Abstract
Hepatocellular carcinoma (HCC) is the most common type of liver cancer. It has a poor prognosis because it is often diagnosed at the advanced stage when treatments are limited. In addition, HCC pathogenesis is not fully understood, and this has affected early diagnosis and treatment of this disease. Human alkaline ceramidase 2 (ACER2), a key enzyme that regulates hydrolysis of cellular ceramides, affects cancer cell survival, however its role in HCC has not been well characterized. Our results showed that ACER2 is overexpressed in HCC tissues and cell lines. In addition, high ACER2 protein expression was associated with tumor growth; ACER2 knockdown resulted in decreased cell growth and migration. Sphingomyelin phosphodiesterase acid‐like 3B (SMPDL3B) promoted HCC cell growth, invasion, and migration; SMPDL3B knockdown had a significant inhibitory effect on HCC tumor growth in vivo. Moreover, ACER2 positively regulated the protein level of SMPDL3B. Of note, ACER2/SMPDL3B promoted ceramide hydrolysis and S1P production. This axis induced HCC survival and could be blocked by inhibition of S1P formation. In conclusion, ACER2 promoted HCC cell survival and migration, possibly via SMPDL3B. Thus, inhibition of ACER2/SMPDL3B may be a novel therapeutic target for HCC treatment.
Keywords: alkaline ceramidase 2, hepatocellular carcinoma, invasion, migration, proliferation, sphingomyelin phosphodiesterase acid‐like 3B
ACER2 induced SMPDL3B resulting in S1P formation and ceramide hydrolysis, which promoted HCC cells growth, invasion and migration.
1. INTRODUCTION
HCC is the one of the most common solid tumors and has a high mortality rate. 1 The 5‐y survival rate after HCC surgical resection is <30%, in large part due to both high recurrence and metastasis rates. 2 Mechanisms responsible for HCC progression and metastasis remain unknown. 3 , 4
Sphingolipids, components of the cell membrane, also have important biological functions in cancer. As the structural backbone of sphingolipids, the endogenous, bioactive sphingolipid ceramide (CER) has been shown to suppress tumor growth in various cancers.
Alkaline ceramidases (ACERs), including ACER1, ACER2, and ACER3, convert CER to sphingosine (SPH), which is further transformed into sphingosine‐1‐phosphate (S1P). 5 , 6 , 7 ACERs mediate cell survival, tumor initiation, and development by regulating the balance of CER, SPH, and S1P. 8 ACER2 is highly expressed in the placenta; it has 7 putative transmembrane domains with its amino‐terminus in the lumen of the Golgi and its carboxyl‐terminus in the cytosol. 5 In mice, ACER2 downregulation significantly decreases plasma SPH and S1P levels. 9 Under some conditions, ACER2 works as a novel transcriptional target of p53. 10 Moreover, ACER2 was shown to regulate cancer cell survival, but its role in HCC is not fully understood. 11
Sphingomyelin phosphodiesterase acid‐like 3B (SMPDL3B) is involved in regulation of the Toll‐like receptor signaling pathway and in ATP hydrolysis. 12 In addition, downregulation‐induced CER are associated with radiation‐induced damage in renal podocytes. 13 , 14 SMPDL3B potentially regulates sphingolipid metabolism, as it functions as a hydrolase and sphingomyelin phosphodiesterase. 15 However, the role of SMPDL3B in sphingolipid metabolism has not been fully elucidated. As described above, sphingolipid metabolism has a great effect on cancer cell survival, but how SMPDL3B works in cancer, especially in HCC, is not well known. Therefore, in this study, the roles of ACER2 and SMPDL3B in HCC were investigated.
2. MATERIALS AND METHODS
2.1. Reagents
The following antibodies were used: ACER2 (Origene), SMPDL3B (Novus), green fluorescent protein (GFP; Origene), tubulin (Proteintech Group [PTG], Rosemont, IL, USA), and β‐actin (PTG). Lipofectamine™ 3000 was purchased from Invitrogen. The CER inhibitor D‐erythro‐MAPP (D‐e‐MAPP) was purchased from Abcam. Cell Counting Kit‐8 (CCK‐8) was purchased from Dojindo. SKII was purchased from MCE (China). The C1P ELISA kit was obtained from Jinmei Biotechnology (China).
2.2. Cell culture
HepG2 and Huh‐7 human liver cancer cells were purchased from the American Type Culture Collection (ATCC), and QGS7701 cells were from Shanghai Second Military Medical University. QGS7701, HepG2 and Huh‐7 cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco). All cell lines were grown at 37°C in a 5% CO2 in air atmosphere.
2.3. Transfection and stable cell line establishment
Three small interfering RNAs (siRNAs) were designed and synthesized by GenePharma Co., Ltd. Plasmids were from Origene. Huh‐7 cells were transfected with siRNA or plasmids using Lipofectamine™ 3000 in accordance with the manufacturer's instructions. To establish stable cell lines, Huh‐7 cells were infected with lentivirus containing the indicated plasmid. Puromycin was added at 48 h after infection. Sequences of the indicated siRNAs are as follows:
SMPDL3B siRNA: 5′‐GGUGGUCCGGAAGCAUCAUTT‐3′, 5′‐AUGAUGCUUCCGGACCACCTT‐3′;
Negative control (NC siRNA): 5′‐UUCUCCGAACGUGUCACGUTT‐3′, 5′‐ACGUGACACGUUCGGAGAATT‐3′.
2.4. Western blot analysis
Cells were lysed. After quantification, proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrotransferred to PVDF membranes. Membranes were blocked in 5% fat‐free milk, then membranes were incubated with primary antibody overnight at 4°C following by secondary antibody for another 1 h. Next, membranes were washed again with TBS‐Tween (TBST). Finally, the proteins were visualized.
2.5. Immunohistochemistry analysis
Formalin‐fixed paraffin‐embedded HCC specimens were treated with xylene and then rehydrated in alcohol at decreasing concentrations. Then, the sections were boiled in citrate antigenic retrieval buffer (pH = 6.0) for 3 min and cooled to room temperature (RT), after which, endogenous peroxidase activity was quenched for 10 min by addition of 3% hydrogen peroxide. Sections were blocked in 10% goat serum at RT for 30 min, then sections were incubated with ACER2 antibody (1:200; Origene) at 4°C overnight, washed, and then incubated with biotinylated secondary antibody at RT for 30 min, followed by a final wash with PBS. Sections were then incubated with streptavidin‐horseradish peroxidase conjugate at RT for 20 min, developed with 3,3‐diaminobenzidine tetrahydrochloride, and counterstained with hematoxylin. The ACER2 protein levels were evaluated independently by 2 pathologists who had no knowledge of the clinical data for the HCC patients. The proportion of ACER2 staining in the tumor was scored as follows: Grade 0, ≤5% positive cells; Grade 1, ≤25% positive cells; Grade 2, ≤50% positive cells; Grade 3, ≤75% positive cells; and Grade 4, ≤100% positive cells. The staining intensity was scored as follows: Grade 0, blue; Grade 1, light yellow; Grade 2, brown yellow; and Grade 3, sepia. Final score was the average score from the 2 pathologists.
2.6. Cell proliferation assay
Huh‐7 cells (5 × 103) were seeded in 96‐well plates. After transfection with the indicated plasmids or at 24 h after D‐e‐MAPP treatments, the cells were incubated with 100 µL of culture medium and 10 µL of CCK‐8 reaction mixtures for 1 h at 37°C. Then, cell proliferation was determined by measuring absorbance at 450 nm. All experiments were repeated 3 times.
2.7. Cell invasion and migration assay
Before use, an invasion chamber (Corning) and transwell chambers with 8‐μm pore inserts (Corning) in a 24‐well plate were activated with serum‐free medium (30 min, 37°C). Cells (100 μL, 2 × 104) were seeded in serum‐free medium to the upper chamber, and 600 μL of DMEM (with 20% FBS) was added to the lower chamber. After 24 h, the cells in the inner surface of the chamber were wiped away. The invasive and migrated cells were fixed in 4% paraformaldehyde for 15 min and stained with 0.1% crystal violet overnight. Then, cells were imaged and counted.
2.8. Wound‐healing assay
Cells were seeded in 6‐well plates, and the cell monolayer was wounded by scratching with 10‐μL micropipette tips. Then, the cells were cultured in serum‐free medium. Cell migration distances were observed and imaged under a microscope (Olympus) at 0, 24, and 48 h.
2.9. PCR array experiment
An equal amount of total RNA purified in the cells was reverse‐transcribed to cDNA. PCR array experiments were performed using the human Sphingolipids Signaling Pathway Custom RT2 PCR Array kit (Qiagen). Gene expression was calculated. Then, the fold change of each gene was calculated using the gene expression value of the sample from ACER2 shRNA‐treated cells divided by the gene expression value of the control sample.
2.10. Subcutaneous tumorigenesis model
Four‐wk‐old male athymic BALB/c nude mice were kept under pathogen‐free conditions. Huh‐7 ACER2 shRNA stable or SMPDL3B‐knockdown cells (1 × 106) were injected into the right flanks of mice. Mice were sacrificed 2 wk later. Weights of the mice and tumors as well as tumor size were measured. Tumor volume was calculated using the formula (L × W2)/2 (L: length of tumor; W: width of tumor). Subcutaneous tumor tissues were subjected to western blot analysis. Animal experiments for this study were approved by the Medical Experimental Animal Care Commission of Guilin Medical University, China.
3. RESULTS
3.1. ACER2 is upregulated in HCC, and its expression is positively associated with tumor size
CER is known to promote cancer cell death. Therefore, as the key enzyme regulating CER, ACER2 might affect the survival of liver cancer cells. To determine the role of this enzyme in HCC, we investigated ACER2 expression in HCC cells and tissues. The protein level of ACER2 was higher in the liver cancer cells Huh‐7 and HepG2 than in the normal liver cell line QSG7701 (Figure 1A). ACER2 expression was also increased in HCC tissues, as determined by western blot (Figure 1B). ACER2 expression in liver tissues from HCC patients was visualized by immunohistochemistry (IHC) and scored (Figure 1C,D). The results showed that HCC tissue had obvious ACER2 expression (Figure 1C), and statistical analysis confirmed that its expression was significantly upregulated in HCC tissues (Figure 1D). To further investigate the relationship between ACER2 and HCC, chi‐square analysis between the histopathologic score of ACER2 expression and the respective clinical parameter was performed. ACER2 expression correlated with tumor size (χ2 = 4.123, P = .041), however there were no correlations between ACER2 protein expression and other clinicopathological parameters, including age, sex, tumor‐node‐metastasis stage, cirrhosis, portal vein tumor thrombus, hepatitis B surface antigen, alcohol abuse, and alpha fetoprotein (all P > .05; Table 1). Together, these data demonstrated that ACER2 expression was upregulated in HCC and positively associated with HCC tumor size.
Figure 1.
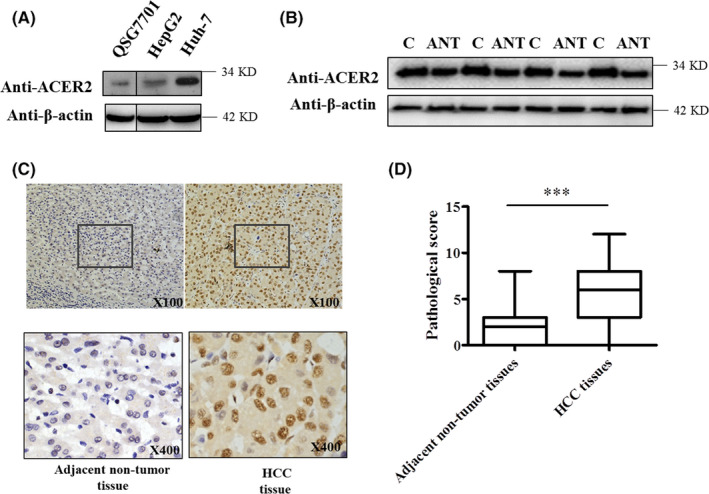
ACER2 is upregulated in HCC. QSG7701, HepG2, and Huh‐7 cells (A) or adjacent non‐tumor tissues and HCC tissues from patients (B) were lysed, and the lysates were subjected to western blot with an ACER2 antibody. “ANT” indicates adjacent non‐tumor tissue; “C” indicates HCC tissue. C, ACER2 levels in tissues were detected by IHC. D, The histopathologic score of ACER2 expression in tissue was evaluated. Statistical analysis was performed using Student t test (***P < .001)
Table 1.
The relationship between ACER2 expression and clinicopathologic variables of patients with hepatocellular carcinoma
| No. of patient | ACER2 | χ2 | P value | ||
|---|---|---|---|---|---|
| <3 | ≥3 | ||||
| Gender | |||||
| Male | 122 | 25(20.49) | 97(79.51) | 0.276 | .383 |
| Female | 28 | 7(25) | 21(75) | ||
| Age (y) | |||||
| <50 | 69 | 16(23.19) | 53(76.81) | 0.262 | .377 |
| ≥50 | 81 | 16(19.75) | 65(80.25) | ||
| TNM stage | |||||
| I II | 106 | 19(17.92) | 87(82.08) | 2.502 | .088 |
| III IV | 44 | 13(29.55) | 31(70.45) | ||
| HBsAg | |||||
| Negative | 52 | 10(19.23) | 42(80.77) | 0.21 | .407 |
| Positive | 98 | 22(22.45) | 76(77.55) | ||
| Size (cm) | |||||
| <3 | 32 | 11(34.38) | 21(65.62) | 4.123 | .041 |
| ≥3 | 118 | 21(17.80) | 97(82.20) | ||
| AFP (μg/L) | |||||
| <20 | 127 | 28(22.05) | 99(77.95) | 0.252 | .426 |
| ≥20 | 23 | 4(17.39) | 19(82.61) | ||
| Liver cirrhosis | |||||
| Negative | 79 | 19(24.05) | 60(75.95) | 0.734 | .256 |
| Positive | 71 | 13(18.31) | 58(81.69) | ||
| PVTT | |||||
| Negative | 79 | 19(24.05) | 60(75.95) | 0.734 | .256 |
| Positive | 71 | 13(18.31) | 58(81.69) | ||
| Drink alcohol | |||||
| Yes | 83 | 17(20.48) | 66(79.52) | 0.08 | .465 |
| No | 67 | 15(22.39) | 52(77.61) | ||
The value in bold was of statistical significance. Abbreviations: AFP, alpha fetoprotein; PVTT, portal vein tumor thrombus; TNM, tumor node metastasis.
3.2. ACER2 promotes HCC cell proliferation
Next, we studied the function of ACER2 in HCC cells. To this end, Huh‐7 cells were treated with the ACER2 inhibitor D‐e‐MAPP, and cell growth was determined using the CCK‐8 assay after 24 h. After D‐e‐MAPP treatment, ACER2 protein levels in Huh‐7 cells decreased (Figure 2A). Consequently, the content of ceramides, which are substrates of ACER2, increased, indicating a downregulation of ACER2 activity (Figure 2B). Meanwhile, Huh‐7 cell proliferation was inhibited after D‐e‐MAPP treatment (Figure 2C). Next, ACER2 was stably knocked down or overexpressed in Huh‐7 cells to confirm its effects on liver cancer cell proliferation. Consistent with a previous report, 8 the downregulation of ACER2 led to ACER2 activity inhibition, as indicated by the increase in its substrates (Figure 2D,E). In addition, ACER2 knockdown inhibited the growth of Huh‐7 cells (Figure 2F). Conversely, upregulation of ACER2 increased its activity and promoted Huh‐7 cell proliferation (Figure 2G‐I). These results indicated that ACER2 promoted HCC cell proliferation, and this effect was related to the activity of ACER2.
Figure 2.
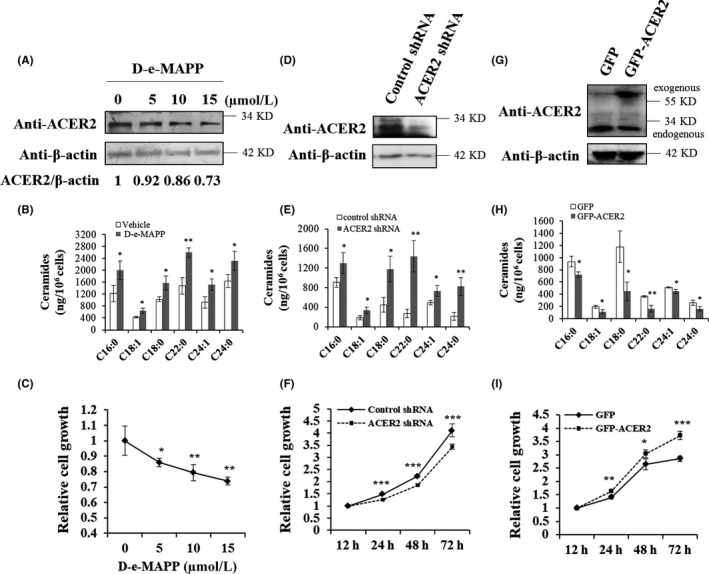
ACER2 promotes HCC cell proliferation. Huh‐7 cells were treated with D‐e‐MAPP at the indicated concentrations for 24 h (A‐C). Cell lysates were subjected to western blot with indicated antibodies, and the relative protein level of ACER2 was quantified by ImageJ software (A). Intracellular ceramide was detected by UHPLC‐QTOF‐MS (B). Cell proliferation was investigated using the CCK8 assay (C). Lentivirus containing plasmid encoding ACER2 shRNA (D‐F) or full‐length ACER2 (G‐I) was used to infect Huh‐7 cells for 48 h, and then, cells were treated with puromycin until stable cell lines were established. The efficiency of ACER2 knockdown (D) or overexpression (G) was confirmed by western blot with an anti‐ACER2 antibody. Cells lysates were subjected to ceramide analysis (E, H). Huh‐7 cells with stable ACER2 knockdown (F) or overexpression (I) were seeded, and cell survival was measured at 12, 24, 48, and 72 h using the CCK‐8 assay (F, I). Error bars represent the standard deviation (SD) from 3 independent experiments. Statistical significance was analyzed using Student t test (*P < .05; **P < .01; ***P < .001)
3.3. ACER2 promotes HCC cell invasion and migration
To evaluate the role of ACER2 in HCC invasion and migration, we measured the invasion and migration ability of Huh‐7 cells after ACER2 knockdown or overexpression using the transwell chamber assay. As shown, ACER2 knockdown significantly inhibited Huh‐7 cell invasion and migration (Figure 3A,C). In contrast, cells stably overexpressing ACER2 showed stronger invasion and migration ability (Figure 3B,D). The wound‐healing assay was also performed to confirm the effects of ACER2 on cancer cell migration. The results showed that the wound areas in Huh‐7 cells upon ACER2 downregulation were significantly larger than those in cells with control shRNA (Figure 3E). Opposite results were obtained upon ACER2 overexpression (Figure 3F). These results indicated that ACER2 promoted HCC cell invasion and migration.
Figure 3.
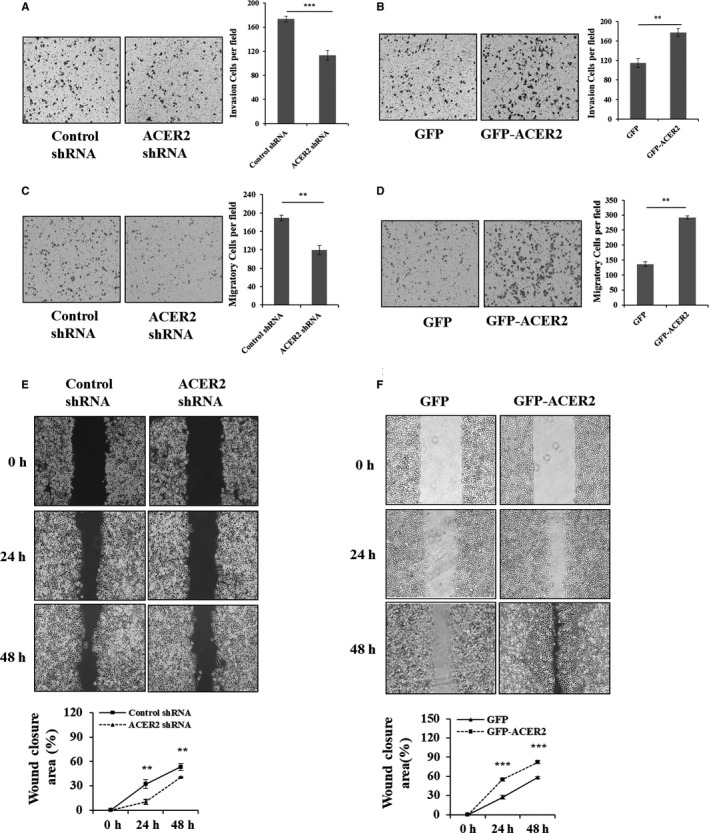
ACER2 promotes HCC cell invasion and migration in vitro. Stable ACER2 knockdown (A, C, E) or overexpressing (B, D, F) cells were seeded. Transwell assays (A‐D) and wound‐healing assay (scale bar: 50 μm) (E, F) were performed in Huh‐7 cells. Cell numbers (A‐D) were counted using ImageJ software. The histograms (A‐D) represent the mean values of invasive and migrated cells per high‐power field (from at least 5 fields, mean ± SD) (** P < .01; *** P < .001). The wound closure (E, F) area was quantified by ImageJ software. Error bar represents the SD from 3 independent experiments, and statistical significance was analyzed using Student t test (** P < .01; *** P < .001)
3.4. ACER2 knockdown inhibits tumor growth
The above results showed that ACER2 promoted liver cancer cell survival and migration in vitro and that ACER2 expression was upregulated in HCC tissue. To confirm these results in vivo, mice were injected with ACER2‐knockdown Huh‐7 cells and were found to have smaller tumors than control mice (Figure 4A‐D).
Figure 4.
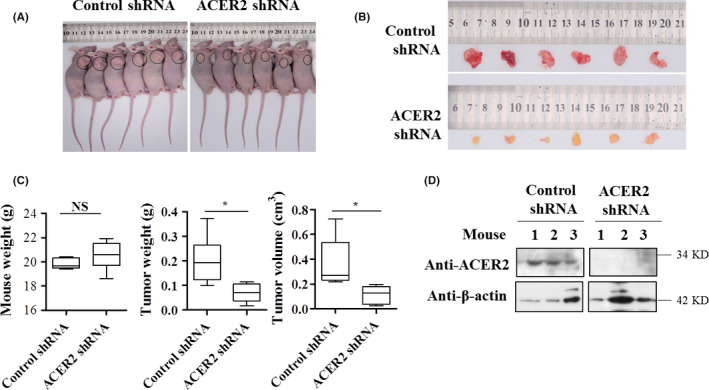
ACER2 knockdown significantly inhibits tumor growth. A, Nude mice with tumors were imaged; the tumors are marked with a circle. B, Tumor tissues were collected and imaged. C, Weight of mouse (left), the weight (middle) and size (right) of the tumor and were collected and analyzed. D, Protein levels of ACER2 in tumor tissues were investigated by western blot; representative results are shown. Data were analyzed using Student t test (*P < .05)
3.5. SMPDL3B promotes HCC cell proliferation, invasion, and migration
S1P is known to promote cancer cell survival, therefore the levels of intracellular S1P were also detected. Consistent with a previous study, 8 S1P in Huh‐7 cells was found to be positively regulated by ACER2 (Figure 5A,B). Moreover, it has been reported that sphingosine kinase 1 and S1P lyase both affect HCC development. 16 Therefore, we postulated that other sphingolipid‐metabolizing enzymes may also play roles in ACER2‐mediated HCC proliferation. In PCR array assay, SMPDL3B showed the largest fold change in mRNA expression when ACER2 was knocked down (Table 2). Meanwhile, SMPDL3B was found to be upregulated in HCC tissue (Figure 5C). Next we investigated how SMPDL3B regulated HCC. The results showed that SMPDL3B knockdown inhibited HCC cell proliferation (Figure 5D,E), while SMPDL3B overexpression promoted HCC cell proliferation (Figure 5F,G). Similar to ACER2, SMPDL3B knockdown inhibited HCC cell invasion (Figure 6A) and migration (Figure 6C,E), while SMPDL3B overexpression promoted HCC cell invasion (Figure 6B) and migration (Figure 6D,F).
Figure 5.
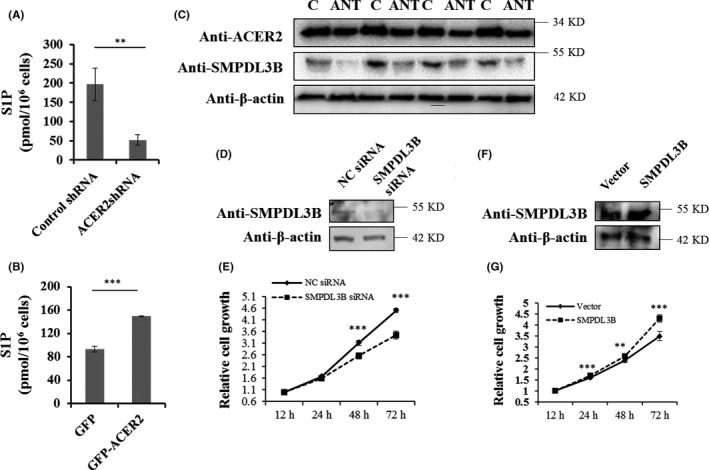
SMPDL3B promotes HCC cell proliferation. The amounts of S1P in stable ACER2 knockdown (A) or overexpression (B) Huh‐7 cells were measured with an ELISA kit. Error bars represent the SD from 3 independent experiments, and statistical significance were analyzed using Student t test (**P < .01; ***P < .001). C, SMPDL3B expression in HCC human samples, same samples from Figure 1(B) and same images of ACER2 and β‐actin shown in Figure 1(B). Huh‐7 cells with SMPDL3B knockdown (D and E) or overexpression (F and G) were seeded, the protein level of SMPDL3B was measured (D, F) and cell survival at 12, 24, 48, and 72 h was assessed using the CCK‐8 assay (E, G). Error bars represent the standard deviation (SD) from 3 independent experiments. Statistical significance was analyzed using Student t test (**P < .01; ***P < .001)
Table 2.
Sphingolipid signaling genes changes after ACER2 downregulation
| Gene | CT in cells with control shRNA | CT in cells with ACER2 shRNA | Fold of control |
|---|---|---|---|
| ACER2 | 28.11 | 29.75 | −3.3 |
| SMPDL3B | 28.24 | 30.94 | −5 |
| FAAH | 32.75 | 34.12 | −2.5 |
| GBA3 | 32.54 | 33.69 | −2 |
| SMPDL3A | 25.94 | 26.96 | −2 |
| FAAH2 | 25.89 | 26.94 | −2 |
| SGMS1 | 22.86 | 23.70 | −1.7 |
| SMPD1 | 24.82 | 24.11 | 1.6 |
| SGPP2 | 31.98 | 31.32 | 1.6 |
| SPHK1 | 23.90 | 23.49 | 1.3 |
The value in bold indicated the largest fold of change. Abbreviations: ACER2, alkaline ceramidase 2; FAAH, fatty‐acid amide hydrolase; FAAH2, fatty‐acid amide hydrolase 2; GBA3, glucocerebrosidase 3; SGMS1, phosphatidylcholine ceramide choline phosphotransferase 1; SGPP2, sphingosine‐1‐phosphate phosphatase 2; SMPD1, sphingomyelin phosphodiesterase 1; SMPDL3A, acid sphingomyelinase‐like phosphodiesterase 3a; SMPDL3B, acid sphingomyelinase‐like phosphodiesterase 3b; SPHK1, sphingosine kinase 1.
Figure 6.
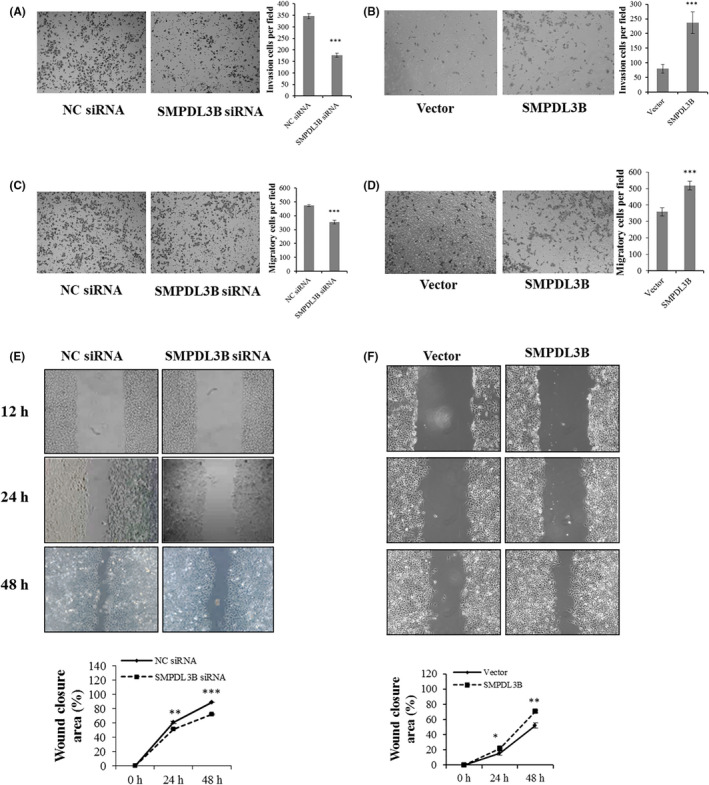
SMPDL3B promotes HCC cell invasion and migration in vitro. SMPDL3B‐knockdown (A, C, E) and SMPDL3B‐overexpression (B, D, F) cells were seeded. Transwell assays (A‐D) and wound‐healing assays (scale bar: 50 μm) (E, F) were performed in Huh‐7 cells. Cell numbers (A‐D) were counted using ImageJ software. The histograms (A‐D) represent the mean values of invasive and migrated cells per high‐power field (from at least 5 fields, mean ± SD) (***P < .001). The wound closure (E, F) area was quantified by ImageJ software. The error bar represents the SD from 3 independent experiments, and statistical significance was analyzed using Student t test (*P < .05; **P < .01; ***P < .001)
3.6. SMPDL3B knockdown inhibits tumor growth
The above results showed that SMPDL3B promoted the survival and the migration of liver cancer cells in vitro and that SMPDL3B expression was upregulated in HCC tissue. To confirm these results in vivo, mice were injected with SMPDL3B‐knockdown (SMPDL3B KD) Huh‐7 cells and were found to have smaller tumors compared with control mice (Figure 7A‐D).
Figure 7.
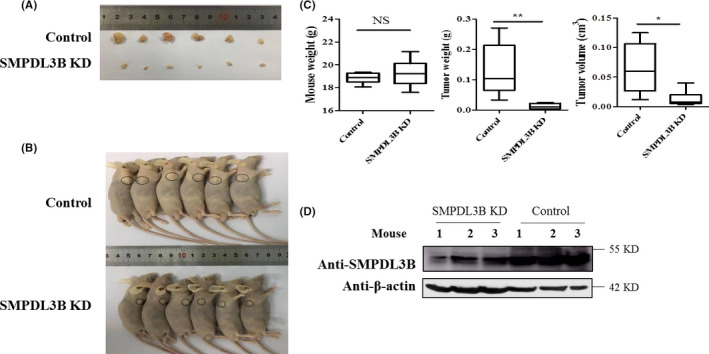
SMPDL3B knockdown (KD) significantly inhibits tumor growth. A, Nude mice with tumors were imaged; tumors are marked with a circle. B, Tumor tissues were collected and imaged. C, The weight of mouse (left), weight (middle) and the size (right) of the tumor and were collected and analyzed. D, The protein levels of SMPDL3B in tumor tissues were investigated by western blot; representative results are shown. Data were analyzed using Student t test (*P < .05; **P < .01)
3.7. SMPDL3B is involved in the ACER2‐mediated proliferation of cancer cells
SMPDL3B acts as oncogene, and mRNA expression of SMPDL3B was positively regulated by ACER2. Therefore, SMPDL3B might act downstream of ACER2. Similar to the PCR results, ACER2 also regulated the protein level of SMPDL3B in Huh‐7 cells (Figure 8A,C). Specifically, ACER2 knockdown led to SMPDL3B downregulation (Figure 8A), and ACER2 overexpression resulted in SMPDL3B upregulation (Figure 8C). Of note, SMPDL3B did not affect ACER2 expression (Figure 8A,C). We further evaluated the role of SMPDL3B in ACER2‐induced liver cancer cell proliferation. As expected, ACER2 knockdown inhibited Huh‐7 cell proliferation, and the reverse results were obtained with SMPDL3B overexpression (Figure 8B). Conversely, ACER2 overexpression promoted cancer cell growth, and SMPDL3B inhibition diminished the increased proliferation induced by ACER2 overexpression (Figure 8D). Taken together, these data demonstrated that ACER2 promoted cancer cell growth through SMPDL3B.
Figure 8.
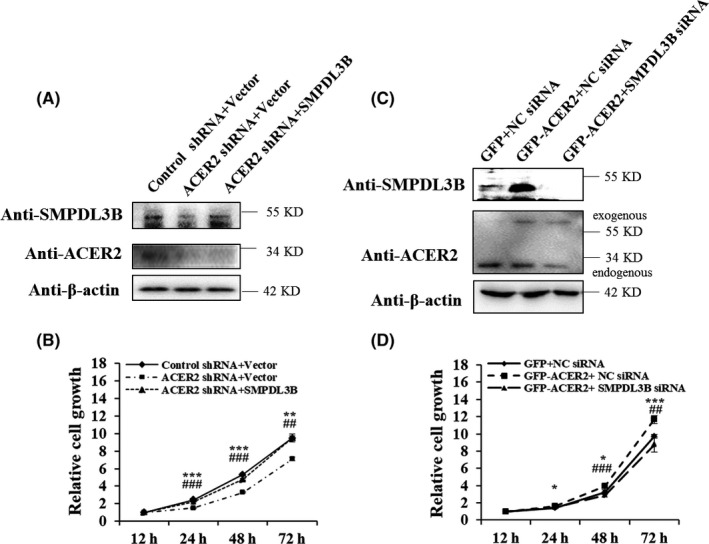
ACER2 affects HCC cell proliferation via SMPDL3B. A, B, Upregulation of SMPDL3B rescued the downregulation of ACER2‐mediated cell growth inhibition. C, D, Downregulation of SMPDL3B inhibited ACER2 overexpression‐mediated cell proliferation. Error bars represent the SD from 3 independent experiments, and statistical significance was analyzed using Student t test (*P < .05, ** or ##P < .01, *** or ###P < .001). Asterisk: Control shRNA + Vector vs ACER2 shRNA + Vector, or GFP + NC siRNA vs GFP‐ACER2 + NC siRNA; octothorpe: ACER2 shRNA + Vector vs ACER2 shRNA + SMPDL3B, or GFP‐ACER2 + NC siRNA vs GFP‐ACER2 + SMPDL3B siRNA (applicable below)
3.8. SMPDL3B is involved in ACER2‐mediated HCC cell invasion and migration
The above results showed that ACER2 promoted HCC cell invasion and migration, and ACER2 regulated HCC cell proliferation through SMPDL3B, indicating that the ACER2/SMPDL3B axis might play an important role in regulation of HCC invasion and migration. To confirm this hypothesis, we investigated the function of SMPDL3B in stable ACER2‐knockdown or ACER2‐overexpression cell lines. Results showed that ectopic SMPDL3B reversed invasion (Figure 9A) and migration inhibition (Figure 9C,E) induced by ACER2 knockdown. Meanwhile, SMPDL3B knockdown reduced the increased invasion (Figure 9B) and migration (Figure 9D,F) ability mediated by ACER2 overexpression. These results confirmed that ACER2 promoted HCC cell invasion and migration through SMPDL3B.
Figure 9.
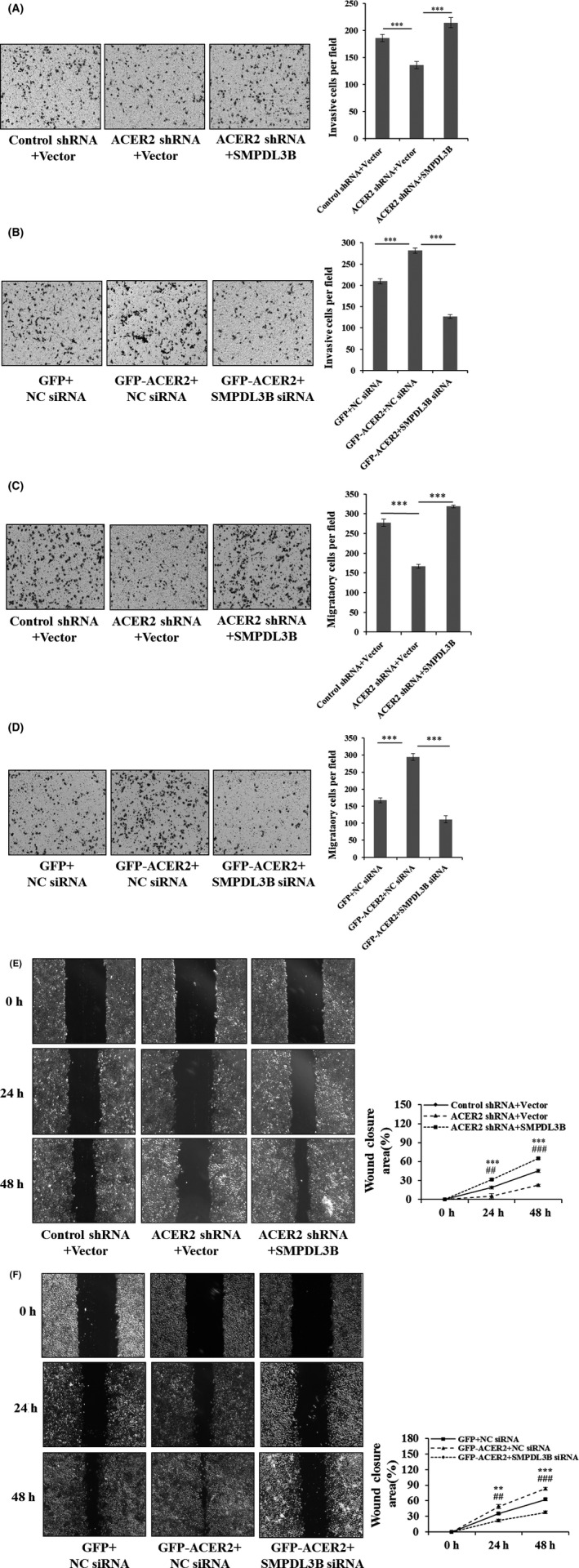
ACER2 promotes HCC cell invasion and migration through SMPDL3B. Huh‐7 cells with stable ACER2 knockdown (A, C, E) or overexpression (B, D, F) were transfected with the indicated plasmids (A, C, E) or siRNA (B, D, F) for 48 h; then, transwell assays (A‐D) and wound‐healing assays (E, F) were performed. The histograms (A‐D) represent the mean values of invasive and migrated cells per field (from at least 5 fields, mean ± SD); cell number was counted using ImageJ software (**P < .01; ***P < .001) (A‐D). The wound closure area (scale bar: 50 μm) was also quantified using ImageJ (E, F). Error bars represent the SD from 3 independent experiments, and statistical significance was analyzed using Student t test (** or ## P < .01, ***or ### P < .001)
3.9. The ACER2/SMPDL3B axis promotes HCC through S1P production
The above results indicated that SMPDL3B was involved in the oncogene function of ACER2; the associated mechanism was then investigated. Intracellular ceramides and S1P were measured to detect ACER2 activity. ACER2 knockdown increased ceramide content and decreased the amount of S1P in Huh‐7 cells, and these effects could be blocked by SMPDL3B overexpression (Figure 10A,B). In contrast, ACER2 overexpression resulted in intracellular ceramide hydrolysis and S1P production, which was reversed by SMPDL3B knockdown (Figure 10C,D). S1P promotes cancer cell survival. Therefore, the sphingosine kinase inhibitor SKII, which reduces S1P production, was used. SKII inhibited S1P formation induced by ACER2 or SMPDL3B overexpression without affecting the protein levels of ACER2 and SMPDL3B (Figure 11A‐D). Moreover, SKII blocked the effects of the ACER2/SMPDL3B axis on HCC cell growth (Figure 11E,F), invasion (Figure 12A, B), and migration (Figure 12C‐F).
Figure 10.
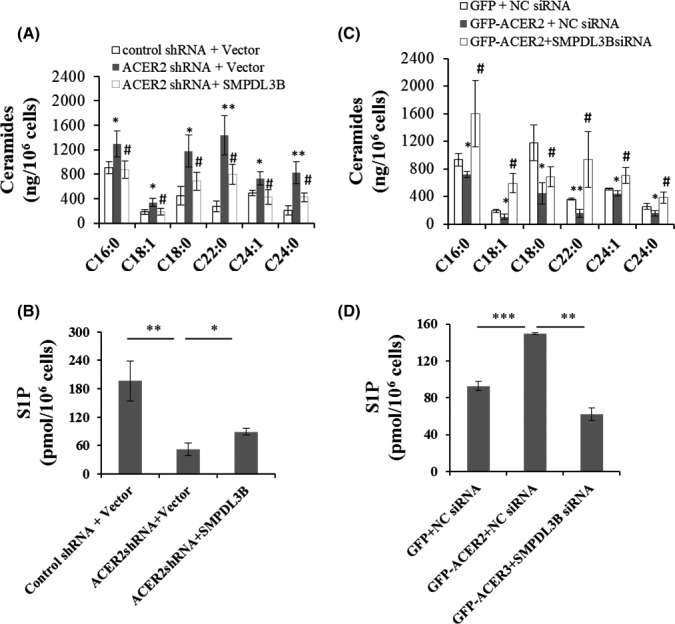
The ACER2/SMPDL3B axis promotes ceramides hydrolysis and S1P production. Huh‐7 cells with stable ACER2 knockdown (A, B) or overexpression (C, D) were transfected with the SMPDL3B expression vector (A, B) or SMPDL3B siRNA (C, D) for 48 h. Intracellular ceramides (A, C) and S1P (B, D) were measured. Error bars represent the SD from 3 independent experiments, and statistical significance was analyzed using Student t test. *, #P < .05; **P < .01; ***P < .001
Figure 11.
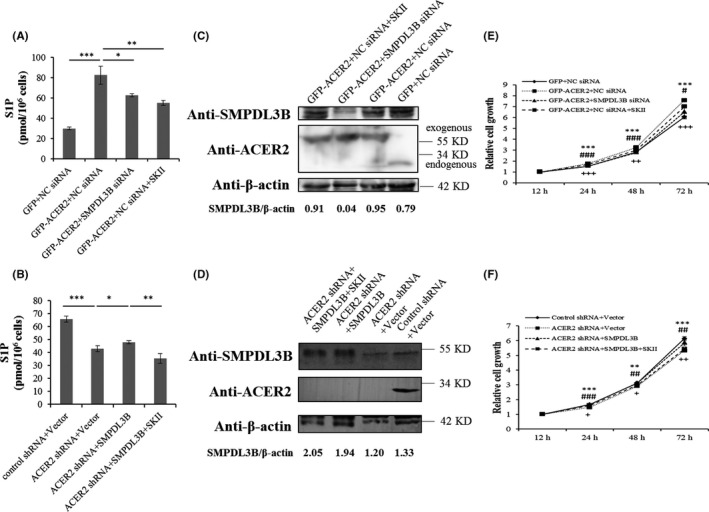
The ACER2/ SMPDL3B axis regulates HCC cell growth via S1P. A‐D, Hunh‐7 cells under the indicated treatments were lysed. One portion of the lysate was subjected to an S1P ELISA (A, B). Error bars represent the SD from 3 independent experiments, and statistical significance was analyzed using Student t test (*P < .05; **P < .01; ***P < .001). The other portion of the lysate was analyzed by western blotting with the indicated antibodies (C, D). E, F, SKII rescued the cell growth mediated by ACER2 overexpression (E) or SMPDL3B overexpression in the presence of ACER2 knockdown (F). Error bars represent the SD from 3 independent experiments, and statistical significance was analyzed using Student t test (#, +P < .05; **, ## or ++P < .01; ***, ### or +++P < .001). plus sign: ACER2 shRNA + SMPDL3B vs ACER2 shRNA + SMPDL3B+SKII or GFP‐ACER2 + NC siRNA vs GFP‐ACER2 + NC siRNA + SKII (applicable below)
Figure 12.
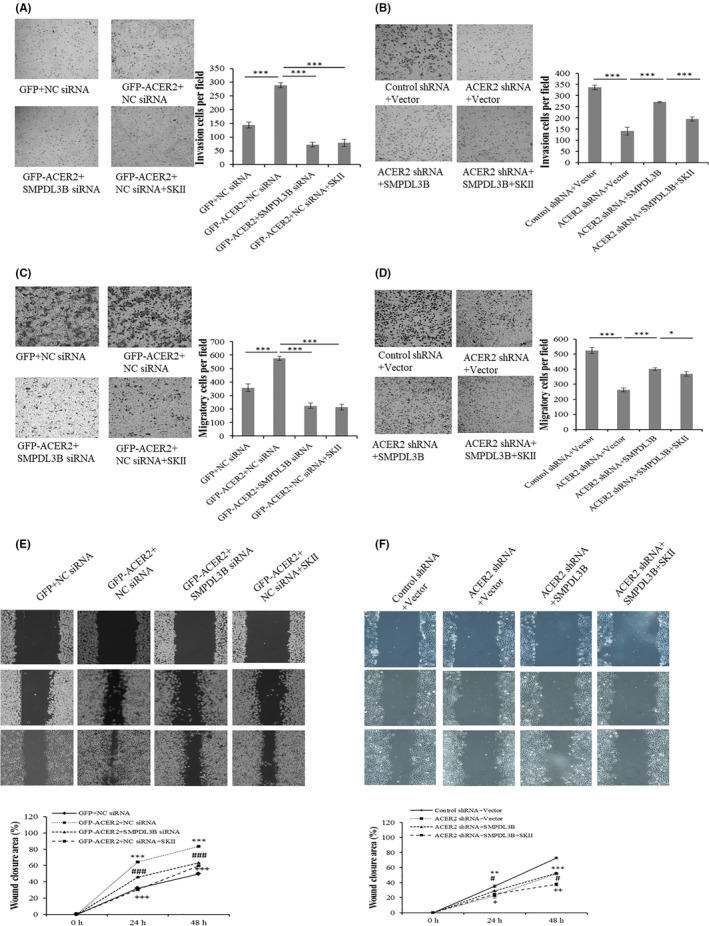
The ACER2/SMPDL3B axis regulates HCC cell invasion and migration via S1P. Stable ACER2 overexpression (A, C, E) or knockdown (B, D, F) Huh‐7 cells were transfected with the siRNA (A, C, E) or indicated plasmids (B, D, F) for 48 h during which SKII was added. Then, transwell assays (A‐D) and wound‐healing assays (E, F) were performed. The histograms (A‐D) represent the mean values of invasive and migrated cells per field (from at least 5 fields, mean ± SD); cell number was counted using ImageJ software (*P < .05; ***P < .001) (A‐D). The wound closure area (scale bar: 50 μm) was also quantified using ImageJ (E, F). Error bars represent the SD from 3 independent experiments, and statistical significance were analyzed using Student t test (# or +P < .05; ** or ++P < .01; ***, ### or +++P < .001)
4. DISCUSSION
In this study, we found that ACER2 expression was upregulated in livers of HCC patients and was positively correlated with tumor size. In addition, nude mouse xenograft experiments confirmed that ACER2 knockdown inhibited HCC tumor growth. Moreover, ACER2 promoted liver cancer cell growth, invasion, and migration via the sphingolipid‐metabolizing enzyme SMPDL3B.
ACER2 is well known to hydrolyze CER to produce sphingosine, both of which are stimuli for cell death. ACER2 was also recently found to mediate DNA damage, 10 , 17 and induce autophagy and apoptosis through reactive oxygen species. 17 In our previous study, ACER2 was also shown to promote tumor cell growth. 8 However, the precise effects of ACER2 on tumor cell proliferation and death have not been fully understood. ACER2 appears to have a dual role in tumor cell survival, as a low level of ectopic ACER2 promoted cancer cell growth and a high level of ectopic expression induced cell death, 8 this might explain the paradoxical phenomenon of its dual role in tumor cell growth.
Little information is known about the roles of ACER2 in HCC. In this study, there were higher levels of ACER2 in HCC tumor tissues compared with the adjacent non‐tumor tissues, and expression was positively related with tumor size. The IHC results revealed that ACER2 protein was localized to the cytoplasm and nucleus and, compared with adjacent non‐tumor tissues, both cytosolic and nuclear ACER2 were increased in HCC. However, HCC tissue expressed more nuclear ACER2, which indicated that ACER2 translocation might occur in HCC, but the underlying mechanisms remain unclear. Thus, ACER2 might serve as a prognostic indicator of HCC diagnosis. Our in vivo studies confirmed that ACER2 knockdown inhibited tumor growth, suggesting that ACER2 might be a novel target for HCC therapy. Our in vitro studies revealed that ACER2 affected liver cancer cell migration, but there was no significant association between ACER2 expression and tumor metastasis in the clinical samples from HCC patients, possibly due to the different microenvironments in vivo and in vitro.
In our study, we found that ACER2 expression negatively regulated the level of CER and positively regulated S1P content. Ceramides are known to promote cancer cell death, while S1P facilitates cell survival. Therefore, the promotion of HCC progression by ACER2 is probably related to CER as well as S1P production. Sphingosine kinase inhibited the oncogenic function of ACER2, suggesting that ACER2 promotes HCC through S1P.
Interestingly, SMPDL3B was found to promote HCC proliferation, invasion, and migration. Meanwhile, SMPDL3B knockdown inhibited HCC tumor growth in vivo. Therefore, SMPDL3B might be treated as a potential predictor for HCC.
It is worth noting that SMPDL3B was recently reported to generate the bioactive lipid ceramide‐1‐phosphate (C1P) in kidney cells. 18 , 19 However, in our study, we did not observe any significant change in the level of C1P when SMPDL3B was knocked down or overexpressed (Supporting Information Figure S1). Meanwhile, SMPDL3B overexpression reversed the HCC cell growth inhibited by ACER2 knockdown. However, this phenomenon disappeared in the presence of SKII. These results indicated that a ACER2/SMPDL3B/S1P axis exists during HCC development.
Apart from the hydrolysis of sphingomyelin, SMPDL3B recognizes ATP as its potential substrate 20 ; SMPDL3B hydrolyzes ATP to promote cancer cell growth, which may be another reason for ACER2 involvement in HCC. In addition, SMPDL3B blocks the Toll‐like receptor signaling pathway and negatively regulates innate immunity. 12 Because an increasing amount of evidence has demonstrated that innate immunocytes are very important in the body's defense against cancer cells, the induction of tumorigenesis by ACER2 via SMPDL3B is reasonable. Taken together, these data revealed that the ACER2/SMPDL3B axis is upregulated in HCC.
In conclusion, the results of this study showed that ACER2 expression was higher in HCC than in adjacent non‐tumor tissue. In vitro, increased ACER2 expression promoted cancer cell growth and migration. SMPDL3B has a similar function as ACER2. In addition, ACER2 in HCC cells upregulated the protein level of SMPDL3B, which may serve as a downstream effector of ACER2. Furthermore, ACER2/SMPDL3B regulated CER and S1P production, and the ACER2/SMPDL3B/S1P axis promoted HCC. Additional studies are needed on the physiological targets of ACER2 and its specific roles in the pathogenesis of HCC that will facilitate the development of novel therapeutic strategies for HCC.
DISCLOSURE STATEMENT
The authors have no conflicts of interest to declare.
Supporting information
Figure S1
ACKNOWLEDGMENTS
The present study was supported in part by the National Natural Science Foundation of China (81572738, 81960520), the Science and Technology Planned Project in Guilin (20190206‐1), the Lijiang Scholar Award in Guilin (2017‐004), the High Level of Innovation Team and Outstanding Scholars Program in Colleges and Universities in Guangxi (2017‐38‐08), and the Guangxi Distinguished Experts Special Fund (2019‐13‐12).
Liu B, Xiao J, Dong M, Qiu Z, Jin J. Human alkaline ceramidase 2 promotes the growth, invasion, and migration of hepatocellular carcinoma cells via sphingomyelin phosphodiesterase acid‐like 3B. Cancer Sci. 2020;111:2259–2274. 10.1111/cas.14453
REFERENCES
- 1. Zhu Y, Qu C, Hong X, et al. Trabid inhibits hepatocellular carcinoma growth and metastasis by cleaving RNF8‐induced K63 ubiquitination of Twist1. Cell Death Differ. 2019;26:306‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leonardi GC, Candido S, Cervello M, et al. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol. 2012;40:1733‐1747. [DOI] [PubMed] [Google Scholar]
- 4. Song R, Song H, Liang Y, et al. Reciprocal activation between ATPase inhibitory factor 1 and NF‐kappaB drives hepatocellular carcinoma angiogenesis and metastasis. Hepatology. 2014;60:1659‐1673. [DOI] [PubMed] [Google Scholar]
- 5. Coant N, Sakamoto W, Mao C, Hannun YA. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul. 2017;63:122‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao C, Xu R, Szulc ZM, Bielawska A, Galadari SH, Obeid LM. Cloning and characterization of a novel human alkaline ceramidase. A mammalian enzyme that hydrolyzes phytoceramide. J Biol Chem. 2001;276:26577‐26588. [DOI] [PubMed] [Google Scholar]
- 7. Xu R, Sun W, Jin J, Obeid LM, Mao C. Role of alkaline ceramidases in the generation of sphingosine and its phosphate in erythrocytes. FASEB J. 2010;24:2507‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu R, Jin J, Hu W, et al. Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. FASEB J. 2006;20:1813‐1825. [DOI] [PubMed] [Google Scholar]
- 9. Li F, Xu R, Low BE, et al. Alkaline ceramidase 2 is essential for the homeostasis of plasma sphingoid bases and their phosphates. FASEB J. 2018;32:3058‐3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu R, Garcia‐Barros M, Wen S, et al. Tumor suppressor p53 links ceramide metabolism to DNA damage response through alkaline ceramidase 2. Cell Death Differ. 2018;25:841‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mao Z, Sun W, Xu R, et al. Alkaline ceramidase 2 (ACER2) and its product dihydrosphingosine mediate the cytotoxicity of N‐(4‐hydroxyphenyl)retinamide in tumor cells. J Biol Chem. 2010;285:29078‐29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinz LX, Baumann CL, Koberlin MS, et al. The Lipid‐modifying enzyme SMPDL3B negatively regulates innate immunity. Cell Rep. 2015;11:1919‐1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoo TH, Pedigo CE, Guzman J, et al. Sphingomyelinase‐like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol. 2015;26:133‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmad A, Mitrofanova A, Bielawski J, et al. Sphingomyelinase‐like phosphodiesterase 3b mediates radiation‐induced damage of renal podocytes. FASEB J. 2017;31:771‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abou Daher A, El Jalkh T, Eid AA, Fornoni A, Marples B, Zeidan YH. Translational aspects of sphingolipid metabolism in renal disorders. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uranbileg B, Ikeda H, Kurano M, et al. Increased mRNA levels of sphingosine kinases and S1P lyase and reduced levels of S1P were observed in hepatocellular carcinoma in association with poorer differentiation and earlier recurrence. PLoS ONE. 2016;11:e0149462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Zhang C, Jin Y, et al. Alkaline ceramidase 2 is a novel direct target of p53 and induces autophagy and apoptosis through ROS generation. Sci Rep. 2017;7:44573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitrofanova A, Mallela SK, Ducasa GM, et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat Commun. 2019;10:2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mallela SK, Mitrofanova A, Merscher S, Fornoni A. Regulation of the amount of ceramide‐1‐phosphate synthesized in differentiated human podocytes. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:158517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gorelik A, Heinz LX, Illes K, Superti‐Furga G, Nagar B. Crystal structure of the acid sphingomyelinase‐like phosphodiesterase SMPDL3B provides insights into determinants of substrate specificity. J Biol Chem. 2016;291:24054‐24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


