Abstract
The transmembrane receptors integrins are the bridges for cell‐cell or cell‐ECM interaction, which is strictly correlated to cancer development in several tumor types. Here, we revealed that integrin β8 serves as a driver to mediate sustained growth of bladder cancer and development of drug resistance. The elevated expression of integrin β8 was observed in highly malignant bladder tumor tissues from patients. The in vitro and in vivo results further indicated that integrin β8 overexpression in Biu87/T24 bladder cancer could mediate and strengthen cell proliferation and resistance to mitomycin C and hydroxycamptothecin. Mechanistically, integrin β8 on the cellular surface might recruit phosphorylated Y‐box binding protein 1, leading to the activation of c‐Myc and nuclear factor‐κB signals. Pharmacological targeting of integrin β8 by Arg‐Gly‐Asp‐Ser efficiently suppressed sustained growth and drug resistance in bladder cancer cells. Our findings identified integrin β8 as a marker of bladder cancer diagnosis and development, and provides an innovative approach for clinical bladder cancer therapy.
Keywords: bladder cancer, c‐Myc, integrin β8, NF‐κB, YBX1
We found that integrin β8 expression induces bladder cancer sustained growth and drug resistance through a Y‐box binding protein 1‐associated signaling pathway. Blockade of integrin β8 efficiently improves anticancer effects, revealing a new target for bladder cancer therapy.
1. INTRODUCTION
The incidence of malignant bladder carcinoma continues to rise. 1 Despite extensive efforts invested in clinical bladder cancer therapies, current therapeutic regimens, including surgical resection or chemotherapy, have produced only modest long‐term efficacy due to ultimate tumor relapse or sustained tumor growth. 2 Increasing evidence indicates that cancer stem cells, also named tumor repopulating cells or tumor initiating cells, are capable of self‐renewal and multilineage differentiation, resulting in the tumor reoccurrence and sustained growth. 3 , 4 However, the underlying mechanisms in cancer stem cell‐induced bladder cancer progression remain unclear.
The integrin family, consisting of the integrin α and β subunits, is known to mediate contact between the ECM and stroma cells or tumor cells. 5 , 6 Previous reports have implied that several types of integrins participate in tumor progression. Ligation of α3 and β1 integrins promotes cell survival and migration in gastric cancer. 7 Additionally, integrin αvβ3 is reported to drive tumor stemness upregulation and facilitate tumor cell proliferation or migration in multiple tumor types, which implies the crucial role of integrins in carcinoma progression. 8 , 9 However, little information is available concerning integrin β8‐associated tumor development, even though high expression of integrin β8 has been detected in several tumor cells. 10 , 11
In this study, we observed elevated expression of integrin β8 in highly malignant bladder tumor tissues from patients. We provided evidence that integrin β8 plays a critical role in bladder cancer cell proliferation and drug resistance development. Furthermore, we identified Y‐box binding protein 1 (YBX1) is downstream of integrin β8 to mediate the c‐Myc prosurvival signal and the nuclear factor‐κB (NF‐κB)/B‐cell lymphoma 2 (BCL2) antiapoptosis pathway activation, eventually causing multidrug resistance development. This study described the mechanism by which integrin β8 regulates bladder cancer progression and mediates drug resistance development in the clinic. Blockade of integrin β8 significantly improved the anticancer effects of chemotherapy, which provides an innovative approach for bladder cancer therapy.
2. MATERIALS AND METHODS
2.1. Cell lines and reagents
Human bladder cancer cells Biu87 and T24 were purchased from ATCC. All cells were maintained in RPMI‐1640 complete medium (Gibco) supplemented with 10% FCS (Gibco) at 37°C in a humidified, 5% CO2 atmosphere. Mitomycin C (MMC) and hydroxycamptothecin (HCPT) were purchased from Sangon. C‐Myc inhibitor 10058‐F4, NF‐κB inhibitor E330 and integrin inhibitor Arg‐Gly‐Asp‐Ser (AGAS) were purchased from MedChemExpress. Other reagents were of HPLC standard and purchased from Solarbio.
2.2. Tumor tissue collection from patients
Primary bladder tumor tissues were sterilely obtained after the surgery at The First Affiliated Hospital of the University of South China. Samples were divided into low malignant (L‐M, stage T0, Ta, Tis) and high malignant (H‐M, stage T3, T4) groups according to clinical bladder cancer stages. The study was approved by the Ethics Committee of the First Affiliated Hospital of the University of South China. All sample collection and processing were carried out respecting the Declaration of Helsinki. All experiments were carried out under the monitor of the Ethics Committee of the First Affiliated Hospital of the University of South China.
2.3. Cell proliferation analysis
For cell proliferation detection, 2000 tumor cells were seeded into 96‐well plates and cultured at 37°C in a 5% CO2 incubator. After 72 hours, 10 μL CCK‐8 solution (Solarbio) was added into the 96‐well plates and incubated at 37°C for 2 hours. The absorbance at 450 nm was measured by a microplate reader (Bio‐Rad). Each experiment was undertaken independently at least 3 times.
2.4. Colony formation analysis
For colony formation analysis, 250 tumor cells were seeded into 6‐well culture plates in RPMI‐1640 complete culture medium. After a week, crystal violet solution (Solarbio) was used to stain the cells and the colony numbers were calculated. Each experiment was undertaken independently at least 3 times.
2.5. Western blot analysis
Cell pellets were lysed by RIPA Lysis Buffer (Solarbio), and the protein suspension was boiled with loading buffer for 10 minutes, separated by SDS‐PAGE, and transferred onto PVDF membranes. Then the samples were blocked with 5% BSA and incubated overnight at 4°C with primary Abs against: integrin β8 (1:300), p‐YBX1 (1:500), YBX1 (1:500), NF‐κB (c‐rel) (1:500), BCL2 (1:500), and actin (1:500; all Abcam). Samples were incubated with HRP‐conjugated secondary Ab (1:1000, Abcam) for 1 hour at room temperature and then visualized by the ECL Detection Kit (Thermo Fisher Scientific).
2.6. Small interfering RNA interference and plasmid vector
Silencing of YBX1/BCL2 in Biu87 and T24 cells was undertaken using siRNA technology. The siRNA transfections were carried out with Lipofectamine siRNA (Ruibo) in RPMI‐1640 medium according to the manufacturer’s instructions. Sequences of YBX1 siRNA were as follows: siRNA#1, 5′‐GGUUCCCACCUUACUACAU‐3′ and siRNA#2, 5′‐GGUCAUCGCAACGAAGGUU‐3′. Sequences of BCL2 siRNA were as follows: siRNA#1, 5′‐GCAUGCGGCCUCUGUUUGAUU‐3′ and 5′‐GGGAGAUAGUGAUGAAGUAUU‐3′. The control vector (pcDNA3.1‐vector) plasmids were purchased from GenePharma and the overexpression vector of integrin β8 (pcDNA3.1‐ITGB8) was designed.
2.7. Immunofluorescence staining
The pathological sections of tumor tissues were retrieved by EDTA antigen retrieval (Solarbio). The tumor cells were fixed with 4% paraformaldehyde and permeated by 0.5% Triton‐X100. The tissues or cell samples were then blocked by 5% BSA, followed by incubating with anti‐c‐Myc primary Ab (1:400, Abcam) for 4°C overnight, and followed by secondary Abs (1:600; Abcam). The nucleus was stained with DAPI. The immunofluorescence images were captured from FV1000 laser scanning confocal microscope (Leica, Barnack).
2.8. Immunohistochemistry staining
The pathological sections of tumor tissues were retrieved by EDTA antigen retrieval (Solarbio), blocked by 5% BSA, followed by incubating with anti‐integrin β8 Ab (1:100, Abcam) for 4°C overnight, signal amplification staining using the ABC HRP Kit (Thermo) and counter‐staining with hematoxylin. The images were captured with microscope (Leica, Barnack).
2.9. Animal protocol
For s.c. tumor‐bearing mice analysis, 2 × 106 BIU87, T24, or Biu87/ITGB8 cells in 50 μL PBS were s.c. injected into nude mice. On day 14, 100 μL PBS, MMC (2 mg/kg), gemcitabine (GEM) (2 mg/kg), and AGAS (0.1 mg/mL) combined with MMC/HCPT (2 mg/kg) were injected into mice twice a week through the tail vein. The treatment lasted for 2 weeks. The mice of control groups received an equal volume of saline. The incidence of tumor in mice and the survival of mice were recorded. Tumor volume was calculated according to the formula: tumor volume = length × width2/2. All our animal experiments were undertaken in accordance with guidelines approved by the Institute Ethics Committee of the First Affiliated Hospital of the University of South China.
2.10. Statistical analysis
Each experiment was undertaken at least 3 times, independently. Results are presented as the mean ± SEM and statistical significance was analyzed using GraphPad 6.0 software. Statistical significance between groups was calculated by Student’s t test for 2 groups or by one‐way ANOVA for more than 2 groups. The survival rates were determined by Kaplan‐Meier survival analysis (*P < .05; **P < .01; ***P < .001; ns, no significant difference).
3. RESULTS
3.1. Integrin β8 promotes bladder cancer growth and development of drug resistance
Increasing evidence has indicated that integrins serve as the cellular surface receptors to transduce the prosurvival signals from ECM, resulting in sustained tumor growth. 9 , 12 Herein, to investigate the potential role of integrin β8 in bladder cancer development, we examined the expression of integrin β8 in tumor tissues from low malignant (LM, stage T0, Ta, Tis) and high malignant (HM, stage T3, T4) bladder cancer patients. Notably, elevated expression of integrin β8 was observed in those tumor tissues from patients with highly malignant bladder cancer (Figure 1A). This pattern was observed in multiple patients (Figure 1B), reminding us that integrin β8 might participate in tumor development in bladder cancer. Thereby, we established integrin β8‐overexpressing bladder cancer cell lines Biu87/ITGB8 and T24/ITGB8 (Figure S1A,B). Notably, integrin β8 expression significantly facilitated bladder cancer cell proliferation in vitro (Figures 1C and S1C) and tumor growth in nude mice (Figures 1D and S1D). The same results were observed in colony formation analysis (Figures 1E and S1E), indicating that elevated expression of integrin β8 could strengthen the capability of proliferation and tumorigenesis in bladder cancer. Sustained tumor growth is also correlated with chemoresistance in multiple tumor types, causing poor outcome and intensive tumor progression in the clinic. 13 , 14 We further examined the cytotoxicity of clinical therapeutic agents MMC and HCPT to Biu87/ITGB8 and T24/IGTB8 cells. Intriguingly, enhanced drug resistance was observed in integrin β8‐overexpressing cells Biu87/ITGB8 and T24/IGTB8 (Figures 1F,G and S1F,G). Those results suggested that integrin β8 promotes tumor sustained growth and development of drug resistance in bladder cancer.
FIGURE 1.
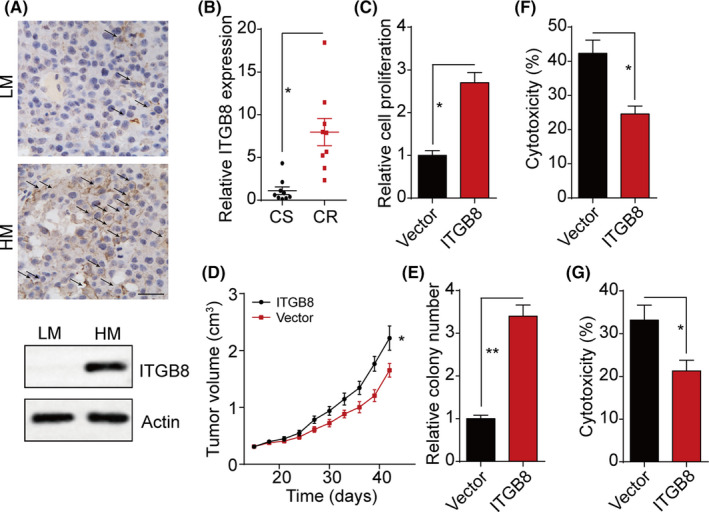
Integrin β8 promotes bladder cancer cell proliferation and drug resistance. A, Immunohistochemistry and western blot analysis of integrin β8 in low malignant (LM; stage T0, Ta, Tis) and high malignant (HM; stage T3, T4) tumor tissues from bladder cancer patients. Scale bar = 50 μm. B, Relative intensity of integrin β8 (ITGB8) expression in LM and HM tumor tissues (n = 10). CR, chemo‐resistance; CS, chemo‐sensitive. C, Relative cell proliferation of Biu87 and Biu87/ITGB8 for 48 h. D, Tumor volumes of Biu87‐ and Biu87/ITGB8‐bearing mice (2 × 106 cells per mouse). E, Relative colony formation of Biu87 and Biu87/ITGB8 cells. F, Cytotoxicity of Biu87 and Biu87/ITGB8 cells treated with mitomycin C (MMC; 0.5 μg/mL, 24 h). F, Cytotoxicity of Biu87 and Biu87/ITGB8 cells treated with hydroxycamptothecin (HCPT; 0.5 μg/mL, 24 h). G, Cytotoxicity of Biu87 and Biu87/ITGB8 cells treated with MMC (0.5 μg/mL, 24 h).*P < .05; **P < .01
3.2. Integrin β8 promotes bladder cancer development through YBX1
Y‐box binding protein 1 is a member of the family of DNA/RNA binding proteins that regulate cell proliferation/differentiation and mediate biodynamic signal responses. 15 We examined the expression of YBX1 in integrin β8‐overexpressing bladder cancer cells. Intriguingly, both phosphorylated YBX1 and total TBX1 were upregulated in Biu87/ITGB8 and T24/IGTB8 cells (Figures 2A and S2A), indicating activation of YBX1 in integrin β8‐overexpressing bladder cancer cells. To further investigate the role of YBX1 in bladder cancer progression, we used siRNA to silence YBX1 in Biu87/ITGB8 and T24/IGTB8 cells. We found that blockade of YBX1 results in proliferation and colony formation suppression in Biu87/ITGB8 (Figure 2B,C) and T24/IGTB8 cells (Figure S2B,C). Similarly, the drug resistance of bladder cancer cells induced by integrin β8 was reversed in YBX1 silenced Biu87/ITGB8 (Figure 2D,E) and T24/IGTB8 cells (Figure S2D,E), indicating that integrin β8 regulates bladder cancer development through YBX1. Consistent with our in vitro results, elevated expression of phosphorylated YBX1 and total YBX1 was observed in those tumor tissues from patients with highly malignant bladder cancer (Figure 2F).
FIGURE 2.
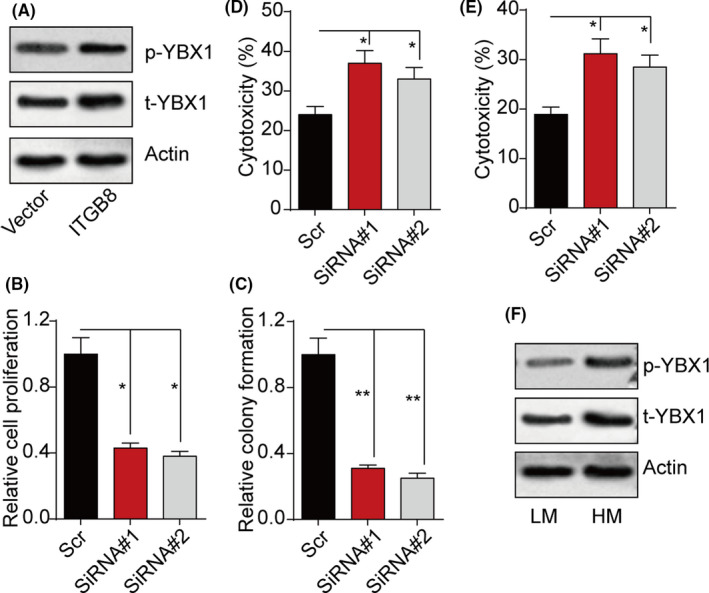
Integrin β8 (ITGB8) regulates tumor progression through Y‐box binding protein 1 (YBX1). A, Expression of phosphorylated (p‐)YBX1 and total (t‐)YBX1 in Biu87 and Biu87/ITGB8 cells. B, C, Relative proliferation (B) and relative colony formation (C) of Biu87/ITGB and YBX1 silenced Biu87/ITGB8 cells. D, E, Cytotoxicity of Biu87/ITGB8 and YBX1 silenced Biu87/ITGB8 cells treated with mitomycin C (0.5 μg/mL, 24 h) (D) or hydroxycamptothecin (0.5 μg/mL, 24 h) (E). F, Expression of p‐YBX1 and t‐YBX1 in low malignant (LM; stage T0, Ta, Tis) and high malignant (HM; stage T3, T4) tumor tissues from bladder cancer patients. *P < .05; **P < .01
3.3. Y‐box binding protein 1 upregulates c‐Myc to induce bladder cancer cell proliferation
Previous studies have indicated that YBX1 could stabilize the c‐Myc mRNA to mediate the upregulation of c‐Myc, 16 resulting in stemness upregulation in cancer cells. We examined c‐Myc expression in integrin β8‐overexpressing bladder cancer cells. As expected, integrin β8 overexpression efficiently mediated c‐Myc activation, whereas YBX1 suppression reversed the phenomenon (Figures 3A and S3A), indicating that integrin β8 mediated c‐Myc activation through YBX1. To further investigate the role of c‐Myc in integrin β8‐induced tumor progression, we used 10058‐F4, a c‐Myc inhibitor, to treat the integrin β8‐overexpressing bladder cancer cells. Blockade of c‐Myc significantly suppressed the proliferation (Figures 3B and S3B) and colony formation (Figures 3C and S3C) of Biu87/ITGB8 and T24/IGTB8 cells, indicating that activation of c‐Myc is essential in integrin β8‐induced tumor growth. The enhanced expression of c‐Myc was also observed in highly malignant bladder tumor tissues from patients (Figure 3D). However, c‐Myc has been reported to be the transcription factor participating in the regulation of cell stemness, which did not mediate drug resistance development in tumor cells directly. The cell cytotoxicity analysis also confirmed that blockade of c‐Myc had no influence on development of drug resistance in bladder cancer cells (Figures 3E,F and S3D,E). Together, those results indicated that YBX1 mediates c‐Myc upregulation to facilitate bladder tumor growth.
FIGURE 3.
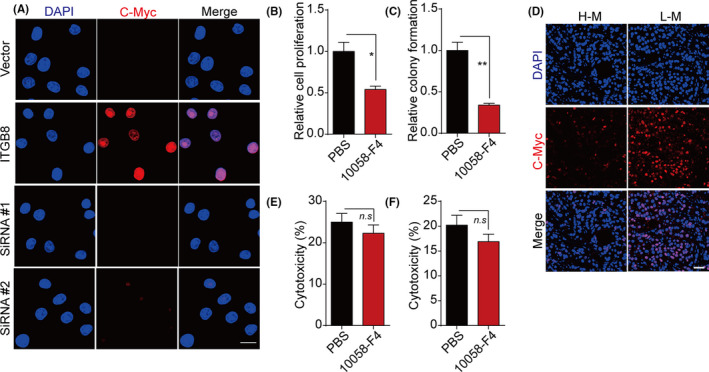
Y‐box binding protein 1 (YBX1) induced c‐Myc activation to facilitate bladder cancer proliferation. A, Immunofluorescence staining of c‐Myc in Biu87, Biu87/integrin β8 (ITGB8), and YBX1 silenced Biu87/ITGB8 cells. Scale bar = 10 μm. B, C, Relative proliferation (B) and of relative colony formation (C) of Biu87/ITGB and Biu87/ITGB8 cells treated with 10058‐F4 (50 μmol/L, 48 h). D, Immunofluorescence staining of c‐Myc in low malignant (LM; stage T0, Ta, Tis) and high malignant (HM; stage T3, T4) tumor tissues from bladder cancer patients. E, F, Cytotoxicity of Biu87/ITGB8 and 10058‐F4 (50 μmol/L, 48 h) precultured Biu87/ITGB8 cells treated with mitomycin C (0.5 μg/mL, 24 h) (E) or hydroxycamptothecin (0.5 μg/mL, 24 h) (F). *P < .05; **P < .01. n.s, no significant difference
3.4. Y‐box binding protein 1 signal mediates drug resistance through NF‐κB/BCL2 signaling pathway
Increasing evidence has implied that YBX1 could mediate the NF‐κB signal activation to regulate tumor progression. 17 , 18 More importantly, the NF‐κB downstream molecular BCL2, an antiapoptosis protein, has been reported to be associated with the development of drug resistance in several tumor types. 19 , 20 We further examined the expression of NF‐κB and BCL2 in integrin β8‐overexpressing bladder cancer cells. As a result, integrin β8 overexpression efficiently mediates NF‐κB activation and BCL2 upregulation, whereas integrin β8 or YBX1 suppression reversed the phenomenon (Figures 4A and S4A). Additionally, integrin β8 overexpression also caused BCL2 upregulation, whereas blockade of the NF‐κB signal by E330, a NF‐κB inhibitor, reversed this phenomenon (Figures 4B and S4B), indicating the activation of integrin β8/YBX1/NF‐κB/BCL2 signaling pathway in integrin β8‐overexpressing bladder cancer cells. To further determine the role of NF‐κB/BCL2 in the drug resistance induced by integrin β8, we used E330 and siRNA to silence NF‐κB or BCL2 expression. Intriguingly, suppression of NF‐κB or BCL2 significantly reversed the drug resistance to MMC (Figures 4C and S4C) and HCPT (Figures 4D and S4D), reminding us that activation of the integrin β8/YBX1/NF‐κB/BCL2 signal induces drug resistance in bladder cancer development.
FIGURE 4.
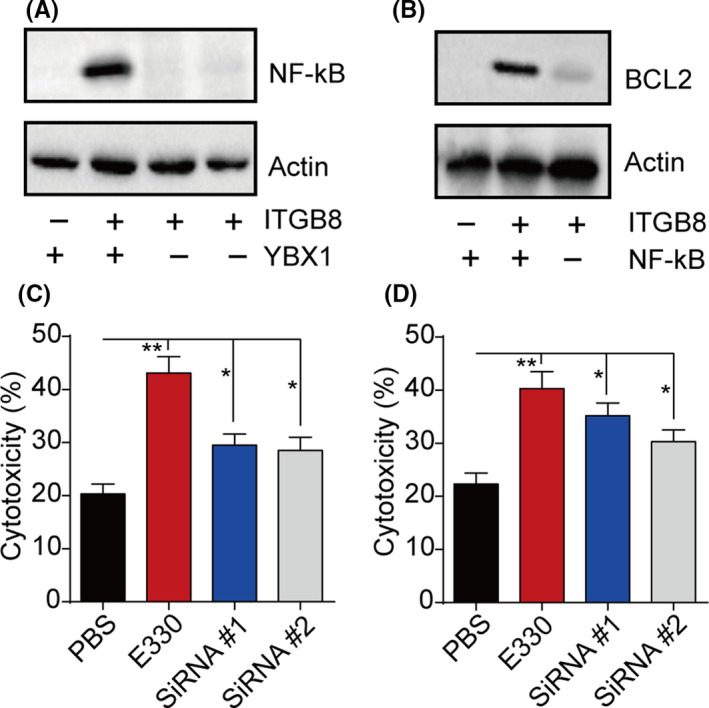
Y‐box binding protein 1 (YBX1) induced development of drug resistance through a nuclear factor‐κB (NF‐κB)/B‐cell lymphoma 2 (BCL2) signaling pathway. A, Expression of NF‐κB in Biu87, Biu87/integrin β8 (ITGB8), and YBX1 silenced Biu87/ITGB8 cells. B, Expression of BCL2 in Biu87, Biu87/ITGB8, and Biu87/ITGB8 cells treated with E330 (20 μmol/L, 48 h). C, D, Cytotoxicity of Biu87/ITGB8 cells, Biu87/ITGB8 cells treated with E330 (20 μmol/L, 48 h), and BCL2 silenced Biu87/ITGB8 cells treated with E330 (20 μmol/L, 48 h) to mitomycin C (0.5 μg/mL, 24 h) (C) or hydroxycamptothecin (0.5 μg/mL, 24 h) (D). *P < .05; **P < .01
3.5. Blockade of integrin β8 signal enhanced anticancer effects of chemotherapy
Our previous results have shown that integrin β8 facilitates tumor growth and development of drug resistance in bladder cancer, which indicated to us that blockade of integrin β8 might improve the anticancer effects in clinical bladder cancer treatment. Herein, we used an integrin β8 inhibitor, AGAS, to codeliver the chemotherapeutic agents by tail vein injection. In combination with MMC treatment, AGAS significantly suppressed the Biu87 tumor growth and prolonged survival time in tumor‐bearing mice (Figure 5A). The same results were observed in T24 tumor‐bearing mice (Figure 5B). More importantly, the results were duplicated in the HCPT combination group (Figure 5C), indicating that AGAS could be combined with multifarious chemotherapeutic agents for improved anticancer effects. To further simulate clinical drug‐resistant bladder carcinoma, we used integrin β8‐overexpressing Biu87 cells to establish the tumor‐bearing mice model. Subsequently, the combination of AGAS and MMC was used for tumor treatment by tail vein injection. Intriguingly, single MMC treatment revealed poor outcome, which might be due to the development of drug resistance induced by integrin β8. However, the combination of AGAS and MMC efficiently suppressed tumor growth and prolonged survival time (Figure 5D). Together, those results indicated that suppression of integrin β8 signals efficiently improved the anticancer effects of traditional chemotherapeutic agents, which provides an innovative approach in bladder cancer therapy.
FIGURE 5.
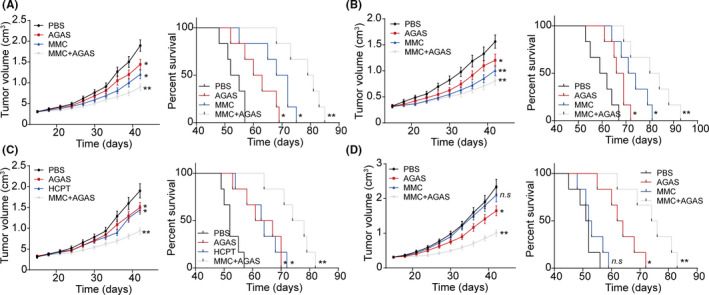
Integrin β8 inhibitor Arg‐Gly‐Asp‐Ser (AGAS) efficiently improved the anticancer effects of chemotherapy to bladder cancer. A, Tumor volume and survival time of Biu87‐bearing mice treated with PBS, mitomycin C (MMC), AGAS, or MMC combined with AGAS (n = 6). B, Tumor volume and survival time of T24‐bearing mice treated with PBS, MMC, AGAS, or MMC combined with AGAS (n = 6). C, Tumor volume and survival time of Biu87‐bearing mice treated with PBS, hydroxycamptothecin (HCPT), AGAS, or HCPT combined with AGAS (n = 6). D, Tumor volume and survival time of Biu87/ITGB8‐bearing mice treated with PBS, MMC, AGAS, or MMC combined with AGAS (n = 6). *P < .05. **P < .01. n.s, no significant difference
4. DISCUSSION
The role of integrins in tumor progression has been reported in several tumor types, including melanoma, 20 colorectal cancer, 21 and breast cancer. 22 Extracellular matrix‐induced integrins could facilitate signal transduction, resulting in tumor cell adhesion, migration, and prosurvival signaling activation. 23 , 24 For example, several reports provided evidence that integrin αvβ3 served as a cancer stem cell driver to regulate melanoma growth and development of drug resistance. 25 , 26 Integrin α2β1 has also been reported to facilitate tumor cell migration through an epithelial‐mesenchymal transition process, leading to tumor invasion and distant metastasis. 27 However, the role of integrin β8 is rarely reported in tumor development despite the high expression detected in several tumor cells. To address this issue, we evaluated the expression of integrin β8 in bladder tumor tissues and found elevated integrin β8 expression in highly malignant bladder tumor tissues. More importantly, expression of integrin β8 in tumor cells is able to promote tumor growth and drug resistance development in vivo. Therefore, the role of integrin β8 that we clarified in bladder cancer cells might be reminiscent of a more fundamental regulator of integrins in tumor progression.
Integrins function as the membrane surface receptors, which are known to transduce extracellular signals to mediate the downstream prosurvival signaling pathway, including KRAS‐, TBK1‐, or YAP‐associated signaling pathways in tumor cells. 28 , 29 , 30 Our study suggests that integrin β8 serves a crucial role in driving bladder cancer development and drug resistance. Integrin β8 facilitated the phosphorylation of YBX1, resulting in the activation of YBX1 downstream c‐Myc and NF‐κB/BCL2 signaling pathways. We showed that phosphorylated YBX1 drives c‐Myc upregulation and NF‐κB signal activation separately, leading to tumor stemness and antiapoptosis characteristics of bladder cancer cells. Consistently, suppression of the integrin β8‐induced YBX1/c‐Myc/NF‐κB/BCL2 signaling pathway is capable of retarding tumor development and improving the outcome of traditional chemotherapy (Figure 6). This highlights the potential of targeting integrin β8 as a mean to disrupt tumor progression.
FIGURE 6.
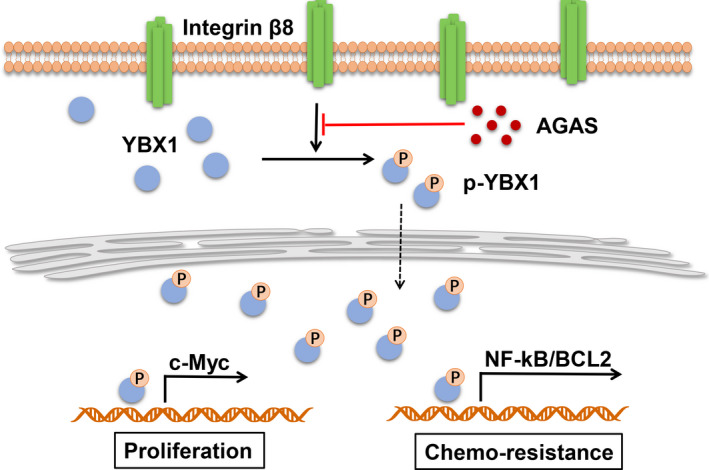
Schematic diagram of integrin β8‐induced bladder cancer cell proliferation and drug resistance. The cellular surface integrin β8 receptor could induce Y‐box binding protein 1 (YBX1) activation through phosphorylation. Phosphorylated (p‐)YBX1 upregulates c‐Myc to promote tumor cell proliferation. Simultaneously, activation of nuclear factor‐κB (NF‐κB)/B‐cell lymphoma 2 (BCL2) signaling through YBX1 causes resistance to chemotherapy in bladder cancer, resulting in poor clinical outcome. AGAS, Arg‐Gly‐Asp‐Ser
Given that integrin β8 is necessary for bladder cancer development and drug resistance, it might be feasible to target and inhibit integrin β8 to kill integrin β8‐positive tumor cells to reverse chemotherapy resistance and suppress tumor growth. However, a better understanding of integrin‐associated mechanisms in tumor progression is necessary for application of integrin inhibitors. A previous clinical trial revealed that the integrin αvβ3 inhibitor cilengitide failed to improve the outcome of carcinoma patients in a phase III study. 30 In terms of mechanism, tumor cells are capable of maintaining physiological activities despite undergoing a low proliferative rate after integrin suppression. Integrin‐negative tumor cells also revealed sustained proliferative characteristics even without development of drug resistance. Herein, targeting bladder cancer cells using the integrin β8 inhibitor AGAS to codeliver chemotherapeutic agents might be particularly advantageous to suppress integrin β8‐positive drug‐resistant tumor cells, as well as integrin β8‐negative tumor cells in tumor tissues. As shown in our results, the combination of AGAS with MMC or HCPT efficiently suppressed bladder tumor growth and simultaneously prolonged the survival time of tumor‐bearing mice, providing a feasible strategy for clinical bladder treatment.
Based on the limitations of previous reports, we further described the role of integrin β8 in bladder cancer progression and drug resistance development. First, we showed the correlation between integrin β8 and bladder cancer, demonstrating that the elevated expression of integrin β8 could result in malignant bladder cancer development. Second, we determined the underlying mechanism of tumor progression induced by integrin β8. We showed that integrin β8 regulates bladder cancer progression through an YBX1‐dependent signaling pathway. Third, the combination of AGAS and chemotherapy could significantly improve outcomes in bladder cancer treatment. Compared to previous integrin inhibitors in cancer therapy, AGAS combined with chemotherapeutic agents could be given by instillation, which is a more efficient and safer clinical treatment. Finally, the expression level of integrin β8 in bladder tumor tissues might serve as potential biomarker for cancer diagnosis or tumor progression analysis.
In conclusion, our studies showed that integrin β8 plays a critical role in bladder cancer cell proliferation and drug resistance development, which is dependent on a YBX1/c‐Myc/NF‐κB/BCL2 signaling pathway. Suppression of integrin β8 efficiently improved the anticancer effects of traditional chemotherapy, which provides new insight into clinical bladder cancer treatment.
DISCLOSURE
The authors declare no conflicts of interest.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Projects of the Health Commission of Hunan Province (20200495 and 20200540) and Project of the Science and Technology Department of Hengyang (2017KJ312).
Liu S, Chen L, Zhao H, Li Q, Hu R, Wang H. Integrin β8 facilitates tumor growth and drug resistance through a Y‐box binding protein 1‐dependent signaling pathway in bladder cancer. Cancer Sci. 2020;111:2423–2430. 10.1111/cas.14439
Shimin Liu and Libo Chen contributed equally to this manuscript.
IRB approval protocol number: 2012B01656.
Contributor Information
Rong Hu, Email: cclb229@foxmail.com.
Hao Wang, Email: cclb229@126.com.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. [DOI] [PubMed] [Google Scholar]
- 2. Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388:2796‐2810. [DOI] [PubMed] [Google Scholar]
- 3. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124‐1134. [DOI] [PubMed] [Google Scholar]
- 4. Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133‐143. [DOI] [PubMed] [Google Scholar]
- 5. Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aoudjit F, Vuori K. Integrin signaling in cancer cell survival and chemoresistance. Chemother Res Pract. 2012;2012:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takatsuki H, Komatsu S, Sano R, Takada Y, Tsuji T. Adhesion of gastric carcinoma cells to peritoneum mediated by α3β1 integrin (VLA‐3). Can Res. 2004;64:6065‐6070. [DOI] [PubMed] [Google Scholar]
- 8. Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91‐100. [DOI] [PubMed] [Google Scholar]
- 9. Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He J, Liu Y, Zhang L, Zhang H. Integrin subunit beta 8 (ITGB8) upregulation is an independent predictor of unfavorable survival of high‐grade serous ovarian carcinoma patients. Med Sci Monit. 2018;24:8933‐8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iordanskaia T, Koeck E, Rossi C, et al. Integrin β‐8, but not β‐5 or‐6, protein expression is increased in livers of children with biliary atresia. J Pediatr Gastroenterol Nutr. 2014;59:679‐683. [DOI] [PubMed] [Google Scholar]
- 12. Subramani D, Alahari SK. Integrin‐mediated function of Rab GTPases in cancer progression. Mol Cancer. 2010;9:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth‐factor‐driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X, Wang Y, Xia H, et al. Loss of E‐cadherin promotes the growth, invasion and drug resistance of colorectal cancer cells and is associated with liver metastasis. Mol Biol Rep. 2012;39:6707‐6714. [DOI] [PubMed] [Google Scholar]
- 15. Lyabin DN, Eliseeva IA, Ovchinnikov LP. YB‐1 protein: functions and regulation. Wiley Interdiscip Rev RNA. 2014;5:95‐110. [DOI] [PubMed] [Google Scholar]
- 16. Cobbold L, Wilson L, Sawicka K, et al. Upregulated c‐myc expression in multiple myeloma by internal ribosome entry results from increased interactions with and expression of PTB‐1 and YB‐1. Oncogene. 2010;29:2884‐2891. [DOI] [PubMed] [Google Scholar]
- 17. Martin M, Hua L, Wang B, et al. Novel serine 176 phosphorylation of YBX1 activates NF‐κB in colon cancer. J Biol Chem. 2017;292:3433‐3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prabhu L, Mundade R, Wang B, et al. Critical role of phosphorylation of serine 165 of YBX1 on the activation of NF‐κB in colon cancer. Oncotarget. 2015;6:29396‐29412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen PM, Cheng YW, Wu TC, Chen CY, Lee H. MnSOD overexpression confers cisplatin resistance in lung adenocarcinoma via the NF‐κB/Snail/Bcl‐2 pathway. Free Radic Biol Med. 2015;79:127‐137. [DOI] [PubMed] [Google Scholar]
- 20. Nehra R, Riggins RB, Shajahan AN, Zwart A, Crawford AC, Clarke R. BCL2 and CASP8 regulation by NF‐κB differentially affect mitochondrial function and cell fate in antiestrogen‐sensitive and‐resistant breast cancer cells. FASEB J. 2010;24:2040‐2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsu RY, Chan CH, Spicer JD, et al. LPS‐induced TLR4 signaling in human colorectal cancer cells increases β1 integrin‐mediated cell adhesion and liver metastasis. Can Res. 2011;71:1989‐1998. [DOI] [PubMed] [Google Scholar]
- 22. Yao ES, Zhang H, Chen YY, et al. Increased β1 integrin is associated with decreased survival in invasive breast cancer. Can Res. 2007;67:659‐664. [DOI] [PubMed] [Google Scholar]
- 23. Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1‐20. [DOI] [PubMed] [Google Scholar]
- 24. Ruoslahti E. Integrins. J Clin Investig. 1991;87:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hofmann UB, Westphal JR, Waas ET, Becker JC, Ruiter DJ, van Muijen GN. Coexpression of integrin αvβ3 and matrix metalloproteinase‐2 (MMP‐2) coincides with MMP‐2 activation: correlation with melanoma progression. J Invest Dermatol. 2000;115:625‐632. [DOI] [PubMed] [Google Scholar]
- 26. Weis SM, Cheresh DA. αV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med. 2011;1:a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katoh D, Nagaharu K, Shimojo N, et al. Binding of αvβ1 and αvβ6 integrins to tenascin‐C induces epithelial–mesenchymal transition‐like change of breast cancer cells. Oncogenesis. 2013;2:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seguin L, Kato S, Franovic A, et al. An integrin β 3–KRAS–RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16:457‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chu P, Yang M, Kulp S, et al. Regulation of oncogenic KRAS signaling via a novel KRAS‐integrin‐linked kinase‐hnRNPA1 regulatory loop in human pancreatic cancer cells. Oncogene. 2016;35:3897‐3908. [DOI] [PubMed] [Google Scholar]
- 30. Wang L, Luo JY, Li B, et al. Integrin‐YAP/TAZ‐JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579‐582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material


