Abstract
Background:
Cost-efficient and non-invasive predictors of antidepressant response to repetitive transcranial magnetic stimulation (rTMS) are required. The personality vulnerabilities—neuroticism and self-criticism—are associated with antidepressant outcomes in other modalities; however, self-criticism has not been examined in response to rTMS, and the literature on neuroticism and rTMS is inconsistent.
Methods:
This naturalistic, 4-week study involved daily dorsolateral prefrontal cortex (DLFPC) rTMS for major depression (15 unipolar, 2 bipolar). Participants completed the Big Five Inventory (neuroticism) and the Depressive Experiences Questionnaire (self-criticism) at baseline and at the end of treatment. Changes in depressive symptoms, as rated by the clinician, were quantified using the 21-item Hamilton Depression Rating Scale. Given the inconsistencies in data regarding the stability of neuroticism in patients receiving rTMS, we performed a systematic review and quantitative meta-analysis of trials examining rTMS and neuroticism.
Results:
rTMS significantly improved depressive symptoms, and this was predicted by higher levels of self-criticism but not neuroticism. Self-criticism was stable over the 4 weeks of rTMS; however, neuroticism decreased, and this was not related to decreases in depressive symptoms. Our quantitative meta-analysis of 4 rTMS trials in major depression (n = 52 patients) revealed decreases in neuroticism, with a moderate effect size.
Limitations:
Our results are limited by a small sample size, and the absence of a sham-rTMS group. Our meta-analysis included only 4 trials.
Conclusion:
Highly self-critical patients appear to benefit more from rTMS than less self-critical patients. Neuroticism, a conceptually similar but distinct personality domain, does not appear to predict antidepressant response, yet this vulnerability factor for depression decreases after rTMS.
Keywords: repetitive transcranial magnetic stimulation, major depression, five-factor personality, self-criticism, neuroticism, meta-analysis
Abstract
Contexte:
Les prédicteurs rentables et non invasifs de la réponse antidépressive à la stimulation magnétique transcrânienne répétitive (SMTr) sont nécessaires. Les vulnérabilités de la personnalité que sont le neuroticisme et l’autocritique sont associées à des résultats antidépressifs dans d’autres modalités; toutefois, l’autocritique n’a pas été examinée dans la SMTr et la littérature sur le neuroticisme et la SMTr n’est pas concluante.
Méthodes:
Cette étude naturaliste de 4 semaines comportait une SMTr quotidienne du cortex préfrontal dorsolatéral (CPFDL) pour la dépression majeure (15 unipolaires, 2 bipolaires). Les participants répondaient à l’inventaire Big Five (neuroticisme) et au questionnaire sur les expériences dépressives (autocritique) au départ et à la fin du traitement. Les changements des symptômes dépressifs évalués par un clinicien étaient quantifiés à l’aide de l’échelle de dépression de Hamilton en 21 items. Étant donné les données inégales sur la stabilité du neuroticisme chez les patients recevant une SMTr, nous avons effectué une revue systématique et une méta-analyse quantitative des essais portant sur la SMTr et le neuroticisme.
Résultats:
La SMTr améliorait significativement les symptômes dépressifs, ce qui était prédit par des niveaux élevés d’autocritique, mais pas de neuroticisme. L’autocritique était stable pendant les 4 semaines de SMTr, cependant, le neuroticisme diminuait et ce n’était pas relié à des réductions des symptômes dépressifs. Notre méta-analyse quantitative des 4 essais de SMTr pour la dépression majeure (n = 52 patients) révélait des diminutions du neuroticisme, et une ampleur de l’effet modérée.
Limitations:
Nos résultats sont limités par une ampleur de l’effet modeste, et l’absence d’un groupe SMTr témoin. Notre méta-analyse ne comprenait que 4 essais.
Conclusion:
Les patients chez qui l’autocritique est élevée semblent bénéficier davantage de la SMTr que les patients moins portés sur l’autocritique. Le neuroticisme, un domaine de la personnalité de conception semblable mais distinct, ne semble pas prédire de réponse antidépressive, mais toutefois, ce facteur de vulnérabilité à la dépression diminue après la SMTr.
Major depression is common and debilitating. Unfortunately, a large proportion of patients do not benefit from existing psychotherapeutic and/or pharmacological interventions, or find available treatments intolerable.1 For treatment-resistant depression (TRD),2 non-invasive brain stimulation techniques have emerged as therapeutic alternatives. Among them, repetitive transcranial magnetic stimulation (rTMS) has the largest evidence base to date for TRD,3 with moderate-to-large antidepressant treatment effects, as compared with sham interventions, and relatively high levels of acceptability to patients.4–10
Although this efficacious treatment is approved in many jurisdictions, delivery remains capital intensive, and the time investiture for the patient and the provider are considerable. Although novel stimulation protocols, such as theta-burst stimulation4 and accelerated delivery,11,12 aim to minimise the overall costs and disruption to patients’ lives, these factors still remain significant. Moreover, not all patients experience clinically meaningful improvements (clinical response and clinical remission) in their depressive symptoms following rTMS treatment.4–10 Accordingly, the identification of readily implementable, low-cost predictors that can guide resource allocation is important. Personality and temperament are attractive in this regard, for they are well-studied in terms of their relation to depression and are relatively stable attributes that can be measured efficiently, economically, and non-invasively.13
Two of the most widely studied personality vulnerability factors for depression are neuroticism, stemming from the 5-factor model of personality14, and self-criticism, stemming from Blatt’s15–17 two-polarities model of personality and psychopathology. Individuals with elevated levels of neuroticism are characterised by being prone to anger, sadness, fearfulness, and negative emotions in general, and tend to be highly emotionally reactive to stress.14 Highly self-critical individuals are permeated with feelings of low self-worth and guilt, and are excessively concerned with maintaining social status and value in the eyes of important others.16,18,19 Both neuroticism and self-criticism have been cross-sectionally and longitudinally associated with subthreshold and pathological depressive symptoms13 and they predict the onset of depressive disorders.20 Accumulating evidence suggests that these factors may serve as clinically useful predictors of antidepressant response to psychotherapeutic and psychopharmacological interventions;21–23 albeit, with divergence in findings.23 Moreover, evidence has linked these traits to activity in the neural circuitry involved in depressive disorders,23,24 suggesting they may have utility as proxy biomarkers for treatment response.
To our knowledge, no research to date has examined the trait of self-criticism in relation to antidepressant treatment response to rTMS. However, both depression and self-criticism are associated with activation in the dorsolateral prefrontal cortex (DLPFC) during error processing25 and when monitoring internal emotional states24,26,27 (for a review, see Kopala-Sibley and Zuroff, 201927). Doerig et al. (2013)26 suggest that self-criticism, or perhaps coping with criticism, involves a top-down pathway between prefrontal regions and the limbic system, representing cognitive reappraisal strategies for dealing with evoked negative affect. As such, individuals high in self-criticism may particularly benefit from rTMS targeting the DLPFC as this may upregulate their emotional regulation and cognitive abilities. Alternatively, altering DLPFC connectivity may directly impact self-criticism; however, this has not been tested.
Similar to self-criticism, elevated neuroticism is associated with alterations in both DLPFC structure,28–30 resting state function,31,32 and activation during a working memory task.33 With data linking neuroticism to depression and to the DLPFC, neuroticism is a putative predictor of rTMS antidepressant response. Yet, we and others have failed to find support for its predictive utility in rTMS for depression.34,35,36 Instead, neuroticism appears to change as a result of rTMS, and in one sample this was proportional to the improvement in depressive symptoms.34 Though the utility of neuroticism in predicting clinical response to rTMS is questionable, the literature to date has predominantly underpowered samples. We therefore sought to synthesize the literature through a meta-analysis.
Here, we examine the predictive utility and stability of neuroticism and self-criticism in rTMS for TRD.
Methods
In this 4-week naturalistic study, we prospectively recruited 17 adults with TRD who were referred to the Vancouver General Hospital Neurostimulation Service for consideration for rTMS. All participants had a diagnosis of a major depressive episode, as confirmed by clinical interview and then by a score of ≥9 on the 21-item Hamilton Depression Rating Scale (HDRS-2137). The primary diagnosis was major depressive disorder (MDD) for 15 patients and bipolar disorder type II for 2 patients. Treatment resistance was determined as having failed at least 2 adequate courses of antidepressants, 2 mood stabilizers or antipsychotics, or having failed to tolerate pharmacological trials. All patients consented and completed the initial study package; however, 2 participants did not return the post-treatment study package.
This study was reviewed and approved by the University of British Columbia Clinical Research Ethics Board.
rTMS Treatment
rTMS treatment was delivered using a Magstim Rapid2 magnetic stimulator (Magstim Company Ltd, UK) connected to a figure-of-eight–shaped coil. Over 4 weeks, patients received 20 treatment sessions, composed of 3,000 pulses delivered at 120% of the resting motor threshold (10 Hz, trains of magnetic pulses for 4 sec, followed by 26-sec inter-train intervals) over the left DLPFC.9 The DLPFC was localised using the 6-cm “rule”. The resting motor threshold was determined weekly.
For 3 patients, high-frequency rTMS (HF-rTMS) of the left DLPFC was discontinued in favour of low-frequency rTMS (LF-rTMS) of the right DLPFC8 (1,800 pulses at 1 Hz) due to a lack of improvement. Sensitivity analyses were performed without these participants, which did not impact our findings.
Clinical Instruments
A psychiatrist completed medical and psychiatric history and safety screenings. The HDRS-21 was used as a clinician-rated measure of depressive symptoms. Participants also completed the self-report Sheehan Disability Scale, where 3 domains of function (work/school, social life, and family life/home responsibilities) were assessed. Scores of ≥5 on any of these domains reflect impaired function.
Personality Predictors
We used the Big Five Inventory to quantify 5-factor constructs of personality.38 This is one of the most widely used, well-validated measures of the Big Five Personality traits. This 44-item self-report questionnaire was completed at baseline and then again at the conclusion of 4 weeks of rTMS treatment. Internal consistency for neuroticism was α = 0.86 at baseline and 0.80 at follow-up.
We used the 66-item self-report Depressive Experiences Questionnaire (DEQ18) to quantify self-criticism. Patients completed this instrument at baseline and at the conclusion of 4 weeks of rTMS treatment. Self-criticism is conceptualised as a continuous—not categorical—dimension of individual difference.39 This scale has shown excellent test-retest reliability and displays high retest stability even following stressful life events.39 In this sample, internal consistency for self-criticism was 0.87 at baseline and 0.89 at follow-up.
Statistical Analyses
We performed statistical analyses using SPSS Statistics version 24 (IBM, Armonk, NY, USA), and factor weighting of the DEQ was completed using R (R Foundation for Statistical Computing). Internal consistency was measured with Cronbach’s alpha. We performed paired t-tests to examine changes in clinical variables pre- and post-rTMS treatment. We then computed multiple regression models testing the ability of prospectively assessed personality traits to predict antidepressant response, defined as the percentage change in HDRS-21 scores from baseline to the end of treatment. Models controlled for baseline depressive symptom severity, as assessed by the HDRS-21. Given the conceptual and empirical overlap between self-criticism and neuroticism,40 we performed separate regression analyses for each. For all results, we present both unstandardised (b) and standardised (β) betas. We also report R2 for the full model. Statistical significance was set at α < 0.05.
Meta-Analytic Procedure
This systematic review was registered with PROSPERO. We searched MEDLINE, EMBASE and PsycINFO from database inception to September 10, 2018. Our search of MEDLINE utilised the following search terms: ((neuroticism) AND ((depression) OR Major Depressive Disorder)) AND ((rTMS) OR Repetitive transcranial magnetic stimulation). Our search of EMBASE utilised the following search terms: (((neuroticism) OR neurosis) AND ((repetitive transcranial magnetic stimulation) OR transcranial magnetic stimulation) AND ((major depression) OR depression))). Our search of PsycINFO used the following search terms: ((depression) AND ((rTMS) OR repetitive transcranial magnetic stimulation) AND (neuroticism)).
Studies were included if they 1) included prospective assessment of neuroticism, 2) assessed neuroticism at the conclusion of treatment, and 3) included participants aged 18-75 years, with a major depressive episode treated with rTMS. Open-label and randomised sham-controlled trials were eligible for inclusion.
The primary outcome was a change in neuroticism from pre- to post-rTMS. Mean scores and standard deviations were extracted in duplicate (AM, SB) and any discrepancies were resolved by consensus together with MTB. To extract graphically depicted data, we utilised freely available digital extraction software (WebPlotDigitizer). As we principally identified non-randomised studies of interventions, we employed the Risk of Bias in Non-randomised Studies – of Interventions (ROBINS-I).
Analyses were performed using Comprehensive Meta-Analyses Version 2.0 (Biostat, Englewood, NJ, USA). Because true treatment effects are likely to vary between studies given different methodological characteristics, such as patient selection, degree of treatment resistance, or concomitant medications, we employed a random effects model.41 Standardised mean differences (SMD) were utilised for changes in neuroticism pre- and post-treatment.42 With respect to SMDs, we conservatively assumed a correlation coefficient of 0.7 if we were unable to obtain the correlation between pre- and post-treatment scores.43 To quantify statistical heterogeneity, we employed Q-statistics, I2 and τ2. Threshold statistical heterogeneity was deemed present if the P value for the Q-statistic was <0.1 or the I2 > 35%.44 Finally, we used Funnel Plots, Rosenthal’s Fail-Safe N,45 and Egger’s Regression Intercept46 to test for the presence of publication bias.44,47
Results
The sociodemographic and clinical characteristics of this sample at baseline are presented in Table 1. This was a moderately depressed sample with a high degree of anxiety and disability. Several patients (n = 6) refused ongoing pharmacological intervention, while polypharmacy was evident in the remainder (n = 6 had 3 or more psychotropics).
Table 1.
Sociodemographic and clinical characteristics
| Characteristic |
N (%) or Mean [SD] |
|---|---|
| Age | 49.59 [10.97] years |
| Sex | 8F/9M (47.1% v. 52.9%) |
| Married | 11 (64.7%) |
| Currently employed | 6 (35.2%) |
| Primary diagnosis | |
| Major depressive disorder | 15 (88.2%) |
| Bipolar disorder type II | 2 (11.7%) |
| HDRS-21 baseline | 16.88 [4.63] |
| Sheehan Disability Scale | 21.35 [6.28] |
| Medications | |
| None | 6 (35.2%) |
| SSRI Monotherapy | 1 (5.8%) |
| Antidepressant(s) + mood stabilizer | 3 (17.6%) |
| Antidepressant(s) + antipsychotic | 5 (29.4%) |
| Benzodiazepine or zopiclone | 8 (47.1%) |
| ≥3 Psychotropic medications | 6 (35.2%) |
rTMS significantly improved clinician-rated depressive symptoms (Figure 1A) and self-reported disability (Figure 1B). rTMS was associated with a decrease in neuroticism over the treatment period (Figure 1C) but did not change self-criticism (Figure 1D). Contrary to previous findings,34 the decrease in neuroticism in this sample was not in proportion to the decrease in depressive symptoms (b = −0.01, β(standardised) = −0.21, t = 1.15; not significant).
Figure 1.
Change in clinical and personality factors after 4 weeks of DLPFC rTMS treatment. (A) Change in depressive symptoms as measured by the 21-item Hamilton Depression Rating Scale (HDRS-21) (16.88 [4.63] v. 12.35 [5.86], t(16) = 4.82, P < 0.001). (B) Change in function as measured by the Sheehan Disability Scale (21.35 [6.28] v. 15.08 [8.07], t(16) = 3.91, P = 0.001). (C) Change in the personality domain Neuroticism (31.92 [5.39] v. 29.85 [4.68], t(13) = 2.32, P = 0.037). (D) Self-Criticism remained stable after 4 weeks of rTMS (0.51 [1.35] v. 0.56 [1.31], t(13) = −0.19, P = 0.85). (E) Change in depressive symptoms as a percentage of baseline was predicted by baseline self-criticism (F(2,16) = 3.88, P = 0.046).
We next examined baseline neuroticism and self-criticism as predictors of antidepressant response. In these analyses, we controlled for baseline HDRS-21 scores. High levels of self-criticism strongly predicted percent improvement in depressive symptoms in this sample (b = −0.11, β(standardised) = −0.57, t = −2.54, P = 0.023; Figure 1E). Self-criticism and baseline HDRS-21 scores explained 43.7% of the variance in percentage change in HDRS-21 scores. Neuroticism did not predict antidepressant response in this sample (b = 0.01, β(standardised) = 0.23, t = 0.81; not significant).
Meta-Analysis
Our literature search is detailed in Figure 2. Our systematic literature review identified 3 eligible studies,34,35,36 all of which were open-label, to which we added the data from the current study. The first study involved 8 patients (3F/5M) with MDD who received 15 to 25 daily treatments of 10 Hz stimulation of the left DLPFC at 100% RMT until they achieved clinical response.35 The second involved 14 patients (8F/6M) with MDD who received 20 daily treatments of 10 Hz stimulation of the left DLPFC at 120% RMT.34 The third involved 15 patients (12F/3M) who received 20 daily treatments of deep rTMS of the left DLPFC at 120% RMT.36 The second risk of bias according to ROBINS-I overall was low (Table 2). Thus, we included a total of 4 studies and 52 depressed participants who completed prospective characterisation of neuroticism before receiving DLPFC rTMS and then again at the conclusion of treatment.
Figure 2.
PRISMA systematic review and meta-analysis flow-chart
Table 2.
Risk of Bias in Included Studies (ROBINS-I).
| Bias Type | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Confounding | Participant selection process | Classification of interventions | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of the reported result | Overall bias |
| Spronk et al., 200835 | No | No | No | No | No | No | No | Low |
| Berlim et al., 201334 | No | No | No | No | Possible, Yes | No | No | Low |
| McGirr et al., 201448 | No | No | No | No | Possible, Yes | No | No | Low |
| Kopala-Sibley et al. (This study) | No | No | No | No | Possible, Yes | No | No | Low |
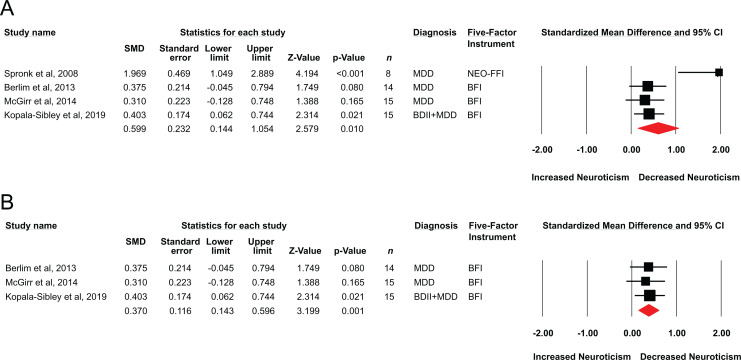
Overall, a standardised mean difference of 0.59 (95% CI = 0.14 to 1.05, z = 2.57, P = 0.010; Figure 3A) was observed, indicating a moderate treatment effect size of rTMS on neuroticism. The classic fail-safe N indicated that 21 null or missing studies would be required to render this finding statistically non-significant. Although Egger’s regression intercept failed to reveal statistical evidence of publication bias (t(2) = 2.98, P = 0.096), the funnel plot revealed an outlying study.35 There was evidence of statistical heterogeneity, as quantified using the Q statistic (Q-value = 11.03, df = 3, P = 0.012), by I2 (72.80), and by τ2 = 0.14, and this appeared to be driven by the same outlying study.35 We therefore repeated our analyses excluding data from Spronk et al.,35 and found a similar statistically significant effect (SMD = 0.37; 95% CI, 0.14 to 0.59, z = 3.19, P = 0.001; Figure 3B), with minimal evidence of statistical heterogeneity (Q-value = 0.10, df = 2, P = 0.94) by I2 (0.00) and by τ2 (0.00).
Figure 3.
(A) Standardised mean differences for change in neuroticism scores in prospective studies of DLPFC rTMS in major depression. (B) Sensitivity analysis showing illustrating standardised mean differences after excluding the data by Spronk et al., 2008.35
Discussion
This study investigated whether rTMS treatment for TRD can be predicted by personality vulnerabilities—neuroticism and self-criticism—and whether rTMS treatment results in changes in depression. To our knowledge, this is the first study to examine whether the trait of self-criticism predicts response to rTMS. Our analyses revealed that improvement in depressive symptoms is related to participants’ degree of self-criticism at baseline, and that self-criticism remains stable with rTMS. Meanwhile, rTMS results in decreases in neuroticism, which we then sought to confirm using a quantitative synthesis of the available literature. This meta-analysis revealed a moderate effect of rTMS on this personality trait in 4 open-label trials of rTMS in the treatment of TRD (n = 52). These results may have clinical utility, and further inform our understanding of the antidepressant mechanism of rTMS.
Our findings may also have implications both for our understanding of factors that predict rTMS treatment efficacy and for our understanding of the role of personality factors in TRD. Our findings are consistent with previous evidence that self-criticism predicts response to pharmacotherapy or psychotherapy.22,23 Moreover, whereas neuroticism and self-criticism have some conceptual overlap, these preliminary results suggest that self-criticism is more stable than neuroticism during rTMS treatment for depression and better able to predict antidepressant response. This is consistent with neuroimaging research, which has found that engaging in self-critical thoughts or receiving critical feedback to task performance is associated with activation in the lateral prefrontal brain regions, potentially indicating involvement of brain regions linked to error processing and the monitoring of internal emotional states.24,26 As such, these results may have clinical utility: pre-treatment screening for self-criticism may be useful in determining who is most likely to respond well to rTMS for depression; albeit, our findings require replication with a larger cohort before clinical implementation.
Where neuroticism is concerned, there is some evidence to suggest the stability of this personality dimension during pharmacological and psychotherapeutic intervention.48 Meanwhile, others have reported that this personality trait may change during antidepressant treatment,49 raising the possibility of state influences during acute depression or a modifiable vulnerability to TRD. To date, neuroticism has not demonstrated predictive utility of the antidepressant effects of rTMS; the conceptually similar character trait of harm avoidance has also failed to show predictive utility.50 Our analyses demonstrating that neuroticism is modifiable by rTMS are consistent with previous DLPFC rTMS studies, where neuroticism decreased as a function of treatment.34,35 Although we have not consistently found this effect,36 our meta-analysis reveals that rTMS has a robust effect on neuroticism, and that insufficient statistical power, together with natural variability, may account for previous inconsistencies. Neuroticism has been linked to the DLPFC both anatomically28–30 and functionally,31–33 and therefore it will be important for future studies to examine neuroticism as a proxy biomarker for pathology that responds to DLPFC rTMS.
Limitations
This study is limited by a small convenience sample. Although the results are also limited by the absence of a sham or another appropriate control group, the clinical utility of these findings is unaffected by this limitation. Our assessment of personality traits was during a major depressive episode and, while it is reflective of information that may be used for clinical decision making and prognostication, it is complicated by both trait- and state-dependent influences. In addition, although the study is prospective, the duration of follow-up is limited, and therefore the stability of clinical improvement is unclear. This is an important question for future research, given the literature linking high self-criticism with a vulnerability to depression and the stability of this trait at the conclusion of rTMS treatment. Conversely, it will be important to determine if changes in neuroticism, a factor that also affects vulnerability to depression, can confer protection against future depressive episodes.
Our systematic review and meta-analysis also have limitations; notably, a small number of trials and the absence of a sham-comparison group to determine specificity to DLPFC rTMS. Moreover, our group has contributed 3 of the 4 studies in this field and have done so each time with the same instrument, the Big Five Inventory. Replication by other groups and using other instruments is needed.
Conclusion
Understanding efficient, non-invasive, and economically viable factors that predict who will respond to rTMS in the treatment of refractory depression is vital for treatment selection and resource allocation. The personality trait of self-criticism may provide a useful, efficient, and cost-effective predictor of response to rTMS for TRD. Neuroticism does not appear to provide prognostic value, yet rTMS has a moderate-to-large treatment effect on vulnerability to depression. Further research is required to determine whether this confers protection against future episodes of depression.
Acknowledgements
We are very appreciative of Sharon Willan’s assistance with providing treatments and to participants for dedicating their time.
Data Access
Data is available on communication with the corresponding author.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: GBC was supported through Vancouver Coastal Health and the UBC Psychiatry Research Track Program.
ORCID iD: Alexander McGirr  https://orcid.org/0000-0002-8425-3958
https://orcid.org/0000-0002-8425-3958
References
- 1. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. [DOI] [PubMed] [Google Scholar]
- 2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23–29. [PubMed] [Google Scholar]
- 3. McGirr A, Berlim MT. Clinical usefulness of therapeutic neuromodulation for major depression: a systematic meta-review of recent meta-analyses. Psychiatr Clin North Am. 2018;41(3):485–503. [DOI] [PubMed] [Google Scholar]
- 4. Berlim MT, McGirr A, Rodrigues Dos Santos N, et al. Efficacy of theta burst stimulation (TBS) for major depression: an exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res. 2017;90:102–109. [DOI] [PubMed] [Google Scholar]
- 5. Berlim MT, Van den Eynde F, Daskalakis ZJ. High-frequency repetitive transcranial magnetic stimulation accelerates and enhances the clinical response to antidepressants in major depression: a meta-analysis of randomized, double-blind, and sham-controlled trials. J Clin Psychiatry. 2013;74(2):e122–e129. [DOI] [PubMed] [Google Scholar]
- 6. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614–623. [DOI] [PubMed] [Google Scholar]
- 7. Berlim MT, Van den Eynde F, Daskalakis ZJ. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychol Med. 2013;43(11):2245–2254. [DOI] [PubMed] [Google Scholar]
- 8. Berlim MT, Van den Eynde F, Jeff Daskalakis Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berlim MT, Van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225–239. [DOI] [PubMed] [Google Scholar]
- 10. Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74(2):143–152. [DOI] [PubMed] [Google Scholar]
- 11. Desmyter S, Duprat R, Baeken C, et al. Accelerated intermittent theta burst stimulation for suicide risk in therapy-resistant depressed patients: a randomized, sham-controlled trial. Front Hum Neurosci. 2016;10:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGirr A, Van den Eynde F, Tovar-Perdomo S, et al. Effectiveness and acceptability of accelerated repetitive transcranial magnetic stimulation (rTMS) for treatment-resistant major depressive disorder: an open label trial. J Affect Disord. 2015;173:216–220. [DOI] [PubMed] [Google Scholar]
- 13. Klein DN, Kotov R, Bufferd SJ. Personality and depression: explanatory models and review of the evidence. Annu Rev Clin Psychol. 2011;7:269–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costa PT, McCrae RR. The Neo Inventories as instruments of psychological theory In: The Oxford Handbook of The Five Factor Model, Widiger TA, ed. Oxford: Oxford University Press; 2017:11–37. [Google Scholar]
- 15. Blatt SJ. Experiences of Depression: Theoretical, Research and Clinical Perspectives. Washington, DC: American Psychological Association; 2004. [Google Scholar]
- 16. Blatt SJ, Luyten P. A structural-developmental psychodynamic approach to psychopathology: two polarities of experience across the life span. Dev Psychopathol. 2009;21(3):793–814. [DOI] [PubMed] [Google Scholar]
- 17. Blatt SJ, Zuroff DC. Interpersonal relatedness and self-definition: two prototypes for depression. Clin Psychol Rev. 1992;12:527–562. [Google Scholar]
- 18. Blatt SJ, D’Afflitti JP, Quinlan DM. Experiences of depression in normal young adults. J Abnorm Psychol. 1976;85(4):383–389. [DOI] [PubMed] [Google Scholar]
- 19. Kopala-Sibley DC, Zuroff DC. The developmental origins of personality factors from the self-definitional and relatedness domains: a review of theory and research. Rev Gen Psychol. 2014;18(3):137. [Google Scholar]
- 20. Kopala-Sibley DC, Klein DN, Perlman G, et al. Self-criticism and dependency in female adolescents: Prediction of first onsets and disentangling the relationships between personality, stressful life events, and internalizing psychopathology. J Abnorm Psychol. 2017;126(8):1029–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bulmash E, Harkness KL, Stewart JG, et al. Personality, stressful life events, and treatment response in major depression. J Consult Clin Psychol. 2009;77(6):1067–1077. [DOI] [PubMed] [Google Scholar]
- 22. Marshall MB, Zuroff DC, McBride C, et al. Self-criticism predicts differential response to treatment for major depression. J Clin Psychol. 2008;64(3):231–244. [DOI] [PubMed] [Google Scholar]
- 23. Rector NA, Bagby M, Zindel VS, et al. Self-criticism and dependency in depressed patients treated with cognitive therapy or phamacotherapy. Cognit Therapy Res. 2000;24(5):571–584. [Google Scholar]
- 24. Longe O, Maratos FA, Gilbert P, et al. Having a word with yourself: neural correlates of self-criticism and self-reassurance. Neuroimage. 2010;49(2):1849–1856. [DOI] [PubMed] [Google Scholar]
- 25. Wittfoth M, Schardt DM, Fahle M, et al. How the brain resolves high conflict situations: double conflict involvement of dorsolateral prefrontal cortex. Neuroimage. 2009;44(3):1201–1209. [DOI] [PubMed] [Google Scholar]
- 26. Doerig N, Schlumpf Y, Spinelli S, et al. Neural representation and clinically relevant moderators of individualised self-criticism in healthy subjects. Soc Cogn Affect Neurosci. 2014;9(9):1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kopala-Sibley DC, Zuroff DC. The self and depression: four psychological theories and their potential neural correlates. J Pers. 2019. DOI: 10.1111/jopy.12456. [DOI] [PubMed] [Google Scholar]
- 28. Schultz CC, Warziniak H, Koch K, et al. High levels of neuroticism are associated with decreased cortical folding of the dorsolateral prefrontal cortex. Eur Arch Psychiatry Clin Neurosci. 2017;267(6):579–584. [DOI] [PubMed] [Google Scholar]
- 29. Wright CI, Williams D, Feczko E, et al. Neuroanatomical correlates of extraversion and neuroticism. Cereb Cortex. 2006;16(12):1809–1819. [DOI] [PubMed] [Google Scholar]
- 30. Kapogiannis D, Sutin A, Davatzikos C, et al. The five factors of personality and regional cortical variability in the Baltimore longitudinal study of aging. Hum Brain Mapp. 2013;34(11):2829–2840.22610513 [Google Scholar]
- 31. Hsu WT, Rosenberg MD, Scheinost D, et al. Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc Cogn Affect Neurosci. 2018;13(2):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang R, Calhoun VD, Zuo N, et al. Connectome-based individualized prediction of temperament trait scores. Neuroimage. 2018;183:366–374. [DOI] [PubMed] [Google Scholar]
- 33. Dima D, Friston KJ, Stephan KE, et al. Neuroticism and conscientiousness respectively constrain and facilitate short-term plasticity within the working memory neural network. Hum Brain Mapp. 2015;36(10):4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berlim MT, McGirr A, Beaulieu MM, et al. Are neuroticism and extraversion associated with the antidepressant effects of repetitive transcranial magnetic stimulation (rTMS)? An exploratory 4-week trial. Neurosci Lett. 2013;534:306–310. [DOI] [PubMed] [Google Scholar]
- 35. Spronk D, Arns M, Bootsma A, et al. Long-term effects of left frontal rTMS on EEG and ERPs in patients with depression. Clin EEG Neurosci. 2008;39(3):118–124. [DOI] [PubMed] [Google Scholar]
- 36. McGirr A, Van den Eynde F, Chachamovich E, et al. Personality dimensions and deep repetitive transcranial magnetic stimulation (DTMS) for treatment-resistant depression: a pilot trial on five-factor prediction of antidepressant response. Neurosci Lett. 2014;563:144–148. [DOI] [PubMed] [Google Scholar]
- 37. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. John OP, Srivastava S. The big-five trait taxonomy: history, measurement, and theoretical perspectives In: Pervin LA, John OP, eds. Handbook of Personality: Theory and Research. Vol 2 New York: Guilford Press; 1999:102–138. [Google Scholar]
- 39. Zuroff DC, Mongrain M, Santor DA. Conceptualizing and measuring personality vulnerability to depression: comment on Coyne and Whiffen (1995). Psychol Bull. 2004;130(3):489–511; discussion 512–422. [DOI] [PubMed] [Google Scholar]
- 40. Zuroff DC. Depressive personality styles and the five-factor model of personality. J Pers Assess. 1994;63(3):453–472. [DOI] [PubMed] [Google Scholar]
- 41. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. Bmj. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 42. Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21(11):1575–1600. [DOI] [PubMed] [Google Scholar]
- 43. Rosenthal R. Meta-Analytic Procedures for Social Research. Newbury Park, CA: Sage Publications; 1993. [Google Scholar]
- 44. Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis. West Sussex: Wiley & Sons Ltd; 2009. [Google Scholar]
- 45. Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638–641. [Google Scholar]
- 46. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta-Analysis. New York, U.S: Russell Sage Foundation Publications; 2009. [Google Scholar]
- 48. De Fruyt F, Van Leeuwen K, Bagby RM, et al. Assessing and interpreting personality change and continuity in patients treated for major depression. Psychol Assess. 2006;18(1):71–80. [DOI] [PubMed] [Google Scholar]
- 49. Costa PT, Jr, Bagby RM, Herbst JH, et al. Personality self-reports are concurrently reliable and valid during acute depressive episodes. J Affect Disord. 2005;89(1–3):45–55. [DOI] [PubMed] [Google Scholar]
- 50. Baeken C, Desmyter S, Duprat R, et al. Self-directedness: an indicator for clinical response to the HF-rTMS treatment in refractory melancholic depression. Psychiatry Res. 2014;220(1–2):269–274. [DOI] [PubMed] [Google Scholar]