Abstract
Cryoprobe is a novel transbronchial biopsy (TBB) tool that yields larger tissue samples than forceps. Pathological diagnosis and biomarker analysis, such as genetic alterations and programmed death‐ligand 1 (PD‐L1) expression, are paramount for precision medicine against lung cancer. We evaluated the safety and usefulness of cryoprobe TBB for lung cancer diagnosis and biomarker analysis. In this single‐center, prospective single‐arm study, patients suspected of having or diagnosed with primary lung cancer underwent cryoprobe TBB using flexible bronchoscopy after conventional forceps TBB from the same lesion. Cryoprobe TBB was performed in 121 patients. The incidence rate of severe bleeding and serious adverse events (4% [90% confidence interval: 2%‐9%]) was significantly lower than the expected rate (20% with 30% threshold, P < 0.01). Combining both central and peripheral lesions, the diagnostic yield rate of cryoprobe samples was 76% and that of forceps samples was 84%. Compared with forceps TBB samples, cryoprobe TBB samples were larger (cryoprobe 15 mm2 vs forceps 2 mm2) and resulted in a larger proportion of definite histomorphological diagnosis (cryoprobe 86% vs forceps 74%, P < 0.01), larger amounts of DNA extracted from samples (median: cryoprobe, 1.60 µg vs forceps, 0.58 µg, P = 0.02) and RNA (median: cryoprobe, 0.62 µg vs forceps, 0.17 µg, P < 0.01) extracted from samples, and tended to yield greater rates of PD‐L1 expression >1% (51% vs 42%). In conclusion, cryoprobe is a safe and useful tool for obtaining lung cancer tissue samples of adequate size and quality, which allow morphological diagnosis and biomarker analysis for precision medicine against lung cancer.
Keywords: biomarker, cryobiopsy, cryoprobe, immunohistochemistry, lung cancer
In this study, cryoprobe biopsy was performed in 121 patients. The incidence rate of severe bleeding and serious adverse events was 4%. Compared with forceps biopsy samples, cryoprobe biopsy samples were larger and resulted in a larger proportion of definite histomorphological diagnoses and larger amounts of DNA/RNA extracted from samples.

1. INTRODUCTION
Tissue sampling is not only an essential aspect of the pathological diagnosis of patients with lung cancer, but it is also crucial for biomarker analysis of tumors, 1 such as epidermal growth factor receptor (EGFR) gene mutations, anaplastic lymphoma kinase (ALK) gene fusions, ROS‐1 gene fusions and programmed death ligand 1 (PD‐L1) expression, which can allow the individualized selection of the best therapeutic approach (precision medicine) for advanced non–small cell lung cancer patients. 2 , 3 Currently used sampling methods, such as sputum cytology, bronchoscopy, percutaneous or transthoracic fine needle aspiration, mediastinoscopy, surgical lung biopsy, and transbronchial biopsy (TBB) with forceps, 4 , 5 , 6 have several limitations. These include low diagnostic yields for small (<2 cm) and peripherally located tumors, 7 inadequate tissue samples (ie, insufficient quantities of tumor tissue, poor quality of tumor tissue and crush artifacts), 1 , 8 , 9 and procedure‐related complications (eg, pneumothorax, infections and interstitial pneumonia). 10 Thus, sampling methods that are safe and allow for better accuracy (higher diagnostic rate), as well as the collection of appropriate tissue samples for biomarker analysis, are needed.
A cryoprobe is a novel biopsy tool based on the Joule‐Thomson principle that extracts tissue by a cryoadhesive effect. 11 TBB using a cryoprobe is an emerging tumor sampling procedure that is reportedly safe and suitable as a diagnostic tool for patients who are post‐lung transplant, 8 and for those with interstitial lung disease. 12 , 13 , 14 , 15 Recent trials that assessed the diagnostic yields of cryoprobe biopsy for endobronchial tumors showed encouraging safety and diagnostic yield results compared with forceps biopsy. 16 , 17 However, as the cryoprobe was only approved in Japan in 2017, related safety and utility data in Japanese patients are limited. Furthermore, the effectiveness of cryobiopsy as a sampling method for biomarker analysis (eg, EGFR gene mutation test and immunostaining for PD‐L1) is not clear. Therefore, the present study aimed to prospectively evaluate the safety and utility of TBB by cryoprobe for the diagnosis of primary lung cancer and biomarker analysis.
2. MATERIALS AND METHODS
2.1. Study design
This was a single‐center, prospective single‐arm study conducted at the National Cancer Center Hospital East at Kashiwa, Japan between February 2016 and February 2018.
2.2. Ethical considerations
The study protocol and associated documents were approved by the Institutional Review Board of the study center (National Cancer Center Japan: 2015‐309). The study was conducted according to the Declaration of Helsinki and local regulations. All patients provided informed consent at enrollment.
2.3. Patients
The main eligibility criteria were as follows: patients aged 20‐80 years with suspected or diagnosed primary lung cancer by chest computed tomography scheduled to undergo TBB by bronchoscopy, Eastern Cooperative Oncology Group (ECOG) performance status (PS) between 0 and 2, and adequate organ function (ie, neutrophil count ≥ 1500/mm3, platelet count ≥100 000/mm3, hemoglobin ≥9.0 g/dL, aspartate aminotransferase/alanine aminotransferase ≤100 U/L, total bilirubin ≤1.5 mg/dL, serum creatinine ≤ 1.5 mg/dL, prothrombin time‐ international normalized ratio ≤1.5, saturation of peripheral oxygen [SpO2] ≥90%, and forced expiratory volume in 1 second ≥1.0 L).
The main exclusion criteria were: hypersensitivity to lidocaine, midazolam, flumazenil and pethidine hydrochloride or serious hypersensitivity to other drugs; current treatment with antiplatelet agents or anticoagulants; history of bloody sputum (>1 teaspoon) within 1 month; radiographic evidence of obvious cavitary lung lesions and/or obvious tumor invasion into blood vessels in the hilum, heart or great arteries; serious complications or comorbidities (eg, heart disease, interstitial pneumonia and poorly controlled hypertension); clinically problematic infections; and women of childbearing potential who were not using adequate contraception, or who were pregnant or breastfeeding.
2.4. Transbronchial biopsy method
The bronchoscopy procedure was performed as previously described. 16 , 17 In this study, the bronchoscopy was conducted by HU and KK. Both investigators had experience in over 100 cases of TBB. One of the following flexible bronchoscopes was used: BF‐1TQ290, BF‐1T260, BF‐260, BF‐F260 or BF‐P260F (Olympus). Bronchial blockers were not used for prevention of bleeding.
All patients were given topical anesthesia for the larynx and pharynx with a 2% lidocaine (5 mL) spray. Pethidine hydrochloride (35 mg) and midazolam (2‐3 mg) were administered for intravenous anesthesia. During the bronchoscopy, 2% lidocaine (1‐2 mL) was injected via the forceps or midazolam (1 mg) was intravenously injected when the anesthesiologist considered it necessary.
The patients’ heart rate, blood pressure, and SpO2 were monitored via electrocardiogram, sphygmomanometer, and SpO2 monitor. To keep SpO2 ≥90%, oxygen inhalation was implemented as needed. During the bronchoscopy, a tracheal tube was inserted if airway control was necessary.
Initially, tissue sample collection by biopsy was performed using common forceps to secure sufficient tissue sample collection. Once the bleeding due to forceps TBB ceased, TBB was performed using the cryoprobe (1.9 mm dimension) (Erbe Elektromedizin GmbH).
The cryoprobe was inserted through the working channel of the bronchoscope and placed so that it was in contact with the lesion. Under direct vision or X‐ray fluoroscopy, the lesion was frozen for 3‐5 seconds, causing the tissue to attach to the cryoprobe tip. While still frozen and attached to the bronchoscope, the cryoprobe was retracted from the trachea, along with the tissue. The frozen tissue sample was then released from the cryoprobe point as previously described 18 and fixed in formalin. The cryoprobe was removed from the forceps opening of the bronchoscope and the bronchoscope was immediately re‐inserted to check for bleeding. The cryoprobe TBB was repeated, when no or a very small biopsy sample, or a sample that was expected to include few malignant cells, such as blood clots or necrotic tissue, was obtained and the bleeding ceased.
The total duration of the cryoprobe TBB was the time from cryoprobe insertion through the forceps opening of the bronchoscope up to the removal of the bronchoscope from the body. The total duration of the bronchoscopy was the time from the bronchoscope insertion through the vocal cords up to the removal of the bronchoscope from the body. The observation period was from the first consultation day up to more than 1 week from the bronchoscopy.
Inflation of tissue samples was not performed. Just after biopsy, forceps and cryoprobe samples were fixed in 10% neutral buffered formalin at room temperature for 18‐20 hours. There was no difference in formalin fixation time between the forceps and cryoprobe samples in each patient.
2.5. Endpoints
The primary endpoint of the study was the incidence rate of severe bleeding and serious adverse events (SAE). Bleeding events were assessed by severity as mild, moderate and severe based on previously reported definitions. 17 Other adverse events (AE) were graded using the Japanese version of Common Terminology Criteria for Adverse Events v.4.0 (CTCAE v4.0 – JCOG).
The secondary endpoints were the definite diagnostic yield rate, the tumor tissue sample size (the length of major and minor axes, the tissue area and the proportion of tumor cells contained), the success rate of cryobiopsy, the amount of DNA and RNA extracted from a tissue sample, the DNA and RNA integrity number, and the success rate of analysis of whole‐exome sequencing and RNA sequencing.
The definition of each term is as follows. The patients included in cryoprobe biopsy analysis were those whose tissue samples were taken by cryoprobe TBB. Patients with a definite diagnosis were those whose biopsy samples were obtained using forceps or cryoprobe, and whose definite pathological diagnosis was based on the analysis of tumor cells in biopsy samples. The diagnostic rate was defined as the rate of patients for whom a definite pathological diagnosis was available based on tissue samples obtained by forceps or cryoprobe biopsy. The size of the tissue sample was calculated by multiplying the length of the major and minor axes of the H&E‐stained sample. For tissue sample size measurement in virtual slides, the tissue areas were measured using a high‐resolution digital slide scanner (Aperio AT2, Leica Biosystems). The success rate of cryobiopsy was defined as the rate of patients whose tissue samples obtained by cryoprobe biopsy.
2.6. Immunohistochemistry
Immunostaining for thyroid transcription factor 1 (TTF‐1) (SP141, Roche/Ventana Medical Systems), p40 (BC28, Abcam) and PD‐L1 (22C3, Dako) was performed in non–small cell lung cancer (NSCLC) samples. The biopsy samples were fixed in neutral 10% buffered formalin and embedded in paraffin. For immunostaining, all formalin‐fixed paraffin‐embedded (FFPE) tissue biopsy samples were cut into 4‐μm‐thick sections. For TTF‐1 and p40 staining, the automated staining system Benchmark ULTRA (Roche) was used. For PD‐L1 staining, the Dako Autostainer Link 48 system (Dako) was used.
2.7. Genetic analysis
As a post‐hoc analysis, whole‐exome sequencing and RNA sequencing were performed in all 20 NSCLC patients who provided written informed consent for genetic analysis and had enough samples using both forceps and cryoprobe samples. From FFPE samples, 20 slides with 10‐µm sliced tissue were prepared for macrodissection. DNA and RNA were extracted using an AllPrep DNA/RNA Kit (Qiagen). The concentration of DNA and RNA were evaluated by Qubit 3.0 Fluorometer (Thermo Fisher Scientific) and Agilent 4200 TapeStation (Agilent Technologies Japan), respectively. Whole‐exome sequencing was performed in tissue samples, from which ≥0.7 µg of DNA was extracted. For targeting and capturing, a SureSelectXT Human All Exon V6 Kit (Agilent Technologies Japan) was used, and HiSeq4000 (Illumina) was used to perform whole‐exome sequencing. RNA sequencing was performed using HiSeq2500 (Illumina) in specimens, from which ≥0.25 µg of RNA was extracted. Libraries were prepared using TruSeq Stranded Total RNA LT (Illumina).
2.8. Statistical analysis
Based on previously published data in non–Japanese populations, 17 the expected value and threshold of the incidence rate for severe bleeding and serious AE were set at 20% and 30%. With a one‐sided significance level of 5% and power of 90%, the tissue sample size was estimated to be 175 patients. Although no formal interim analysis was planned, the incidence of severe bleeding and SAE seemed extremely infrequent with the enrollment of a total of 128 patients (February 2018). A discussion with study investigators reached a consensus that patient accrual should be discontinued because the Bayesian predictive probability that the incidence rate was to be lower than 30% (threshold value) at the time of final analysis (175 patients) was high. Statistical significance was established if the upper limit of the 90% confidence interval (CI) of the primary endpoint calculated for the safety analysis set did not exceed the threshold value of 30%. The Clopper–Pearson method was used to calculate the CI for proportion.
The analysis sets were the safety analysis set (all enrolled patients regardless of whether or not cryoprobe biopsies performed were evaluated for safety) and the efficacy analysis set (all cases that underwent a predetermined cryoprobe biopsy regardless of the diagnosis). We also performed safety assessments limited to those who underwent cryoprobe biopsy. Efficacy was also assessed in cases in which bronchoscopy was performed but excluded those in which cryoprobe biopsy was not performed.
Comparisons between forceps and cryoprobe biopsies for the severity of bleeding, diagnostic yield rate, histomorphological diagnostic rate, PD‐L1 expression evaluation, whole‐exome sequencing success rate, and RNA sequencing success rate were assessed using a McNemar or Bowker test. Comparisons of the size of the tissue samples between forceps and cryoprobe biopsies, which were calculated by multiplying the length of the major and minor axes, were assessed using the Wilcoxon rank‐sum test. Comparisons of the size of the tissue samples measured in virtual slides, the quantity of DNA and RNA, and the DNA and RNA integrity number, were assessed using the Wilcoxon signed‐rank test or paired t test. The definite diagnostic rate was calculated for both the forceps and cryoprobe biopsy samples, and the Pearson χ 2 or Fisher’s exact test was used for comparisons. Statistical analyses were performed using JMP version 11.1.1 (SAS Institute) and SAS Release 9.4 (SAS Institute).
3. RESULTS
3.1. Patients and procedure
Of the 128 patients enrolled, one patient presented hemoptysis before the bronchoscopy procedure and was found to be ineligible for the study. Two patients did not undergo TBB because in those cases, the peripheral lesions were not detectable by endobronchial ultrasound. These two patients were also discontinued from the study. Thus, 125 patients underwent bronchoscopy and TBB by forceps. TBB by cryoprobe was not performed in three patients due to moderate bleeding after forceps biopsy and in one patient due to severe bleeding after forceps biopsy; thus, for these patients, the bronchoscopy procedure was completed without cryoprobe TBB. A total of 121 patients underwent the procedure, including cryoprobe TBB (Figure 1).
Figure 1.
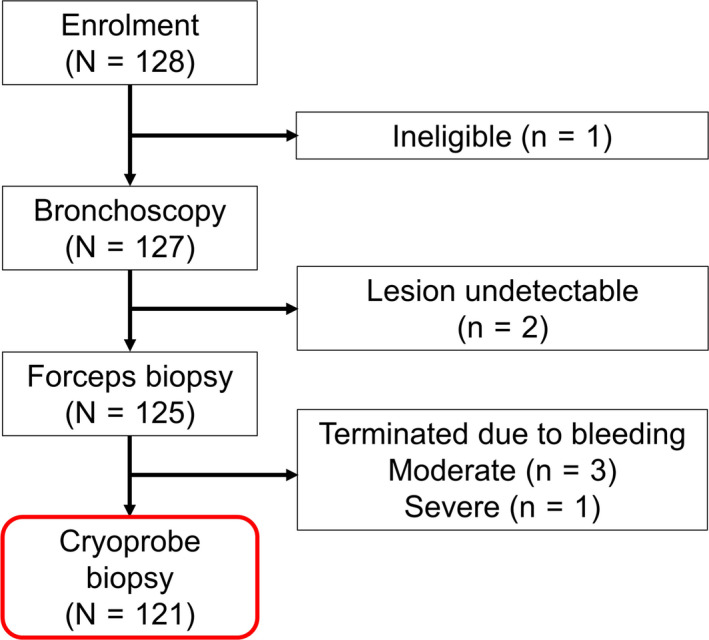
Patient disposition
The main patient characteristics are shown in Table 1. Of the 121 patients who underwent the complete cryoprobe TBB procedure, 69% were male. The median age was 68 years, 83% had a history of smoking, and all patients had an ECOG PS of 0‐1. Central lesions were slightly more frequent (53%) than peripheral lesions (47%). The median of the major axis of the tumor was 38 mm.
Table 1.
Patient characteristics
| Patient characteristics | Cryoprobe TBB performed N = 121 N (%) |
|---|---|
| Sex, male | 84 (69) |
| Age, years, median (range) | 68 (31‐79) |
| With smoking history | 101 (83) |
| ECOG PS 0‐1 | 121 (100) |
| Location of target lesion | |
| Central lesion | 64 (53) |
| Main/intermediate bronchus | 10/4 |
| Lobar bronchi | 42 |
| Segmental bronchi | 8 |
| Peripheral lesion (lobar bronchus) | 57 (47) |
| Right superior/left superior | 16/7 |
| Right middle/left lingular | 4/5 |
| Right inferior/left inferior | 14/11 |
| Tumor diameter, mm, median (range) | 38 (11‐90) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; TBB, transbronchial biopsy.
The median total duration of the bronchoscopy was 20.1 (7.2‐41.2) minutes and the median duration of the cryoprobe TBB was 7.5 (1.8‐26.8) minutes. The median number of forceps and cryoprobe biopsies performed were 5 (2‐12) and 2 (1‐5), respectively. BF‐1TQ290 was used in 115 patients (95%). Forceps TBB with a guide sheath was performed in 49 patients (40%).
3.2. Safety analysis
The proportion of moderate or severe bleeding events due to TBB was significantly higher with the cryoprobe (75%) compared with the forceps (61%) (Bowker test; P = 0.01) (Table 2). After the procedure, the most frequent AE reported was grade 1 bloody sputum, with six events (5%), followed by four (3%) events of grade 3 pulmonary infection and two (2%) grade 1 events of fever. There was no incidence of pneumothorax.
Table 2.
Adverse events during and after the procedure
| Cryoprobe TBB performed (N = 121) | ||||
|---|---|---|---|---|
| Cryoprobe n (%) | Forceps n (%) | P‐value | ||
| During the procedure | ||||
| Bleeding after biopsy | None | 2 (2) | 4 (3) | 0.01 |
| Mild | 28 (23) | 43 (36) | ||
| Moderate | 90 (74) | 74 (61) | ||
| Severe | 1 (1) a | ‐ | ||
| After the procedure | ||||
| Bloody sputum hemoptysis | Grade1 | 6 (5) | ||
| Pulmonary infection | Grade2 | 1 (1) | ||
| Grade3 | 4 (3) a | |||
| Fever | Grade1 | 2 (2) | ||
| Chest pain | Grade1 | 1 (1) | ||
Abbreviations: CI, confidence interval; TBB, transbronchial biopsy.
Severe bleeding/serious adverse event, 4% (90% CI 2‐8), P < 0.01.
Severe bleeding was reported in one patient after cryoprobe biopsy, and SAE (grade 3 lung infection) were reported in four patients; thus, the incidence rate of severe bleeding and SAE, assessed as the primary endpoint, was 4% (5/121 patients, 90% CI: 2%‐9%), resulting in a significantly lower than expected rate (expected value 20% with a 30% threshold).
3.3. Success rate of cryobiopsy
For central lesions, the success rate was 97% and for peripheral lesions, the success rate was 86%. The success rate up to the first 1‐20 cases of central lesions was 90%, and the success rate after the 21st case was 100%. The success rate for the first 1‐20 cases of peripheral lesions was 60%, and the success rate for the 21st and subsequent cases was 100% (Figure 2). There was no difference in the success rate of cryobiopsy depending on the lesion site.
Figure 2.
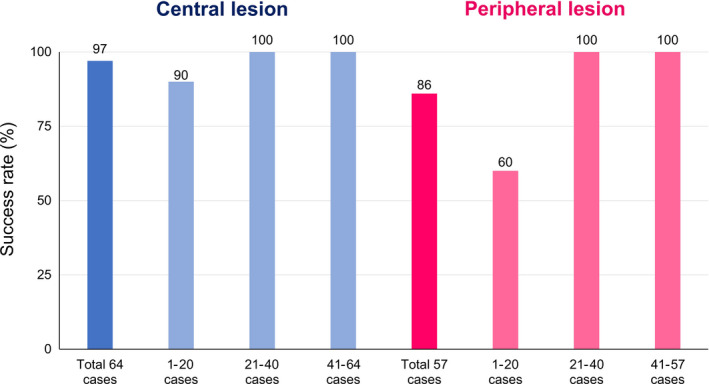
Success rate of cryoprobe transbronchial biopsy
3.4. Tissue sample size
Median tumor tissue sample size was significantly larger in cryoprobe TBB compared with forceps TBB, both overall (cryoprobe 15 mm2 vs forceps 2 mm2, Wilcoxon rank‐sum test; P < 0.01) and for central and peripheral lesions (both, Wilcoxon rank‐sum test; P < 0.01) (Table 3 and Figure 3). Similar findings were obtained in the virtual slides of 81 patients whose slides contained tumor cells (median; cryoprobe 11.4 mm2 vs forceps 2.0 mm2, Wilcoxon signed rank test; P < 0.01).
Table 3.
Size of samples
| Size of sample a (mm2) in glass slides | ||
|---|---|---|
| Median (range) | P‐value | |
| Total patient number (N = 111) | ||
| Cryoprobe: 177 samples | 15 (0.3‐273) | <0.001 |
| Forceps: 358 samples | 2 (0.3‐28) | |
| Central lesion (N = 62) | ||
| Cryoprobe: 108 samples | 15 (0.3‐273) | <0.001 |
| Forceps: 160 samples | 2 (0.3‐20) | |
| Peripheral lesion (N = 49) | ||
| Cryoprobe: 69 samples | 15 (1‐42) | <0.001 |
| Forceps: 198 samples | 2 (0.3‐28) | |
| Size of sample (mm2) in virtual slides | ||
|---|---|---|
| Median (range) | P‐value | |
| Total patient number (N = 81) b | ||
| Cryoprobe | 11.4 (1.8‐135.0) | <0.01 |
| Forceps | 2.0 (0.6‐8.4) | |
Size of pathology specimen = minor axis × major axis.
Both forceps and cryoprobe specimens contained tumor cells.
Figure 3.
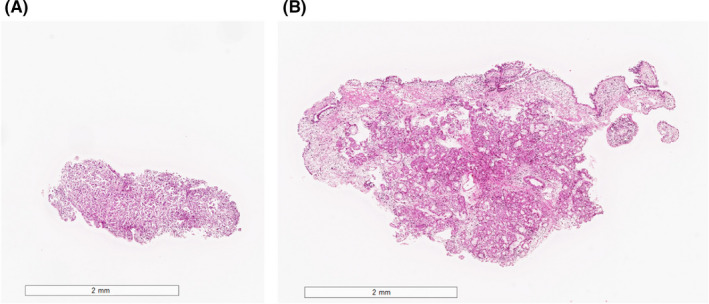
Size of tissue samples in a representative case. A, Forceps biopsy. B, Cryoprobe biopsy
3.5. Diagnostic yield and histomorphological assessment
In total, 121 patients underwent cryoprobe TBB. Among them, 113 patients were diagnosed with lung cancer (adenocarcinoma, 49; squamous‐cell carcinoma, 24; small‐cell carcinoma, 17; NSCLC‐not other specified [NOS], 20; carcinoid, 2; and adenoid cystic carcinoma, 1) in the forceps or cryoprobe sample. Among 8 patients who were not diagnosed in the forceps or cryoprobe sample, 7 patients were finally diagnosed with lung cancer (adenocarcinoma, 4; squamous‐cell carcinoma, 1; large‐cell carcinoma, 1; and NSCLC‐NOS, 1), and 1 patient with sarcoidosis. The diagnostic yield rates of cryoprobe and forceps samples, combining both central and peripheral lesions, were 76% and 84%, respectively, without significant difference (McNemar test; P = 0.08, Table 4). In patients with peripheral lesions (n = 57), the diagnostic yield rates of cryoprobe and forceps biopsy were 67% and 86%, respectively, and the diagnostic yield of cryoprobe was significantly lower (McNemar test; P < 0.01, Table 4), while for patients with central lesions (n = 64), the diagnostic yield rates of cryoprobe and forceps biopsy were 84% and 83%, respectively, without significant differences (McNemar test; P = 0.80, Table 4). However, among patients whose tissue samples were obtained by cryoprobe biopsy (n = 111), the diagnostic yield rates for cryoprobe and forceps samples, combining both central and peripheral lesions, were 83% and 84%, respectively, without significant differences (McNemar test; P = 0.83, Table 4). Compared with the forceps TBB, the cryoprobe TBB resulted in a significantly larger proportion of definite morphological diagnosis (McNemar test; P < 0.01, Table 5 and Figures 4, 5).
Table 4.
Diagnostic yield
| Cryoprobe | |||
|---|---|---|---|
| Patients who underwent cryoprobe biopsy (N = 121) | |||
| Diagnostic | Non–diagnostic | Total, n (%) | |
| Forceps | |||
| Diagnostic | 81 | 21 | 102 (84) |
| Non–diagnostic | 11 | 8 | 19 (16) |
| Total, n (%) | 92 (76) | 29 (24) | P = 0.08 |
| Central lesion (N = 64) | |||
| Forceps | |||
| Diagnostic | 46 | 7 | 53 (83) |
| Non–diagnostic | 8 | 3 | 11 (17) |
| Total, n (%) | 54 (84) | 10 (16) | P = 0.80 |
| Peripheral lesion (N = 57) | |||
| Forceps | |||
| Diagnostic | 35 | 14 | 49 (86) |
| Non–diagnostic | 3 | 5 | 8 (14) |
| Total, n (%) | 38 (67) | 19 (33) | P < 0.01 |
| Patients with successful cryoprobe sampling (N = 111) | |||
|---|---|---|---|
| Diagnostic | Non–diagnostic | Total, n (%) | |
| Forceps | |||
| Diagnostic | 81 | 12 | 93 (84) |
| Non–diagnostic | 11 | 7 | 18 (16) |
| Total, n (%) | 92 (83) | 19 (17) | P = 0.83 |
| Central lesion (N = 62) | |||
| Forceps | |||
| Diagnostic | 46 | 5 | 51 (82) |
| Non–diagnostic | 8 | 3 | 11 (18) |
| Total, n (%) | 54 (87) | 8 (13) | P = 0.41 |
| Peripheral lesion (N = 49) | |||
| Forceps | |||
| Diagnostic | 35 | 7 | 42 (86) |
| Non–diagnostic | 3 | 4 | 7 (14) |
| Total, n (%) | 38 (78) | 11 (22) | P = 0.21 |
Table 5.
Morphological diagnosis
| Patients who underwent cryoprobe biopsy (N = 121) | Cryoprobe N (%) | Forceps N (%) |
|---|---|---|
| Definite morphological diagnosis | 78 (64) | 75 (62) |
| Adenocarcinoma | 41 | 39 |
| Squamous cell carcinoma | 20 | 19 |
| SCLC | 14 | 14 |
| Other | 3 | 3 |
| Morphological diagnosis unavailable | 14 (12) | 27 (22) |
| NSCLC‐NOS | 14 | 26 |
| Carcinoma | 0 | 1 |
| Non–diagnostic | 29 (24) | 19 (16) |
| Both forceps and cryoprobe specimens contained tumor cells (N = 81) | Cryoprobe N (%) | Forceps N (%) | P‐value |
|---|---|---|---|
| Definite morphological diagnosis | 70 (86) | 60 (74) | <0.01 |
| Adenocarcinoma | 35 | 30 | |
| Squamous cell carcinoma | 19 | 15 | |
| SCLC | 12 | 11 | |
| Other | 4 | 4 | |
| Morphological diagnosis unavailable | 11 (14) | 21 (26) | |
| NSCLC‐NOS | 11 | 20 | |
| Carcinoma | 0 | 1 |
Abbreviations: NSCLC‐NOS, non–small cell lung cancer not otherwise specified; SCLC, small cell lung cancer.
Figure 4.
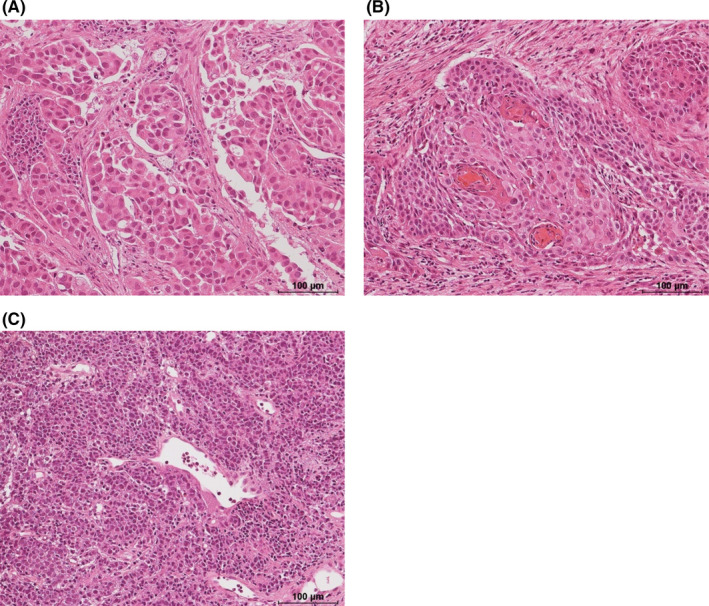
Histomorphological assessment in representative cases. A, Adenocarcinoma. B, Squamous‐cell carcinoma. C, Small‐cell carcinoma
Figure 5.

Representative cases diagnosed as adenocarcinoma in a cryoprobe sample (A) but non–small cell carcinoma in a forceps sample (B), and squamous‐cell carcinoma in a cryoprobe sample (C) but non–small cell carcinoma in a forceps sample (D)
3.6. Immunohistochemical analysis
Table 6 shows the results of the immunohistochemical analysis. The rate of concordance of TTF‐1 immunostaining for forceps samples and cryoprobe samples was 98% and that of p40 was 97%. Among patients with NSCLC, there was no difference between the success rate of PD‐L1 expression evaluation in cryoprobe samples and forceps samples (97% and 98%, respectively). The rate of PD‐L1 expression >1% for cryoprobe samples was 51% and that for forceps samples was 42%. There was no significant difference between samples, but the rate for cryoprobe samples tended to be higher (McNemar test; P = 0.06) (Table 6).
Table 6.
Concordance with TTF‐1 and p40 immunostaining and PD‐L1 expression evaluation
| Concordance with immunostaining | |||
|---|---|---|---|
| NSCLC (N = 62) | Cryoprobe | ||
| Positive | Negative | Total | |
| TTF‐1 | |||
| Forceps | |||
| Positive | 29 | 0 | 29 |
| Negative | 1 | 32 | 33 |
| Total | 30 | 32 | 62 |
| Concordance rate = 98% | |||
| p40 | |||
| Forceps | |||
| Positive | 24 | 1 | 24 |
| Negative | 1 | 37 | 38 |
| Total | 25 | 37 | 62 |
| Concordance rate = 97% | |||
| PD‐L1 expression | |||
|---|---|---|---|
| NSCLC (N = 62) | Evaluable | Unevaluable | Total, n (%) |
| Forceps | |||
| Evaluable | 59 | 2 | 61 (98) |
| Unevaluable | 1 | 0 | 1 (2) |
| Total, n (%) | 60 (97) | 2 (3) | P = 0.56 |
| Evaluable (N = 59) | ≥1% | <1% | Total, n (%) |
|---|---|---|---|
| Forceps | |||
| ≥1% | 24 | 1 | 25 (42) |
| <1% | 6 | 28 | 34 (58) |
| Total, n (%) | 30 (51) | 8 (49) | P = 0.06 |
| Evaluable (N = 59) | ≥50% | <50% | Total, n (%) |
|---|---|---|---|
| Forceps | |||
| ≥50% | 8 | 1 | 9 (15) |
| <50% | 4 | 46 | 50 (85) |
| Total, n (%) | 12 (20) | 47 (80) | P = 0.18 |
Abbreviations: NSCLC, non–small cell lung cancer; PD‐L1, programmed death ligand 1; TTF‐1, thyroid transcription factor 1.
3.7. Genetic analysis
Compared with the forceps TBB, cryoprobe TBB resulted in significantly larger median amounts of DNA extracted from a tissue sample (cryoprobe, 1.60 µg vs forceps, 0.58 µg, Wilcoxon signed‐rank test; P = 0.02) and RNA extracted from a tissue sample (0.62 vs 0.17 µg, P < 0.01), as well as DNA and RNA amounts available for analysis (both, McNemar test; P < 0.01). The DNA integrity number of DNA extracted from the cryoprobe samples was higher than that from the forceps samples (median; 3.1 vs 2.4, paired t test; P < 0.01). There was no difference in the RNA integrity number between RNA extracted from the cryoprobe and forceps samples (median; 2.1 vs 2.2, paired t test; P = 0.07). There were also significantly higher success rates for whole‐exome sequencing (cryoprobe, 90% vs forceps, 15%, respectively, McNemar test; P < 0.01) and RNA sequencing (75% vs 10%, respectively, McNemar test; P < 0.01) (Table 7). Eighteen tissue samples taken using a cryoprobe and three tissue samples taken using forceps contained sufficient DNA to successfully perform whole‐exome sequencing. The calculated tumor mutation burden from the whole‐exome sequencing of cryoprobe samples (N = 18) was 84 (range: 3‐2396). Furthermore, 15 tissue samples obtained by cryoprobe and two tissue samples obtained by forceps contained sufficient RNA to successfully perform RNA sequencing.
Table 7.
Rate of analyses available for NGS
| NSCLC (N = 20) | Cryoprobe N (%) | Forceps N (%) | P‐value |
|---|---|---|---|
| DNA amount (µg), median (range) | 1.60 (0.21‐15.8) | 0.58 (0.01‐1.76) | 0.02 |
| DNA integrity number, median (range) | 3.1 (2.4‐3.8) | 2.4 (0‐2.9) | <0.01 |
| Analysis available for whole‐exome sequencing | 18 (90) | 3 (15) | <0.01 |
| Successfully performed whole‐exome sequencing | 18 (90) | 3 (15) | <0.01 |
| RNA amount (µg), median (range) | 0.62 (0.05‐10.1) | 0.17 (0.00‐0.37) | <0.01 |
| RNA integrity number, median (range) | 2.1 (1.9‐2.5) | 2.2 (1.7‐5.1) | 0.07 |
| Analysis available for RNA sequencing | 15 (75) | 2 (10) | <0.01 |
| Successfully performed RNA sequencing | 15 (75) | 2 (10) | <0.01 |
Formalin‐fixed paraffin‐embedded slides 10 µm × 20 sheets, microdissection.
Abbreviations: NGS, next‐generation sequencing; NSCLC, non–small cell lung cancer.
4. DISCUSSION
Our study showed that cryoprobe TBB is safe and relatively useful for sampling tumor tissue, resulting in larger tissue sample sizes, adequate histomorphological diagnosis and immunohistochemistry analysis, and a greater quantity of DNA and RNA extracted from a tissue sample compared with forceps TBB. This study is the first prospective study to evaluate the safety and usefulness of cryoprobe TBB in Japanese individuals suspected of having or diagnosed with lung cancer. In addition, to the best of our knowledge, this is the first study that shows the utility for biomarker analysis of a cryoprobe TBB sample.
The incidence of severe bleeding and SAE, assessed as the primary endpoint, was 4%, which was much lower than the estimated threshold rate of 30%, indicating that cryoprobe TBB is a safe biopsy method. However, the number of patients with moderate and severe bleeding was higher among patients undergoing cryoprobe TBB than those undergoing forceps biopsy. Therefore, checking for and treating bleeding periprocedurally is crucial.
Compared with the sizes of tissue samples obtained by forceps TBB, larger tissue sample sizes were obtained by cryoprobe TBB. The success rates of histomorphologic diagnosis were also higher for tissue samples obtained by cryoprobe TBB than by forceps TBB. The present findings are consistent with previous studies comparing cryoprobe biopsy with endobronchial biopsy in other populations. 16 , 17 , 19 In addition, in our study, the success rates of whole‐exome sequencing and RNA sequencing were higher for tissue samples obtained by cryoprobe TBB than by forceps TBB. Tissue samples taken using cryoprobe TBB were appropriate for PD‐L1, TTF‐1 and p40 immunostaining.
The cryoprobe TBB success rates in total, and in patients with central and peripheral lesions were 92%, 97% and 86%, respectively. However, the success rates were 90% and 60% for central and peripheral lesions, respectively, among the first 20 patients who underwent the procedure. Conversely, the 21st patient and all subsequent patients had a success rate of 100% for both central and peripheral lesions. Of note, all procedures were performed by two bronchoscopists with experience of over 100 bronchoscopy cases. However, at the beginning of this study, the cryoprobe had not yet been approved in Japan and the operators did not perform cryoprobe biopsy as part of their daily clinical routine. This resulted in insufficient cryoprobe TBB training for the bronchoscopists and may be the reason for the low success rates in the first 20 patients registered in the study. As the success rates remarkably improved after 20 cases of cryoprobe TBB, we can speculate that approximately 20 procedures may be required to become proficient in this technique. It was inferred that greater technical expertise may be needed to sample peripheral lesions compared with central lesions.
A total of 121 patients underwent cryoprobe TBB, and the diagnostic yield rates of cryoprobe and forceps samples were 76% and 84%, respectively. Although the difference was not significant, the diagnostic yield rates for tissue samples obtained by cryoprobe TBB tended to be lower (P = 0.08) than those obtained by forceps. We consider that the success rate of cryoprobe TBB was low in the initial 20 patients enrolled in this study, and this may have affected the overall results of the study. We attribute the initially low success rates to operator‐related technical issues rather than mechanical issues related to the cryoprobe. In fact, in patients successfully sampled with the cryoprobe (n = 111), the diagnostic yield rate was not significantly different (83% and 84%, respectively; P = 0.83) to that in patients sampled using forceps. Another relevant factor that may have affected the present results was that the cryoprobe TBB was performed after forceps biopsy. Especially for central lesions, identifying the lesion became difficult because of bleeding at the site of forceps biopsy, and, thus, it was challenging to correctly collect a tissue sample with the cryoprobe. For peripheral lesions, it is physically more difficult to maneuver the cryoprobe than the forceps and this makes it more difficult to guide the cryoprobe to the bronchus where the lesion is located. Although this study did not show the superiority of the cryoprobe over forceps for TBB, a previous study did demonstrate the superiority of cryoprobe TBB over forceps TBB in terms of diagnostic yield rates. 17 Moreover, a meta‐analysis of eight studies of cryobiopsy versus forceps biopsy involving 916 patients indicated that overall the diagnostic rate for cryobiopsy was significantly higher (risk ratio 1.36; P = 0.0002). 20 More recent clinical data have also reported that diagnostic yield with cryobiopsy is equivalent or better 21 than with forceps biopsy, and is superior to other techniques such as bronchoalveolar lavage for successfully diagnosing lung malignancy. 22
Cryobiopsy has been previously reported to provide both larger size and greater depth of tissue specimens compared with forceps biopsy. 19 In the comparative meta‐analysis of cryobiopsy vs forceps biopsy, a significantly larger specimen area (mm2) was reported for cryobiopsy samples (standard mean difference 1.21; P < 0.00001). This increase in quality and tissue sample size may improve the success rate in establishing a definite diagnosis for patients compared with traditional sampling techniques. 19 The large quantity and high quality of DNA extracted from tissue samples produced by cryobiopsy has previously been shown to be suitable for next‐generation sequencing and rapid diagnosis, 23 which is consistent with the results of the current analysis.
Importantly, our prospective data, combined with previously reported Japanese analyses, support the use of cryobiopsy in the Japanese population. In a retrospective analysis of patients with peripheral pulmonary lesions, cryobiopsy provided a diagnostic rate of 91.6% and facilitated the measurement of multiple biomarkers. 24 A retrospective pathologic analysis of tissue samples obtained during biopsy of Japanese patients with pleural effusion found that cryobiopsy samples produced higher quality samples for immunohistochemistry and improved diagnostic accuracy. 25 A recent small‐scale prospective analysis in 23 Japanese patients with various lung diseases, including pneumonia, pneumonitis, and malignant and benign tumors, also found that cryobiopsy was useful, safe and feasible, providing a high diagnostic yield and high‐quality DNA for next‐generation sequencing. 26
The main limitations of the study are that the sample population was enrolled at a single center, patients underwent both interventions during the same surgical period, which could have influenced the rate of complications, and the procedures were performed by two different operators, which may have also influenced the results. In addition, inflation of tissue samples was not performed in this study. Tissue sample size of forceps biopsy might be underestimated due to no inflation of tissue samples.
In conclusion, the present results indicate that TBB with cryoprobe is a safe procedure and, compared with TBB with forceps, is relatively useful for obtaining tumor tissue samples of suitable size and quality to allow for morphological diagnosis as well as biomarker analysis. This technique should improve the ability to make clinical decisions for precision medicine and optimal treatment against lung cancer.
Disclosure
The authors have no conflict of interest.
ACKNOWLEDGMENTS
We would like to thank: Shinji Sasada, Department of Respiratory Medicine, Tokyo Saiseikai Central Hospital, Tokyo, Japan for participating in the advisory committee on safety; Takashi Ikeno, Clinical Research Support Office, National Cancer Center Hospital East, Kashiwa, Japan for providing statistical analysis support; Yuka Nakamura, Division of Pathology, Exploratory Oncology Research and Clinical Trial Center, National Cancer Center Hospital East, Kashiwa, Japan for providing immunohistochemical analysis support; the Japan Agency for Medical Research and Development for supporting the conduct of this study; and Keyra Martinez Dunn, MD, of Edanz Medical Writing for providing medical writing support. This study was funded by grants from the Japan Agency for Medical Research and Development (AMED) for research projects for innovative medical practice in cancer “Development of a New Treatment for Small Cell Lung Cancer harboring Rare Molecular Alterations (JP16ck0106041, principal researcher: Koichi Goto, National Cancer Center Hospital East)” and “Large‐scale prospective observational study to evaluate the clinical significance of predictive biomarker using innovative technology for the development of future cancer precision medicine (JP18ck0106411, principal researcher: Shingo Matsumoto, National Cancer Center Hospital East).”
Udagawa H, Kirita K, Naito T, et al. Feasibility and utility of transbronchial cryobiopsy in precision medicine for lung cancer: Prospective single‐arm study. Cancer Sci. 2020;111:2488–2498. 10.1111/cas.14489
REFERENCES
- 1. Kim L, Tsao MS. Tumour tissue sampling for lung cancer management in the era of personalised therapy: what is good enough for molecular testing? Eur Respir J. 2014;44:1011‐1122. [DOI] [PubMed] [Google Scholar]
- 2. Korpanty GJ, Graham DM, Vincent MD, Leighl NB. Biomarkers that currently affect clinical practice in lung cancer: EGFR, ALK, MET, ROS‐1, and KRAS. Front Oncol. 2014;4:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aguiar PN Jr, De Mello RA, Hall P, et al. PD‐L1 expression as a predictive biomarker in advanced non–small‐cell lung cancer: updated survival data. Immunotherapy. 2017;9:499‐506. [DOI] [PubMed] [Google Scholar]
- 4. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S‐e165S. [DOI] [PubMed] [Google Scholar]
- 5. Stamatis G. Staging of lung cancer: the role of noninvasive, minimally invasive and invasive techniques. Eur Respir J. 2015;46:521‐531. [DOI] [PubMed] [Google Scholar]
- 6. Herth FJ, Eberhardt R, Ernst A. The future of bronchoscopy in diagnosing, staging and treatment of lung cancer. Respiration. 2006;73:399‐409. [DOI] [PubMed] [Google Scholar]
- 7. McLean AEB, Barnes DJ, Troy LK. Diagnosing lung cancer: the complexities of obtaining a tissue diagnosis in the era of minimally invasive and personalised medicine. J Clin Med. 2018;7:E163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yarmus L, Akulian J, Gilbert C, et al. Cryoprobe transbronchial lung biopsy in patients after lung transplantation: a pilot safety study. Chest. 2013;143:621‐626. [DOI] [PubMed] [Google Scholar]
- 9. Brooks DR, Austin JH, Heelan RT, et al. Influence of type of cigarette on peripheral versus central lung cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:576‐581. [DOI] [PubMed] [Google Scholar]
- 10. Kondoh Y, Taniguchi H, Kitaichi M, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med. 2006;100:1753‐1759. [DOI] [PubMed] [Google Scholar]
- 11. Franke KJ, Szyrach M, Nilius G, et al. Experimental study on biopsy sampling using new flexible cryoprobes: influence of activation time, probe size, tissue consistency, and contact pressure of the probe on the size of the biopsy specimen. Lung. 2009;187:253‐259. [DOI] [PubMed] [Google Scholar]
- 12. Hagmeyer L, Theegarten D, Wohlschläger J, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J. 2016;10:589‐595. [DOI] [PubMed] [Google Scholar]
- 13. Hernández‐González F, Lucena CM, Ramírez J, et al. Cryobiopsy in the diagnosis of diffuse interstitial lung disease: yield and cost‐effectiveness analysis. Arch Bronconeumol. 2015;51:261‐271. [Article in English, Spanish.] [DOI] [PubMed] [Google Scholar]
- 14. Dhooria S, Sehgal IS, Aggarwal AN, Behera D, Agarwal R. Diagnostic yield and safety of cryoprobe transbronchial lung biopsy in diffuse parenchymal lung diseases: systematic review and meta‐analysis. Respir Care. 2016;61:700‐712. [DOI] [PubMed] [Google Scholar]
- 15. Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: Expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration. 2018;95:188‐200. [DOI] [PubMed] [Google Scholar]
- 16. Schumann C, Hetzel J, Babiak AJ, et al. Cryoprobe biopsy increases the diagnostic yield in endobronchial tumor lesions. J Thorac Cardiovasc Surg. 2010;140:417‐421. [DOI] [PubMed] [Google Scholar]
- 17. Hetzel J, Eberhardt R, Herth FJ, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J. 2012;39:685‐690. [DOI] [PubMed] [Google Scholar]
- 18. Dhooria S, Bal A, Sehgal IS, et al. Pleural cryobiopsy versus flexible forceps biopsy in subjects with undiagnosed exudative pleural effusions undergoing semirigid thoracoscopy: a crossover randomized trial (COFFEE trial). Respiration. 2019;98:133‐141. [DOI] [PubMed] [Google Scholar]
- 19. Schiavo D, Batzlaff C, Maldonado F. Pulmonary parenchymal lymphoma diagnosed by bronchoscopic cryoprobe lung biopsy. J Bronchology Interv Pulmonol. 2016;23:174‐176. [DOI] [PubMed] [Google Scholar]
- 20. Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta‐analysis. Respirology. 2016;21:834‐841. [DOI] [PubMed] [Google Scholar]
- 21. Ehab A, Khairy El‐Badrawy M, Abdelhamed Moawad A, et al. Cryobiopsy versus forceps biopsy in endobronchial lesions, diagnostic yield and safety. Adv Respir Med. 2017;85:301‐306. [DOI] [PubMed] [Google Scholar]
- 22. Sánchez‐Cabral O, Martínez‐Mendoza D, Fernandez‐Bussy S, et al. Utility of transbronchial lung cryobiopsy in non–interstitial diseases. Respiration. 2017;94:285‐292. [DOI] [PubMed] [Google Scholar]
- 23. Arimura K, Tagaya E, Akagawa H, et al. Cryobiopsy with endobronchial ultrasonography using a guide sheath for peripheral pulmonary lesions and DNA analysis by next generation sequencing and rapid on‐site evaluation. Respir Investig. 2019;57:150‐156. [DOI] [PubMed] [Google Scholar]
- 24. Imabayashi T, Uchino J, Yoshimura A, et al. Safety and usefulness of cryobiopsy and stamp cytology for the diagnosis of peripheral pulmonary lesions. Cancers (Basel). 2019;11:E410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakai T, Matsumoto Y, Sasada S. Cryobiopsy during flex‐rigid pleuroscopy: an emerging alternative biopsy method in malignant pleural mesothelioma. A comparative study of pathology. Jpn J Clin Oncol. 2019;49:559‐566. [DOI] [PubMed] [Google Scholar]
- 26. Kuse N, Inomata M, Awano N, et al. Management and utility of transbronchial lung cryobiopsy in Japan. Respir Investig. 2019;57:245‐251. [DOI] [PubMed] [Google Scholar]


