during the initial pandemic wavea | |||
| Incidence of AKI 3D requiring CKRT among hospitalized patients with COVID-19 | 5.2% | 4.8%-6.9% | 4-8 |
| Time from hospitalization to AKI 3D requiring CKRT among hospitalized patients with COVID-19 | 7 days | 5-10 days | 20b |
| Duration of CKRT among hospitalized patients with COVID-19 | 6 days | 6-9 days | 8, 21c |
| Non–COVID-19 CKRT demand multiplier during the pandemic | 0.40 | 0.25-0.75 | 22d |
| IHME model version | 06/10/2020 | 04/22/2020, 06/10/2020 | 16e |
| CKRT capacity: CKRT capacity multiplier | 1.50 | 1.25-1.75 | –f |
| CKRT demand and capacity; prevalence of AKI 3D among ICU patients pre–COVID-19 | 8.8% | 6.6%-11.0% | 3g |
Abbreviations: AKI 3D, acute kidney injury stage 3 requiring dialysis; CKRT, continuous kidney replacement therapy; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IHME, Institute for Health Metrics and Evaluation; SEIR, susceptible, exposed, infectious, recovered.
February 6, 2020, to August 4, 2020.
In sensitivity analysis, we varied this parameter from 5 to 10 days based on presumed time from hospitalization to ICU transfer and time from ICU transfer to development of AKI 3D requiring CKRT.20
We assumed an unadjusted duration of CKRT of 8 days based on the Acute Renal Failure Trial Network Study.8 Assuming patients who died had an average CKRT duration of 4 days, we adjusted this duration to 6 days to account for the high mortality rate among patients with COVID-19 (55%).21
We assumed a base-case value of 0.40 based on a review of assumptions made in the Harvard Global Health Institute COVID-19 model.22 We chose a range of 0.25 to 0.75 based on expert opinion.
The original IHME model (including the 04/22/2020 version) estimated daily hospitalizations due to COVID-19 from COVID-19 death rates with assumptions made on the impact of social interventions on COVID-19 transmission.16 This model has been criticized as it did not specifically account for COVID-19 transmission characteristics, traditionally modeled under an SEIR framework.23 IHME updated its model and the 06/10/2020 IHME version uses a multistage hybrid model, incorporating COVID-19 transmission characteristics, death rates, and the impact of social interventions. To assess the impact of this SEIR framework and other IHME updates to the model on outcomes, we varied the IHME version between the 04/22/2020 and 06/10/2020 versions.
This assumption was based on clinical experience informed by local capacity. We confirmed the face validity of this assumption with nephrologists at 2 hospitals.
Data were obtained from a meta-analysis including 17 US studies and more than 415,000 patients with acute kidney injury in medical and surgical ICUs.3 Although this sample size provided a very narrow confidence interval, we chose a range of 6.6% to 11.0% based on expert opinion.
For the time from hospitalization to the development of AKI 3D, the same study reported a median of 2 hours with an interquartile range of −1.63 to +141 hours.8 Because the range included a negative value for time, these data were unsuitable for the model value. With insufficient US data, we estimated the time from hospitalization to the development of AKI 3D requiring CKRT from data by Zhou et al20 in China. In their study, patients developed dyspnea by day 8 and AKI 3D requiring the ICU around day 15. Assuming that patients in their study were admitted to the hospital when they developed dyspnea, we estimated a time from hospitalization to the development of AKI 3D in the ICU as 7 days (15 − 8 = 7 days).20
To estimate CKRT duration, we first used an estimate of 8 days based on the Acute Renal Failure Trial Network Study, conducted between 2003 and 2007.21 To account for a high mortality rate (55%)8 among patients with COVID-19 who require CKRT, we assumed that CKRT duration for nonsurvivors was 50% that of survivors, and the adjusted CKRT duration used in the model was estimated as follows: (4 days × 0.55) + (8 days × 0.45) ≈ 6 days.8
Non–COVID-19 CKRT Demand
Model Structure
We developed a second model to estimate non–COVID-19 CKRT demand and CKRT capacity. Within this model, we simulated the average number of occupied ICU beds across the United States between 2011 and 2016, before the COVID-19 pandemic. We estimated pre–COVID-19 CKRT demand and daily non–COVID-19 CKRT demand as follows:
-
(2)Daily non–COVID-19 CKRT demand = (pre–COVID-19 CKRT demand) × (non–COVID-19 CKRT demand multiplier), where
-
(i)Pre–COVID-19 CKRT demand = (occupied ICU beds) × (prevalence of AKI 3D among ICU patients pre–COVID-19)
-
(i)
Input Parameters
We obtained estimates of occupied ICU beds across the United States from the Harvard Global Health Institute (HGHI) model, a model that uses ICU bed numbers and occupancy rates before the COVID-19 pandemic to provide ICU bed capacity projections during the US COVID-19 pandemic.22 The HGHI model uses data for total and occupied inpatient and ICU beds from the 2018 American Hospital Association (AHA) database and the American Hospital Directory.24 The AHA database incorporates 5-year (2011-2016) hospital use trends across the United States through an annual survey. In the HGHI model, ICU bed count data missing from the AHA database were resolved using data from the American Hospital Directory.25
For this simulation of occupied ICU beds, we estimated a prevalence of AKI 3D among ICU patients pre–COVID-19 of 8.8% from a meta-analysis that included more than 415,000 patients with AKI in medical and surgical ICUs across 17 US studies (Table 1).3 Due to an anticipated decline in elective procedures and trauma surgeries during the COVID-19 pandemic, we assumed non–COVID-19 CKRT demand during the COVID-19 pandemic would decrease to 40% of pre–COVID-19 CKRT demand (through the non–COVID-19 CKRT demand multiplier of 0.40).22
CKRT Capacity
Model Structure
We used the model developed for non–COVID-19 CKRT demand to estimate CKRT capacity before the COVID-19 pandemic. With insufficient data for CKRT capacity across the United States, we estimated CKRT capacity as follows:
-
(3)CKRT capacity = (pre–COVID-19 CKRT demand) × (CKRT capacity multiplier), where
-
(i)Pre–COVID-19 CKRT demand = (occupied ICU beds) × (prevalence of AKI 3D among ICU patients pre–COVID-19)
-
(i)
Input Parameters
Our literature review revealed no publicly available data for the number of CKRT machines in the United States. Therefore, we assumed capacity was 1.50 times the pre–COVID-19 (or historical) CKRT demand. That is, for every 2 CKRT machines in use in a health care system, we assumed there was 1 additional CKRT machine available before the COVID-19 pandemic. This assumption was based on clinical experience informed by local capacity in Boston. We confirmed the face validity of this assumption with nephrologists at 2 hospitals.
CKRT Shortages During the COVID-19 Pandemic
Using these models, we estimated daily total CKRT demand as the sum of daily CKRT demand due to COVID-19 and daily non–COVID-19 CKRT demand. We compared daily total CKRT demand with daily CKRT capacity by US state to estimate CKRT shortages as follows:
-
(1)
CKRT shortage: if CKRT capacity was less than the 95% UI of CKRT demand.
-
(2)
Possible CKRT shortage: if CKRT capacity was within the 95% UI of CKRT demand.
-
(3)
No CKRT shortage: if CKRT capacity was greater than the 95% UI of CKRT demand.
Accordingly, the models projected the number of states encountering CKRT shortages, the number of states encountering possible CKRT shortages, the magnitude of shortage at peak resource use during the initial wave of the COVID-19 pandemic, and the initial date of shortage in each state. Because peak resource use occurs at different times in different states, the projected nationwide combined shortage in CKRT machines at peak resource use refers to the sum of these statewide shortages that occur at different times.
Sensitivity Analysis
To assess the impact of uncertainty in model input parameters on model outcomes, we conducted 1-way and multiway deterministic sensitivity analysis. In 1-way sensitivity analysis, key parameters influencing our estimates of CKRT demand and capacity were varied across a range of plausible values (Table 1). For example, we varied the incidence of AKI 3D requiring CKRT among hospitalized patients with COVID-19 between 4.8% and 6.9% based on data from 3 hospital systems in New York.4, 5, 6, 7, 8 Based on expert opinion, we varied the non–COVID-19 CKRT demand multiplier and the CKRT capacity multiplier between 0.25 and 0.75 and between 1.25 and 1.75, respectively. We also conducted a sensitivity analysis of the IHME model by projecting outcomes using the 06/10/2020 IHME model (base-case) and the 04/22/2020 IHME model. Additionally, in multiway deterministic sensitivity analyses, all input parameters influencing CKRT demand and capacity were simultaneously varied to examine the best-case (lowest demand, highest capacity) and worst-case (highest demand, lowest capacity) scenarios.
Results
Base-Case
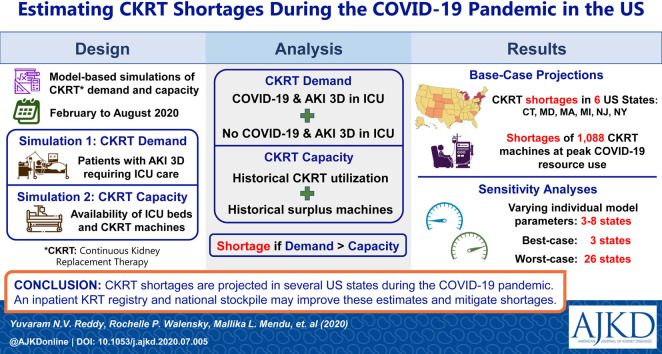
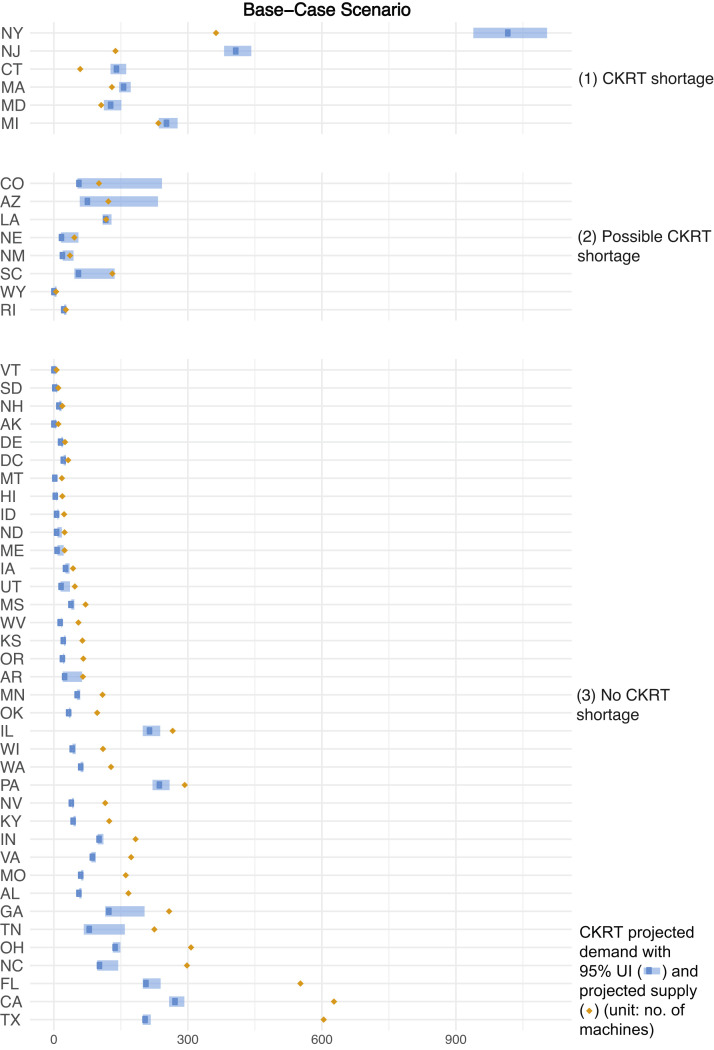
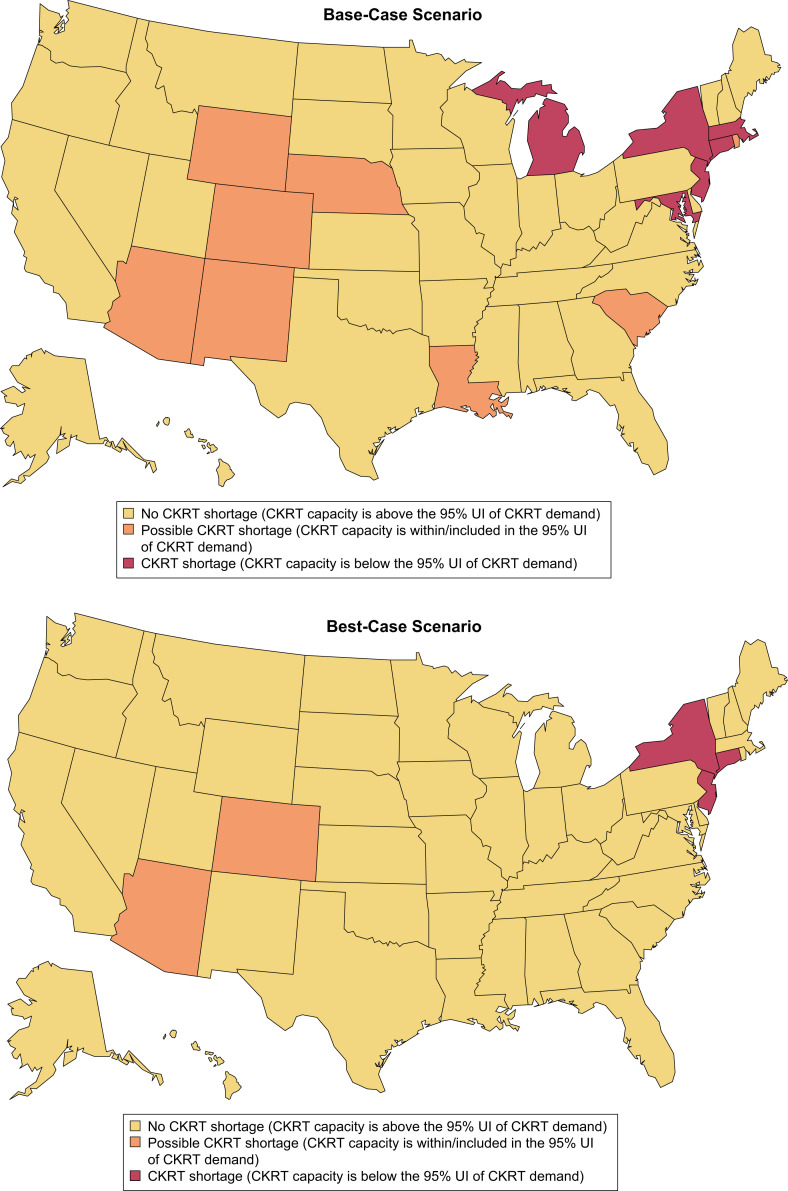
The models projected that from February 6, 2020, to August 4, 2020, cumulatively, 28,479 (95% UI, 21,974-39,338) patients with COVID-19 in the United States would require CKRT. We estimated a nationwide daily capacity of 7,032 CKRT machines (Table S1). A state-by-state comparison of CKRT demand and capacity demonstrated a combined shortage of 1,088 (95% UI, 910-1,568) machines, with shortages projected in 6 states—Connecticut, Maryland, Massachusetts, Michigan, New Jersey, and New York—at peak resource use during the initial wave of the COVID-19 pandemic. Additionally, possible CKRT shortages were projected in 8 states—Arizona, Colorado, Louisiana, Nebraska, New Mexico, Rhode Island, South Carolina, and Wyoming (Table 2 ; Fig 1 ). Model-projected dates of initial CKRT shortage are in Table S2.
Table 2.
Model-Generated CKRT Demand, Capacity, and Shortage at Peak Resource Use During the Initial Wave of the COVID-19 Pandemic
| State | CKRT Demand at Peak Resource Use (95% UI)a | CKRT Capacity | Projected CKRT Shortage | CKRT Shortage at Peak Resource Use (95% UI)a |
|---|---|---|---|---|
| Alabama | 59 (57-62) | 167 | No | — |
| Alaska | 3 (3-4) | 10 | No | — |
| Arizonab | 78 (58-233) | 122 | Possible | 0 (0-110) |
| Arkansas | 27 (20-63) | 65 | No | — |
| California | 274 (258-292) | 627 | No | — |
| Coloradob | 59 (53-242) | 101 | Possible | 0 (0-141) |
| Connecticutc | 143 (127-162) | 59 | Yes | 85 (68-104) |
| Delaware | 18 (17-20) | 25 | No | — |
| District of Columbia | 24 (22-27) | 32 | No | — |
| Florida | 209 (199-239) | 552 | No | — |
| Georgia | 126 (115-203) | 258 | No | — |
| Hawaii | 6 (6-7) | 19 | No | — |
| Idaho | 9 (8-10) | 23 | No | — |
| Illinois | 217 (199-238) | 266 | No | — |
| Indiana | 104 (98-111) | 183 | No | — |
| Iowa | 29 (26-35) | 43 | No | — |
| Kansas | 24 (23-26) | 64 | No | — |
| Kentucky | 46 (44-49) | 124 | No | — |
| Louisianab | 119 (109-129) | 117 | Possible | 2 (0-12) |
| Maine | 10 (8-22) | 24 | No | — |
| Marylandc | 130 (112-151) | 106 | Yes | 24 (5-45) |
| Massachusettsc | 159 (146-172) | 130 | Yes | 29 (16-42) |
| Michiganc | 255 (235-277) | 234 | Yes | 21 (1-43) |
| Minnesota | 55 (52-59) | 109 | No | — |
| Mississippi | 41 (39-46) | 71 | No | — |
| Missouri | 63 (61-66) | 161 | No | — |
| Montana | 5 (5-5) | 18 | No | — |
| Nebraskab | 20 (16-55) | 46 | Possible | 0 (0-9) |
| Nevada | 42 (41-44) | 115 | No | — |
| New Hampshire | 14 (12-17) | 19 | No | — |
| New Jerseyc | 410 (381-442) | 138 | Yes | 272 (243-304) |
| New Mexicob | 22 (20-44) | 36 | Possible | 0 (0-8) |
| New Yorkc | 1,019 (939-1,104) | 363 | Yes | 656 (576-741) |
| North Carolina | 105 (97-144) | 298 | No | — |
| North Dakota | 9 (8-18) | 24 | No | — |
| Ohio | 140 (132-149) | 307 | No | — |
| Oklahoma | 36 (34-37) | 97 | No | — |
| Oregon | 22 (21-23) | 66 | No | — |
| Pennsylvania | 239 (221-259) | 293 | No | — |
| Rhode Islandb | 25 (23-28) | 27 | Possible | 0 (0-1) |
| South Carolinab | 58 (46-136) | 131 | Possible | 0 (0-6) |
| South Dakota | 5 (5-6) | 10 | No | — |
| Tennessee | 82 (67-159) | 225 | No | — |
| Texas | 207 (199-217) | 604 | No | — |
| Utah | 19 (15-36) | 47 | No | — |
| Vermont | 3 (2-3) | 6 | No | — |
| Virginia | 89 (84-94) | 173 | No | — |
| Washington | 34 (60-66) | 128 | No | — |
| West Virginia | 17 (17-18) | 55 | No | — |
| Wisconsin | 44 (42-49) | 110 | No | — |
| Wyomingb | 3 (2-6) | 5 | Possible | 0 (0-1) |
Note: This analysis uses the base-case values for the input parameters listed in Table 1. Minor discrepancies in numerical values in the table are due to rounding.
Abbreviations: CKRT, continuous kidney replacement therapy; COVID-19, coronavirus disease 2019; UI, uncertainty interval.
We derived these estimates from the Institute for Health Metrics and Evaluation model and present them as means with 95% UI.16
Represents states that could possibly encounter a shortage (where CKRT capacity is within the 95% UI of CKRT demand).
Represents states that are projected to encounter a shortage (where CKRT capacity is below the 95% UI of CKRT demand).
Figure 1.
Continuous kidney replacement therapy (CKRT) shortages by state during the initial wave of the coronavirus disease 2019 (COVID-19) pandemic; base-case scenario. Estimates were model-generated. Group (1) represents all states projected to encounter a CKRT shortage, where CKRT capacity is below the 95% uncertainty interval (UI) of CKRT demand; group (2), states that may encounter a CKRT shortage, where CKRT capacity is within the 95% UI of CKRT demand; group (3), states not anticipated to encounter a CKRT shortage, where CKRT capacity is above the 95% UI of CKRT demand.
One-Way Sensitivity Analysis
Sensitivity analysis of the CKRT demand input parameters demonstrated shortages in 4 to 8 states (945-1,723 machines) when the incidence of AKI 3D requiring CKRT among hospitalized patients with COVID-19 was varied between 4.8% and 6.9%, shortages in 4 to 8 states (986-1,388 machines) when the non–COVID-19 CKRT demand multiplier during the COVID-19 pandemic was varied between 0.25 and 0.75, shortages in 6 to 8 states (1,088-2,067 machines) when the duration of CKRT among hospitalized patients with COVID-19 was varied between 6 and 9 days, and no change in the number of states with shortages (or the number of machines in shortage) when the time from hospitalization to AKI 3D requiring CKRT among hospitalized patients with COVID-19 was varied between 5 and 10 days (Tables S3-S6).
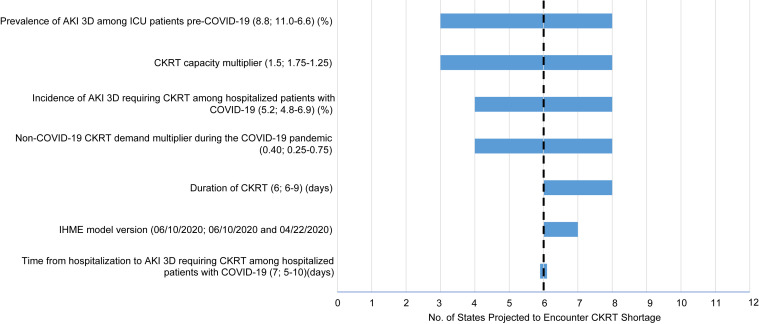
Similarly, sensitivity analysis demonstrated shortages in 3 to 8 states (919-1,302 machines) when the prevalence of AKI 3D among ICU patients pre–COVID-19 (influencing CKRT demand and capacity) was varied between 11.0% and 6.6%, shortages in 3 to 8 states (933-1,282 machines) when the CKRT capacity multiplier (influencing CKRT capacity) was varied between 1.75 and 1.25, and shortages in 6 to 7 states (1,088-1,239 machines) when the IHME model estimates used were varied between the June 10, 2020 (base-case), and the April 22, 2020, version (Tables S7-S9). The impact of uncertainty in these input parameters on the outcome of number of states encountering CKRT shortages is summarized in Figure 2 .
Figure 2.
One-way sensitivity analysis of the number of states projected to encounter continuous kidney replacement therapy (CKRT) shortage during the initial wave of the coronavirus disease 2019 (COVID-19) pandemic. The horizontal axis of this tornado diagram shows the number of states projected to encounter a CKRT shortage. The vertical axis shows key input parameters. The base-case value for each input parameter is listed in parentheses before the semicolon. The range across which we varied each parameter is listed after the semicolon. The number on the left in the range corresponds to the left end of the horizontal bar, and the number on the right in the range corresponds to the right end of the horizontal bar. The dashed vertical line represents the base-case scenario. As shown, the CKRT capacity multiplier has the greatest impact on the outcome of number of states projected to encounter CKRT shortage during the initial wave of the COVID-19 pandemic. Abbreviations: AKI 3D, acute kidney injury stage 3 requiring dialysis; ICU, intensive care unit; IHME, Institute for Health Metrics and Evaluation.
Multiway Sensitivity Analysis
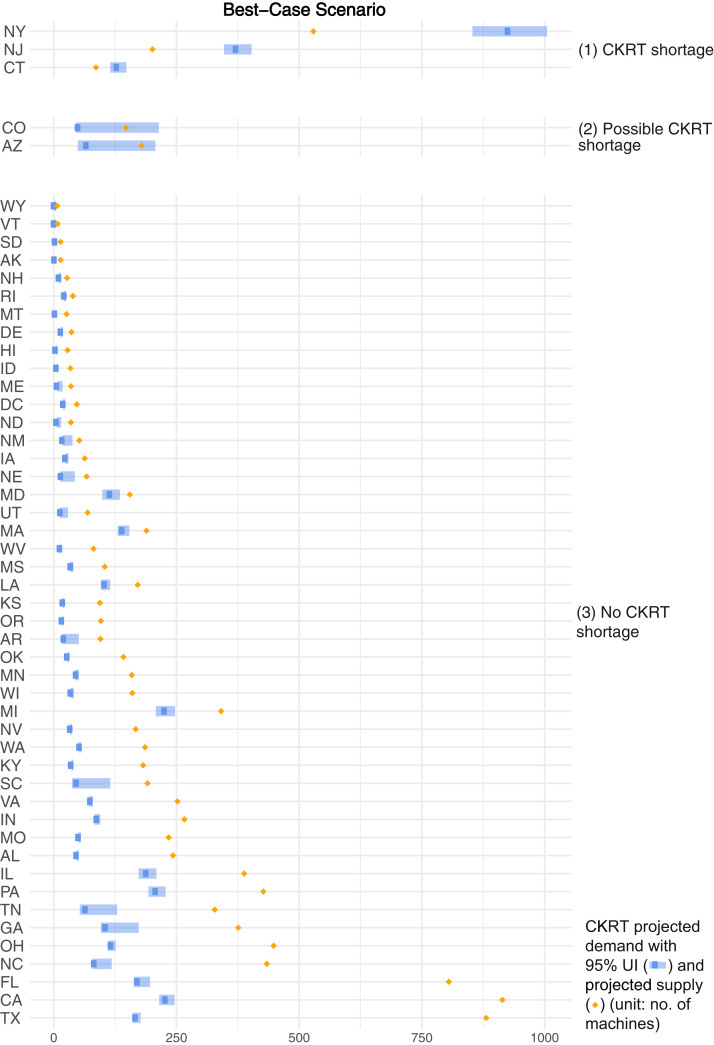
In the best-case scenario (lowest demand, highest capacity), projections demonstrated that from February 6, 2020, to August 4, 2020, a total of 26,053 (95% UI, 20,229-35,523) patients with COVID-19 in the United States would require CKRT. We estimated a nationwide daily capacity of 10,254 CKRT machines (Table S10). A state-by-state comparison demonstrated a combined shortage of 614 (95% UI, 498-834) machines, with shortages projected in 3 states—Connecticut, New Jersey, and New York—at peak resource use during the initial wave of the COVID-19 pandemic. Additionally, there were possible shortages in 2 states—Arizona and Colorado (Fig 3 ).
Figure 3.
Continuous kidney replacement therapy (CKRT) shortages by state during the initial wave of the coronavirus disease 2019 (COVID-19) pandemic; best-case scenario. Estimates were model-generated. Group (1) represents all states projected to encounter a CKRT shortage, where CKRT capacity is below the 95% uncertainty interval (UI) of CKRT demand; group (2), states that may encounter a CKRT shortage, where CKRT capacity is within the 95% UI of CKRT demand; group (3), states not anticipated to encounter a CKRT shortage, where CKRT capacity is above the 95% UI of CKRT demand. The best-case scenario projected by the model is obtained when the input parameters are varied simultaneously as follows: (1) incidence of acute kidney injury stage 3 requiring dialysis (AKI 3D) requiring CKRT among hospitalized patients with COVID-19: 4.8%; (2) time from hospitalization to AKI 3D: 10 days; (3) duration of CKRT: 6 days; (4) non–COVID-19 CKRT demand multiplier during the initial wave of the COVID-19 pandemic: 0.25; (5) prevalence of AKI 3D among intensive care unit patients pre–COVID-19: 11.0%; and (6) CKRT capacity multiplier: 1.75.
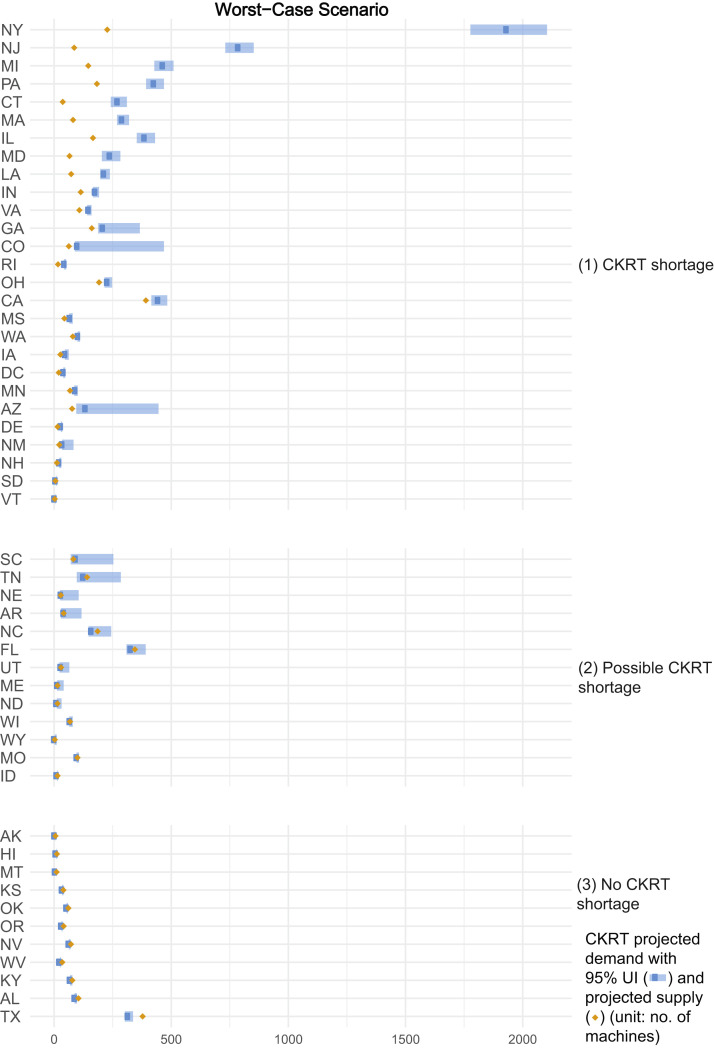
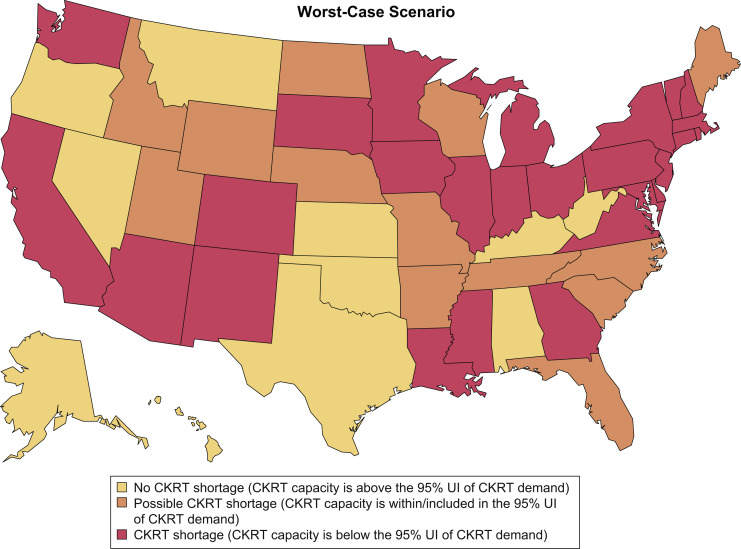
In the worst-case scenario (highest demand, lowest capacity), projections demonstrated that from February 6, 2020, to August 4, 2020, a total of 38,013 (95% UI, 29,208-52,978) patients with COVID-19 in the United States would require CKRT. We estimated a nationwide daily capacity of 4,395 CKRT machines (Table S11). A state-by-state comparison demonstrated a combined shortage of 4,540 (95% UI, 3,886-6,692) machines, with shortages projected in 26 states at peak resource use during the initial wave of the COVID-19 pandemic. There were possible shortages in 13 other states (Fig 4 ). The impact of uncertainty in multiway sensitivity analysis on the outcome of number of states encountering CKRT shortages is summarized in heat maps of the base-case, best-case, and worst-case scenarios in Figure 5 .
Figure 4.
Continuous kidney replacement therapy (CKRT) shortages by state during the initial wave of the coronavirus disease 2019 (COVID-19) pandemic; worst-case scenario. Estimates were model-generated. Group (1) represents all states projected to encounter a CKRT shortage, where CKRT capacity is below the 95% uncertainty interval (UI) of CKRT demand; group (2), states that may encounter a CKRT shortage, where CKRT capacity is within the 95% UI of CKRT demand; group (3), states not anticipated to encounter a CKRT shortage, where CKRT capacity is above the 95% UI of CKRT demand. The worst-case scenario projected by the model is obtained when the input parameters are varied simultaneously as follows: (1) incidence of acute kidney injury stage 3 requiring dialysis (AKI 3D) requiring CKRT among hospitalized patients with COVID-19: 6.9%; (2) time from hospitalization to AKI 3D: 5 days; (3) duration of CKRT: 9 days; (4) non–COVID-19 CKRT demand multiplier during the initial wave of the COVID-19 pandemic: 0.75; (5) prevalence of AKI 3D among intensive care unit patients pre–COVID-19: 6.6%; and (6) CKRT capacity multiplier: 1.25.
Figure 5.
Heat maps demonstrating states with continuous kidney replacement therapy (CKRT) shortages during the initial wave of the coronavirus disease 2019 (COVID-19) pandemic in the base-case, best-case, and worst-case scenario. The base-case scenario uses input parameters listed in the base-case value column of Table 1. The best-case scenario uses the highest CKRT capacity estimate and lowest CKRT demand estimate, which is obtained when the input parameters are varied simultaneously as detailed in the legend to Figure 3. The worst-case scenario uses the lowest CKRT capacity estimate and highest CKRT demand estimate, which is obtained when the input parameters are varied simultaneously as detailed in the legend to Fig 4. Abbreviation: UI, uncertainty interval.
Discussion
Our models provide estimates of CKRT demand and capacity in the United States. The models projected a nationwide shortage of 1,088 CKRT machines (95% UI, 910-1,568) across 6 US states—Connecticut, Maryland, Massachusetts, Michigan, New Jersey, and New York—with possible shortages in 8 additional states—Arizona, Colorado, Louisiana, Nebraska, New Mexico, Rhode Island, South Carolina, and Wyoming—during the initial wave of the COVID-19 pandemic. Concordant with model findings, hospital systems in New York, Massachusetts, and Louisiana encountered shortages in CKRT machines, solutions, cartridges, and/or trained personnel that were managed through the expansion of intermittent dialysis modalities and a decrease in CKRT dose and duration.8 , 13 , 26 , 27 However, although individual hospital systems reported shortages, due to a lack of consistent reporting of CKRT demand and capacity, it is unclear whether these shortages occurred throughout each state with a projected shortage in our models. Apart from anecdotal data from the press, webinars, and social media, little is otherwise known about the actual state of CKRT demand and capacity in the United States.13 , 26 , 28
Within these models, limited US data led to uncertainty. In sensitivity analysis, uncertainty in CKRT demand input parameters (such as the incidence of AKI 3D and the duration of CKRT among hospitalized patients with COVID-19) had the largest impact on the model outcome of the number of machines in shortage at peak resource use during the COVID-19 pandemic. For example, the range of the incidence of AKI 3D requiring CKRT among hospitalized patients with COVID-19 in the models (4.8%-6.9%) was derived from 3 New York counties, where the incidence was considerably higher than in other regions such as China (1.45%-2.3%).4, 5, 6, 7, 8, 9 , 29 In the absence of data from other US states, it is unclear whether this high incidence is reflective of the rest of the United States.
Similarly, uncertainty in CKRT capacity input parameters (such as the CKRT capacity multiplier) had the largest impact on the model outcome of number of states projected to encounter CKRT shortages. This is not unexpected because a lack of data on the number of CKRT machines in each state forced the use of assumptions to estimate CKRT capacity as a multiple of pre–COVID-19 (or historical) CKRT demand. Varying this parameter between 1.25 and 1.75 predictably resulted in a lower or higher surplus of CKRT machines, changing the threshold at which a state may encounter a CKRT shortage. More data from US states on the number of CKRT machines available (capacity) and in use (demand) would allow future model-based analyses to provide more precise estimates of CKRT shortages.
Although the assumptions made on CKRT demand and capacity allowed projections of plausible results at a nationwide and statewide level, these projections are insufficiently granular to hold true at the county, health care system, and hospital levels. As such, these models may not be useful for county- or hospital-level decision making. Instead, these models provide high-level projections of CKRT shortages and highlight the need for reliable nationwide and local data on the number of CKRT machines available and in use in each system.
In the absence of reliable data on CKRT machine availability, recommendations during the COVID-19 pandemic have been for all systems to conserve KRT (CKRT, HD, and PD) supplies and standardize lower dialysate patient prescriptions in fear of an imminent shortage.10 , 11 This has led hospitals to race to purchase more KRT machines and supplies, creating a competition for machines.11 , 28 , 30 If publicly available data on KRT capacity existed, hospitals could collaborate during health care crises to mitigate shortages while continuing to provide the standard of care. Although this analysis focused on CKRT machines, estimates of CKRT demand and capacity could be further improved if data were available for all inpatient KRT machines, supplies, and personnel.11
The current lack of standardized reporting of data on inpatient KRT machines, supplies, and personnel is an impediment to emergency preparedness; strategies to improve data collection are urgently needed. Creating a national multidisciplinary task force comprising key stakeholders—the federal government, the nephrology community, industry, and patients—could improve data collection and emergency preparedness planning for KRT. Considerations for a task force include: (1) developing a national registry of inpatient KRT machines, supplies, and personnel; (2) creating a national stockpile of KRT machines and supplies; and (3) adding questions about the number of CKRT, HD, and PD machines in each hospital to the American Hospital Association annual hospital survey.
Notably, as hospitals return to standard capacities toward the eventual end of the COVID-19 pandemic, many will be left with surplus CKRT machines. This creates a unique opportunity to improve emergency preparedness because the federal government could repurpose these surplus machines to provide relief for future waves of the COVID-19 pandemic and other health care crises.11 , 30 With these strategies in place to collect data on the number and availability of KRT machines, subsequent iterations of mathematical models could help determine the optimal number of KRT machines needed for a national stockpile, inform triage of machines to areas of need, and prompt early manufacturing of KRT supplies for future health care crises.
In the interim, pragmatic research is needed to study new practices borne out of necessity from the COVID-19 pandemic. For example, concerns of CKRT shortages led to recommendations to standardize CKRT dosing and duration.9 Prior studies have shown a benefit to adopting standardized criteria for initiation of KRT.31 If the outcome of these CKRT recommendations during the COVID-19 pandemic suggests no harm, this standardization of dosing can help conserve dialysis solutions. Similarly, due to shortages, urgent-start PD has also expanded in the inpatient setting.12 , 32, 33, 34 Although short-term outcomes of urgent-start PD during the COVID-19 pandemic suggest safety, longer-term results on peritonitis, technique failure, and mortality are needed to assess the benefit of this program.12 , 32 , 33 Successful practices from the COVID-19 pandemic, if studied appropriately, could help avoid shortages and improve patient outcomes during future health care crises.
There are limitations to this analysis. First, the model results are subject to simplifications and assumptions. Sensitivity analysis demonstrates the influence of these assumptions on the results. The models use IHME model estimates and are subject to that model’s limitations.23 In particular, early versions of the IHME model did not account for viral transmission characteristics, traditionally done with a susceptible, exposed, infectious, recovered (SEIR) framework. This study used estimates from the 06/10/2020 IHME model, which is an improved multistage hybrid model that incorporates an SEIR framework. The impact of this SEIR framework on model outcomes can be seen in the sensitivity analyses, in which the absence of this framework in the 04/22/2020 IHME model resulted in 1 additional state (Louisiana) encountering a CKRT shortage, with 151 additional machines in shortage at peak resource use during the initial wave of the COVID-19 pandemic.
Second, due to the dynamic nature of the COVID-19 pandemic, subtle characteristics of model results from IHME such as the exact date of peak resource use should be interpreted cautiously.35 Fortunately, because the IHME model is updated periodically, we anticipate future IHME iterations will allow for more precise projections over time.16
Third, we assumed that all patients with AKI 3D in the ICU receive CKRT. As hospitals are faced with a surge in AKI 3D, the use of intermittent dialysis modalities in the ICU have expanded.23 To the extent that supplies and personnel for these modalities are available, results may underestimate total KRT capacity in the ICU.10
Finally, although we conducted a deterministic multiway sensitivity analysis, this approach tends to overweight extreme values compared with probabilistic sensitivity analysis.36 Given the evolving nature of COVID-19 and the limited data on these input parameters, we were unable to generate more specific distributions for the model input parameters at the time of manuscript submission. Policymakers are cautioned to avoid overvaluing the likelihood of the best-case and worst-case scenarios presented in this report.
In conclusion, several US states could encounter CKRT shortages at peak resource use during the initial wave of the COVID-19 pandemic. More complete and reliable data on CKRT demand and capacity would improve the estimates of future model-based analyses. Strategies such as the creation of an inpatient KRT national registry and a national stockpile to bolster state capacity should be considered to mitigate CKRT shortages during the COVID-19 pandemic and future health care crises.
Article Information
Authors’ Full Names and Academic Degrees
Yuvaram N.V. Reddy, MBBS, Rochelle P. Walensky, MD, MPH, Mallika L. Mendu, MD, MBA, Nathaniel Green, MBA, MS, and Krishna P. Reddy, MD, MS.
Authors’ Contributions
Study concept: YNVR; study design: YNVR, RPW, KPR; data acquisition: YNVR, NG; model generation: YNVR, NG, KPR; data analysis and interpretation: YNVR, RPW, MLM, NG, KPR; supervision: RPW, MLM, KPR. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) under award number T32DK007527 supporting Dr Y. Reddy and the Massachusetts General Hospital Executive Committee on Research Stephen and Deborah Gorlin Research Scholar Award supporting Dr Walensky. The NIH and Massachusetts General Hospital had no role in the study design; collection, analysis, and interpretation of the data; writing the report; or the decision to submit the report for publication. Mr Green works at American Biomedical Group, Inc, a company with diversified product/R&D arms. One aim of this company is to limit expenditures on biomedical equipment while providing higher quality biomedical support services. Mr Green analyzes capital expenditures for biomedical equipment. Within this company, he also works on AllTraq, a tracking technology product that locates equipment and personnel in GPS-denied environments. His company, American Biomedical Group, Inc, had no role in study design; collection, analysis, and interpretation of data; writing the report; funding for the study (apart from employment of Mr Green); or the decision to submit the report for publication. Specifically, the company did not impose any limits on authors’ access to the study data or the content of the report.
Financial Disclosure
Dr Mendu provides consulting services to Bayer AG. The remaining authors declare that they have no other relevant financial interests.
Acknowledgements
The authors thank Drs Andrew Allegretti and Finnian Mc Causland, who provided face validity on the assumptions used in the model in the midst of their busy clinical commitments during the COVID-19 pandemic, and Ms Giulia E. Park, Ms Tijana Stanic, and Mr Aditya R. Gandhi for technical assistance.
Disclaimer
This work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Peer Review
Received May 21, 2020. Evaluated by 3 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form July 22, 2020.
Footnotes
Complete author and article information provided before references.
Table S1: Model-generated statewide estimates of occupied ICU beds and CKRT demand and capacity before the pandemic.
Table S2: Model-generated initial CKRT shortage date in the base-case, best-case, and worst-case scenarios during the initial wave of the pandemic.
Table S3: Effect of varying the incidence of AKI 3D requiring CKRT among hospitalized patients with COVID-19 on a) nationwide CKRT shortage at peak resource use in each state and b) number of states projected to encounter CKRT shortage during the initial wave of the pandemic.
Table S4: Effect of varying the non–COVID-19 CKRT demand multiplier during the pandemic on model-generated a) nationwide CKRT shortage at peak resource use in each state and b) number of states projected to encounter CKRT shortage during the initial wave of the pandemic.
Table S5: Effect of varying the duration of CKRT among hospitalized patients with COVID-19 on a) nationwide CKRT shortage at peak resource use in each state and b) number of states projected to encounter CKRT shortage during the initial wave of the pandemic.
Table S6: Effect of varying the time from hospitalization to AKI 3D requiring CKRT among hospitalized patients with COVID-19 on a) nationwide CKRT shortage at peak resource use in each state and b) number of states projected to encounter CKRT shortage during the initial wave of the pandemic.
Table S7: Effect of varying the prevalence of AKI 3D among ICU patients pre–COVID-19 on model-generated a) nationwide CKRT shortage at peak resource use in each state and b) number of states projected to encounter CKRT shortage during the initial wave of the pandemic.
Table S8: Effect of varying the CKRT capacity multiplier on a) nationwide CKRT shortage at peak resource use in each state and b) number of states projected to encounter CKRT shortage during the initial wave of the pandemic.
Table S9: Effect of varying the IHME model version on a) nationwide CKRT shortage at peak resource use in each state and b) number of states projected to encounter CKRT shortage during the initial wave of the pandemic.
Table S10: Multiway sensitivity analysis assessing CKRT demand, capacity, and shortage at peak resource use during the initial wave of the pandemic; best-case scenario.
Table S11: Multiway sensitivity analysis assessing CKRT demand, capacity, and shortage at peak resource use during the initial wave of the pandemic; worst-case scenario.
Supplementary Material
Tables S1-S11.
References
- 1.Center for Systems Science and Engineering Coronavirus COVID-19 global cases. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 2.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages - the need for ventilators and personal protective equipment during the COVID-19 pandemic. N Engl J Med. 2020;382(18):e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 3.Melo F.A.F., Macedo E., Fonseca Bezerra A.C., et al. A systematic review and meta-analysis of acute kidney injury in the intensive care units of developed and developing countries. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0226325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argenziano M.G., Bruce S.L., Slater C.L., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naicker S., Yang C.W., Hwang S.J., Liu B.C., Chen J.H., Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgner A., Ikizler T.A., Dwyer J.P. COVID-19 and the inpatient dialysis unit. Managing resources during contingency planning pre-crisis. Clin J Am Soc Nephrol. 2020;15(5):720–722. doi: 10.2215/CJN.03750320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfarb D.S., Benstein J.A., Zhdanova O., et al. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol. 2020;15(6):880–882. doi: 10.2215/CJN.05180420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sourial M.Y., Sourial M.H., Dalsan R., et al. Urgent Peritoneal dialysis in patients with COVID-19 and acute kidney injury: a single-center experience in a time of crisis in the United States. Am J Kidney Dis. 2020;76(3):401–406. doi: 10.1053/j.ajkd.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.New York Times An overlooked, possibly fatal coronavirus crisis: a dire need for kidney dialysis. https://www.nytimes.com/2020/04/18/health/kidney-dialysis-coronavirus.html
- 14.New York Times A life and death battle: 4 days of kidney failure but no dialysis. https://www.nytimes.com/2020/05/01/health/coronavirus-dialysis-death.html
- 15.New York Times These places could run out of hospital beds as coronavirus spreads. https://www.nytimes.com/interactive/2020/03/17/upshot/hospital-bed-shortages-coronavirus.html
- 16.Murray C.J. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator-days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1 Preprint at.
- 17.Emanuel E.J., Persad G., Upshur R., et al. Fair allocation of scarce medical resources in the time of COVID-19. N Engl J Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 18.Truog R.D., Mitchell C., Daley G.Q. The toughest triage - allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973–1975. doi: 10.1056/NEJMp2005689. [DOI] [PubMed] [Google Scholar]
- 19.Reuters GM Accelerates ‘Project V’ to Build Ventilators in Indiana. https://www.reuters.com/article/us-health-coronavirus-autos-ventilators/gm-accelerates-project-v-to-build-ventilators-in-indiana-idUSKBN21A2Y4
- 20.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palevsky P.M., Zhang J.H., O’Connor T.Z., et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvard Global Health Institute COVID-19 model. https://docs.google.com/spreadsheets/d/1XUVyZF3X_4m72ztFnXZFvDKn5Yys1aKgu2Zmefd7wVo/edit#gid=1576394115
- 23.Jewell N.P., Lewnard J.A., Jewell B.L. Caution warranted: using the Institute for Health Metrics and Evaluation model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173(3):226–227. doi: 10.7326/M20-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Hospital Association . American Hospital Association; Chicago, IL: 2018. AHA Hospital Statistics. [Google Scholar]
- 25.American Hospital Directory Hospital statistics by state. https://www.ahd.com/state_statistics.html
- 26.Kliger A., Mokrzycki M., Vijayan A., et al. Hospital care and treatment options for COVID-19 positive patients with ESKD and AKI. American Society of Nephrology Webinar presented April 2, 2020. https://www.asn-online.org/g/blast/files/ASN_Webinar_Hospital_Care_And_Treatment_Options_for_COVID19_Positive_Patients_with_ESKD_and_AKI_04.02.2020a.pdf
- 27.Sise M.E., Baggett M.V., Shepard J.-A.O., Stevens J.S., Rhee E.P. Case 17-2020: a 68-year-old man with COVID-19 and acute kidney injury. N Engl J Med. 2020;382(22):2147–2156. doi: 10.1056/NEJMcpc2002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijayan A., Koyner J., Heung M., et al. Overcoming challenges to the provision of acute dialysis for COVID-19 positive patients. American Society of Nephrology Webinar presented April 30, 2020. https://www.asn-online.org/g/blast/files/COVID19%20Overcoming_Challenges%2004.30.20%20Slide%20Handout.pdf
- 29.Zhang G., Hu C., Luo L., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Propublica Without Federal Help, New York Doctors Had to Ask Medical Supply Execs for Dialysis Supplies. https://www.propublica.org/article/without-federal-help-new-york-doctors-had-to-ask-medical-supply-execs-for-dialysis-supplies
- 31.Mendu M.L., Ciociolo G.R., McLaughlin S.R., et al. A decision-making algorithm for initiation and discontinuation of RRT in severe AKI. Clin J Am Soc Nephrol. 2017;12(2):228–236. doi: 10.2215/CJN.07170716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Shamy O., Patel N., Abdelbaset M.H., et al. Acute start peritoneal dialysis during the COVID-19 pandemic: outcomes and experiences. J Am Soc Nephrol. 2020;31(8):1680–1682. doi: 10.1681/ASN.2020050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigiola Cruz M., Bellorin O., Srivatana V., Afaneh C. Safety and efficacy of bedside peritoneal dialysis catheter placement in the COVID-19 era: initial experience at a New York City Hospital. World J Surg. 2020;44(8):2464–2470. doi: 10.1007/s00268-020-05600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Shamy O., Sharma S., Winston J., Uribarri J. Peritoneal dialysis during the coronavirus 2019 (COVID-19) pandemic: acute inpatient and maintenance outpatient experiences. Kidney Med. 2020;2(4):377–380. doi: 10.1016/j.xkme.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jewell N.P., Lewnard J.A., Jewell B.L. Predictive mathematical models of the COVID-19 pandemic: underlying principles and value of projections. JAMA. 2020;323(19):1893–1894. doi: 10.1001/jama.2020.6585. [DOI] [PubMed] [Google Scholar]
- 36.Baio G., Dawid A.P. Probabilistic sensitivity analysis in health economics. Stat Methods Med Res. 2015;24(6):615–634. doi: 10.1177/0962280211419832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S11.