Abstract
The interaction between CD47 and signal‐regulatory protein‐α (SIRPα) inhibits phagocytosis, thus affecting the clinical outcomes of neoplastic diseases. Although CD47 upregulation is associated with poor prognosis in several malignancies, the effect of SIRPα expression and its coexpression with CD47 remains unclear. This study aimed to investigate the clinicopathologic effect of CD47 and SIRPα expression in diffuse large B‐cell lymphoma (DLBCL). Immunostaining of 120 biopsy samples showed that CD47 is primarily expressed in tumor cells, whereas SIRPα is expressed in nonneoplastic stromal cells, mostly macrophages. CD47high cases showed higher MYC protein expression and lower MYC translocation. The SIRPαhigh cases presented significantly shorter overall survival (OS) and progression‐free survival (PFS) than SIRPαlow cases in the activated B‐cell (ABC) subtype of DLBCL (P = .04 and P = .02, respectively). Both CD47high and SIRPαhigh presented significantly shorter OS and PFS than other cases among all DLBCL patients (P = .01 and P = .004, respectively), and the ABC type (P = .04 and P = .008, respectively) but not the germinal center B‐cell type. Both CD47high and SIRPαhigh yielded a constant independent prognostic value for OS and PFS in multivariate analysis (hazard ratio [HR], 2.93; 95% confidence interval [CI], 1.20‐7.43; P = .02; and HR, 2.87; 95% CI, 1.42‐5.85; P = .003, respectively). To the best of our knowledge, this is the first study to report that combinatorial CD47 and SIRPα expression is a potential independent prognostic factor for DLBCL. Evaluation of CD47 and SIRPα expression could be useful before CD47 blockade therapy.
Keywords: hematology, immunohistochemistry, non‐Hodgkin lymphoma, pathology, prognosis
This study investigated the clinicopathologic effect of CD47 and signal‐regulatory protein‐α (SIRPα) in diffuse large B‐cell lymphoma by immunohistochemical staining. CD47 and SIRPα coexpression is associated with a poor prognosis in the activated B‐cell type, but not in the germinal center B‐cell type.

1. INTRODUCTION
Diffuse large B‐cell lymphoma (DLBCL) is the most prevalent histologic subtype of non‐Hodgkin lymphomas (NHLs), accounting for 25%‐35% of NHL cases. 1 Although a subset of patients could be successfully treated with regimens including anti‐CD20 Abs, the median overall survival (OS) among patients with refractory DLBCL is 6 months. 2 Gene expression profiling studies have reported distinct expression profiles in both tumor cells and the tumor microenvironment, providing novel insights into the prognostic prediction or potential novel therapies for DLBCL. 3 , 4 , 5 Immunotherapy, including immune checkpoint inhibitors or chimeric antigen receptor T cells, is a potentially novel approach for treating DLBCL. 6
CD47, originally reported as an integrin‐associated protein, constitutes the “don’t eat me signal” in numerous cell types. 7 , 8 , 9 Signal‐regulatory protein‐α (SIRPα), a CD47 ligand, is expressed on myeloid cells, including monocytes, macrophages, and granulocytes. 7 , 8 , 9 The CD47‐SIRPα interaction initiates a dephosphorylation cascade that inhibits phagocytosis. 7 , 8 , 9 CD47 is overexpressed in different cancers, preventing tumor cell phagocytosis and promoting tumor progression. 10 , 11 , 12 , 13 , 14 Numerous methods and agents targeting the CD47‐SIRPα interaction are under investigation. 9
CD47 mRNA upregulation is associated with poor prognosis in NHL, including DLBCL. 15 An anti‐CD47 Ab is reportedly safe and induces durable complete responses in patients with relapsed or refractory DLBCL. 16 Detailed analysis of the clinicopathologic characteristics of CD47 expression in DLBCL is required to determine the effectiveness of anti‐CD47 therapies. Furthermore, SIRPα expression in the tumor microenvironment in DLBCL has not been widely examined.
In this study, we retrospectively investigated CD47 and SIRPα expression in DLBCL and evaluated the association between their expression and the clinicopathologic features of DLBCL.
2. MATERIALS AND METHODS
2.1. Patients and samples
We investigated 120 cases of DLBCL, 6 cases of double‐hit lymphoma (DHL), and 2 cases of triple‐hit lymphoma (THL) from 2005 to 2018 newly diagnosed at the Department of Pathology, Kurume University and treated by experienced hematologists at institutions including Kurume University, Kyushu University, and Niigata University. All cases were reviewed by hematopathologists (KO and HM) and diagnosed in accordance with the WHO classification. 17 Double‐hit lymphoma includes high‐grade B‐cell lymphoma with MYC and BCL2 rearrangement. Triple‐hit lymphoma is composed of B‐cell lymphoma with translocation of MYC, BCL2, and BCL6. Cases of recurrent lymphoma or indolent lymphoma were not included. Thirty‐three cases of extranodal DLBCL were included. A tissue microarray containing 120 samples from these patients in a formalin‐fixed paraffin block was prepared, with each sample occupying a 2‐mm diameter space. Clinical data were obtained through reviewing medical charts. The use of materials and clinical information was approved by the Research Ethics Committee of Kurume University in accordance with the tenets of the Declaration of Helsinki.
2.2. Classification of DLBCL patients based on CD47 and SIRPα expression
Primary Abs used for immunohistochemical (IHC) staining were anti‐CD47 Ab (SP279, 1:400 dilution, ab226837; Abcam) and anti‐SIRPα/SHPS1 Ab (D6I3M, 1:200 dilution, 13 379; Cell Signaling Technology). Immunohistochemical staining was carried out using 2.5‐µm thick, formalin‐fixed, paraffin‐embedded tissue sections from the tissue microarray of patient samples, except for cases of DHL and THL, for which IHC staining was undertaken using whole sections.
CD47 and SIRPα were primarily localized on the membrane and/or cytoplasm of tumor cells and stromal cells, respectively. The positive rate of CD47 expression in tumor cells and number of nonmalignant stromal cells expressing SIRPα were evaluated in a representative high‐power field, including the hotspot of CD47 or SIRPα expression (Figure 1). Patients with DLBCL were categorized in accordance with the patterns of CD47 and SIRPα expression. The cut‐off values for CD47 and SIPRα were defined as the median for each protein.
Figure 1.
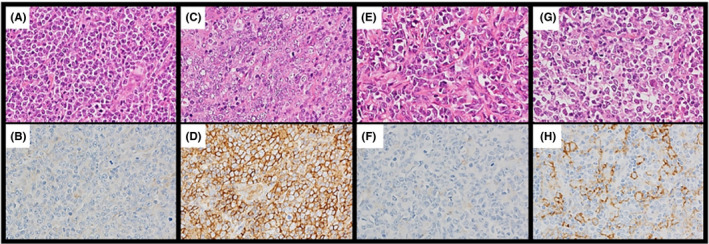
Representative immunohistochemical analysis of CD47 and signal‐regulatory protein‐α (SIRPα) expression in diffuse large B‐cell lymphoma (DLBCL). A, B, CD47low DLBCL. Neoplastic cells were negative for CD47. C, D, CD47high DLBCL. The plasma membranes and/or cytoplasm of neoplastic cells were positive for CD47 (brown staining). E, F, SIRPαlow DLBCL. The plasma membranes of neoplastic cells were negative for SIRPα. G, H, SIRPαhigh DLBCL. SIRPα‐positive nonmalignant stromal cells were observed. Most of those cells were morphologically consistent with monocyte‐derived cells, including macrophages. Some neoplastic cells were positive for SIRPα and excluded from enumeration. Original magnification, ×400 for all panels
2.3. Immunohistochemical staining of other proteins
Paraffin‐embedded sections of each sample were subjected to IHC staining, using anti‐CD10 (56C6; Leica Microsystems), anti‐CD20 (L‐26; DakoCytomation), anti‐B‐cell lymphoma 2 (BCL2) (124; DakoCytomation), anti‐BCL6 (P1F6; Leica Microsystems), anti‐MUM1 (MUM1p; DakoCytomation), and anti‐c‐MYC (Y69; Abcam) Abs. Each case was considered as positive if more than approximately 30% neoplastic cells were positive, except for BCL2 and MYC, for which each case was considered positive if more than approximately 50% and 40% neoplastic cells were positive, respectively. 17 Tumors coexpressing the MYC and BCL2 proteins were considered as double expressor lymphomas (DELs). 17 Immunohistochemical staining was also undertaken with the anti‐CD68 Ab (KP‐1; DakoCytomation) as the panmacrophage marker and the anti‐CD163 Ab (10D6; Leica Microsystems) for the M2 macrophage. The number of CD68‐ and CD163‐positive cells was evaluated in a representative high‐power field corresponding to that of SIRPα.
2.4. Classification of DLBCL subtypes
The cell‐of‐origin of the tumors was established using the Hans algorithm 18 : germinal center B‐cell (GCB) type or non‐GCB type, and the Lymph2Cx assay (NanoString Technologies) 19 : GCB, activated B‐cell (ABC), or unclassified.
2.5. In situ hybridization for Epstein‐Barr virus‐encoded RNA
Epstein‐Barr virus (EBV) was detected by in situ hybridization with a fluorescein‐conjugated EBV peptide nucleic acid probe kit (DakoCytomation) following the manufacturer’s instructions. This probe was complementary to the 2 nuclear EBV‐encoded RNAs.
2.6. Fluorescence in situ hybridization
Fluorescence in situ hybridization was undertaken using 4‐μm tissue sections with the MYC FISH DNA Probe, Split Signal (Dako) as described previously. 20 The slides were evaluated using a fluorescent microscope (BX51; Olympus). One hundred nuclei were scored, and cases were considered positive if more than 15% of the tumor cells showed a break‐apart signal.
2.7. Statistical analysis
Clinicopathologic characteristics were compared using the χ2 test or Fisher’s 2‐sided exact test. Pearson product‐moment correlation coefficient was used to determine the correlation between CD47 and SIRPα expression. The prognostic analyses included only patients who received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) or R‐CHOP‐like chemotherapy regimens. The end‐points of OS or progression‐free survival (PFS) were defined as the time to all‐cause mortality or relapse owing to DLBCL, respectively. Kaplan‐Meier analysis was carried out to estimate the OS and PFS distributions, and the log‐rank test was applied to identify significant differences. Univariate and multivariate Cox proportional regression models were used to evaluate the proposed prognostic factors. The variables included in the multivariate analysis were the DLBCL subtype, international prognostic index (IPI), MYC rearrangement, DEL, and CD47/SIRPα expression. 19 , 21 , 22 , 23 Results at P less than .05 were considered significant. JMP version 13.0 software (SAS Institute) was used for statistical analysis.
3. RESULTS
3.1. Immunohistochemical analysis of CD47 and SIRPα in DLBCL
CD47 was expressed in tumor cells. Signal‐regulatory protein‐α was expressed primarily in nonneoplastic stromal cells, most of which contained ovoid nuclei and a rich cytoplasm morphologically consistent with monocyte‐derived cells, including macrophages. The median CD47‐positive rate was 70%, and the median number of SIRPα‐positive stromal cells was 108 (Figure 2). Complete evaluation of SIRPα expression in stromal cells was interrupted among patients with an extremely high positive rate of 80% or greater in tumor cells; thus, 5 cases were excluded from the analyses including evaluation of the SIRPα expression status. The correlation between CD47 and SIRPα expression was not significant (Figure 3; r 2 = .03, P = .06). Seventy‐one cases (71/120, 59%) had the positive rate of CD47 expression greater than or equal to the median, which were categorized as CD47high. In 57 cases (57/115, 50%), the number of SIRPα‐positive stromal cells was greater than the median, and thus were categorized as SIRPαhigh.
Figure 2.
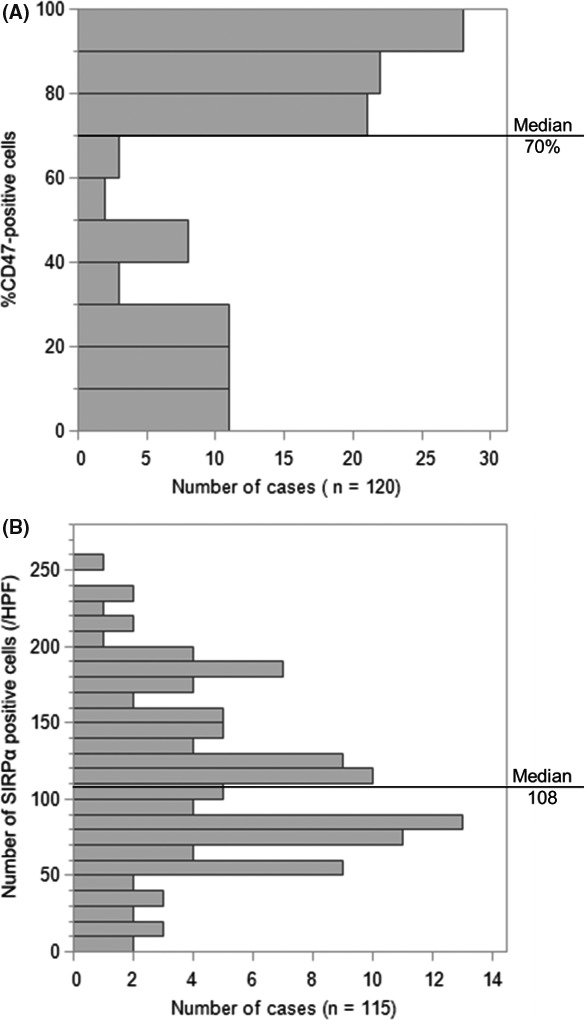
Histograms for immunohistochemistry in diffuse large B‐cell lymphoma. A, Distributions of the rate of CD47‐positive tumor cells. B, Distributions of the number of signal‐regulatory protein‐α (SIRPα)‐positive stromal cells. Cut‐off points are shown as the median. HPF, high power field
Figure 3.

Correlation between the rate of CD47‐positive tumor cells and the number of signal‐regulatory protein‐α (SIRPα)‐positive stromal cells in diffuse large B‐cell lymphoma. The coefficient of determination was 0.03 (P = .06)
The mean ratio of SIRPα‐positive stromal cells to CD68‐ and CD163‐positive cells was 0.81 and 3.19, respectively. The number of SIRPα‐positive stromal cells was significantly correlated with the number of CD68‐ and CD163‐positive cells (Figure 4; r 2 = .46, P < .0001 and r 2 = .35, P < .0001, respectively).
Figure 4.

Correlation among the number of signal‐regulatory protein‐α (SIRPα)‐positive stromal, CD68‐positive, and CD163‐positive cells in diffuse large B‐cell lymphoma. A, Correlation between the number of SIRPα‐positive stromal cells and the number of CD68‐positive cells. The coefficient of determination was 0.46 (P < .0001). B, Correlation between the number of SIRPα‐positive stromal cells and the number of CD163‐positive cells. The coefficient of determination was 0.35 (P < .0001). HPF, high power field
3.2. Immunohistochemical analysis of CD47 and SIRPα in DHL and THL
In DHL cases, the CD47‐positive rate was 80% or more in 5 cases, and the number of SIRPα‐positive stromal cells was greater than 108 in 1 case. In THL cases, the CD47‐positive rate was 80% or more in 2 cases and the number of SIRPα‐positive stromal cells was less than 108 in 2 cases. The distribution of the CD47‐positive rate and the number of SIRPα‐positive stromal cells in cases of DHL and THL are shown in Figure 5.
Figure 5.
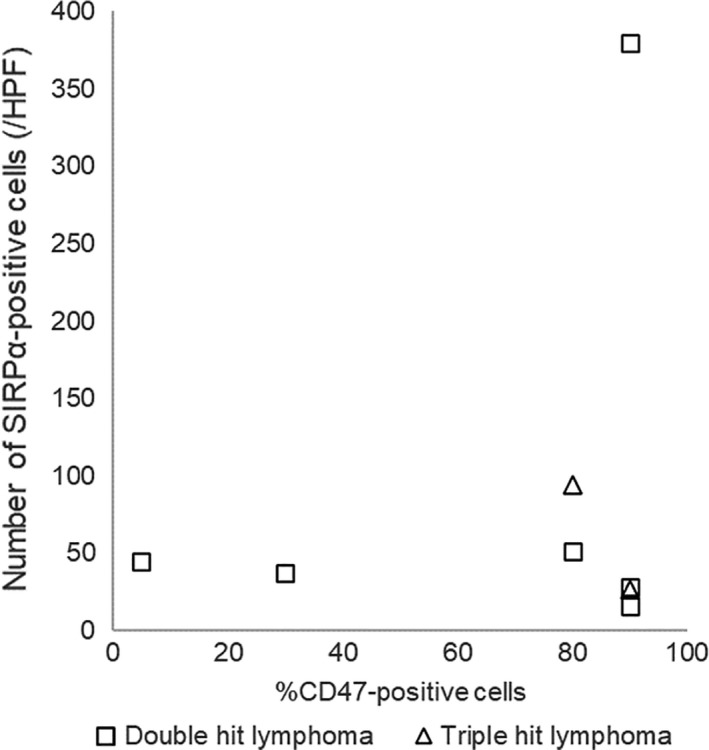
Distributions of the rate of CD47‐positive tumor cells and the number of signal‐regulatory protein‐α (SIRPα)‐positive stromal cells in cases of double‐hit lymphoma (squares) and triple‐hit lymphoma (triangles). HPF, high power field
3.3. Clinicopathologic comparison between CD47high and CD47low
Table 1 summarizes the statistical comparisons between CD47 expression patterns for different clinical parameters of patients with DLBCL. The number of male patients exceeded that of female patients in CD47low cases (P = .005). Based on Hans criteria and Lymph2Cx, the number of GC‐type DLBCL was lower in CD47high cases than in CD47low cases (P = .01 and P < .0001, respectively). In CD47low cases, a significantly larger number of cases with MYC translocation was detected (P = .03), whereas the number of MYC protein‐positive cases was significantly higher among CD47high cases (P = .04). The number of DELs was significantly higher in CD47high cases than in CD47low cases (P = .005). Significant differences were observed between the 2 groups following initial therapy (P = .004). Six cases were treated with chemotherapy other than the R‐CHOP or R‐CHOP‐like regimen, including in CD47low cases: 2 patients were treated with rituximab, cyclophosphamide, vincristine, and prednisone; 1 patient was given rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; 1 patient was given cyclophosphamide, doxorubicin, and vincristine; 1 patient was given rituximab and etoposide; and 1 patient was given rituximab and etoposide. Other characteristics, including IPI and the rate of complete response to initial therapy, did not significantly differ between groups.
Table 1.
Clinicopathologic comparison among CD47 expression patterns in diffuse large B‐cell lymphoma
| Characteristic | CD47high (n = 71) | CD47low (n = 49) | P value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex (male/female) | 34/37 | 48/52 | 36/13 | 73/27 | 0.005 |
| Age (y) | |||||
| Median | 72 | 68 | |||
| Range | 29‐87 | 23‐85 | |||
| >60 | 57/69 | 83 | 31/44 | 70 | 0.13 |
| ECOG PS > 1 | 18/66 | 27 | 9/41 | 22 | 0.53 |
| Extranodal sites > 1 | 4/69 | 6 | 8/43 | 19 | 0.06* |
| Ann Arbor stage > 2 | 37/65 | 57 | 22/41 | 54 | 0.74 |
| IPI risk group | 0.62 | ||||
| Low (0‐2) | 31/66 | 47 | 23/42 | 55 | |
| High (3‐5) | 35/66 | 53 | 19/42 | 45 | |
| Hans criteria | 0.01 | ||||
| GCB | 27/68 | 40 | 29/46 | 63 | |
| Non‐GCB | 41/68 | 60 | 17/46 | 37 | |
| Lymph2Cx | <0.0001 | ||||
| GCB | 19/71 | 27 | 32/49 | 65 | |
| ABC | 36/71 | 51 | 10/49 | 20 | |
| Unclassified | 16/71 | 23 | 7/49 | 14 | |
| MYC translocation, positive | 3/71 | 4 | 8/48 | 17 | 0.03* |
| MYC protein, positive | 38/71 | 54 | 17/49 | 35 | 0.04 |
| DEL | 22/71 | 31 | 5/49 | 10 | 0.005 |
| EBER‐ISH, positive | 2/71 | 3 | 2/49 | 4 | 1.00* |
| Treatment | 0.004* | ||||
| R‐CHOP/R‐CHOP‐like | 70/71 | 99 | 42/49 | 86 | |
| Other chemotherapies | 0/71 | 0 | 6/49 | 12 | |
| RT alone | 1/71 | 1 | 1/49 | 2 | |
| Treatment response | 0.91 | ||||
| CR/CRu | 53/70 | 76 | 36/47 | 77 | |
| Not CR | 17/70 | 24 | 11/47 | 23 | |
Abbreviations: ABC, activated B‐cell; CR, complete response; CRu, uncertain complete response; DEL, double expressor lymphoma; EBER‐ISH, in situ hybridization for Epstein‐Barr virus‐encoded RNA; GCB, germinal center B‐cell; IPI, international prognostic index; PS, performance status; R‐CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; RT, radiotherapy.
Fisher’s exact test.
3.4. Overall survival and PFS curves in accordance with CD47 expression
The OS and PFS curves in patients with DLBCL treated with R‐CHOP or R‐CHOP‐like regimens are shown in Figure 6A,B. The results of subgroup analyses for Lymph2Cx are also shown, with ABC in Figure 6C,D, and GCB in Figure 6E,F. No significant differences in OS and PFS were observed in accordance with the positive rate of CD47.
Figure 6.
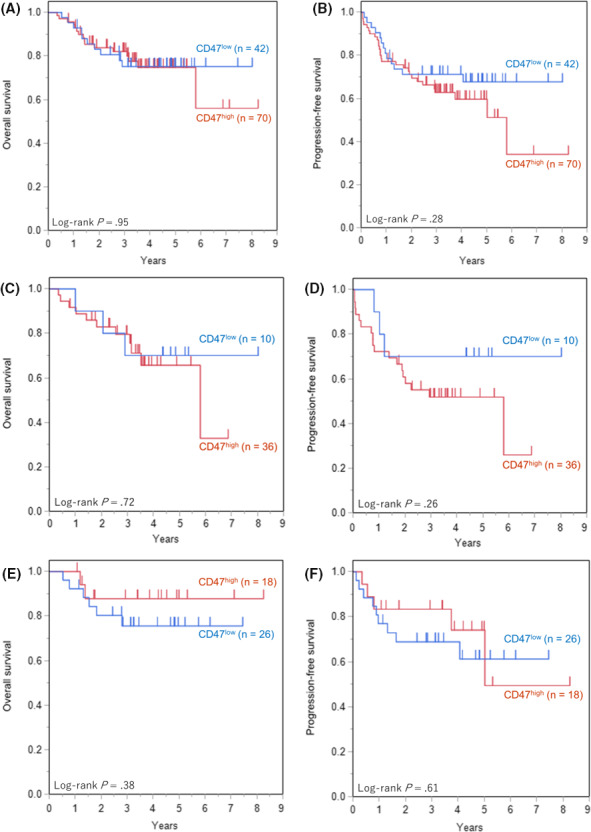
Overall survival (OS) and progression‐free survival (PFS) of diffuse large B‐cell lymphoma (DLBCL) patients among the CD47 expression groups. A, OS of all patients. B, PFS of all patients. C, OS of activated B‐cell (ABC) subtype. D, PFS of ABC subtype. E, OS of germinal center B‐cell (GCB) subtype. F, PFS of GCB subtype. There were no significant differences in OS or PFS according to CD47 expression
3.5. Clinicopathologic comparison between SIRPαhigh and SIRPαlow
Table 2 summarizes the statistical comparisons between SIRPα expression patterns for different clinical parameters of patients with DLBCL. Pathological characteristics including Hans criteria, Lymph2Cx, and the positivity of MYC translocation or MYC protein, did not significantly differ between groups. No significant differences were observed between the 2 groups upon initial therapy. Other characteristics, including IPI and the rate of complete response to initial therapy, did not significantly differ between groups.
Table 2.
Clinicopathologic comparison among signal‐regulatory protein‐α (SIRPα) expression patterns in diffuse large B‐cell lymphoma
| Characteristic | SIRPαhigh (n = 57) | SIRPαlow (n = 58) | P value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex (male/female) | 33/24 | 58/42 | 33/25 | 57/43 | 0.91 |
| Age (y) | |||||
| Median | 72 | 70.5 | |||
| Range | 29‐87 | 23‐85 | |||
| >60 | 39/52 | 75 | 45/56 | 80 | 0.50 |
| ECOG PS > 1 | 15/50 | 30 | 11/53 | 21 | 0.28 |
| Extranodal sites > 1 | 5/51 | 10 | 6/56 | 11 | 1.00 |
| Ann Arbor stage > 2 | 28/46 | 61 | 28/55 | 51 | 0.32 |
| IPI risk group | 0.38 | ||||
| Low (0‐2) | 23/50 | 46 | 29/53 | 55 | |
| High (3‐5) | 27/50 | 54 | 24/53 | 45 | |
| Hans criteria | 0.91 | ||||
| GCB | 25/53 | 47 | 27/56 | 48 | |
| Non‐GCB | 28/53 | 53 | 29/56 | 52 | |
| Lymph2Cx | 0.26 | ||||
| GCB | 19/57 | 33 | 28/58 | 48 | |
| ABC | 25/57 | 44 | 20/58 | 34 | |
| Unclassified | 13/57 | 23 | 10/58 | 17 | |
| MYC translocation, positive | 5/57 | 9 | 6/57 | 11 | 1.00* |
| MYC protein, positive | 26/57 | 46 | 29/58 | 50 | 0.64 |
| DEL | 13/57 | 23 | 14/58 | 24 | 0.87 |
| EBER‐ISH, positive | 2/57 | 4 | 2/58 | 3 | 1.00* |
| Treatment | 0.26* | ||||
| R‐CHOP/R‐CHOP‐like | 51/57 | 89 | 56/58 | 97 | |
| Other chemotherapies | 4/57 | 7 | 2/58 | 3 | |
| RT alone | 2/57 | 4 | 0/58 | 0 | |
| Treatment response | 0.66 | ||||
| CR/CRu | 41/56 | 73 | 43/56 | 77 | |
| Not CR | 15/56 | 27 | 13/56 | 23 | |
Abbreviations: ABC, activated B‐cell; CR, complete response; CRu, uncertain complete response; DEL, double expressor lymphoma; EBER‐ISH, in situ hybridization for Epstein‐Barr virus‐encoded RNA; GCB, germinal center B‐cell; IPI, international prognostic index; PS, performance status; R‐CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; RT, radiotherapy.
Fisher’s exact test.
3.6. Overall survival and PFS curves in accordance with SIRPα expression
The OS and PFS curves in patients with DLBCL treated with R‐CHOP or R‐CHOP‐like regimens are shown in Figure 7A,B. Subgroup analyses for Lymph2Cx are also shown, with ABC in Figure 7C,D, and GCB in Figure 7E,F. In the ABC subtype, the OS and PFS of the SIRPαhigh cases were significantly inferior to those of SIRPαlow cases (P = .04 and P = .02, respectively). No significant differences in OS and PFS were observed in accordance with SIRPα expression in all cases, nor the GCB subtype with R‐CHOP or R‐CHOP‐like treatment.
Figure 7.
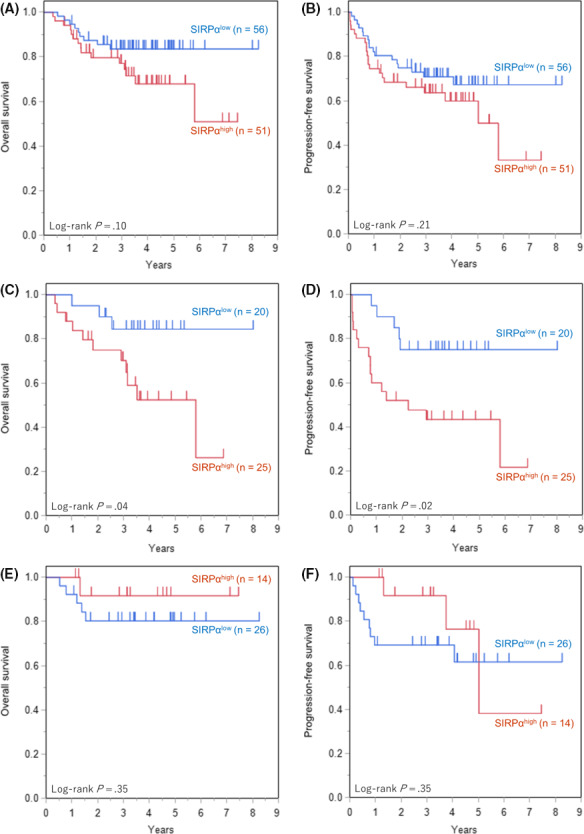
Overall survival (OS) and progression‐free survival (PFS) of diffuse large B‐cell lymphoma (DLBCL) patients among the signal‐regulatory protein‐α (SIRPα) expression groups. A, OS of all patients. B, PFS of all patients. C, OS of activated B‐cell (ABC) subtype. D, PFS of ABC subtype. E, OS of germinal center B‐cell (GCB) subtype. F, PFS of GCB subtype. Significantly worse OS and PFS of SIRPαhigh were observed in ABC subtype (P = .04 and P = .02, respectively). There were no significant differences in OS or PFS according to SIRPα expression in all patients and in GCB subtype
3.7. Overall survival and PFS curves between CD47 and SIRPα expression and others
For patients with DLBCL treated with R‐CHOP or R‐CHOP‐like regimens, the OS and PFS curves are shown in Figure 8A,B. Thirty‐six cases (36/108, 34%) were categorized as CD47high/SIRPαhigh, whereas 71 cases (71/108, 66%) were categorized as others, including CD47high/SIRPαlow, CD47low/SIRPαhigh, and CD47low/SIRPαlow. The OS and PFS of the CD47high/SIRPαhigh group were significantly inferior to those of the other groups (P = .01 and P = .004, respectively). In the ABC subtype, significantly inferior OS and PFS were observed in the CD47high/SIRPαhigh group compared to that in the other groups (Figure 8C,D; P = .04 and P = .008, respectively). No significant differences in OS or PFS were observed in the GCB subtype (Figure 1, 4, 8
Figure 8.
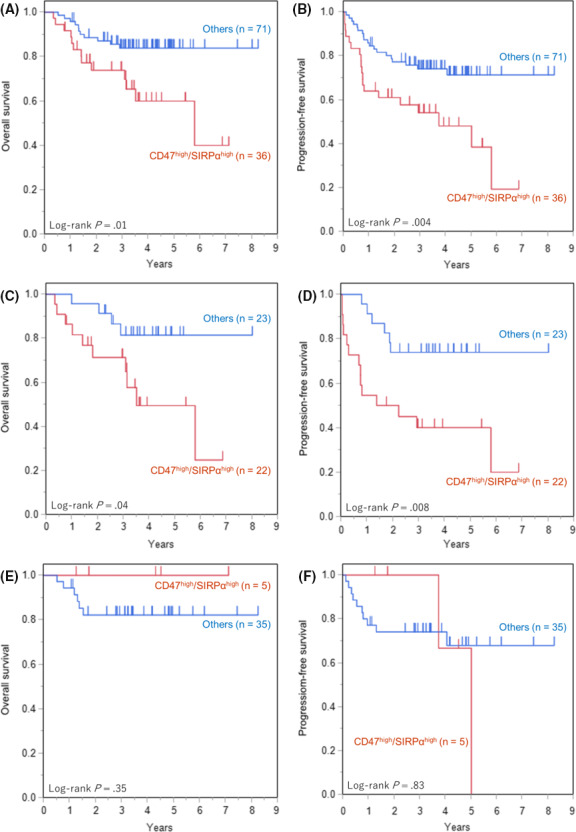
Overall survival (OS) and progression‐free survival (PFS) of diffuse large B‐cell lymphoma (DLBCL) patients among the CD47/signal‐regulatory protein‐α (SIRPα) expression groups. A, OS of all patients. B, PFS of all patients. C, OS of activated B‐cell (ABC) subtype. D, PFS of ABC subtype. E, OS of germinal center B‐cell (GCB) subtype. F, PFS of GCB subtype. Significantly worse OS and PFS of CD47high/SIRPαhigh were observed in all patients (P = .01 and P = .004, respectively) and in ABC subtype (P = .04 and P = .008, respectively). There were no significant differences in OS or PFS according to CD47/SIRPα expression in GCB subtype. “Others” include CD47high/SIRPαlow, CD47low/SIRPαhigh, and CD47low/SIRPαlow
E,F).
3.8. Univariate and multivariate analyses of OS and PFS in patients with DLBCL in accordance with CD47 and SIRPα expression
Univariate analysis identified the following variables as prognostic factors for OS: high IPI (hazard ratio [HR], 3.64; 95% confidence interval [CI], 1.51‐10.13; P = .004), DEL (HR, 2.34; 95% CI, 1.05‐5.09; P = .04), and CD47high/SIRPαhigh (HR, 2.68; 95% CI, 1.20‐6.12; P = .02) (Table 3). The following variables were identified as prognostic factors for PFS: high IPI (HR, 2.26; 95% CI, 1.17‐4.53; P = .02); and CD47high/SIRPαhigh (HR, 2.46; 95% CI, 1.29‐4.68; P = .007) (Table 4).
Table 3.
Prognostic factors affecting the overall survival of patients with diffuse large B‐cell lymphoma
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Lymph2Cx ABC (vs GCB, unclassified) | 1.69 (0.78‐3.72) | 0.18 | 0.92 (0.27‐3.05) | 0.89 |
| IPI high (3‐5) (vs low [0‐2]) | 3.64 (1.51‐10.13) | 0.004 | 3.68 (1.39‐10.87) | 0.008 |
| MYC translocation positive (vs negative) | 3.13 (0.91‐8.25) | 0.07 | 1.13 (0.17‐4.69) | 0.88 |
| DEL (vs non‐DEL) | 2.34 (1.05‐5.09) | 0.04 | 2.27 (0.76‐7.22) | 0.14 |
| CD47high/SIRPαhigh (vs others†) | 2.68 (1.20‐6.12) | 0.02 | 2.93 (1.20‐7.43) | 0.02 |
Abbreviations: ABC, activated B‐cell; CI, confidence interval; DEL, double expressor lymphoma; GCB, germinal center B‐cell; HR, hazard ratio; IPI, international prognostic index.
Including CD47high/signal‐regulatory protein‐α (SIRPα)low, CD47low/SIRPαhigh, and CD47low/SIRPαlow.
Table 4.
Prognostic factors affecting the progression‐free survival of patients with diffuse large B‐cell lymphoma
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Lymph2Cx ABC (vs GCB, unclassified) | 1.61 (0.87‐3.00) | 0.13 | 1.09 (0.44‐2.64) | 0.85 |
| IPI high (3‐5) (vs low [0‐2]) | 2.26 (1.17‐4.53) | 0.02 | 2.19 (1.04‐4.69) | 0.04 |
| MYC translocation positive (vs negative) | 2.60 (0.89‐6.10) | 0.08 | 1.27 (0.28‐4.12) | 0.72 |
| DEL (vs non‐DEL) | 1.84 (0.93‐3.47) | 0.08 | 1.55 (0.64‐3.80) | 0.33 |
| CD47high/SIRPαhigh (vs others†) | 2.46 (1.29‐4.68) | 0.007 | 2.87 (1.42‐5.85) | 0.003 |
Abbreviations: ABC, activated B‐cell; CI, confidence interval; DEL, double expressor lymphoma; GCB, germinal center B‐cell; IPI, international prognostic index.
†Including CD47high/signal‐regulatory protein‐α (SIRPα)low, CD47low/SIRPαhigh, and CD47low/SIRPαlow.
Multivariate analysis based on CD47 and SIRPα expression revealed that CD47high/SIRPαhigh is a significant independent prognostic factor for OS (HR, 2.93; 95% CI, 1.20‐7.43; P = .02) (Table 3) and PFS (HR, 2.87; 95% CI, 1.42‐5.85; P = .003) (Table 4).
4. DISCUSSION
The present study shows that patients with DLBCL with a high expression of SIRPα on stromal cells and those with coexpression of CD47 on DLBCL tumor cells and SIRPα on stromal cells have a poor prognosis, which was observed only in the ABC subtype of DLBCL and not in the GCB subtype. Coexpression of CD47 and SIRPα was also an independent poor prognostic factor for OS and PFS in patients with DLBCL.
Numerous previous studies reported that CD47 upregulation on tumor cells is associated with poor prognosis in various neoplasms, including DLBCL; however, few studies have investigated SIRPα expression in the tumor microenvironment. 10 , 11 , 12 , 13 , 14 , 15 In the current study, upregulation of CD47 on tumor cells alone did not predict poor prognosis, but upregulation of SIRPα on stromal cells, mostly macrophages, and combinatorial upregulation of CD47 and SIRPα were well correlated with poor outcomes. Macrophages and other phagocytes can inhibit tumor growth through phagocytosis and play an important role in Ab‐dependent phagocytosis during treatment with anti‐CD20 Abs, a key component of DLBCL treatment. 24 , 25 The interaction of CD47 on tumor cells with SIRPα on phagocytes inhibits this immune function, thus enabling tumor cells to evade phagocytosis and leading to tumor growth and progression. 7 , 8 , 9
In the present study, a significantly worse prognosis was observed in patients with DLBCL with a high SIRPα expression on stromal cells, most of which were morphologically consistent with monocyte‐derived cells, including macrophages. Correlation among SIRPα, CD68, and CD163 expression was also observed, suggesting that SIRPα‐positive cells accounted for some parts of CD68‐ and CD168‐positive macrophages. Tumor‐infiltrating macrophages, also known as tumor‐associated macrophages (TAMs), tend to acquire the M2 immunosuppressive phenotype, rather than the M1 phenotype, which has strong tumoricidal activity. 26 Several studies reported that an increased number of CD163‐positive M2 type TAMs is associated with poor prognosis in DLBCL. 27 , 28 , 29 An in vitro study using bone marrow‐derived macrophages reported M2 polarization with SIRPα overexpression and M1 polarization with interference of CD47 or SIRPα signaling, suggesting that the interaction between SIRPα and CD47 polarizes macrophages to the M2 phenotype. 30 A study of endometrial cancer reported that CD47 blockade results in increased phagocytosis among M2 macrophages without affecting the function of M1 macrophages, indicating the importance of the CD47‐SIRPα interaction in M2 type TAMs. 31 Our results regarding the association between high expression of SIRPα and poor therapeutic outcomes in DLBCL can be explained on the basis of the M2 type TAM‐rich microenvironment provided by the interaction between CD47 and SIRPα.
The OS and PFS of patients with DLBCL with a high expression of both CD47 and SIRPα were significantly inferior to those of the other groups in the present study. These inferior results were also observed in the ABC subtype but not in the GCB subtype. Our results were partly consistent with those of a study showing that the negative effect of high CD47 expression on OS after R‐CHOP treatment was only evident in the non‐GCB subtype of DLBCL. 32 The ABC or non‐GCB subtypes of DLBCL show significantly poorer outcomes than the GCB subtype. 17 , 19 Different subtypes show different gene profiles in the tumor and its microenvironment. 3 , 4 A significant correlation between the non‐GCB subtype and high‐M2 type TAM density was also reported. 28 The interaction between CD47 and SIRPα could influence the prognostic difference between DLBCL subtypes.
CD47 is expressed on numerous cell types in all tissues, modulating various cellular functions through interactions with other proteins, including SIRPα. 7 The transcription factor MYC directly interacts with the promoters of CD47. 33 In a study of DLBCL cell lines, high levels of surface CD47 were observed in DHL, which was reduced by transcriptional repression of MYC. 34 MYC translocation reportedly occurs in approximately 10% of lymphomas with DLBCL‐like morphology, and their presence is associated with poor outcomes, whereas some of these tumors are classified as high‐grade B‐cell lymphoma when accompanied by BCL2 and/or BCL6 translocations. 17 , 22 , 35 , 36 CD47 upregulation could partly account for the aggressiveness of DLBCL with high expression of MYC. In the present study, high expression of CD47 in tumor cells was correlated with positivity for the MYC protein, not MYC translocation, which might differ among the subtypes of DLBCL.
Novel treatment methods targeting the CD47‐SIRPα interaction have been intensively investigated. 9 An anti‐CD47 Ab, Hu5F9‐G4, in combination with rituximab displayed promising activity against relapsed or refractory DLBCL or follicular lymphoma. 16 The mechanisms underlying the antitumor activity of the Ab have been explained based on synergistic macrophage‐mediated, Ab‐dependent cellular phagocytosis with rituximab. 16 , 32 , 37 Considering the important role of macrophages expressing SIRPα in this novel treatment, evaluation of SIRPα expression along with CD47 could help predict treatment responsiveness and provide insights into the therapeutic indications.
There were some limitations to the present study. First, although the results of the present study are significant, the number of cases was relatively small. Further studies of a large cohort are warranted to validate our results. Second, cut‐off points for CD47 and SIRPα expression have not been determined. Although we considered the median as the cut‐off for positive expression, previous studies adopted other criteria to evaluate positivity in IHC analysis. 13 , 14 Further studies are required to determine the appropriate threshold expression levels on IHC. Third, evaluating the expression of proteins with the immunoenzyme method of IHC, the cases with an extremely high SIRPα expression on tumor cells were excluded from the present study. Although the essential result of the present study could not change by this exclusion, considering the small proportion of those cases, further studies using other methods are warranted. Finally, we did not count the number of stromal cells without expression of SIRPα. Thus, we could not determine whether the small number of cells expressing SIRPα indicated a small overall number of stromal cells or a small proportion of SIRPα‐positive cells. Although correlation among the expression of SIRPα, CD68, and CD163 was revealed, further studies evaluating the types of TAMs or other stromal cells expressing SIRPα will improve the understanding of the association between its expression and the tumor microenvironment in DLBCL.
In conclusion, we showed that higher expression levels of CD47 on tumor cells and SIRPα on stromal cells indicate a poor prognosis in DLBCL, highlighting the importance of the association between tumor cells and stromal cells in DLBCL. Evaluation of CD47 and SIRPα expression could be useful before treatment with CD47 blockade therapy. Additional detailed studies are required to determine the clinical and biological significance of the CD47‐SIRPα interaction in DLBCL.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Kazama R, Miyoshi H, Takeuchi M, et al. Combination of CD47 and signal‐regulatory protein‐α constituting the “don’t eat me signal” is a prognostic factor in diffuse large B‐cell lymphoma. Cancer Sci. 2020;111:2608–2619. 10.1111/cas.14437
REFERENCES
- 1. Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non‐Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non‐Hodgkin's Lymphoma Classification Project. Ann Oncol. 1998;9:717‐720. [DOI] [PubMed] [Google Scholar]
- 2. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B‐cell lymphoma: results from the international SCHOLAR‐1 study. Blood. 2017;130:1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B‐cell lymphoma identified by gene expression profiling. Nature. 2000;403:503‐511. [DOI] [PubMed] [Google Scholar]
- 4. Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large‐B‐cell lymphomas. N Engl J Med. 2008;359:2313‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B‐cell lymphoma. N Engl J Med. 2018;378:1396‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Medeiros LJ, Young KH. Cancer immunotherapy in diffuse large B‐cell lymphoma. Front Oncol. 2018;8:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown EJ, Frazier WA. Integrin‐associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130‐135. [DOI] [PubMed] [Google Scholar]
- 8. Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur J Cancer. 2017;76:100‐109. [DOI] [PubMed] [Google Scholar]
- 9. Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47‐SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276:145‐164. [DOI] [PubMed] [Google Scholar]
- 10. Xiao Z, Chung H, Banan B, et al. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett. 2015;360:302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaur S, Elkahloun AG, Singh SP, et al. A function‐blocking CD47 antibody suppresses stem cell and EGF signaling in triple‐negative breast cancer. Oncotarget. 2016;7:10133‐10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiskopf K, Jahchan NS, Schnorr PJ, et al. CD47‐blocking immunotherapies stimulate macrophage‐mediated destruction of small‐cell lung cancer. J Clin Invest. 2016;126:2610‐2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sudo T, Takahashi Y, Sawada G, Uchi R, Mimori K, Akagi Y. Significance of CD47 expression in gastric cancer. Oncol Lett. 2017;14:801‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Lu S, Xu Y, et al. Overexpression of CD47 predicts poor prognosis and promotes cancer cell invasion in high‐grade serous ovarian carcinoma. Am J Transl Res. 2017;9:2901‐2910. [PMC free article] [PubMed] [Google Scholar]
- 15. Chao MP, Alizadeh AA, Tang C, et al. Anti‐CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non‐Hodgkin lymphoma. Cell. 2010;142:699‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9‐G4 and rituximab in non‐Hodgkin's lymphoma. N Engl J Med. 2018;379:1711‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th edn. Lyon, France: IARC; 2017. [Google Scholar]
- 18. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275‐282. [DOI] [PubMed] [Google Scholar]
- 19. Scott DW, Wright GW, Williams PM, et al. Determining cell‐of‐origin subtypes of diffuse large B‐cell lymphoma using gene expression in formalin‐fixed paraffin‐embedded tissue. Blood. 2014;123:1214‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weremowicz S. Preparation of cells from formalin‐fixed, paraffin‐embedded tissue for use in fluorescence in situ hybridization (FISH) experiments. Curr Protoc Hum Genet. 2015;84(8):8.1‐8.8.10. [DOI] [PubMed] [Google Scholar]
- 21. International Non‐Hodgkin's Lymphoma Prognostic Factors Project . A predictive model for aggressive non‐Hodgkin's lymphoma. N Engl J Med. 1993;329:987‐994. [DOI] [PubMed] [Google Scholar]
- 22. Savage KJ, Johnson NA, Ben‐Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B‐cell lymphoma patients treated with R‐CHOP chemotherapy. Blood. 2009;114:3533‐3537. [DOI] [PubMed] [Google Scholar]
- 23. Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452‐3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J, Jin S, Guo X, Qian W. Targeting the CD47‐SIRPα signaling axis: current studies on B‐cell lymphoma immunotherapy. J Int Med Res. 2018;46:4418‐4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keating GM. Rituximab: a review of its use in chronic lymphocytic leukaemia, low‐grade or follicular lymphoma and diffuse large B‐cell lymphoma. Drugs. 2010;70:1445‐1476. [DOI] [PubMed] [Google Scholar]
- 26. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nam SJ, Go H, Paik JH, et al. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B‐cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma. 2014;55:2466‐2476. [DOI] [PubMed] [Google Scholar]
- 28. Marchesi F, Cirillo M, Bianchi A, et al. High density of CD68+/CD163+ tumour‐associated macrophages (M2‐TAM) at diagnosis is significantly correlated to unfavorable prognostic factors and to poor clinical outcomes in patients with diffuse large B‐cell lymphoma. Hematol Oncol. 2015;33:110‐112. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Gao K, Lei W, et al. Lymphocyte‐to‐monocyte ratio is associated with prognosis of diffuse large B‐cell lymphoma: correlation with CD163 positive M2 type tumor‐associated macrophages, not PD‐1 positive tumor‐infiltrating lymphocytes. Oncotarget. 2017;8:5414‐5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin Y, Zhao JL, Zheng QJ, et al. Notch signaling modulates macrophage polarization and phagocytosis through direct suppression of signal regulatory protein α expression. Front Immunol. 2018;9:1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gu S, Ni T, Wang J, et al. CD47 blockade inhibits tumor progression through promoting phagocytosis of tumor cells by M2 polarized macrophages in endometrial cancer. J Immunol Res. 2018;2018:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bouwstra R, He Y, de Boer J, et al. CD47 expression defines efficacy of rituximab with CHOP in non‐germinal center B‐cell (non‐GCB) diffuse large B‐cell lymphoma patients (DLBCL), but not in GCB DLBCL. Cancer Immunol Res. 2019;7:1663‐1671. [DOI] [PubMed] [Google Scholar]
- 33. Casey SC, Tong L, Li Y, et al. MYC regulates the antitumor immune response through CD47 and PD‐L1. Science. 2016;352:227‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li W, Gupta SK, Han W, et al. Targeting MYC activity in double‐hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J Hematol Oncol. 2019;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dunleavy K, Fanale MA, Abramson JS, et al. Dose‐adjusted EPOCH‐R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B‐cell lymphoma with MYC rearrangement: a prospective, multicentre, single‐arm phase 2 study. Lancet Haematol. 2018;5:e609‐e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott DW, King RL, Staiger AM, et al. High‐grade B‐cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B‐cell lymphoma morphology. Blood. 2018;131:2060‐2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J, Wang L, Zhao F, et al. Pre‐clinical development of a humanized anti‐CD47 antibody with anti‐cancer therapeutic potential. PLoS ONE. 2015;10:e0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]


