Peter Liddle’s insightful paper is a timely revisit of the century-old concept of what he calls “classical” schizophrenia.1 He draws upon Bleulerian symptoms and reflects upon its implications for predicting outcomes of psychotic illness and the much-needed development of newer and effective treatments. Eugene Bleuler had himself renamed the erstwhile Kraepelinian concept of dementia praecox into “the group of Schizophrenias” highlighting the key challenge of heterogeneity of this syndrome. While Liddle’s paper and his work over the last two decades has elegantly addressed the heterogeneity within the boundaries of the Schizophrenia syndrome, this heterogeneity cuts across a broad spectrum of psychotic disorders as evidenced by accumulating literature in recent years.2 We herein address three aspects of this heterogeneity which can help refine our thinking, including (a) symptom overlap between psychotic disorders, (b) biological heterogeneity across the psychosis syndromes, and (c) heterogeneity in longitudinal course of psychotic disorders.
First, there is considerable evidence for overlap in symptomatology, and core cognitive impairments between schizophrenia, schizoaffective disorder, and bipolar disorder3 as well as between these disorders, depression, and personality disorders.4 For example, cognitive impairments are seen in many patients with bipolar disorder, especially those with psychotic features, and when they are, they seem to be associated with worse outcomes and more chronic course.5-7 Several electroencephalographic properties are also related to functional outcomes. Mismatch negativity (MMN) is a biomarker and a neurodegenerative marker of psychosis.8 Deterioration of MMN correlates with impairments in neurocognition and social cognition9 and gray matter volume reduction in both patients10 and in clinical high-risk individuals who later transitioned to psychosis.11,12 MMN is a stronger indicator of functional outcome in early psychosis. Patients with the most impaired MMN amplitudes at baseline showed the most severe disability at follow-up.13 This suggests that the cognitive symptomatic signatures of the so-called “classic” schizophrenia might extend beyond the boundaries of the schizophrenia syndrome.
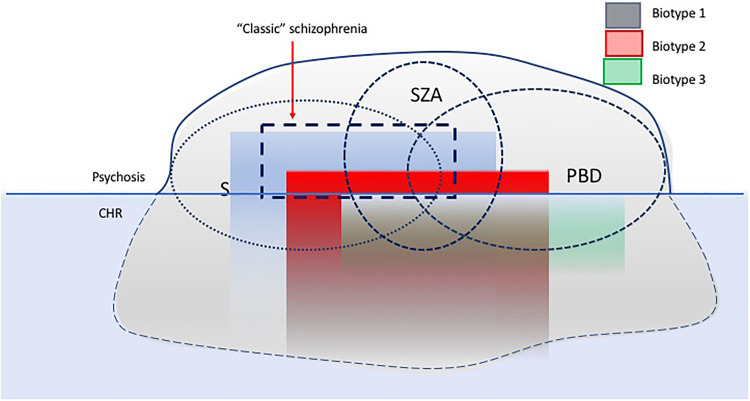
Second, there is considerable biological heterogeneity across the psychosis syndromes. In a recent series of studies, the Bipolar-Schizophrenia Network on Intermediate Phenotypes consortium investigators, using a K-means clustering approach, have identified three categories, termed “biotypes” across the psychosis dimension characterized by distinct alterations in cognition and electrophysiology; these biotypes appear to be orthogonal to the DSM-based clinical categorization.14 Similar clusters have been derived by Hall et al.15 Biotype 1 is characterized by prominent cognitive impairments, pronounced gray matter deficits, diminished neural response to salient stimuli, social dysfunction, and more pronounced negative symptoms similar to Liddle’s articulation of a “classical schizophrenia.” Biotype 2 is characterized by moderate cognitive, social, and brain structural impairments and accentuated neural reactivity, and Biotype 3 differs relatively little from healthy controls.3 These observations point to the possibility that biomarker-derived classifications may better distinguish subtypes within the psychotic spectrum distinct subgroups separable by etiological factors such as genetics, trauma, and substance abuse. A testable prediction is whether Biotype 1 is associated with the kind of reduced neuroplasticity suggested in Peter Liddle’s paper as underlying classical schizophrenia. Thus, within each subtype, individuals may associate with distinct pathophysiological or neurobiological characteristics and functioning impairments. Linking basic biological and behavioral components of normal and abnormal functioning enables us to construct more valid and effective individual-tailored treatment interventions. A similar “diagnostically agnostic” approach is also the guiding principle of the Research Diagnostic Criteria (RDoC) initiative.16,17 However, the RDoC is essentially dimensional and does not yield categories which clinicians in practice need for day-to-day decision-making; the biotype approach offers an alternative way forward and needs to be further validated using external measures of etiology, outcome, and treatment response (Figure 1).
Figure 1.
A schematic diagram illustrating the approaches to classification of psychotic disorders. Circles represent the traditional, symptom-based approach (i.e., schizophrenia, schizoaffective disorder, and psychotic bipolar disorder). Rectangles represent biomarker-based approaches (biotypes) which are orthogonal to traditional clinical diagnoses. Such biotypes may overlap with the subtypes proposed by Liddle and may have better predictive value for outcome across psychotic disorders.
The cross-cutting biology may reflect overlapping etiological factors. Numerous large-scale genetic studies indicate overlap in susceptibility across schizophrenia, schizoaffective disorder, bipolar disorder, and other related disorders.18-20 These cumulative lines of evidence indicate the existence of within disorder genetic heterogeneity as well as a complex and overlapping genetic architecture of psychiatric risk.
Third, there is heterogeneity in the longitudinal course of schizophrenia and related psychotic disorders as well, both following onset of the illness and in the prepsychotic phase. Symptoms alone are not good predictors of outcome. There is an increasing evidence that neurobiological alterations such as gray matter reductions,21 electrophysiological changes,22 and cognitive impairment23 predict unfavorable outcomes in first episode psychotic disorders. Such longitudinal heterogeneity also extends to the premorbid and prodromal phases of the illness, as pointed out by Liddle. The clinical high-risk (CHR) phase is heterogeneous in terms of both risk factors and outcomes, with a significant proportion of CHR converters having nonaffective psychosis (73%) and a smaller group converting to affective psychoses (11%).24 Additionally, the outcome of CHRs who do not convert to psychosis is heterogeneous,25 and data suggest psychopathological26 and neurocognitive heterogeneity in CHR,27 highlighting the need to better understand different clinical and functional profiles in the CHR population. It is well known that longer untreated prodromal illness28 as well as premorbid impairments are associated with poor outcomes.29 This suggests that biological subtypes identified within the psychotic syndromes may have unique antecedents in the prodromal or clinical high-risk syndrome period leading up to the psychotic illness. Systematic studies are needed to identify such prepsychotic biotypes by comprehensive neuropsychiatric assessment and multivariate data analysis agnostic to clinical diagnosis. Such biotypes may allow the identification of some groups that are separable by etiology (genes, trauma, and substance abuse) and thereby facilitate better approaches to early identification and personalized interventions.
Heterogeneity in the longitudinal course of schizophrenia-related diseases limits our progress and ability to predict clinical outcomes, treatment responses, and conversion to psychosis. The power of using cutting-edge data-driven bioinformatics approaches is becoming more important. Such methods can accommodate high-dimensional data types, separating subgroups empirically, and capturing the specific functional trajectories over time. Identifying individuals’ outcome trajectories and significant risk factors associated with a poor outcome trajectory can guide the development of individually tailored treatment that could potentially alter the course of the disease. To date, few individual-level predictors of outcomes are known. A recent longitudinal study using a data-driven machine learning unsupervised approach has identified four homogeneous early function outcome trajectories in patients with first episode of psychosis.30 Longitudinal data-driven trajectory analysis is useful because it looks beyond conventional diagnostic categories to better understand heterogeneity in the longitudinal course of psychosis syndromes and provide insight into clinically meaningful subgroups of patients.31 Such a strategy may likely facilitate personalized approach to prediction and treatment.
In addition, recent advances in machine learning and natural language processing are making detection and reliable prediction of psychosis onset among high-risk youths possible. For example, latent linguistic and behavioral features can be mined to construct a digital phenotype, a characterization of an individual’s knowledge representations and thought processes, to predict the later emergence of psychosis.32,33 Capitalizing on advances of computational methods offers an opportunity to move psychiatry beyond reliance on self-report and clinical observation toward more objective measures of illness at the level of the individual, helping treatment and prognostic decisions, thus providing important information to complement existing methods for patient assessment.34 Such a fine-grained behavioral analysis could allow tighter mapping between psychiatric phenotypes and their underlying biology, leading to greater understanding of the pathophysiology of schizophrenia and other psychiatric disorders, potentially also informing psychiatric nosology.
In summary, we agree with Liddle’s articulation of the need to identify the “core deficit” of classical schizophrenia in our efforts to predict functional outcome in psychotic illness. However, we believe that there may be no one classical schizophrenia but perhaps several pathophysiologically distinct syndromes across the psychosis spectrum disorders. We argue that (a) this trans-diagnostic view may be applicable to all of psychotic spectrum disorders, (b) a neuroscience-based categorization of distinct subtypes in these disorders, as opposed to symptom-based categories, may have even better power to predict outcome and treatment response, and (c) extending such a translational approach to clinical and familial high-risk states may help identify early predictors of illness and enable individually tailored preventive interventions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIMH grant RO1 MH78113 (M.S.K.) and MH109687 (M.H.H.).
References
- 1. Liddle PF. The core deficit of classical schizophrenia: implications for predicting the functional outcome of psychotic illness and developing effective treatments. Can J Psychiatry. 2019;64(10):680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keshavan MS, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA. Reimagining psychoses: an agnostic approach to diagnosis. Schizophr Res. 2013;146(1-3):10–16. [DOI] [PubMed] [Google Scholar]
- 3. Tamminga CA, Ivleva EI, Keshavan MS, et al. Clinical phenotypes of psychosis in the bipolar-schizophrenia network on intermediate phenotypes (B-SNIP). Am J Psychiatry. 2013;170(11):1263–1274. [DOI] [PubMed] [Google Scholar]
- 4. Witt SH, Streit F, Jungkunz M, et al. Genome-wide association study of borderline personality disorder reveals genetic overlap with bipolar disorder, major depression and schizophrenia. Transl Psychiatry. 2017;7(6):e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Rheenen TE, Lewandowski KE, Tan EJ, et al. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol Med. 2017;47(10):1848–1864. [DOI] [PubMed] [Google Scholar]
- 6. Lewandowski KE, Cohen BM, Keshavan MS, Ongur D. Relationship of neurocognitive deficits to diagnosis and symptoms across affective and non-affective psychoses. Schizophr Res. 2011;133(1-3):212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewandowski KE, Sperry SH, Cohen BM, et al. Treatment to enhance cognition in bipolar disorder (TREC-BD): efficacy of a randomized controlled trial of cognitive remediation versus active control. J Clin Psychiatry. 2017;78(9):e1242–e1249. [DOI] [PubMed] [Google Scholar]
- 8. Hall MH, Rijsdijk FV, Picchioni M, et al. Substantial shared genetic influences on schizophrenia and event-related potentials. Am J Psychiatry. 2007;164(5):804–812. [DOI] [PubMed] [Google Scholar]
- 9. Light GA, Naatanen R. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proc Natl Acad Sci U S A. 2013;110(38):15175–15176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69(10):959–966. [DOI] [PubMed] [Google Scholar]
- 12. Shaikh M, Valmaggia L, Broome MR, et al. Reduced mismatch negativity predates the onset of psychosis. Schizophr Res. 2012;134(1):42–48. [DOI] [PubMed] [Google Scholar]
- 13. Lee SH, Sung K, Lee KS, Moon E, Kim CG. Mismatch negativity is a stronger indicator of functional outcomes than neurocognition or theory of mind in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:213–219. [DOI] [PubMed] [Google Scholar]
- 14. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall MH, Smoller JW, Cook NR, et al. Patterns of deficits in brain function in bipolar disorder and schizophrenia: a cluster analytic study. Psychiatry Res. 2012;200(2-3):272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cuthbert BN, Kozak MJ. Constructing constructs for psychopathology: the NIMH research domain criteria. J Abnorm Psychol. 2013;122(3):928–937. [DOI] [PubMed] [Google Scholar]
- 17. Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cross-Disorder Group of the Psychiatric Genomics Consortium, Lee SH, Ripke S, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruderfer DM, Fanous AH, Ripke S, et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2014;19(9):1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cross-Disorder Group of the Psychiatric Genomics Consortium, Smoller JW, Craddock N, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wojtalik JA, Smith MJ, Keshavan MS, Eack SM. A systematic and meta-analytic review of neural correlates of functional outcome in schizophrenia. Schizophr Bull. 2017;43(6):1329–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62(2):127–136. [DOI] [PubMed] [Google Scholar]
- 23. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–588. [DOI] [PubMed] [Google Scholar]
- 24. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Addington J, Cornblatt BA, Cadenhead KS, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168(8):800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fusar-Poli P. Extending the benefits of indicated prevention to improve outcomes of first-episode psychosis. JAMA Psychiatry. 2017;74(7):667–668. [DOI] [PubMed] [Google Scholar]
- 27. Velthorst E, Froudist-Walsh S, Stahl E, et al. Genetic risk for schizophrenia and autism, social impairment and developmental pathways to psychosis. Transl Psychiatry. 2018;8(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris MG, Henry LP, Harrigan SM, et al. The relationship between duration of untreated psychosis and outcome: an eight-year prospective study. Schizophr Res. 2005;79(1):85–93. [DOI] [PubMed] [Google Scholar]
- 29. Malla AK, Norman RM, Manchanda R, Townsend L. Symptoms, cognition, treatment adherence and functional outcome in first-episode psychosis. Psychol Med. 2002;32(6):1109–1119. [DOI] [PubMed] [Google Scholar]
- 30. Hall MH, Holton KM, Ongur D, Montrose D, Keshavan M. Longitudinal trajectory of early functional recovery in patients with first episode psychosis. Schizophr Res. 2019;209(Feb 27):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Honer WG, Jones AA, Thornton AE, Barr AM, Procyshyn RM, Vila-Rodriguez F. Response trajectories to clozapine in a secondary analysis of pivotal trials support using treatment response to subtype schizophrenia. Can J Psychiatry. 2015;60(3 Suppl 2):S19–S25. [PMC free article] [PubMed] [Google Scholar]
- 32. Bedi G, Carrillo F, Cecchi GA, et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophr. 2015;1:15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rezaii N, Walker E, Wolff P. A machine learning approach to predicting psychosis using semantic density and latent content analysis. NPJ Schizophr. 2019;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holderness E, Miller N, Cawkwell P, et al. Analysis of risk factor domains in psychosis patient health records. J Biomed Semantics. 2019;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]