Abstract
Glaucoma is a leading cause of irreversible blindness worldwide. Reducing intraocular pressure is currently the only effective treatment. Elevated intraocular pressure is associated with increased resistance of outflow pathway, mainly the trabecular meshwork (TM). Despite great progress in the field, the development of novel and effective treatment for glaucoma is still challenging. In this study, we reported that human induced pluripotent stem cells (iPSCs) can be cultured as colonies and monolayer cells expressing OCT4, alkaline phosphatase, SSEA4 and SSEA1. After induction to NCCs positive to NGFR and HNK1, the iPSCs can differentiate into TM cells. The induced iPSC-TM cells expressed TM cell marker CHI3L1, were responsive to dexamethasone treatment with increased expression of myocilin, ANGPTL7, and formed CLANs, comparable to primary TM cells. To the best of our knowledge, this is the first study that induces iPSCs to TM cells through a middle neural crest stage, which ensures a stable NCC pool and ensures the high output of same TM cells. This system can be used to develop personalized treatments using patient-derived iPSCs, explore high throughput screening of new drugs focusing on TM response for controlling intraocular pressure, and investigate stem cell-based therapy for TM regeneration.
Keywords: Induced Pluripotent Stem Cells, Glaucoma, Neural Crest, Trabecular Meshwork
Graphical Abstract
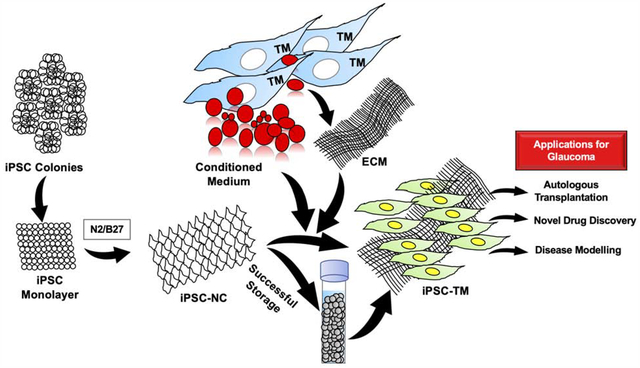
Introduction:
Vision is one of the most critical senses humans require. Glaucoma is a disease that destroys people’s ability to see the world. It is characterized as a chronic optic neuropathy leading to irreversible vision loss. Increased intraocular pressure (IOP) is the main risk factor of glaucoma, and causes retinal ganglion cell death and vision loss[1]. Primarily, increased IOP is related to increased outflow resistance of the conventional outflow pathway, mainly the trabecular meshwork (TM)[2]. In primary open-angle glaucoma patients, the TM cellularity is reduced, which results in reduced physiological function of TM tissue and enhanced aqueous humor outflow resistance[3,4]. Therefore, restitution of TM cellularity represents a promising approach for glaucoma treatment. Researchers are exploring possible stem cell therapies using trabecular meshwork stem cells (TMSCs), mesenchymal stem cells, or iPSCs to restore TM structure and function [5–12]. All these studies used animal models and indicate that stem cell-based therapy for glaucoma is feasible. However, there is a need for an unlimited cell source for in vitro TM cellular models to explore the mechanisms of stem cell therapy. It could be used in the discovery of novel drugs for glaucoma, which can circumvent the need for maintenance and sacrifice of so many animals. iPSCs offer an uninhibited approach to addressing this need as these cells can be derived in large quantities by using any somatic cells and transfecting them with Yamanaka factors OCT4, KLF4, SOX2, and cMyc[13]. These patient-specific iPSCs can be induced to differentiate into TM cells, hence yielding a readily available population of patient-specific TM cells for autologous transplantation or disease modelling to test novel drugs. iPSCs have the ability to differentiate into TM cells and reduce IOP ex vivo or in vivo [6–8]. Since the TM cells arise from the neural crest during embryonic development[14], we hypothesize that iPSCs can be induced to differentiate into neural crest cells (NCCs) first which maintain a stable cell pool for further differentiating into TM cells with high output. This will ensure the same TM cells for developing personized therapies and new drug screening. Researchers have discovered that NCCs can be derived from iPSCs [15], but there have been no studies to date reporting the differentiation of NCCs to TM cells in vitro. In this study, we have used a novel approach to differentiate human iPSCs into TM cells via an intermediate NCC stage, using conditioned media and extracellular matrix (ECM) from primary human TM cells. These differentiated human iPSC-TM cells offer a valuable resource for mechanistic studies of stem cell therapy, high throughput novel drug discovery, and autologous cell transplantation to restore TM cellularity in glaucoma.
Material and Methods:
Cell culture:
Human iPSCs were reprogramed from human dermal fibroblasts and characterized previously[16]. Then, the iPSCs were cultured using mTeSR medium (STEMCELL Technologies) supplemented with FGF2 beads (StemCultures) to maintain a concentration of FGF2 at 100ng/ml, on Matrigel (Corning)-coated plates without feeder cells. Medium was changed every third day or alternate day depending on the colony confluence. iPSC colonies were passaged by manually separating colonies into small pieces using a StemPro EZPassage tool (ThermoFisher). Primary TM cells were cultured as previously described [9]. TM conditioned medium and TM cell-derived ECM were prepared as reported[12]. The study was conducted under an Institutional Biosafety Committee protocol approved by University of Pittsburgh.
Cell differentiation:
For induction into NCCs, iPSC colonies were first dissociated by incubation with Accutase and seeded on Matrigel-coated plates and cultured as a monolayer in mTeSR. The cells were passaged onto plates coated with A549 cells (a kind gift from Dr. James Funderburgh, University of Pittsburgh)-derived ECM in mTeSR for 3 days and switched to N2B27 medium with 100 mM Rho kinase inhibitor Y27632 to induce NCC differentiation for 10 days. N2B27 medium consisted of Neurobasal: DMEM/F12 (1:1) supplemented with 2% B27, 1% N2, 100X Glutamax, 100U/ml penicillin, and 100mg/ml streptomycin (Invitrogen). NCCs were characterized by flow cytometry and immunofluorescent staining for neural crest markers NGFR (CD271) and HNK1. After induction, NCCs were dissociated using Accutase and seeded on TM ECM-coated cell culture plates. TM-ECM was generated by completely lysing confluent TM cells using 0.02 N ammonium hydroxide + 0.05% Triton X 100 for 5 minutes[12]. TM-ECM coated plates were immediately used or stored at −20°C until future use. The medium used for TM differentiation was TM cell conditioned medium: DMEM/F12 (1:1) with 10% FBS. Cells were induced 10–14 days until clear morphological changes were observed. Post differentiation, iPSC-TM cells were characterized by immunofluorescent staining for TM cell marker CHI3L1 and confirmed by responsiveness to dexamethasone (Dex) treatment. iPSC-TM cells were treated with 100nM Dex (Sigma-Aldrich) for 5 days for assessment of CHI3L1, myocilin ANGPTL7 expression by qPCR/immunofluorescent staining, or 14 days to detect cross-link actin networks (CLANs) by phalloidin staining.
Flow cytometry:
Differentiated NCCs were dissociated using Accutase, blocked with 1% bovine serum albumin, and stained with antibodies NGFR-APC and SSEA4-FITC for 30 minutes in dark. The stained cells were acquired using FACS Aria Instrument. At least 2×104 events were recorded per tube. Data analysis was performed using FlowJo_V10 software.
Immunofluorescent staining:
Cells were cultured on coverslips and fixed with 4% paraformaldehyde. Cell permeabilization was achieved using 0.1%Triton X-100 incubation for 20 minutes and blocking with 1% BSA for one hour. Cells were treated overnight at 4°C with primary antibodies for OCT4-FITC (SantaCruz Bioscience), alkaline phosphatase-PE, SSEA4-APC (eBioscience), SSEA1-PerCP (Biolegend), HNK1-PECY7 (Invitrogen), NGFR-APC (BD Pharmingen), CHI3L1 (R&D Systems), and myocilin (SantaCruz). Phalloindin-555 was used to stain F-actin and DAPI was employed as a nuclear stain and samples were acquired using laser scanning confocal microscope (Olympus).
Real-time PCR:
RLT buffer was used for lysis of cultured cells and an RNA purification kit (RNeasy Mini Kit, Qiagen) was used for isolation of total RNAs. RNAs were reverse transcribed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). SYBR green (Applied Biosystems) chemistry was used for qPCR. Primer3 was used to design primers for Housekeeping gene 18S rRNA (Forward: CCCTGTAATTGGAATGAGTCCAC, Reverse: GCTGGAATTACCGCGGCT), myocilin (forward: AAGCCCACCTACCCCTACAC; reverse: TCCAGTGGCCTAGGCAGTAT), CHI3L1 (forward: CCTTGACCGCTTCCTCTGTA; reverse: GTGTTGAGCATGCCGTAGAG), ANGPTL7 (forward: GCACCAAGGACAAGGACAAT; reverse: GATGCCATCCAGGTGCTTAT).
Statistical analysis:
Results were presented as mean ± standard deviation (SD). The statistical differences were analyzed by t-test or one-way ANOVA followed by Tukey posttest using PRISM. p< 0.05 was considered to be statistically significant.
Results:
iPSCs expressed pluripotent stem cell markers in feeder-free culture condition and as monolayer.
iPSCs were initially cultured and passaged on feeder cells and then switched to Matrigel without feeders. After more than ten passages on Matrigel, they still maintained colonies with the diameter of more than 600mm and expressed pluripotent stem cell markers OCT4, alkaline phosphatase, SSEA4, and SSEA1 (Fig 1). After Accutase digestion, iPSCs were cultured as monolayer maintained the expression of OCT4, alkaline phosphatase, SSEA4, and SSEA1, as shown in Fig 2. Within the monolayer cells, a few cell clusters failed to express those pluripotent stem cell markers.
Fig 1. iPSC colonies express pluripotent cell markers.

Immunofluorescent staining shows expression of pluripotency markers OCT4, alkaline phosphatase, SSEA4, and SSEA1 in iPSCs grown as colonies (A). (B) are the magnified pictures of (A) in framed region. DAPI stains nuclei as blue. Scale bars, 100μm.
Fig 2. Monolayer cultured iPSCs express pluripotent cell markers.

Immunofluorescent staining shows expression of pluripotency markers OCT4, alkaline phosphatase, SSEA4, and SSEA1 in iPSCs grown as monolayered cells (A). Isolated cell clusters were differentiated within the monolayer (Arrows). (B) are the magnified pictures of (A) in framed region. DAPI stains nuclei as blue. Scale bars, 100μm.
iPSCs could differentiate into NCCs.
Based on the fact that TM cells originate from neural crest, our plan was to induce monolayer cultured iPSCs into NCCs first and then into TM cells. Monolayer iPSCs were negative to neural crest markers NGFR and HNK1 (Fig 3A) in undifferentiated state. After induced on A549 cell-derived ECM in N2B27 + Y27632 induction medium, iPSCs differentiated into NCCs (iPSC-NC) as indicated by expression of NGFR and HNK1 (Fig 3B) by immunofluorescent staining. After induction, the iPSC-NC cells had reduced expression of SSEA4 and increased expression of NGFR by flow cytometry (Fig 3C). These experiments were repeated twice (n=6 of each cell type) and the percentage of positive expression cells was averaged and compared (Fig 3D). Pluripotent stem cell marker SSEA4 was significantly reduced after neural crest differentiation from 85.8±3.9% to 21.1±6.5% and NCC marker NGFR was significantly increased from 30.4±2.6% to 84.9±6.0% (mean±SD, Fig 3D). These iPSC-NC cells could be successfully passaged while maintaining the expression of NGFR.
Fig 3. iPSCs can differentiate into NCCs (iPSC-NC).

Immunofluorescent staining shows iPSC were negative to neural crest markers NGFR and HNK1 (A), while iPSC-NC cells expressed NGFR and HNK1 (B). DAPI stains nuclei as blue. Scale bars, 100μm. (C) Representative figures of flow cytometry analysis showing most of iPSCs were positive to SSEA4 and negative to NGFR. After induction, iPSC-NC reduced expression of SSEA4 and increased expression of NGFR. (D) Percentage of iPSCs and iPSC-NC cells labeling positive by SSEA4 and NGFR (n = 6, mean ± SD). One way ANOVA followed by Tukey’s multiple comparisons test.
iPSC-NC cells could differentiate into TM-like cells.
After iPSC-NC cells were induced on TM cell-derived ECM with TM cell-conditioned medium, they displayed TM cell characteristics. The differentiated cells (iPSC-TM) expressed TM cell marker CHI3L1 and reduced the CHI3L1 expression after treatment with Dex for 5 days (Fig 4A). Treatment with Dex increased myocilin expression (Fig 4B). The F-actin of iPSC-TM cells after 100 nM Dex treatment for 14 days formed CLANs presenting polygonal actin networks making up spokes (Fig 4C). qPCR results (Fig 4D) show that CHI3L1 expression was reduced in iPSC-TM cells after Dex treatment, similar to primary TM cells treated with Dex. After Dex treatment, primary TM cells dramatically increased the expression of myocilin and ANGPTL7 in comparison with primary TM cells without Dex treatment. The increase of myocilin and ANGPTL7 expression after Dex treatment in iPSC-TM cells was also statistically significant as compared to iPSC-TM cells without Dex treatment, although the increase was not as high as primary TM cells (Fig 4D).
Fig 4. iPSC-NC cells can differentiate into TM-like cells.

(A) Immunofluorescent staining shows iPSC-NC differentiated TM-like cells (iPSC-TM) expressed TM cell marker CHI3L1 and after treatment with Dex, the cells (iPSC-TM-Dex) reduced CHI3L1 expression. (B) iPSC-TM Dex cells had increased expression of myocilin. DAPI stains nuclei as blue. Scale bars, 50μm. (C) Phalloidin staining shows F-actin in the iPSC-TM-Dex cells formed cross-linked actin networks (CLANs) and the enlarged figure. (D) qPCR shows mRNA expression of CHI3L1, myocilin (MYOC), ANGPTL7 comparing iPSC-TM and iPSC-TM-Dex, TM cells and TM-Dex treated cells (n = 6, mean ± SD). One way ANOVA followed by Tukey’s multiple comparisons test.
Discussion:
In this study, we reported that iPSCs could differentiate into NCCs (iPSC-NC) with high efficiency as an intermediate stage which can be further induced to differentiate into trabecular meshwork (iPSC-TM) cells using a novel biological approach. The iPSC-NC cells can be passaged and stored for further induction which ensures the resource of getting same TM cells for further studies, such as drug screening, cell therapy, as well as studying mechanisms of TM regeneration for glaucoma.
iPSCs can be reprogrammed from any type of somatic cells with expression of pluripotency markers OCT4, SSEA4, SSEA1, Notch1 [17,18]. Spontaneous or induced differentiation of iPSCs results in the loss of these markers [19]. Previous reports have demonstrated the maintenance of pluripotency markers in non-colony monolayer cultures of embryonic stem cells [20]. The standard procedure of iPSC/ESC culture in colonies results in slow expansion, introduction of heterogeneity in cell population[21], and chromosomal abnormalities[22,23]. Contrastingly, growth in monolayer for differentiation can produce a more uniform and homogeneous culture which can enhance the efficiency to terminal differentiation[24,25]. In our study, iPSCs maintained the expression of pluripotency markers after monolayer culture, indicating that monolayer iPSCs retained their stem cell characteristics.
For TM induction, we first induced monolayer iPSCs to NCCs since NCCs migrate to the eye to form different eye structures including the TM during ocular development[26,27]. NGFR is an important marker of NCCs[28]. HNK1 is a predominant epitope expressed in migrating NCCs[29]. Coculture with mouse stromal PA6 cells was used to induce human embryonic stem cells into NCCs[30]. Here, we induced neural crest differentiation by culturing monolayer iPSCs on A549 cell-derived ECM in the presence of N2B27 and Y27632 for 10 days. Increased expression of NGFR and HNK1 after induction indicated that iPSCs could successfully differentiate into NCCs in such a condition. A549 cells can secret laminin-511 into conditioned medium[31] and laminin-511 can induce iPSC differentiation into cells of neural crest, neural and retinal origin by interaction with various integrin receptors[32]. Here we report that A549 ECM can effectively induce iPSCs to differentiate into NCCs which might be associated with laminin-511.
Use of paracrine factors in conditioned media offers an attractive biological approach for differentiation of stem cells into desired lineage [12,33]. In this study, we cultured iPSC-NC cells on TM cell-derived ECM with TM cell conditioned medium and successfully induced TM differentiation. These induced iPSC-TM cells expressed CHI3L1 and responded to Dex treatment with increased expression of myocilin and ANGPTL7. CHI3L1 is an important marker of TM cells[34–36], which is employed in ECM remodeling and its expression levels are changed during endoplasmic reticulum stress[37]. Using this approach, iPSC-TM cells showed a robust expression of CHI3L1, indicating the efficient differentiation of iPSC-NC into functional TM cells. Mutations in myocilin gene have been associated with juvenile and adult onset of POAG[38]. In primary TM cells, overexpression of ANGPTL7 modulated ECM proteins and matrix metalloproteases, resulting into increased deposition of ECM, re-orientation of fibrillary assembly of fibronectin, and increased expression of steroid responsiveness genes[39]. Treatment of TM cells with Dex induces glaucoma-like symptoms with increased TM stiffness[40], elevated expression of myocilin, and reorganized F-actin to form CLANs[12,41–44]. After Dex treatment, increased expression levels of both myocilin and ANGPTL7 and CLAN formation in iPSC-TM cells, further indicated the functional differentiation of iPSC-NC to TM cells.
Other groups have reported successful induction of iPSCs into TM cells [6–8,45] by coculturing iPSCs with primary TM cells to induce TM differentiation directly from iPSCs without an intermediate stage. Our study with two steps consisting of iPSCs to neural crest then to TM cells, mimicking the events during embryonic differentiation, offers a more amenable approach for TM cell differentiation and ensures the stable cell resource with the storable neural crest stage. This is consistent with a previous report that human pluripotent stem cells cultured in N2B27 medium with TGF-β inhibition withdrawing for ten days differentiated into promigratory NCCs[28]. These cells maintained their differentiation capacity even after 20 passages, suggesting the generation of stable NCCs. Furthermore, use of TM conditioned medium and biological ECM in this novel approach makes the derivation of TM cells simple and devoid of any chemical factors. Animal models offer valuable insight into ophthalmology research, but exact recapitulation of glaucoma is difficult in animal models and there are numerous differences that exist between animal models and human diseases. An iPSC-based cellular model mimicking primary TM cells can successfully produce a high number of cells and recapitulate human diseases due to the usage of patient specific iPSCs.
Additionally, stem cell therapy for TM regeneration has shown great potential for glaucoma treatment [46]. The ability to derive iPSC-TM cells through a middle neural crest stage that recapitulate TM characteristics offers stable cell sources for stem cell therapy and personalized medicine for glaucoma.
In conclusion, iPSC-TM cells produced by a two-step novel approach offer a valuable resource for studying personalized treatment, drug screening, and autologous transplantation for structural and functional restoration of TM tissue for treating glaucoma.
Highlights:
A novel two-step induction approach from iPSCs to neural crest then to TM cells
Neural crest cells can be stored that ensures same lot and large number of TM cells
Differentiated TM cells expressed TM markers and lost iPSC and neural crest markers
Differentiated TM cells responded to dexamethasone with increased glaucoma genes
A model for cell transplantation, drug discovery and disease modelling in glaucoma
Acknowledgments:
The authors thank Dr. John Danias at SUNY Downstate for helpful discussions and Nancy Zurowski at University of Pittsburgh for Flow Cytometry. This work was supported by NIH grants EY025643 (YD), P30-EY008098, Research to Prevent Blindness; and Eye and Ear Foundation of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- [1].Weinreb RN, Leung CK, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL, Martin KR, Primary open-angle glaucoma, Nat Rev Dis Primers 2 (2016) 16067 10.1038/nrdp.2016.67. [DOI] [PubMed] [Google Scholar]
- [2].Ethier CR, Kamm RD, Palaszewski BA, Johnson MC, Richardson TM, Calculations of flow resistance in the juxtacanalicular meshwork, Invest Ophthalmol Vis Sci 27 (1986) 1741–1750. [PubMed] [Google Scholar]
- [3].Alvarado J, Murphy C, Juster R, Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals, Ophthalmology 91 (1984) 564–579. [DOI] [PubMed] [Google Scholar]
- [4].Gabelt BT, Kaufman PL, Changes in aqueous humor dynamics with age and glaucoma, Prog Retin Eye Res 24 (2005) 612–637. 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- [5].Du Y, Yun H, Yang E, Schuman JS, Stem cells from trabecular meshwork home to TM tissue in vivo, Invest Ophthalmol Vis Sci 54 (2013) 1450–1459. 10.1167/iovs.12-11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Abu-Hassan DW, Li X, Ryan EI, Acott TS, Kelley MJ, Induced pluripotent stem cells restore function in a human cell loss model of open-angle glaucoma, Stem Cells 33 (2015) 751–761. 10.1002/stem.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhu W, Gramlich OW, Laboissonniere L, Jain A, Sheffield VC, Trimarchi JM, Tucker BA, Kuehn MH, Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo, Proc Natl Acad Sci U S A 113 (2016) E3492–3500. 10.1073/pnas.1604153113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhu W, Jain A, Gramlich OW, Tucker BA, Sheffield VC, Kuehn MH, Restoration of Aqueous Humor Outflow Following Transplantation of iPSC-Derived Trabecular Meshwork Cells in a Transgenic Mouse Model of Glaucoma, Invest Ophthalmol Vis Sci 58 (2017) 2054–2062. 10.1167/iovs.16-20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yun H, Wang Y, Zhou Y, Wang K, Sun M, Stolz DB, Xia X, Ethier CR, Du Y, Human stem cells home to and repair laser-damaged trabecular meshwork in a mouse model, Commun Biol 1 (2018) 216 10.1038/s42003-018-0227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stern JH, Tian Y, Funderburgh J, Pellegrini G, Zhang K, Goldberg JL, Ali RR, Young M, Xie Y, Temple S, Regenerating Eye Tissues to Preserve and Restore Vision, Cell Stem Cell 22 (2018) 834–849. 10.1016/j.stem.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Manuguerra-Gagne R, Boulos PR, Ammar A, Leblond FA, Krosl G, Pichette V, Lesk MR, Roy DC, Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment, Stem Cells 31 (2013) 1136–1148. 10.1002/stem.1364. [DOI] [PubMed] [Google Scholar]
- [12].Zhou Y, Xia X, Yang E, Wang Y, Marra KG, Ethier CR, Schuman JS, Du Y, Adipose-derived stem cells integrate into trabecular meshwork with glaucoma treatment potential, FASEB J (2020). 10.1096/fj.201902326R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell 126 (2006) 663–676. 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [14].Williams AL, Bohnsack BL, Neural crest derivatives in ocular development: discerning the eye of the storm, Birth Defects Res C Embryo Today 105 (2015) 87–95. 10.1002/bdrc.21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Saadai P, Wang A, Nout YS, Downing TL, Lofberg K, Beattie MS, Bresnahan JC, Li S, Farmer DL, Human induced pluripotent stem cell-derived neural crest stem cells integrate into the injured spinal cord in the fetal lamb model of myelomeningocele, J Pediatr Surg 48 (2013) 158–163. 10.1016/j.jpedsurg.2012.10.034. [DOI] [PubMed] [Google Scholar]
- [16].Lin B, Kim J, Li Y, Pan H, Carvajal-Vergara X, Salama G, Cheng T, Li Y, Lo CW, Yang L, High-purity enrichment of functional cardiovascular cells from human iPS cells, Cardiovasc Res 95 (2012) 327–335. 10.1093/cvr/cvs185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pan GJ, Chang ZY, Scholer HR, Pei D, Stem cell pluripotency and transcription factor Oct4, Cell Res 12 (2002) 321–329. 10.1038/sj.cr.7290134. [DOI] [PubMed] [Google Scholar]
- [18].Trusler O, Huang Z, Goodwin J, Laslett AL, Cell surface markers for the identification and study of human naive pluripotent stem cells, Stem Cell Res 26 (2018) 36–43. 10.1016/j.scr.2017.11.017. [DOI] [PubMed] [Google Scholar]
- [19].Li T, Zhao H, Han X, Yao J, Zhang L, Guo Y, Shao Z, Jin Y, Lai D, The spontaneous differentiation and chromosome loss in iPSCs of human trisomy 18 syndrome, Cell Death Dis 8 (2017) e3149 10.1038/cddis.2017.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen KG, Mallon BS, Hamilton RS, Kozhich OA, Park K, Hoeppner DJ, Robey PG, McKay RD, Non-colony type monolayer culture of human embryonic stem cells, Stem Cell Res 9 (2012) 237–248. 10.1016/j.scr.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hartung O, Huo H, Daley GQ, Schlaeger TM, Clump passaging and expansion of human embryonic and induced pluripotent stem cells on mouse embryonic fibroblast feeder cells, Curr Protoc Stem Cell Biol Chapter 1 (2010) Unit 1C 10 10.1002/9780470151808.sc01c10s14. [DOI] [PubMed] [Google Scholar]
- [22].Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, Sui G, Cutler DJ, Liu Y, Brimble SN, Noaksson K, Hyllner J, Schulz TC, Zeng X, Freed WJ, Crook J, Abraham S, Colman A, Sartipy P, Matsui S, Carpenter M, Gazdar AF, Rao M, Chakravarti A, Genomic alterations in cultured human embryonic stem cells, Nat Genet 37 (2005) 1099–1103. 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- [23].Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y, Van der Elst J, Liebaers I, Sermon K, Recurrent chromosomal abnormalities in human embryonic stem cells, Nat Biotechnol 26 (2008) 1361–1363. 10.1038/nbt.1510. [DOI] [PubMed] [Google Scholar]
- [24].Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, Mahairaki V, Koliatsos VE, Tung L, Zambidis ET, A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability, PLoS One 6 (2011) e18293 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Menendez L, Kulik MJ, Page AT, Park SS, Lauderdale JD, Cunningham ML, Dalton S, Directed differentiation of human pluripotent cells to neural crest stem cells, Nat Protoc 8 (2013) 203–212. 10.1038/nprot.2012.156. [DOI] [PubMed] [Google Scholar]
- [26].Kish PE, Bohnsack BL, Gallina D, Kasprick DS, Kahana A, The eye as an organizer of craniofacial development, Genesis 49 (2011) 222–230. 10.1002/dvg.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tripathi BJ, Tripathi RC, Neural crest origin of human trabecular meshwork and its implications for the pathogenesis of glaucoma, Am J Ophthalmol 107 (1989) 583–590. 10.1016/0002-9394(89)90253-5. [DOI] [PubMed] [Google Scholar]
- [28].Noisa P, Lund C, Kanduri K, Lund R, Lahdesmaki H, Lahesmaa R, Lundin K, Chokechuwattanalert H, Otonkoski T, Tuuri T, Raivio T, Notch signaling regulates the differentiation of neural crest from human pluripotent stem cells, J Cell Sci 127 (2014) 2083–2094. 10.1242/jcs.145755. [DOI] [PubMed] [Google Scholar]
- [29].Vincent M, Duband JL, Thiery JP, A cell surface determinant expressed early on migrating avian neural crest cells, Brain Res 285 (1983) 235–238. 10.1016/0165-3806(83)90058-5. [DOI] [PubMed] [Google Scholar]
- [30].Chan AA, Hertsenberg AJ, Funderburgh ML, Mann MM, Du Y, Davoli KA, Mich-Basso JD, Yang L, Funderburgh JL, Differentiation of human embryonic stem cells into cells with corneal keratocyte phenotype, PLoS One 8 (2013) e56831 10.1371/journal.pone.0056831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pouliot N, Kusuma N, Laminin-511: a multi-functional adhesion protein regulating cell migration, tumor invasion and metastasis, Cell Adh Migr 7 (2013) 142–149. 10.4161/cam.22125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shibata S, Hayashi R, Okubo T, Kudo Y, Katayama T, Ishikawa Y, Toga J, Yagi E, Honma Y, Quantock AJ, Sekiguchi K, Nishida K, Selective Laminin-Directed Differentiation of Human Induced Pluripotent Stem Cells into Distinct Ocular Lineages, Cell Rep 25 (2018) 1668–1679 e1665 10.1016/j.celrep.2018.10.032. [DOI] [PubMed] [Google Scholar]
- [33].Kumar A, Kumar V, Rattan V, Jha V, Bhattacharyya S, Secretome Cues Modulate the Neurogenic Potential of Bone Marrow and Dental Stem Cells, Mol Neurobiol 54 (2017) 4672–4682. 10.1007/s12035-016-0011-3. [DOI] [PubMed] [Google Scholar]
- [34].Stamer WD, Seftor RE, Snyder RW, Regan JW, Cultured human trabecular meshwork cells express aquaporin-1 water channels, Curr Eye Res 14 (1995) 1095–1100. 10.3109/02713689508995815. [DOI] [PubMed] [Google Scholar]
- [35].Du Y, Roh DS, Mann MM, Funderburgh ML, Funderburgh JL, Schuman JS, Multipotent stem cells from trabecular meshwork become phagocytic TM cells, Invest Ophthalmol Vis Sci 53 (2012) 1566–1575. 10.1167/iovs.11-9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kumar A, Xu Y, Du Y, Stem Cells from Human Trabecular Meshwork Hold the Potential to Develop into Ocular and Non-Ocular Lineages After Long-Term Storage, Stem Cells Dev 29 (2020) 49–61. 10.1089/scd.2019.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Y, Osakue D, Yang E, Zhou Y, Gong H, Xia X, Du Y, Endoplasmic Reticulum Stress Response of Trabecular Meshwork Stem Cells and Trabecular Meshwork Cells and Protective Effects of Activated PERK Pathway, Invest Ophthalmol Vis Sci 60 (2019) 265–273. 10.1167/iovs.18-25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tamm ER, Myocilin and glaucoma: facts and ideas, Prog Retin Eye Res 21 (2002) 395–428. 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- [39].Comes N, Buie LK, Borras T, Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: implications for glaucoma, Genes Cells 16 (2011) 243–259. 10.1111/j.1365-2443.2010.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P, Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix, Invest Ophthalmol Vis Sci 56 (2015) 4447–4459. 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Keller KE, Bhattacharya SK, Borras T, Brunner TM, Chansangpetch S, Clark AF, Dismuke WM, Du Y, Elliott MH, Ethier CR, Faralli JA, Freddo TF, Fuchshofer R, Giovingo M, Gong H, Gonzalez P, Huang A, Johnstone MA, Kaufman PL, Kelley MJ, Knepper PA, Kopczynski CC, Kuchtey JG, Kuchtey RW, Kuehn MH, Lieberman RL, Lin SC, Liton P, Liu Y, Lutjen-Drecoll E, Mao W, Masis-Solano M, McDonnell F, McDowell CM, Overby DR, Pattabiraman PP, Raghunathan VK, Rao PV, Rhee DJ, Chowdhury UR, Russell P, Samples JR, Schwartz D, Stubbs EB, Tamm ER, Tan JC, Toris CB, Torrejon KY, Vranka JA, Wirtz MK, Yorio T, Zhang J, Zode GS, Fautsch MP, Peters DM, Acott TS, Stamer WD, Consensus recommendations for trabecular meshwork cell isolation, characterization and culture, Exp Eye Res 171 (2018) 164–173. 10.1016/j.exer.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W, Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells, Invest Ophthalmol Vis Sci 35 (1994) 281–294. [PubMed] [Google Scholar]
- [43].Filla MS, Schwinn MK, Sheibani N, Kaufman PL, Peters DM, Regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct beta1 and beta3 integrin pathways, Invest Ophthalmol Vis Sci 50 (2009) 5723–5731. 10.1167/iovs.08-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xiong S, Xu Y, Wang Y, Kumar A, Peters DM, Du Y, alpha5beta1 Integrin Promotes Anchoring and Integration of Transplanted Stem Cells to the Trabecular Meshwork in the Eye for Regeneration, Stem Cells Dev (2020). 10.1089/scd.2019.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ding QJ, Zhu W, Cook AC, Anfinson KR, Tucker BA, Kuehn MH, Induction of trabecular meshwork cells from induced pluripotent stem cells, Invest Ophthalmol Vis Sci 55 (2014) 7065–7072. 10.1167/iovs.14-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Castro A, Du Y, Trabecular Meshwork Regeneration - A Potential Treatment for Glaucoma, Curr Ophthalmol Rep 7 (2019) 80–88. 10.1007/s40135-019-00203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


