Abstract
Long noncoding RNAs (lncRNAs) have recently been verified to have significant regulatory functions in many types of human cancers. The lncRNA ANRIL is transcribed from the INK4b‐ARF‐INK4a gene cluster in the opposite direction. Whether ANRIL can act as an oncogenic molecule in cholangiocarcinoma (CCA) remains unknown. Our data show that ANRIL knockdown greatly inhibited CCA cell proliferation and migration in vitro and in vivo. According to the results of RNA sequencing analysis, ANRIL knockdown dramatically altered target genes associated with the cell cycle, cell proliferation, and apoptosis. By binding to a component of the epigenetic modification complex enhancer of zeste homolog 2 (EZH2), ANRIL could maintain lysine residue 27 of histone 3 (H3K27me3) levels in the promoter of ERBB receptor feedback inhibitor 1 (ERRFI1), which is a tumor suppressor gene in CCA. In this way, ERRFI1 expression was suppressed in CCA cells. These data verified the key role of the epigenetic regulation of ANRIL in CCA oncogenesis and indicate its potential as a target for CCA intervention.
Keywords: ANRIL, cholangiocarcinoma, epigenetic regulation, ERRFI1, long noncoding RNA
ANRIL can adjust the proliferation and migration of cholangiocarcinoma (CCA) cells in vitro. Mechanistic investigations indicated that ANRIL could inhibit the expression of ERRFI1 by directly binding to EZH2, which mediated H3K27me3 in the promoter region of ERRFI1, thus accelerating CCA tumorigenesis.

Abbreviations
- ASO
antisense oligonucleotide
- CCA
cholangiocarcinoma
- ERRFI1
ERBB receptor feedback inhibitor 1
- EZH2
enhancer of zeste homolog 2
- H3K27me3
lysine residue 27 of histone 3
- lncRNA
long noncoding RNA
- qRT‐PCR
quantitative RT‐PCR
- RNA‐seq
RNA sequencing
- si‐SC
scrambled negative control siRNA
- SPRY4
sprouty RTK signaling antagonist 4
- TCGA
The Cancer Genome Atlas
1. INTRODUCTION
Cholangiocarcinoma, the most frequently occurring biliary tract cancer, accounts for 3% of all gastrointestinal malignancies and originates from the ductal epithelial cells lining the intrahepatic and extrahepatic biliary ducts. 1 , 2 Cholangiocarcinoma is a destructive malignancy with an extreme overall 5‐year survival rate of less than 10%. 3 These patients have a median survival of 24 months after diagnosis, which indicates that CCA has a poor prognosis. 4 Although surgical resection and liver transplantation are possible curative treatment choices for early‐stage CCA patients, the median 5‐year survival after R0 resection is approximately 30%. 5 However, because of the destructive nature of CCA, most patients already have advanced disease at diagnosis. 3 What is more, patients with CCA are insensitive to conventional chemotherapy or radiotherapy. 6 Therefore, there are no potentially curative clinical therapeutic interventions for CCA, and no targeted molecular therapies have been adopted for use in CCA.
Long noncoding RNAs are over 200 nucleotides in length and represent an enormous RNA family. Long noncoding RNAs have limited protein coding potential and lack detectable ORFs, which are necessary for protein coding potential. 7 , 8 , 9 , 10 , 11 Recently, lncRNAs have been reported to serve as pivotal regulators in many biological processes, such as cellular proliferation, development, and differentiation. Long noncoding RNAs can alter the expression of genes involved in diverse biological functions 12 by binding to transcription factors, 13 chromatin‐modifying factors, 14 , 15 , 16 or heterogeneous nuclear ribonucleoproteins. 17 Long noncoding RNAs can act as regulators of the alternative splicing, translation, or stability of host mRNAs by posttranscriptional mechanisms; they can also serve as scaffolds or guides to regulate protein‐protein or protein‐DNA interactions 18 , 19 and can act as endogenous microRNA sponges to modulate microRNA targets. 20 , 21 , 22 Notably, abnormal lncRNA expression has been proven in many cancers, including CCA. 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30
Among them, the lncRNA ANRIL (CDKN2B antisense RNA 1), which is transcribed from the INK4b‐ARF‐INK4a gene cluster in the opposite direction, has been identified as a shared genetic susceptibility locus associated with intracranial aneurysm, 31 , 32 coronary disease, 33 and various types of cancer. In particular, this susceptibility locus has been associated with the invasive pathophysiology of ovarian cancer, 34 nasopharyngeal carcinoma, 35 hepatocellular carcinoma, 36 lung cancer, 37 , 38 epithelial ovarian cancer, 34 colorectal cancer, 39 , 40 cervical cancer, 41 and gastric cancer. 42 Regarding the underlying molecular mechanism, ANRIL can be induced by the ATM‐E2F1 signaling pathway 43 and is required for silencing the p15(INK4B) tumor suppressor gene. 44 In non‐small cell lung cancer, ANRIL promotes non‐small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression by binding to PRC2. 38 In hepatocellular carcinoma, ANRIL regulates cell apoptosis by epigenetically silencing KLF2. 38
We speculated that many lncRNAs remain unexplored in CCA, particularly those with dysfunctional expression patterns. To comprehensively characterize aberrantly expressed lncRNAs in CCA, we analyzed TCGA CCA and normal tissue RNA sequencing data (9 normal and 36 cancer samples) and 1 independent microarray dataset from the Gene Expression Omnibus database (GSE76297; 92 cancer tissue samples and 91 normal tissue samples). We discovered a CCA‐specific upregulated lncRNA termed ANRIL that was expressed at higher levels in CCA tissues than in normal tissues. The functional association between the underlying molecular mechanism and ANRIL overexpression in CCA had not been determined. Our data showed that ANRIL knockdown greatly inhibited CCA cell proliferation and migration in vitro and in vivo. According to the RNA‐seq analysis results, ANRIL knockdown dramatically altered target genes associated with the cell cycle, cell proliferation, and apoptosis. By binding to a component of EZH2, ANRIL could maintain H3K27me3 levels in the promoter of ERRFI1, which was verified to act as a tumor suppressor gene in CCA. In this way, ANRIL expression was suppressed in CCA cells. These data verified the key role of the epigenetic regulation of the lncRNA ANRIL in CCA oncogenesis and indicate its potential as a target for CCA intervention.
2. MATERIALS AND METHODS
2.1. Tissue collection and ethics statement
A total of 17 samples were collected during resection for CCA at the Second Affiliated Hospital of Nanjing Medical University. After removal, all specimens were instantly frozen in tubes with RNAlater and stored in liquid nitrogen until RNA extraction. Our research was approved by the Ethics Committee of Nanjing Medical University, and written consent was obtained from every patient.
2.2. RNA extraction and qRT‐PCR analyses
All RNA was obtained from cultured cells or specimens with TRIzol reagent (Invitrogen). For RT‐qPCR, 1 μg RNA was reverse transcribed into cDNA with a Reverse Transcription Kit (Takara). Real‐time PCR analyses were carried out with SYBR Green (Takara). The findings were normalized to the expression of GAPDH. The primer sequences are shown in Table S1.
2.3. Cell culture
The CCA cell lines HuCCT1 and RBE were obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). All cell lines were maintained in DMEM (Life Technologies) with 10% FBS (Sciencell), 100 mg/mL streptomycin, and 100 U/mL penicillin (Invitrogen) in humidified air at 37°C with 5% CO2.
2.4. Cell line transfection
Cholangiocarcinoma cells were plated in 6‐well plates and transfected the next day with particular siRNAs (100 nmol/L) or si‐SC (100 nmol/L; Invitrogen) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. A lentiviral shRNA vector targeting ANRIL was generated by inserting stand oligonucleotides into the pENTRTM/U6 vector, HuCCT1 cells were infected with the sh‐ANRIL vectors and selected with puromycin (5 μg/mL) to establish stable shRNA‐ANRIL cell lines. The siRNA and shRNA sequences are shown in Table S1.
2.5. Subcellular fractionation
Nuclear and cytosolic fractions were separated with a PARIS Kit (Life Technologies) according to the manufacturer’s instructions.
2.6. Cell proliferation analysis
Cell viability was determined with CCK‐8 assays according to the manufacturer’s suggestions. HuCCT1 and RBE cells transfected with siRNA or si‐SC (3000 cells/well) were cultivated in 5 96‐well plates with 6 replicate wells. For the colony formation assays, 500 transfected cells were plated in a 6‐well plate and maintained in media with 10% FBS for 2 weeks. The medium was replaced every 4 days. Then colonies were treated with methanol and dyed with a 0.1% crystal violet solution (Sigma‐Aldrich) for 15 minutes. The number of visibly stained colonies was counted for colony formation. The wells were independently measured in triplicate for the different treatment groups.
2.7. Cell migration assays
For the migration assays, after 24 hours of transfection, 3.5 × 104 cells in media with 1% FBS were put into the upper chamber of an insert (Millipore), and medium with 10% FBS was put into the lower chamber. After 24 hours of incubation, the remaining cells on the upper layer of the membrane were removed, while those cells that had migrated through the membrane were dyed with methanol and a 0.1% crystal violet solution and imaged with an IX71 inverted microscope (Olympus). This experiment was carried out in triplicate.
2.8. Flow cytometric analysis
Flow cytometry assays were carried out as previously reported in Xu et al. 45 After the cells were transfected with siRNAs for 48 hours, we harvested the cells and then undertook FITC‐annexin V and propidium iodide staining using an FITC‐annexin V Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s instructions. Cell cycle analysis was determined by propidium oxide staining using a CycleTest Plus DNA Reagent Kit (BD Biosciences) according to the manual. The results were evaluated with a FACScan flow cytometer. The numbers of cells in each phase were assessed.
2.9. Western blot assay and Abs
Cell protein lysates were separated by 10% SDS‐PAGE and transferred onto 0.22‐µm nitrocellulose blotting membranes (Sigma‐Aldrich). The membranes were incubated with the indicated Abs. Densitometry (Quantity One software; Bio‐Rad) was used to quantify the autoradiograms. A GAPDH Ab was used as a control. An anti‐EZH2 Ab was purchased from Proteintech, and an anti‐ERRFI1 Ab was purchased from Genetex.
2.10. In vivo tumor formation assay
Four‐week‐old athymic male mice from the Animal Center of Nanjing University (Nanjing, China) were kept in specific pathogen‐free conditions. HuCCT1 cells were stably transfected with shRNA or empty vector and harvested from cell culture plates. The cells were then washed with PBS and resuspended at 2 × 107 cells/mL. Next, these cells were xenografted into BALB/c male nude mice. The tumor sizes were measured every 3 days, and the volumes were calculated as follows: length × width2 × 0.5. At 16 days postinjection, the mice were subjected to CO2 asphyxiation, and the tumors were weighed and examined. The study was carried out strictly in accordance with the Guidelines for the Care and Use of Laboratory Animals of the NIH. The protocol was approved by the Committee on the Ethics of Animal Experiments of Nanjing Medical University.
2.11. RNA immunoprecipitation assays
To study whether ANRIL could interact with EZH2, we undertook a RIP experiment with a Magna RIP RNA‐Binding Protein Immunoprecipitation Kit (Millipore) following the manufacturer’s instructions. The Ab used for the EZH2 RIP assays was obtained from Millipore (Cat. #17‐662).
2.12. Chromatin immunoprecipitation assays
The ChIP assays were carried out with an EZ‐ChIP Kit according to the manufacturer’s recommendations (Millipore). EZH2 Abs (Cat. #17‐662) and H3K27me3 Abs (Cat. # 07‐449) were purchased from Millipore. The sequences of the ChIP primers are shown in Table S1. The equation used for calculating the ChIP data as a proportion relative to the DNA input was as follows: 2(Input Ct − Target Ct) × 0.1 × 100.
2.13. Deep sequencing of the whole transcriptome
Total RNA from ANRIL knockdown and control HuCCT1 cells was separated and quantified. The concentrations for each specimen were measured with a NanoDrop 2000 (Thermo Scientific) and evaluated with an Agilent2200 (Agilent). The sequence library for each RNA specimen was created with an Ion Proton Total RNA‐Seq Kit version 2 according to the manufacturer’s instructions (Life Technologies). The data are shown in Table S2.
2.14. Statistical analysis
Statistical analyses were carried out with GraphPad Prism 5 (GraphPad Software). The statistical significance of differences between various groups was calculated by Student’s t test or the χ2 test as appropriate. All data are expressed as the means ± SD, and 2‐sided P values of .05 were used to indicate statistical significance.
3. RESULTS
3.1. ANRIL is upregulated in human CCA tissues
To comprehensively characterize aberrantly expressed lncRNAs in CCA, we analyzed TCGA CCA and normal tissue RNA sequencing data (9 normal and 36 cancer samples) (Figure 1A) and 1 independent microarray dataset from the Gene Expression Omnibus database (GSE76297; 92 cancer tissue samples and 91 normal tissue samples) (Figure 1B). The results showed that ANRIL was differentially expressed in CCA tissues and normal tissues (P value = 9.95 * 10−5), which provided a clue for our research. ANRIL, a 3857 nt lncRNA, is transcribed from the antisense strand of the INK4A‐ARF‐INK4B gene, which was overexpressed in many other cancers, such as prostate cancer, 46 cervical cancer, 41 and breast cancer, 47 so we wanted to validate whether it is also important in CCA. To further verify the informatics data, we detected ANRIL expression in a cohort of 17 paired CCA tumors and adjacent tissues by qRT‐PCR. The results confirmed that ANRIL expression was remarkably higher in carcinoma tissues than in normal tissues (Figure 1C). Our data indicated that upregulated ANRIL expression is related to human CCA.
FIGURE 1.

Increased long noncoding RNA (lncRNA) ANRIL levels in cholangiocarcinoma (CCA) tissues. A, Hierarchical clustering analysis of lncRNAs that were differentially expressed (P < .05) in CCA tissues and normal tissues from The Cancer Genome Atlas (TCGA) database. B, ANRIL was overexpressed in Gene Expression Omnibus datasets (GSE76297). C, ANRIL was detected in 17 pairs of CCA tissues by quantitative RT‐PCR. ANRIL levels are significantly higher in CCA tissues than in nontumorous tissues. Error bars indicate means ± SD. ***P < .001
3.2. ANRIL knockdown in CCA cell lines inhibits cell proliferation and migration
We first investigated the effects of increased ANRIL expression in CCA. The qRT‐PCR results showed that ANRIL expression was significantly lower in the siRNA‐mediated knockdown group than in the si‐SC group for the HuCCT1 and RBE cell lines (Figure 2A). Both siRNA and ASO are interference technologies of lncRNA. 48 , 49 Although it is possible that ASO has a better interference efficiency for lncRNA in the nucleus than siRNA, qPCR detection after siRNA interference showed that siRNA could efficiently inhibit the expression of ANRIL. Therefore, siRNA was used for interference in this project. The CCK‐8 assays revealed that, compared to control cells, ANRIL knockdown cells had significantly lower cell viability for both the HuCCT1 and RBE cell lines (Figure 2B). Additionally, the clonogenic formation number was significantly lower in the ANRIL knockdown cells than in the 2 CCA cell lines (Figure 2C). Furthermore, Transwell assays showed that ANRIL knockdown dramatically repressed the migration of cells (Figure 2D). These data showed that ANRIL plays a vital role in CCA cell proliferation and migration.
FIGURE 2.

ANRIL promotes cell proliferation and migration in cholangiocarcinoma (CCA) cells. A, Quantitative RT‐PCR was used to detect ANRIL expression in HuCCT1 and RBE cell lines after siRNA transfection. B, CCK‐8 assays were used to determine cell viability in si‐ANRIL‐transfected CCA cells. C, Colony formation assays were used to determine the cell colony formation ability of si‐ANRIL‐transfected cells. D, Transwell assays showed that ANRIL knockdown inhibits CCA cell migration. Error bars indicate means ± SD. *P < .05, **P < .01; ***P < .001. si‐SC, scrambled negative control siRNA
3.3. ANRIL depletion leads to increased cell apoptosis and delayed cell cycle in CCA cell lines
To further study whether ANRIL could affect apoptosis in CCA cell lines, flow cytometry was carried out. The findings revealed that HuCCT1 and RBE cell lines transfected with ANRIL siRNA showed higher apoptotic rates than the control cells (Figure 3A). Next, to determine whether the effects of ANRIL on CCA cell proliferation and apoptosis are caused by ANRIL‐mediated alterations to cell cycle progression, we undertook flow cytometry assays for both HuCCT1 and RBE cell lines. The flow cytometry assays revealed that the numbers of cells in the G0/G1 phase were higher and that the numbers of cells in the S and G2/M phases were lower in ANRIL knockdown cells than in control cells (Figure 3B). Thus, ANRIL could accelerate cell proliferation, inhibit apoptosis, and regulate cell cycle progression in CCA cell lines.
FIGURE 3.

ANRIL depletion increases cell apoptosis and delays the cell cycle in cholangiocarcinoma (CCA) cell lines. A, FACS analysis of the effect of ANRIL on cell apoptosis. B, FACS analysis of the effect of ANRIL on cell cycle progression. Error bars indicate means ± SD. *P < .05, **P < .01; ***P < .001; n.s., not significant; si‐NC, negative control siRNA; si‐SC, scrambled negative control siRNA
3.4. ANRIL knockdown inhibits CCA cell tumorigenesis in vivo
To determine whether ANRIL influences CCA tumorigenesis in vivo, HuCCT1 cells transfected with sh‐ANRIL or control vector were injected into nude mice. At 16 days postinjection, the tumors established in the sh‐ANRIL group were dramatically smaller than those in the control group (Figure 4A,B). Correspondingly, the average tumor volumes and weights at the final time point were obviously lower in the sh‐ANRIL group than in the control vector group (Figure 4C,D). Our results showed that silencing ANRIL could repress CCA tumor growth in vivo, indicating that the lncRNA ANRIL plays a significant role in CCA tumor growth.
FIGURE 4.

ANRIL promotes cholangiocarcinoma cell tumor growth in vivo. A, B, Scrambled (sh‐SC) or sh‐ANRIL was stably transfected into HuCCT1 cells, which were then injected into nude mice. C, Tumor volumes were calculated every 4 days after injection. Bars indicate SD. D, Tumor weights represent means ± SD *P < .05; **P < .01
3.5. Cell proliferation and apoptosis pathways altered by ANRIL siRNAs
To determine the target genes that could be regulated by ANRIL in CCA, RNA transcriptome sequencing was carried out using control and siRNAs against ANRIL. A set of 1383 mRNAs had a minimum of 1.5‐fold increase in abundance, whereas 1350 genes had a decrease in abundance (less than 1.5‐fold) after ANRIL silencing (Figure 5A, Table S2). An in‐depth investigation of the gene ontology analysis indicated that the most markedly overrepresented biological process pathways involved the cell cycle, cell proliferation, and cell apoptosis (Figure 5B). To prioritize the most strongly ANRIL‐related genes, attention was paid to those most highly expressed following ANRIL knockdown. Prospectively, among the most highly expressed genes, many well‐known genes related to proliferation and migration (eg PTPRH, CXCL2, G0S2, ERRFI1, GDF15, ANGPTL4, and SPRY4) were included. Some of these genes were verified by qRT‐PCR after ANRIL knockdown in HuCCT1 and RBE cells (Figure 5C,D).
FIGURE 5.
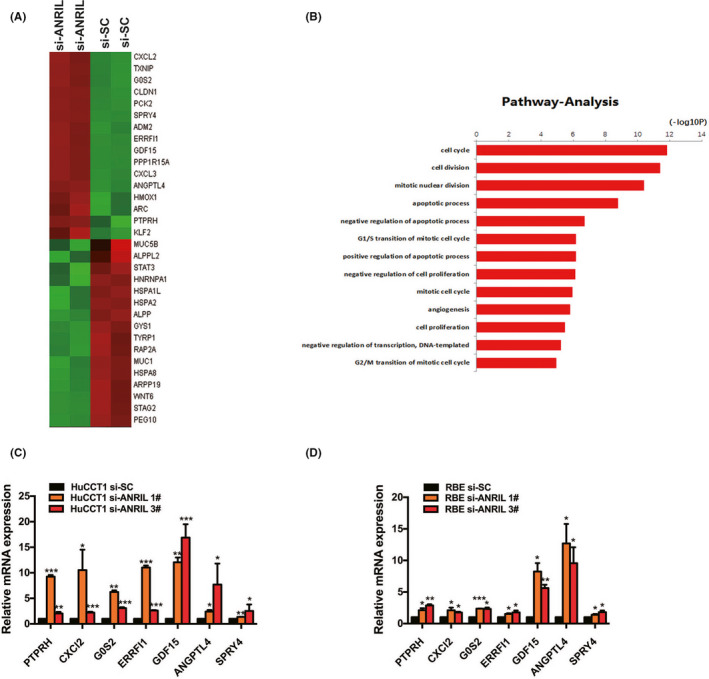
RNA sequencing after ANRIL knockdown in HuCCT1 cholangiocarcinoma cells. A, Mean‐centered, hierarchical clustering of the 2733 transcripts altered (≥1.5‐fold change) in scrambled negative control siRNA (si‐SC)‐treated cells and siRNA‐ANRIL‐treated cells with 2 repeats. B, Gene ontology analysis for all genes with altered expression. C, D, Altered mRNA levels were selectively confirmed by quantitative RT‐PCR in ANRIL knockdown cells. Error bars indicate means ± SD. *P < .05; **P < .01; ***P < .001
3.6. ANRIL epigenetically silenced ERRFI1 transcription through EZH2‐mediated H3K27me3 demethylation
Recent studies have reported that many lncRNAs cooperate with chromatin‐modifying enzymes to accelerate epigenetic activation and thus silence target gene expression. 50 In particular, PRC2, a classical methyltransferase that comprises EZH2, EED, and SUZ12, can serve as a catalyst in both the dimethylation and trimethylation of H3K27me3 to epigenetically repress target gene expression. 51 , 52 In our study, to investigate the mechanism of ANRIL‐mediated regulation, we first carried out subcellular fractionation location assays, which verified ANRIL localization to mainly the nucleus (Figure 6A). In addition, the probability of interaction between EZH2 and ANRIL was predicted on the RNA‐Protein Interaction Prediction website, and the result showed that EZH2 could bind well with ANRIL (random forest = 0.7, support vector machine = 0.97) (http://pridb.gdcb.iastate.edu/RPISeq/index.html) (Figure 6B). As revealed in Figure 6C, endogenous ANRIL was amplified in the anti‐EZH2 RIP fraction relative to the input; the IgG fraction of HuCCT1 and RBE cell lines with an Ab against enhancer of EZH2 was used for comparison. Taken together, these results confirmed that ANRIL could interact with EZH2.
FIGURE 6.

ANRIL binds to EZH2 in the nucleus to epigenetically silence ERRFI1. A, After nuclear and cytosolic separation, RNA expression levels were measured by quantitative (q)RT‐PCR. GAPDH was used as a cytosolic marker, and U1 was used as a nuclear marker. B, Interaction probabilities for EZH2 and ANRIL (http://pridb.gdcb.iastate.edu/RPISeq/index.html). RF, random Forest; SVM, support vector machine. C, RIP experiments for EZH2 were carried out, and the coprecipitated RNA was subjected to qRT‐PCR for ANRIL (GAPDH as the internal control). D, qRT‐PCR was used to detect EZH2 expression in HuCCT1 and RBE cell lines after si‐EZH2 transfection. E, Methylation‐related genes were detected by qRT‐PCR in HuCCT1 and RBE cell lines after EZH2 knockdown. F, Correlation between EZH2 and ERRFI1 expression was detected by analyzing GSE76297 data. G, Altered protein levels of ERRFI1 after EZH2 knockdown were selectively confirmed by western blotting. H, Altered protein levels of ERRFI1 after ANRIL knockdown were selectively confirmed by western blotting. I, ChIP‐qPCR of EZH2/H3K27me3 and in ERRFI1 promoter region after transfection with scrambled negative control siRNA (si‐SC) or ANRIL siRNAs in HuCCT1 cells. Enrichment was quantified with the anti‐IgG Ab as an internal control. Error bars indicate means ± SD. *P < .05; **P < .01; ***P < .001; n.s., not significant
Subsequently, we hypothesized that EZH2 could coregulate the suppression of these ANRIL‐mediated genes by binding with ANRIL. We first investigated the expression of ANRIL‐suppressed genes with EZH2 absent or present using qRT‐PCR. The results showed that the ANRIL‐suppressed genes were increased by also knocking down EZH2 (Figure 6D,E) in HuCCT1 and RBE cell lines. Subsequently, a correlation analysis of the GSE76297 dataset (92 pairs of cancer and 91 normal tissue samples) from the Molecular Signature Database revealed that ERRFI1 was significantly negatively correlated with EZH2 (Figure 6F). Moreover, ERRFI1 protein levels were increased by knocking down EZH2 (Figure 6G) and ANRIL (Figure 6H). To further determine whether ANRIL suppressed the expression of ERRFI1 by interacting with EZH2, a ChIP analysis was carried out. The ChIP assays revealed that ANRIL knockdown reduced the binding of EZH2 to the promoters of ERRFI1 as well as H3K27me3 levels (Figure 6I). These results indicated that EZH2 could bind directly to the promoter of ERRFI1 and then repress ERRFI1 expression directly by mediating H3K27me3 demethylation modifications.
3.7. Overexpression of ERRFI1 suppresses CCA cell proliferation and metastasis, and ERRFI1 is a confirmed target of ANRIL
ERRFI1, also known as Mig‐6 and RALT, can inhibit cancer cell proliferation and migration, including in non‐small cell lung cancer, 53 , 54 endometrial cancer, 55 , 56 hepatocellular carcinoma, 57 , 58 endometrial cancer, 59 and papillary thyroid cancer. 60 In addition, hypermethylation of the ERRFI1 promoter region has been reported to contribute to ERRFI1 transcription inactivation. 61 However, no report has proven that ERRFI1 is a tumor suppressor gene in CCA. Therefore, to test this hypothesis, we first analyzed the GSE76297 dataset (92 pairs of cancer and 91 normal tissue samples) and found that ERRFI1 expression was lower in CCA tissues than in normal tissues (Figure 7A). Subsequently, we detected ERRFI1 expression and found that ERRFI1 levels were lower in CCA tumor tissues than in neighboring tissues according to qRT‐PCR of a cohort of 17 pairs of CCA tumor tissues and adjacent tissues (Figure 7B). Subsequently, ERRFI1 overexpression substantially suppressed the proliferation of HuCCT1 and RBE cell lines (Figure 7C,D); moreover, ERRFI1 overexpression partially reversed ANRIL‐mediated proliferation (Figure 7E).
FIGURE 7.

EFFRI1 overexpression suppresses cholangiocarcinoma (CCA) cell proliferation and metastasis, and ERRFI1 is a confirmed target of ANRIL. A, ERRFI1 expression levels in CCA according to GSE76297 data analysis. B, ERRFI1 was detected in 17 pairs of CCA tissues by quantitative RT‐PCR. ERRFI1 levels were significantly lower in CCA tissues than in nontumorous tissues. C, CCK‐8 assays were used to determine the cell viability of pcDNA‐ERRFI1‐transfected CAA cells. D, Colony formation assays were used to determine the cell colony formation ability of pcDNA‐ERRFI1‐transfected cells. E, HuCCT1 and RBE cells transfected with vector/pcDNA‐ERRFI1/pcDNA‐ANRIL were transfected with ANRIL followed by ERRFI1. After transfection, the cells were analyzed by CCK‐8 assays. F, Proposed model in which ANRIL interacts with EZH2 to suppress ERRFI1 expression and promote CCA tumor growth. Error bars indicate means ± SD. *P < .05; **P < .01; ***P < .001
The probability of interaction between EZH2 and ANRIL was predicted on the RNA‐Protein Interaction Prediction website, and the result showed that EZH2 could bind well with ANRIL. For the RIP experiment, endogenous ANRIL was amplified in the anti‐EZH2 RIP fraction relative to the input. These results confirmed that ANRIL could interact with EZH2. In addition, ChIP assays revealed that ANRIL knockdown reduced the binding of EZH2 to the promoters of ERRFI1 as well as H3K27me3 levels, leading to increased levels of ERRFI1, which could decelerate CCA growth (Figure 7F). In conclusion, our results indicated that ANRIL promotes CCA malignancy by binding to EZH2 and then epigenetically repressing ERRFI1 expression in the nucleus.
4. DISCUSSION
Recent advances in high‐throughput biotechnologies have led to the exponential growth of high‐resolution lncRNA profiles of specimens from various types of cancer. These newly detectable lncRNAs have proven to be critical players in diverse human diseases, particularly human cancers. Research has shown that ANRIL levels are much higher in CCA tissues than in matched nontumor tissues. This led us to hypothesize that ANRIL might play a significant role in CCA malignancy. In fact, ANRIL has been identified as part of a shared genetic susceptibility locus associated with intracranial aneurysm, 31 , 32 coronary disease, 33 and various types of cancers, particularly the invasive pathophysiology of ovarian cancer, 34 nasopharyngeal carcinoma, 35 hepatocellular carcinoma, 36 lung cancer, 37 , 38 epithelial ovarian cancer, 34 colorectal cancer, 39 , 40 cervical cancer, 41 and gastric cancer. 42
We discovered that silencing ANRIL could inhibit CCA cell proliferation and migration in vitro and in vivo. Although ANRIL has been proposed to have oncogenic functions in diverse types of cancer, the global genes regulated by ANRIL have not been discovered. Through RNA‐seq, we noted that the gene ontology analysis results were particularly related to proliferation and migration, which agreed with the prevention of proliferation and migration in CCA cell lines with ANRIL knockdown. Recent studies have shown that many lncRNAs cooperate with chromatin‐modifying enzymes to stimulate epigenetic activation and thus silence the expression of the target genes. 50 For instance, the lncRNA PVT1 can interact with enhancer of EZH2, which is needed to suppress p15 and p16 in gastric cancer. 62 Moreover, lncRNAs can serve as scaffolds for protein complexes. 63 , 64 , 65 For example, the lncRNA CCAT1 can act as a scaffold for double epigenetic modification complexes (the 5′‐ domain of CCAT1 binding PRC2 with the 3′‐ binding domain of SUV39H1) and then mediate histone methylation at the promoter locus of SPRY4 in esophageal squamous cell carcinoma. 66 Our results show that ANRIL could bind to EZH2, a type of histone methylation modification complex in the nucleus, and thus regulate the expression of a series of target genes, including ERRFI1, a novel tumor suppressor in CCA.
In particular, previous studies have shown that ERRFI1 can act as a tumor suppressor gene in many types of cancers. 67 Nevertheless, the function of ERRFI1 in the tumorigenesis of CCA remains unknown. Our data revealed that histone methylation (H3K27me3) modulated by ANRIL could facilitate the lower expression of ERRFI1 in CCA cell lines. In combination with these findings, the functional interaction between ANRIL and EZH2 that we characterized in this study further emphasizes the centrality of lncRNAs in regulating gene expression, including that of ERRFI1, a novel tumor suppressor. Our results also suggest that interaction with chromatin‐modifying complexes is an important mechanism by which ANRIL exerts its functions in CCA.
In summary, our study revealed the regulatory mechanism of ANRIL in tumorigenesis. ANRIL promotes CCA malignancy through epigenetically regulating ERRFI1 transcription in the nucleus, thus facilitating cell survival and metastasis in CCA. To this end, rapid advances in oligonucleotide/nanoparticle technology support the development of siRNA‐based therapeutics to regulate lncRNA levels in vivo. In light of our findings, further investigation into the potential of ANRIL as an informative biomarker and therapeutic target for patients with CCA is warranted.
CONFLICT OF INTEREST
None.
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
This study was supported by the Project of Standard Diagnosis and Treatment of Key Disease of Jiangsu Province (BE2015722), the Project of the Peak of the Six Talents of Jiangsu Province (WSN‐018), and the Scientific Research Foundation for Health of Jiangsu Province (H201408). We also thank NovelBio Bio‐Pharm Technology Co., Ltd. for their support of the next‐generation sequencing and bioinformatics analysis with the NovelBrain Cloud Analysis Platform (www.novelbrain.com)
Yu Y, Chen Q, Zhang X, et al. Long noncoding RNA ANRIL promotes the malignant progression of cholangiocarcinoma by epigenetically repressing ERRFI1 expression. Cancer Sci. 2020;111:2297–2309. 10.1111/cas.14447
Yang Yu, Qiaoyu Chen, Xunlei Zhang, and Jian Yang equal contributors.
Contributor Information
Lei Yang, Email: leiyang.53@163.com.
Lin Miao, Email: linmiao@njmu.edu.cn.
REFERENCES
- 1. Wang W‐T, Ye H, Wei P‐P, et al. LncRNAs H19 and HULC, activated by oxidative stress, promote cell migration and invasion in cholangiocarcinoma through a ceRNA manner. J Hematol Oncol. 2016;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rizvi S, Borad MJ, Patel T, Gores GJ. Cholangiocarcinoma: molecular pathways and therapeutic opportunities. Semin Liver Dis. 2014;34:456‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rizvi S, Gores GJ. Emerging molecular therapeutic targets for cholangiocarcinoma. J Hepatol. 2017;67:632‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu AX. Future directions in the treatment of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:355‐361. [DOI] [PubMed] [Google Scholar]
- 6. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273‐1281. [DOI] [PubMed] [Google Scholar]
- 7. Lee S, Kopp F, Chang T‐C, et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164:69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niazi F, Valadkhan S. Computational analysis of functional long noncoding RNAs reveals lack of peptide‐coding capacity and parallels with 3'UTRs. RNA. 2012;18:825‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao J, Liu Y, Zhang W, et al. Long non‐coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle. 2015;14:3112‐3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jia H, Osak M, Bogu GK, Stanton LW, Johnson R, Lipovich L. Genome‐wide computational identification and manual annotation of human long noncoding RNA genes. RNA. 2010;16:1478‐1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saha A, Bhattacharya S, Bhattacharya A. Serum stress responsive gene EhslncRNA of Entamoeba histolytica is a novel long noncoding RNA. Sci Rep. 2016;6:27476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kugel JF, Goodrich JA. Non‐coding RNAs: key regulators of mammalian transcription. Trends Biochem Sci. 2012;37:144‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu X, Feng YI, Zhang D, et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26:344‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun M, Nie F, Wang Y, et al. LncRNA HOXA11‐AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76:6299‐6310. [DOI] [PubMed] [Google Scholar]
- 15. Guttman M, Donaghey J, Carey BW, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beckedorff FC, Amaral MS, Deocesano‐Pereira C, Verjovski‐Almeida S. Long non‐coding RNAs and their implications in cancer epigenetics. Biosci Rep. 2013;33;e00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atianand MK, Hu W, Satpathy AT, et al. A long noncoding RNA lincRNA‐EPS acts as a transcriptional brake to restrain inflammation. Cell. 2016;165:1672‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simon MD, Pinter SF, Fang R, et al. High‐resolution Xist binding maps reveal two‐step spreading during X‐chromosome inactivation. Nature. 2013;504:465‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Engreitz JM, Pandya‐Jones A, McDonel P, et al. The Xist lncRNA exploits three‐dimensional genome architecture to spread across the X chromosome. Science. 2013;341:1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang H, Liu P, Zhang J, et al. Long noncoding RNA MIR31HG exhibits oncogenic property in pancreatic ductal adenocarcinoma and is negatively regulated by miR‐193b. Oncogene. 2016;35:3647‐3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guttman M, Rinn JL. Modular regulatory principles of large non‐coding RNAs. Nature. 2012;482:339‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi X, Sun M, Wu Y, et al. Post‐transcriptional regulation of long noncoding RNAs in cancer. Tumour Biol. 2015;36:503‐513. [DOI] [PubMed] [Google Scholar]
- 23. Ma SL, Li AJ, Hu ZY, Shang FS, Wu MC. Coexpression of the carbamoylphosphate synthase 1 gene and its long noncoding RNA correlates with poor prognosis of patients with intrahepatic cholangiocarcinoma. Mol Med Rep. 2015;12:7915‐7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu X, Zhou C, Li R, Deng Y, Zhao L, Zhai W. Long noncoding RNA AFAP1‐AS1 promoted tumor growth and invasion in cholangiocarcinoma. Cell Physiol Biochem. 2017;42:222‐230. [DOI] [PubMed] [Google Scholar]
- 25. Jiang XM, Li ZL, Li JL, et al. LncRNA CCAT1 as the unfavorable prognostic biomarker for cholangiocarcinoma. Eur Rev Med Pharmacol Sci. 2017;21:1242‐1247. [PubMed] [Google Scholar]
- 26. Zhang F, Wan M, Xu Y, et al. Long noncoding RNA PCAT1 regulates extrahepatic cholangiocarcinoma progression via the Wnt/beta‐catenin‐signaling pathway. Biomed Pharmacother. 2017;94:55‐62. [DOI] [PubMed] [Google Scholar]
- 27. Tan X, Huang Z, Li X. Long non‐coding RNA MALAT1 interacts with miR‐204 to modulate human hilar cholangiocarcinoma proliferation, migration, and invasion by targeting CXCR4. J Cell Biochem. 2017;118:3643‐3653. [DOI] [PubMed] [Google Scholar]
- 28. Wang C, Mao Z P, Wang L, et al. Long non‐coding RNA MALAT1 promotes cholangiocarcinoma cell proliferation and invasion by activating PI3K/Akt pathway. Neoplasma. 2017;64:725‐731. [DOI] [PubMed] [Google Scholar]
- 29. Zhang C, Li JY, Tian FZ, et al. LncRNA NEAT1 promotes growth and metastasis of cholangiocarcinoma cells. Oncol Res. 2017; 26:879‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang S, Xiao J, Chai Y, et al. LncRNA‐CCAT1 promotes migration, invasion, and EMT in intrahepatic cholangiocarcinoma through suppressing miR‐152. Dig Dis Sci. 2017;62:3050‐3058. [DOI] [PubMed] [Google Scholar]
- 31. Che J. Molecular mechanisms of the intracranial aneurysms and their association with the long noncoding ribonucleic acid ANRIL ‐ a review of literature. Neurol India. 2017;65:718‐728. [DOI] [PubMed] [Google Scholar]
- 32. Foroud T, Koller DL, Lai D, et al. Genome‐wide association study of intracranial aneurysms confirms role of Anril and SOX17 in disease risk. Stroke. 2012;43:2846‐2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhuang J, Peng W, Li H, et al. Methylation of p15INK4b and expression of ANRIL on chromosome 9p21 are associated with coronary artery disease. PLoS ONE. 2012;7:e47193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qiu JJ, Wang Y, Liu YL, Zhang Y, Ding JX, Hua KQ. The long non‐coding RNA ANRIL promotes proliferation and cell cycle progression and inhibits apoptosis and senescence in epithelial ovarian cancer. Oncotarget. 2016;7:32478‐32492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu X, Jiang H, Jiang X. Downregulation of lncRNA ANRIL inhibits proliferation, induces apoptosis, and enhances radiosensitivity in nasopharyngeal carcinoma cells through regulating miR‐125a. Cancer Biol Ther. 2017;18:331‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hua L, Wang CY, Yao KH, Chen JT, Zhang JJ, Ma WL. High expression of long non‐coding RNA ANRIL is associated with poor prognosis in hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:3076‐3082. [PMC free article] [PubMed] [Google Scholar]
- 37. Lin L, Gu ZT, Chen WH, Cao KJ. Increased expression of the long non‐coding RNA ANRIL promotes lung cancer cell metastasis and correlates with poor prognosis. Diagn Pathol. 2015;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nie F‐Q, Sun M, Yang J‐S, et al. Long noncoding RNA ANRIL promotes non‐small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther. 2015;14:268‐277. [DOI] [PubMed] [Google Scholar]
- 39. Sun Z, Ou C, Ren W, Xie X, Li X, Li G. Downregulation of long non‐coding RNA ANRIL suppresses lymphangiogenesis and lymphatic metastasis in colorectal cancer. Oncotarget. 2016;7:47536‐47555. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Sun Y, Zheng ZP, Li H, Zhang HQ, Ma FQ. ANRIL is associated with the survival rate of patients with colorectal cancer, and affects cell migration and invasion in vitro. Mol Med Rep. 2016;14:1714‐1720. [DOI] [PubMed] [Google Scholar]
- 41. Zhang D, Sun G, Zhang H, Tian J, Li Y. Long non‐coding RNA ANRIL indicates a poor prognosis of cervical cancer and promotes carcinogenesis via PI3K/Akt pathways. Biomed Pharmacother. 2016;85:511‐516. [DOI] [PubMed] [Google Scholar]
- 42. Zhang EB, Kong R, Yin DD, et al. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR‐99a/miR‐449a. Oncotarget. 2014;5:2276‐2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen S, Zhang J‐Q, Chen J‐Z, et al. The over expression of long non‐coding RNA ANRIL promotes epithelial‐mesenchymal transition by activating the ATM‐E2F1 signaling pathway in pancreatic cancer: an in vivo and in vitro study. Int J Biol Macromol. 2017;102:718‐728. [DOI] [PubMed] [Google Scholar]
- 44. Kotake Y, Nakagawa T, Kitagawa K, et al. Long non‐coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30:1956‐1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu Y, Ge Z, Zhang E, et al. The lncRNA TUG1 modulates proliferation in trophoblast cells via epigenetic suppression of RND3. Cell Death Dis. 2017;8:e3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao B, Lu YL, Yang Y, et al. Overexpression of lncRNA ANRIL promoted the proliferation and migration of prostate cancer cells via regulating let‐7a/TGF‐beta1/Smad signaling pathway. Cancer Biomark. 2018;21:613‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mehta‐Mujoo PM, Cunliffe HE, Hung NA, Slatter TL. Long non‐coding RNA ANRIL in the nucleus associates with periostin expression in breast cancer. Front Oncol. 2019;9:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhuo W, Liu Y, Li S, et al. Long noncoding RNA GMAN, up‐regulated in gastric cancer tissues, is associated with metastasis in patients and promotes translation of Ephrin A1 by competitively binding GMAN‐AS. Gastroenterology. 2019;156(676–691):e611. [DOI] [PubMed] [Google Scholar]
- 49. Micheletti R, Plaisance I, Abraham BJ, et al. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci Transl Med. 2017;9:eaai9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marchese FP, Huarte M. Long non‐coding RNAs and chromatin modifiers: their place in the epigenetic code. Epigenetics. 2014;9:21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shen X, Liu Y, Hsu Y‐J, et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamaguchi H, Hung MC. Regulation and role of EZH2 in cancer. Cancer Res Treat. 2014;46:209‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Z, Dong Q, Wang Y, Qu L, Qiu X, Wang E. Downregulation of Mig‐6 in nonsmall‐cell lung cancer is associated with EGFR signaling. Mol Carcinog. 2012;51:522‐534. [DOI] [PubMed] [Google Scholar]
- 54. Li Z, Qu L, Zhong H, Xu K, Qiu X, Wang E. Low expression of Mig‐6 is associated with poor survival outcome in NSCLC and inhibits cell apoptosis via ERK‐mediated upregulation of Bcl‐2. Oncol Rep. 2014;31:1707‐1714. [DOI] [PubMed] [Google Scholar]
- 55. Kim TH, Lee D‐K, Cho S‐N, et al. Critical tumor suppressor function mediated by epithelial Mig‐6 in endometrial cancer. Cancer Res. 2013;73:5090‐5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim TH, Yoo JY, Kim HI, et al. Mig‐6 suppresses endometrial cancer associated with Pten deficiency and ERK activation. Cancer Res. 2014;74:7371‐7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li Z, Qu L, Luo W, et al. Mig‐6 is down‐regulated in HCC and inhibits the proliferation of HCC cells via the P‐ERK/Cyclin D1 pathway. Exp Mol Pathol. 2017;102:492‐499. [DOI] [PubMed] [Google Scholar]
- 58. Reschke M, Ferby I, Stepniak E, et al. Mitogen‐inducible gene‐6 is a negative regulator of epidermal growth factor receptor signaling in hepatocytes and human hepatocellular carcinoma. Hepatology. 2010;51:1383‐1390. [DOI] [PubMed] [Google Scholar]
- 59. Yoo J‐Y, Yang WS, Lee JH, et al. MIG‐6 negatively regulates STAT3 phosphorylation in uterine epithelial cells. Oncogene. 2018;37:255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xia T, Liao QI, Jiang X, et al. Long noncoding RNA associated‐competing endogenous RNAs in gastric cancer. Sci Rep‐Uk. 2014;4:6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang YW, Staal B, Dykema KJ, Furge KA, Vande Woude GF. Cancer‐type regulation of MIG‐6 expression by inhibitors of methylation and histone deacetylation. PLoS ONE. 2012;7:e38955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kong R, Zhang E‐B, Yin D‐D, et al. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol Cancer. 2015;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsai M‐C, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yoon J‐H, Abdelmohsen K, Kim J, et al. Scaffold function of long non‐coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Puvvula PK, Desetty RD, Pineau P, et al. Long noncoding RNA PANDA and scaffold‐attachment‐factor SAFA control senescence entry and exit. Nat Commun. 2014;5:5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang E, Han L, Yin D, et al. H3K27 acetylation activated‐long non‐coding RNA CCAT1 affects cell proliferation and migration by regulating SPRY4 and HOXB13 expression in esophageal squamous cell carcinoma. Nucleic Acids Res. 2017;45:3086‐3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Y‐W, Staal B, Su Y, et al. Evidence that MIG‐6 is a tumor‐suppressor gene. Oncogene. 2007;26:269‐276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


