Abstract
Objective:
To review the current evidence for efficacy of cannabidiol in the treatment of mood disorders.
Methods:
We systematically searched PubMed, Embase, Web of Science, PsychInfo, Scielo, ClinicalTrials.gov, and The Cochrane Central Register of Controlled Trials for studies published up to July 31, 2019. The inclusion criteria were clinical trials, observational studies, or case reports evaluating the effect of pure cannabidiol or cannabidiol mixed with other cannabinoids on mood symptoms related to either mood disorders or other health conditions. The review was reported in accordance with guidelines from Preferred Reporting Items for Systematic reviews and Meta-Analyses protocol.
Results:
Of the 924 records initially yielded by the search, 16 were included in the final sample. Among them, six were clinical studies that used cannabidiol to treat other health conditions but assessed mood symptoms as an additional outcome. Similarly, four tested cannabidiol blended with Δ-9-tetrahydrocannabinol in the treatment of general health conditions and assessed affective symptoms as secondary outcomes. Two were case reports testing cannabidiol. Four studies were observational studies that evaluated the cannabidiol use and its clinical correlates. However, there were no clinical trials investigating the efficacy of cannabidiol, specifically in mood disorders or assessing affective symptoms as the primary outcome. Although some articles point in the direction of benefits of cannabidiol to treat depressive symptoms, the methodology varied in several aspects and the level of evidence is not enough to support its indication as a treatment for mood disorders.
Conclusions:
There is a lack of evidence to recommend cannabidiol as a treatment for mood disorders. However, considering the preclinical and clinical evidence related to other diseases, cannabidiol might have a role as a treatment for mood disorders. Therefore, there is an urgent need for well-designed clinical trials investigating the efficacy of cannabidiol in mood disorders.
Keywords: cannabidiol, cannabinoids, mood disorders, affective disorders, major depressive disorder, depressive disorders, bipolar disorder, mania
Abstract
Objectif:
Examiner les données probantes actuelles sur l’efficacité du cannabidiol dans le traitement des troubles de l’humeur.
Méthodes:
Nous avons systématiquement recherché dans PubMed, Embase, Web of Science, PsychInfo, Scielo, ClinicalTrials.gov et CENTRAL des études publiées jusqu’au 31 juillet 2019. Les critères d’inclusion étaient des essais cliniques, des études par observation ou des rapports de cas qui évaluent l’effet du cannabidiol pur ou du cannabidiol mélangé avec d’autres cannabinoïdes sur les symptômes de l’humeur liés aux troubles de l’humeur ou à d’autres états de santé. La revue a été rédigée conformément aux lignes directrices du protocole PRISMA.
Résultats:
Sur les 924 études d’abord produites par la recherche, 16 ont été incluses dans l’échantillon final. Sur celles-ci, six étaient des études cliniques qui utilisaient le cannabidiol pour traiter d’autres états de santé mais qui évaluaient les symptômes de l’humeur comme résultat additionnel. De même, quatre études testaient le cannabidiol mélangé avec du Δ-9-tétrahydrocannabinol dans le traitement d’états de santé généraux et évaluaient les symptômes affectifs comme résultats secondaires. Deux étaient des rapports de cas testant le cannabidiol. Quatre études étaient des études par observation qui évaluaient l’utilisation du cannabidiol et ses corrélats cliniques. Cependant, aucun essai clinique n’investiguait l’efficacité du cannabidiol, spécifiquement dans les troubles de l’humeur, ni n’évaluait les symptômes affectifs comme résultat principal. Bien que certains articles laissent présager les avantages du cannabidiol pour traiter les symptômes dépressifs, la méthodologie variait à plusieurs égards et le niveau des données probantes ne suffit pas à l’indiquer comme traitement des troubles de l’humeur.
Conclusions:
Les données probantes actuelles ne suffisent pas pour recommander le cannabidiol comme traitement des troubles de l’humeur. Toutefois, compte tenu des données probantes précliniques et cliniques liées à d’autres maladies, le cannabidiol pourrait jouer un rôle comme traitement des troubles de l’humeur. Il y a donc un urgent besoin d’essais cliniques bien conçus qui recherchent l’efficacité du cannabidiol dans les troubles de l’humeur.
Introduction
Mood disorders are common psychiatric conditions, with major depressive disorder (MDD) reaching a lifetime prevalence of up to 14.6%,1 affecting more than 300 million people worldwide,2 while bipolar disorder reaches a prevalence of 4.4%, including subthreshold cases.3 Both are chronic mental disorders that usually start early in life and follow a chronic course, commonly resulting in significant functional impairment and are associated with premature death due to comorbid medical illness and suicide.4–6 Both conditions are also among the leading causes of years lived with disability directly and of years of life lost due to suicide.7–9 Long-term treatment strategies are, therefore, the mainstay of management. However, especially in the case of bipolar disorder, the most efficacious medications are associated with adverse effects, which can lead to noncompliance with treatment and poor clinical and functional outcomes.10,11 In this context, new treatments with novel mechanisms of action and good tolerability profile which are suitable for long-term use are urgently needed.
The plant Cannabis sativa contains more than 600 compounds, among which 100 share similar structures and are known as cannabinoids.12 Cannabidiol is one of these phytocannabinoids; preclinical and clinical studies have suggested its therapeutic utility in brain disorders. While Δ-9-tetrahydrocannabinol (THC), the main active component of the plant, is related to psychotomimetic effects, inducing anxiety and psychotic symptoms, cannabidiol can reverse these effects due to its impact on CB1 and 5-hydroxytryptamine (5-HT1A) receptors.13,14 Recent clinical studies have shown the anticonvulsant,15,16 antipsychotic,17,18 and anxiolytic19–21 properties of cannabidiol; preclinical studies have also shown antidepressant-like effects in several animal models of depression.22–25 Acting in the endocannabinoid system, cannabidiol has a unique pharmacological profile with promising results in neuropsychiatric disorders.
Briefly, the endocannabinoid system consists of the CB1 and CB2 receptors and their endogenous ligands. While the CB1 receptors are primarily present in the cortex, basal ganglia, hippocampus, and cerebellum,26 the CB2 receptors are less prevalent in the central nervous system but can be found in microglia and vascular elements.27,28 The endocannabinoid system is a neuromodulator in both the central and peripheral nervous systems influencing several biological functions including mood, anxiety, appetite, sleep, memory processing, pain sensation, and immune functions.29,30 There is evidence that schizophrenia is associated with alterations in the endocannabinoid system,17,31,32 which could explain favorable results with cannabidiol in some clinical trials in patients with schizophrenia.17,18 Furthermore, with receptors and ligands present in neurons and immune cells, the endocannabinoid system is especially interesting in the context of mood disorders since both MDD and bipolar disorder are illnesses whose pathophysiology involves changes in the brain33–37 and the immune system.36–39
Cannabidiol has been studied in several animal models of depression such as forced swimming test,23,40,41 learned helplessness paradigm,25 olfactory bulbectomy mouse model,22 and genetic models24,42 showing promising results in improving the depressive-like behaviors. Also, it shows antidepressant-like effects in models that are interesting for clinical purposes, for example, models of chronic stress22 and anhedonia.24,42 Its antidepressant effects have been shown not only when compared with vehicle solutions but also with active and established antidepressant medications, such as imipramine and fluoxetine.23,41,43 Moreover, these studies have shown that cannabidiol exerts acute and subchronic and chronic effects and can maintain antidepressant-like effects.22,25,40,43 In contrast, the only published study investigating the effects of cannabidiol in an animal model of mania induced by d-amphetamine failed to show improvement in locomotion, although it showed neuroprotective effects increasing brain-derived neurotrophic factor (BDNF) expression in the hippocampus.44
Regarding its psychophysiological basis, the preclinical studies have suggested that the cannabidiol antidepressant-like effect is probably mediated by indirect activation of CB1 receptors,45 activation of 5-HT1A receptors,22,23,45 and increased serotonin levels in the central nervous system but are not related to noradrenergic levels.22,41 For example, in one study, the administration of cannabidiol into the ventral medial prefrontal cortex (vmPFC) induced antidepressant-like effects possibly through indirect activation of CB1 and 5-HT1A receptors.45 Another study with in vivo microdialysis revealed that the administration of cannabidiol significantly enhanced serotonin and glutamate levels in vmPFC, with the increase in neurotransmitters levels occurring immediately after the first injection of cannabidiol.22 Moreover, adaptive changes in pre- and postsynaptic 5-HT1A receptor functionality were also found after chronic cannabidiol exposure.22 Some studies have also shown effects on neuroplasticity. For instance, a study showed that chronic administration of cannabidiol increased BDNF levels in the amygdala of rats treated for depression-like symptoms.43 Another study showed that the acute antidepressant effects were associated with increased BDNF levels in both medial prefrontal cortex (mPFC) and the hippocampus, as well as increased spine density in the mPFC.25
In spite of the clinical and preclinical evidence described earlier, a literature search indicated that there is no systematic review on clinical studies of cannabidiol and mood disorders to date. Therefore, the objective of this study is to systematically review the available literature and clarify the current level of evidence for efficacy of cannabidiol in the treatment of mood disorders.
Methods
Search Strategy and Identification of Eligible Articles
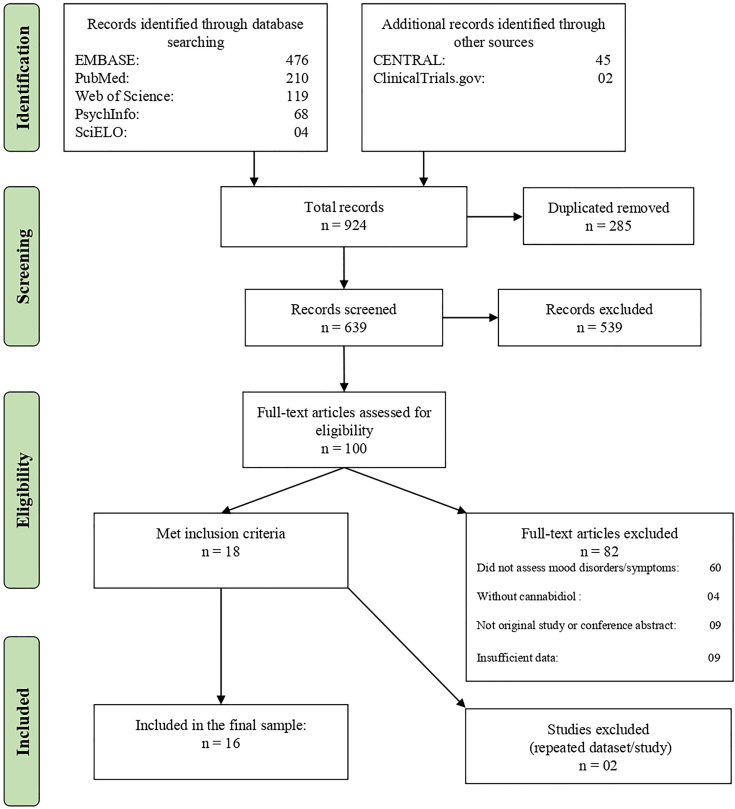
This systematic review has been reported following guidance from Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement46 and was registered at International Prospective Register of Systematic Reviews (PROSPERO) (CRD: 42019119766). The PRISMA flowchart is given in Figure 1, and the PRISMA checklist can be found in the Supplementary Materials. The authors searched PubMed, Web of Science, Embase, and PsychInfo databases for articles published in any language up to July 31, 2019, using the following search terms: cannabidiol AND (mania OR hypomania OR depression OR euphoria OR irritability OR irritable mood OR dysphoria OR anhedonia OR bipolar disorder OR bipolar affective disorder OR bipolar I disorder OR bipolar II disorder OR manic depressive OR manic depression OR major depression OR major depressive disorder OR depressive disorders OR depressive episode OR recurrent depressive disorder OR dysthymia OR dysthymic disorder OR cyclothymia OR cyclothymic disorder OR mood disorder OR mood disorders OR affective disorder OR affective disorders OR affective symptoms OR emotions). A complementary search using the same terms was performed on ClinicalTrials.gov and The Cochrane Central Register of Controlled Trials. The Medical Subject Headings terms were used in PubMed. The reference lists of the included studies were also examined in order to find more results.
Figure 1.
Flowchart of the review process and study selection.
Selection Criteria
Two reviewers (JVP and GS) independently screened and selected the studies, and a third investigator (LNY) made the final decision in cases of disagreement. The inclusion criteria were double-blind randomized clinical trials (RCTs) with and without placebo conditions, open-label clinical trials, observational studies (longitudinal or cross-sectional), or case series that evaluated the effect of pure cannabidiol or cannabidiol mixed with other cannabinoids on affective symptoms. These symptoms could be discrete hypomanic, manic, mixed, or depressive episodes in mood disorders or mood symptoms associated with other psychiatric illnesses and neuropsychiatric disorders. The exclusion criteria were studies not reporting mood symptoms, not using cannabidiol and nonoriginal studies (reviews, editorials, book chapters). The authors did not specifically search gray literature. The methodological quality appraisal of included clinical studies was carried out using the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials, while the quality of the observational studies was evaluated using the Newcastle-Ottawa Quality Assessment Scale.47
Results
Figure 1 summarizes the selection process according to the PRISMA protocol. The literature search identified a total of 924 records, but, after the exclusion of duplicates, a final sample of 639 remained. Then, the abstracts and their titles were screened independently, and a total of 539 abstracts were excluded after initial screening. Finally, 100 full texts were reviewed, and 84 were excluded. A final sample of 18 full texts met the inclusion criteria. However, only 16 were included in the final sample because two papers were from the same study or shared the same data set of other included articles. In these cases, the papers with the largest sample size were included. The characteristics of the studies included are described in Tables 1 and 2. Seven papers were clinical studies that used cannabidiol to treat other medical and psychiatric conditions but assessed mood symptoms as an additional outcome. Five studies tested medical cannabis, nabiximols, or other blends of cannabinoids that included cannabidiol in the treatment of medical or psychiatric conditions and assessed affective symptoms as secondary outcomes. Among the clinical studies with cannabidiol, two were case reports or case series using cannabidiol to treat psychiatric disorders. Four were surveys or observational studies that assessed the cannabidiol use and its clinical correlates.
Table 1.
Interventional Studies.
| Study | Intervention | CBD Dose (Per Day) | Health Condition | Design | Outcomes | Sample (Total) | Main Findings—Affective Symptoms | Adverse Effects and Other Findings |
|---|---|---|---|---|---|---|---|---|
| Wade et al. (2004) | CBD + THC | 2.5 to 120 mg | Multiple sclerosis | RCT | BDI | 160 | No significant differences | Most common adverse effect: dizziness (active: 32.5%, placebo: 12.5%), application site discomfort (active: 26%, placebo: 22.5%) |
| Notcutt et al. (2004) | CBD, THC or CBD + THC | 2.5 mg+ | Chronic pain | Open-label/RCT | BDI and GHQ-28 | 24 | Reduction in depression from a median of 16 at baseline to 8 at the end of the study | Most common adverse effect: Dry mouth, drowsiness, dysphoria/euphoria |
| Aragona et al. (2009) | CBD + THC | 2.5 mg+ | Multiple sclerosis | RCT | SCL-90-R | 17 | No significant differences | Blood THC levels positively correlated with SCL-90-R scores (interpersonal sensitivity, aggressive behavior, paranoid tendencies) |
| Zuardi et al. (2010) | CBD | 600 to 1,200 mg | Bipolar disorder—mania | Case series | YMRS | 02 | CBD monotherapy showed no therapeutic benefits to maintaining the achievements of the association with olanzapine. | No adverse effects were observed. |
| Martin-Santos et al. (2012) | CBD or THC | 600 mg | Healthy individuals | RCT | ARCI 49 | 16 | No significant differences in euphoria or dysphoria | THC was associated with anxiety, dysphoria, positive psychotic symptoms, physical and mental sedation, subjective intoxication, increased heart rate |
| Portenoy et al. (2012) | CBD + THC | 2.5 mg+ | Chronic pain | RCT | MADRS | 360 | No significant differences | Pain was reduced with low and medium doses. Only high doses had more adverse effects than placebo |
| Crippa et al. (2013) | CBD | 300 to 600 mg | Cannabis use disorder | Case report | BDI | 01 | Improvement in depressive symptoms associated with cannabis withdrawal syndrome | Improvement in withdrawal symptoms, discomfort, anxiety, and abstinence |
| Morgan et al. (2013) | CBD | 400 μg+ | Nicotine addiction | RCT | MRS | 24 | No significant differences | Reduction in craving and cigarettes smoked (∼40%) |
| Allsop et al. (2014) | CBD + THC | 5 to 80 mg | Cannabis use disorder | RCT | DASS | 51 | Significantly associated with reductions in withdrawal-related irritability and depressive symptoms | Reduction in withdrawal intensity including cravings, sleep disturbance, anxiety, appetite loss, physical symptoms, and restlessness. |
| Jadoon et al. (2016) | CBD, THCV or CBD + THCV | 200 mg | Diabetes type II | RCT | BDI | 62 | Only the group of 20:1 CBD/THCV had a significant improvement in depressive symptoms | THCV decrease fasting plasma glucose levels, pancreatic β-cell function, adiponectin, and apolipoprotein A. CBD decreased resistin and improved glucose-dependent insulinotropic peptide |
| Solowij et al. (2018) | CBD | 200 mg | Cannabis use | Open-label | BDI | 20 | Significant reduction in depressive symptoms after the treatment | No cognitive impairment or side effects but reduced euphoria when smoking cannabis. Reduced psychotic-like symptoms. Improvement in attentional switching, verbal memory, and memory |
| Gaston et al. (2019) | CBD | 5 mg/kg/day to 50 mg/kg/day | Treatment-resistant epilepsy | Open-label | POMS | 53 | Improvement in mood symptoms | Improvement in quality of life. |
Note. ARCI-49 = Addiction Research Centre Inventory 49 item; BDI = Beck Depression inventory; CBD = cannabidiol; DASS = Depression, Anxiety, and Stress Scale; GHQ-28 = General Health Questionnaire 28; MADRS = Montgomery-Åsberg Depression Rating Scale; MRS = 16-item Mood Rating Scale; POMS = Profile of Moods States; RCT = randomized clinical trial; SCL-90-R = Symptom Checklist-90-Revised; THC = Δ-9-tetrahydrocannabinol; THCV = tetrahydrocannabivarin; YMRS = Young Mania Rating Scale.
Table 2.
Observational Studies.
| Study | CBD Concentration | Design | Sample | Outcomes | Main Findings—Affective Symptoms | Adverse Effects and Other Findings |
|---|---|---|---|---|---|---|
| Schubart et al. (2011) | Cannabis (CBD concentration based on the type of cannabis used) | Cross-sectional | 1,877 | CAPE | Depressive symptoms were smaller in the high CBD group than in the high THC group, but the difference was not significant. | Association between using products with low CBD content and higher levels of psychotic symptoms. |
| Morgan et al. (2012) | Cannabis (CBD measured in hair samples) | Cross-sectional | 120 | BDI | Users of “high THC cannabis,” with no or less CBD than “low THC cannabis,” had significantly higher depression scores than the low THC group | CBD improved the psychotic-like of cannabis. THC negatively impacted the memory and psychological well-being. |
| Corroon and Phillips (2018) | Cannabidiol | Cross-sectional | 2,409 | Reasons for CBD use and self-report treatment efficacy | 380 used for treatment of depression. Among them, 250 users reported that CBD treated “very well by itself” or “moderately well by itself” the depressive symptoms. | Adverse effects in 30.8% of the sample. Most common: dry mouth (11.12%), euphoria (6.43%), hunger (6.35%), and red eyes (2.74%) |
| Cuttler et al. (2018) | Cannabis (CBD concentration based on the type of cannabis used) | Longitudinal | 2,057 | Change in mood after consumption of cannabis through a self-report app. | Significant reduction in ratings of depression after using cannabis relatively low levels of THC and high levels of CBD. No significant perceived tolerance effects on depression. | Reduction in anxiety and stress |
Note. BDI = Beck Depression Inventory; CAPE = Community Assessment of Psychic Experience; CBD = cannabidiol; THC = Δ-9-tetrahydrocannabinol.
Interventional Studies With Cannabidiol
Placebo-controlled trials
A randomized, double-blind, cross-over, placebo-controlled trial with 16 healthy male participants observed the pharmacological effects of both THC and cannabidiol separately in the same subjects. They all received THC 10 mg, cannabidiol 600 mg, and placebo in three individual sessions at 1 month intervals. Physiological parameters, as well as symptoms ratings, were collected before and at 1, 2, and 3 hr after drug administration. Cannabidiol was not different from placebo in physiological measurements and clinical rating scales such as Visual Analogue Mood Scale,48 State Anxiety Inventory (STAI),49 Positive and Negative Syndrome Scale,50 and Addiction Research Centre Inventory-49.51 In contrast, THC increased anxiety, sedation, dysphoria, positive psychotic symptoms, negative psychotic symptoms, general psychopathology, and heart rate.52
A 13-week randomized, double-blind, placebo-controlled, parallel-group study examined the effect of cannabidiol and/or D9-tetrahydrocannabivarin (THCV) on lipid and glucose metabolism in 62 patients with type II diabetes. Secondary measurements included the Beck Depression Inventory-II (BDI-II),53 which showed no difference from the placebo group except for the 20:1 cannabidiol/THCV compound, which had a statistically significant, though mild, increase in depressive symptoms, with an estimated mean difference on the BDI-II of 4.77 (P < 0.01).54 The other compounds tested in this study were pure cannabidiol, pure THCV, and a blend of 1:1 cannabidiol/THCV, but the BDI-II mean changes from screening to the end of treatment were not statistically significantly different from placebo.
A pilot randomized double-blind placebo-controlled study examined the effect of inhaled cannabidiol 400 μg dissolved in absolute ethanol (≈5%) or placebo (ethanol alone) in 24 tobacco smokers wanting to quit. The 16-item Mood Rating Scale55 was used at baseline and after the 7-day trial period. No significant difference was measured in depressive symptoms or sedation. Both conditions showed a marked decrease in anxiety scores over time, but there was no significant effect of condition.56
Open-label trials and case series
A pragmatic open-label clinical trial assessed the effects of cannabidiol 200 mg/day for 12 weeks on psychiatric symptoms and cognitive functioning in 20 chronic cannabis users. The scores on BDI,57 STAI, Community Assessment of Psychic Experiences (CAPE),58 global and social functioning (Global Assessment of Functioning [GAF], Social and Occupational Functioning Assessment Scale [SOFAS]),59 verbal learning (Rey Auditory Verbal Learning Test),60 and attention switching scales (from the Cambridge Neuropsychological Test Automated Battery)61 were compared between post-treatment and baseline. The severity of depressive symptoms as assessed by BDI was significantly lower after treatment, compared to the baseline scores (P = 0.017). In addition, the frequency of positive psychotic-like symptoms (P = 0.025) and the distress associated with positive symptoms (P = 0.025) on CAPE were both significantly reduced, especially in patients with dependence for cannabis. Notably, state anxiety according to STAI was increased after treatment (P < 0.015). Cognitive functioning also improved, and participants reported a reduction in cannabis-induced euphoria during the trial.62
A 1-year follow-up open-label study included 53 participants with treatment-resistant epilepsy and assessed the effect of cannabidiol (starting at 5 mg/kg/day and titrated to 50 mg/kg/day) on the quality of life using the Quality of Life in Epilepsy–89 (QOLIE-89).63,64 As an additional outcome, the Profile of Moods States (POMS) was included.65 The POMS is a 65-item validated questionnaire commonly used to assess mood symptoms in epilepsy clinical trials. It consists of six subscales, among which the Depression/Dejection subscale correlates with BDI.66 The results showed improvement in mood symptoms with a significant reduction in POMS scores (9.7, P = 0.01), as well as there was an improvement in the QOLIE-89 scores. Furthermore, in the regression model, POMS scores at follow-up was the only variable that had an independent and negative association with QOLIE-89 at follow-up (b = −0.325, SE = 0.134, P = .020), indicating mood problems being associated with lower quality of life in this population.64
A case study described two patients with bipolar I disorder without comorbidity, hospitalized for a manic episode with psychotic symptoms, who were treated with cannabidiol (initially 600 mg/day, then 900 mg/day, and 1200 mg/day).67 The first patient received cannabidiol plus olanzapine for 2 weeks. After this period, olanzapine was withdrawn to observe whether the cannabidiol alone would maintain the improvement but cannabidiol alone did not maintain the improvements achieved through the combination with olanzapine. The second patient received monotherapy with cannabidiol but had no symptoms improvement with any dose. In both cases, the symptoms were evaluated through the Young Mania Rating Scale68 and Brief Psychiatric Rating Scale,69 and adverse effects were evaluated with the Udvalg for Kliniske Undersogelser Scale.70 Tolerability was very good, and no side effects were reported, even with doses as high as 1200 mg/day.67
In another case study, a 19-year-old woman with cannabis dependence (according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria) and no other psychiatric comorbidities received a 10-day regime of cannabidiol (300 mg on day 1 and 11; 600 mg, days 2 to 10) to treat cannabis withdrawal syndrome. The patient was monitored with the BDI, Beck Anxiety Inventory,71 Withdrawal Discomfort Score, and Marijuana Withdrawal Symptom Checklist.72 This approach led to a quick and progressive reduction in withdrawal, depression, anxiety, and dissociative symptoms.73
Interventional Studies With Medical Cannabis, Nabiximols, and Other Blends of Cannabinoids
Placebo-Controlled Trials
A double-blind RCT randomized 51 inpatients treated for cannabis dependence with nabiximols (86.4 mg THC and 80 mg cannabidiol) or placebo for 6 days to alleviate withdrawal symptoms. The symptoms were measured with the Cannabis Withdrawal Scale74; with a subscale from the Depression, Anxiety, and Stress Scale75; and with the Anxiety Sensitivity Index–Revised,76 as well as retention from withdrawal and adverse events. Adjusting for covariates, nabiximols use was significantly associated with reductions in withdrawal-related irritability and depressive symptoms. Positive effects on sleep, anxiety, appetite loss, physical symptoms, and restlessness were also observed but less pronounced and nonsignificant. Treatment participation was longer in the nabiximols group but self-reported cannabis use, cannabis-related problems, cannabis dependence, and adverse events frequency and severity did not differ from placebo.77
An initially open-label “N of 1” trial, which then converted to a randomized, double-blind, placebo-controlled, crossover study of 34 patients, examined the effects of sublingually applied medicinal cannabis extracts for chronic pain. Patients received either THC 2.5 mg, cannabidiol 2.5 mg, or a 1:1 mixture (cannabidiol 2.5 mg and THC 2.5 mg) over a 12-week period. Each extract contained >95% of the specified cannabinoid, the remainder being a mixture of other plant compounds. Twenty-four patients completed the crossover period without cannabinoid rescue medication and, in this group, there was an improvement in depression severity from a median BDI score of 16 at baseline to 8 at the end of the study. Furthermore, there were significant improvements in pain symptoms measured with the visual analogue scale with THC alone and a mixture of THC and cannabidiol groups. In addition, these patients showed improvements in the total scores of the General Health Questionnaire 2878 as well as in each subdomain of this instrument (Severe Depression, Anxiety, and Insomnia, Somatic Symptoms and Social Dysfunction).79
In an 8-week crossover, double-blind, placebo-controlled, randomized controlled trial, 17 patients with multiple sclerosis were given nabiximols (THC 2.7 mg and cannabidiol 2.5 mg) to evaluate psychopathological and cognitive effects.80 The Symptom Checklist–90 Revised81 showed no differences in depressive symptoms between the active and the placebo period. In addition, no correlations were found between the plasma levels of cannabidiol and the depressive symptoms.80
Another randomized, double-blind, placebo-controlled, parallel-group trial examined 160 patients with multiple sclerosis who were given cannabis-based medicinal extracts with a THC/cannabidiol ratio of 1:1 (max dose 120 mg/day each) over a 6-week period. Among other outcome measures, the BDI showed no significant difference between the placebo and the cannabis-based medicinal extracts group after the trial.82
A large randomized, double-blind, placebo-controlled, graded-dose, parallel-group trial with 360 opioid-treated cancer patients with poorly controlled chronic pain observed the effects of nabiximols (cannabis extract containing 2.7 mg of THC and 2.5 mg of cannabidiol) on their pain.83 Patients were excluded from study participation if they had a major psychiatric disorder. The patients were split into three dosage groups and assessed after 4 weeks. There were no significant differences between groups regarding depressive symptoms assessed with the Montgomery-Åsberg Depression Rating Scale (MADRS).83,84
Surveys and Observational Studies
An Internet survey aimed to investigate the reported reasons for self-described cannabidiol use in 2,490 cannabis users. A questionnaire was developed to assess broad characteristics of self-described cannabidiol users and consisted of structured questions answered by either yes/no or multiple-choice responses. Questions included reasons for use, duration and frequency of use, method of administration, perceived clinical efficacy, and adverse effects. Both anxiety and depression were commonly self-reported reasons for cannabidiol use, with around 380 participants reporting use of cannabidiol for treatment of depression. In addition, the survey investigated the self-report treatment efficacy asking how well the user felt cannabidiol treated their medical conditions. In this regard, around 250 participants reported that cannabidiol treated their symptoms “very well by itself” or “moderately well by itself” (the other two possible responses were “well in combination with conventional medicine” and “not very well”).85
In an observational study, 120 cannabis smokers (recreational and daily users) were examined with regard to the THC and cannabidiol content in their consumed cannabis (measured in hair samples) in relation to cognition, psychotic-like symptoms, and psychological well-being. All participants were tested twice, when intoxicated and when nonintoxicated. Depressive symptoms were examined with the BDI and anxiety symptoms with the STAI. Overall, users of high THC cannabis, which tended to contain no or less cannabidiol than low THC cannabis, had significantly higher depression and anxiety scores than the low THC group, independent of daily or recreational use.86
A Web-based cross-sectional study evaluated 1,877 cannabis users with respect to the type of cannabis (including THC/cannabidiol ratios) consumed and their subclinical psychiatric experiences. This was achieved through the use of the CAPE. High cannabidiol contents in the consumed cannabis were statistically associated with fewer positive psychotic symptoms. The scores of negative psychotic symptoms and depressive symptoms were smaller in the high-cannabidiol group than in the high-THC group, but the difference was not significant.87
A large longitudinal naturalistic study examined 1,399 medical cannabis users with regard to the change in mood after consumption through a self-report app called Strainprint™, in which the user could rate the severity of their symptom on a scale of 0 (none) to 10 (extreme). Participants had to inform the reasons they were using cannabis through a list of health conditions or symptoms. There was a significant reduction in ratings of depression from before to after using cannabis, with the greatest reduction in ratings of depression being reported after using cannabis with relatively low levels of THC and high levels of cannabidiol. Furthermore, analyses examining changes in perceived efficacy of cannabis across time revealed no significant perceived tolerance effects on depression.88
Discussion
Current Evidence
To the best of our knowledge, this is the first study to systematically review the current evidence of cannabidiol as a treatment for mood disorders and affective symptoms associated with other health conditions. We did not find any published clinical trial investigating the efficacy of cannabidiol in patients with mood disorders. In other health conditions, there were no studies that examined affective symptoms as a primary outcome, although 10 clinical studies evaluated affective symptoms, mainly depressive, as secondary outcomes. In general, these studies showed a good safety profile of cannabidiol, without inducing dysphoria, anxiety, or psychotic symptoms and with few adverse effects.52,77,79,82 However, regarding the mood symptoms, the results were conflicting, showing both significant improvements in depressive symptoms and absence of significant differences.54,56,64,77,79,80,82,83 These results are probably due to the methodological differences among the studies.
Our review revealed considerable heterogeneity and methodological variations among the trials in several aspects. Among the eight studies including at least a phase of randomization and blinding, one evaluated the effects of pure cannabidiol or THC in healthy participants and the affective symptoms assessed were dysphoria and euphoria.52 All the others assessed depressive symptoms, but in patients with different health conditions.54,56,77,79,80,82,83 Furthermore, some studies investigated pure cannabidiol,52,54,56,79 and others evaluated combinations of cannabidiol and THC.77,80,82,83 The daily doses of cannabidiol also varied among the studies, ranging from 2.5 mg to 600 mg/day. In terms of clinical rating scales, the clinician-rated MADRS,83 a rating scale widely used as an outcome measure in RCTs of mood disorders, was used in only one study while the self-rated BDI was used in three studies,54,79,82 and different measures were used in remaining studies.52,56,77,80
The methodology also varied among studies with other levels of evidence. For example, an open-label trial and a case report assessed depressive symptoms in cannabis use disorder through BDI.62,73 Only one of the open-label studies testing purified cannabidiol assessed symptoms with the POMS.64 A cross-sectional study that assessed the concentration of cannabidiol in cannabis used by evaluating hair samples included BDI as an outcome.86 Similarly, in another survey investigating depressive symptoms, the cannabidiol content was estimated by getting information from the subjects about the type of cannabis consumed instead of an objective measurement.87 A cross-sectional study assessed the reasons for use and self-reported perception of improvement of depressive symptoms in people using only cannabidiol, but the symptoms were assessed without a validated scale.85 The only longitudinal study investigated the concentration of cannabidiol based on the type of cannabis consumed and rated the depressive symptoms through a self-report app, without any validated scale, although it showed improvement in symptoms.88 The only study done in patients with mania was a case series that included two patients with bipolar disorder and assessed whether cannabidiol monotherapy could maintain the improvement achieved with an initial combination of cannabidiol and olanzapine in the first patient and whether cannabidiol alone could improve the manic symptoms in the second patient.67 Considering all these aspects, the results were, as expected, diverse. Some papers showed no difference while others demonstrated improvements in depressive symptoms. Because of this conflicting evidence, it is essential to discuss the studies investigating the relationship between the endocannabinoid system and mood disorders to try to understand the possible role of cannabidiol as a treatment for bipolar disorder and MDD.
Clinical evidence linking the endocannabinoid system and mood disorders
As presented before, the endocannabinoid system has its receptors and ligands present in neurons and immune cells, an interesting fact in the context of mood disorders considering the interplay between the immune and the nervous system in their pathophysiological basis. Apart from that, studies evaluating specifically the relations of endocannabinoid system and affective symptoms and emotional functions are of interest too. For instance, both preclinical and clinical studies have shown the role of endocannabinoid system components on reactions to traumatic events89,90 as well as its relation with emotional processing.91 In addition, some clinical studies also showed the endocannabinoids as markers of improvement in schizophrenia and mood disorders.17,92 Besides, it is well known that cannabis use affects the endocannabinoid system, leading to a wide variety of neurobiological processes, including brain development and emotions as well as other neuropsychiatric functions.93,94
Notwithstanding these theoretical digressions and experimental evidence, the clinical impact of cannabis use on mood disorders is well-known and indirectly shows the relationship of these illnesses and the endocannabinoid system. For example, longitudinal studies have associated cannabis use during adolescence with later development of MDD, depressive symptoms,95,96 and suicidal ideation.97–100 In addition, a prospective study also suggested that adolescent cannabis use may be an independent risk factor for the development of hypomania in young adulthood.101 Although there is uncertainty about causal effects, in general, recent cannabis use is associated with more severe symptoms (manic, depressive, and especially anhedonia) and long-term cannabis use is associated with negative outcomes such as higher rates of recurrence in mood disorders.102,103 Particularly, cannabis use in bipolar disorder is associated with earlier onset of illness, psychotic symptoms, suicide, and misuse of other substances.104 Although these can be considered outcomes related to the use of cannabis, it is essential to note that these results are derived from cross-sectional studies which are not conducive to causal inference. In addition, it important to note that, as mentioned earlier, the plant Cannabis sativa contains more than 600 chemical compounds12 and its main compounds, namely THC and cannabidiol, have distinct and often opposing effects on mood, anxiety, psychosis, psychomotricity, and neurocognition.93,94
Potential use of cannabidiol in mood disorders
Considering the evidence discussed so far, what would be the potential benefits of further testing the efficacy of cannabidiol as a treatment in mood disorders? Cannabidiol represents a potentially novel therapeutic strategy for depression, with mechanism of action distinct from the traditional antidepressant medication which target the monoaminergic pathways.13,14,45 It is well established that about a third of patients with MDD are treatment resistant to such medications; cannabidiol could be a new therapeutic approach for this population either as monotherapy or an augmentation strategy.105,106 Regarding bipolar disorder, a medication with anxiolytic,19–21 anticonvulsant,15,16 and antipsychotic17,18 properties could help reduce the polypharmacy so common in this illness.107,108 Furthermore, the neuroprotective effects of cannabidiol, widely described in the literature,12,44,109–112 could help arrest the neuroprogression that occurs over the long-term in bipolar disorder.113,114 Given the excellent tolerability of cannabidiol, this might be more acceptable for bipolar disorder in the early stages of the illness and in those at high-risk for BD, where tolerability and treatment acceptability are very important considerations.115–117
An important potential advantage of testing cannabidiol in mood disorders is its well-known effect on anxiety, including physiological anxiety,52,118,119 social phobia,19,120 and even anxiety associated with heroin abstinence.121 It can be useful for populations with mood disorders, since 45.7% of patients with MDD and 34.7% of patients with bipolar disorder have a comorbid diagnosis of anxiety disorder and many more have anxiety symptoms associated with depressive episodes.122,123 In addition, comorbid anxiety disorders have been associated with pernicious outcomes in mood disorders, such as lower remission rates of depressive symptoms, increased suicidal ideation, and occupational disabilities.123–126 However, treatment of anxiety in mood disorders challenging and therapeutic options are limited, particularly in the light of the safety concerns such as the risk of dependence and cognitive impairment associated with benzodiazepines127–130 and the risk of manic switches in the case of antidepressants when prescribed to patients with bipolar disorder.131,132
Conclusions and Future Directions
A strength of this systematic review was the search strategy employed, including a broad range of search terms and databases. However, the review also has limitations. The synthesis of existing studies did not lend itself to a meta-analysis due to the lack of consistent outcome measures and rating scales used across studies. This highlights the fact that there is a lack of clinical evidence about the potential role of cannabidiol as a treatment of mood disorders. Given this lack of evidence, it is not surprising that cannabidiol is not recommended as a treatment for mood disorders according to the treatment guidelines, for example, the Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for treatment of MDD133 and the CANMAT and International Society for Bipolar Disorders guidelines for the management of patients with bipolar disorder.134
Nevertheless, considering that evidence from preclinical studies suggests antidepressants effects and that well-conducted RCTs in other health conditions have shown antipsychotic, anticonvulsant, and anxiolytic effects, as well as a favorable adverse effects profile, cannabidiol may still have a role in the treatment of mood disorders. Hence, there is an urgent need for well-conducted RCTs of cannabidiol in the treatment of acute episodes of MDD and bipolar depression. In this sense, future studies in this field could start with studies testing cannabidiol as add-on drug, similar to what was done in schizophrenia,18 and then, depending on the results, this could be tested further for its utility as monotherapy for mood disorders.
Another important point for future studies is defining a dose window for mood disorders since studies have shown an inverted U-curve profile of cannabidiol20,21 and the doses used in clinical trials vary between anxiety disorders,19,120 schizophrenia,17,18 and epilepsy.15,16 In addition, despite its general safety and good tolerability profile, the studies testing cannabidiol in epilepsy have shown critical drug–drug interactions with medications commonly used to treat mood disorders, such as benzodiazepines, valproate, and other anticonvulsants.135,136 This fact highlights the necessity for careful drug monitoring when testing cannabidiol in mood disorders, especially bipolar disorder. In this regard, it is noteworthy that, given the risks associated with THC in particular in the induction of psychosis93,137 and mania,101,103 future studies should focus primarily on pure cannabidiol produced according to Good Manufacturing Practices rather than medicinal cannabis or other products containing blends of cannabinoids. However, even products purportedly containing only cannabidiol should be carefully evaluated, as there are many artisanal cannabidiol oil extracts that may contain unknown amounts of this substance as well as other cannabinoids, especially THC, which may worsen the outcomes.138–140 It is critically important that clinicians be aware of this fact when providing education to patients.
Supplemental Material
Supplemental Material, 19039-d-CJP-2019-218-SR.R1-_abstract_for_translation for Cannabidiol as a Treatment for Mood Disorders: A Systematic Review by Jairo Vinícius Pinto, Gayatri Saraf, Christian Frysch, Daniel Vigo, Kamyar Keramatian, Trisha Chakrabarty, Raymond W. Lam, Márcia Kauer-Sant’Anna and Lakshmi N. Yatham in The Canadian Journal of Psychiatry
Acknowledgments
JVP acknowledges the financial support by the National Council for Scientific and Technological Development, Ministry of Science and Technology, Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq). This work was conducted during a scholarship supported by the CNPq.
Authors’ Note: Data available upon request.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LNY has been on speaker/advisory boards for or has received research grants from Alkermes, AstraZeneca, Bristol Myers Squibb, Canadian Network for Mood and Anxiety Treatments (CANMAT), Canadian Institutes of Health Research (CIHR), DSP, Eli Lilly, GlaxoSmithKline, Janssen, the Michael Smith Foundation for Health Research, Pfizer, Servier, Sunovion, and the Stanley Foundation. MK-S has received consulting or speaking fees from Daichii Sankyo and Shire. RWL has received ad hoc speaking/consulting fees or research grants from Akili, Allergan, Asia-Pacific Economic Cooperation, BC Leading Edge Foundation, CANMAT, Canadian Psychiatric Association, CIHR, CME Institute, Hansoh, Healthy Minds Canada, Janssen, Lundbeck, Lundbeck Institute, Medscape, Mind.Me, MITACS, Ontario Brain Institute, Otsuka, Pfizer, St. Jude Medical, University Health Network Foundation, and VGH-UBCH Foundation. DV, GS, JVP, KK, and TC report no biomedical financial interests or potential conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JVP has received a scholarship from CNPq, Brazil. MK-S has received research grants from CNPq and CAPES, Brazil.
ORCID iD: Jairo Vinícius Pinto, MD  https://orcid.org/0000-0001-6990-6749
https://orcid.org/0000-0001-6990-6749
Raymond W. Lam, MD  https://orcid.org/0000-0001-7142-4669
https://orcid.org/0000-0001-7142-4669
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bromet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Key facts. Department of Agriculture and Water Resources. 2018;(January):15–16. [Google Scholar]
- 3. Merikangas KR, Jin R, He J, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2012;68(3):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourne C, Aydemir O, Balanza-Martinez V, et al. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr Scand. 2013;128(3):149–162. [DOI] [PubMed] [Google Scholar]
- 5. Passos IC, Mwangi B, Vieta E, Berk M, Kapczinski F. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134(2):91–103. [DOI] [PubMed] [Google Scholar]
- 6. Osby U, Westman J, Hallgren J, Gissler M. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet (London, England). 2017;390(10100):1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mokdad AH, Forouzanfar MH, Daoud F, et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England). 2016;387(10036):2383–2401. [DOI] [PubMed] [Google Scholar]
- 9. Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3(2):171–178. [DOI] [PubMed] [Google Scholar]
- 10. Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170(3):265–274. [DOI] [PubMed] [Google Scholar]
- 11. Bond DJ, Kauer-Sant’Anna M, Lam RW, Yatham LN. Weight gain, obesity, and metabolic indices following a first manic episode: prospective 12-month data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM). J Affect Disord. 2010;124(1-2):108–117. [DOI] [PubMed] [Google Scholar]
- 12. Crippa JA, Guimaraes FS, Campos AC, Zuardi AW. Translational investigation of the therapeutic potential of cannabidiol (CBD): Toward a new age. Front Immunol. 2018;9:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szkudlarek HJ, Desai SJ, Renard J, et al. Delta-9-tetrahydrocannabinol and cannabidiol produce dissociable effects on prefrontal cortical executive function and regulation of affective behaviors. Neuropsychopharmacology. 2019;44(4):817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of delta9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95(4):434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–2020. [DOI] [PubMed] [Google Scholar]
- 16. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888–1897. [DOI] [PubMed] [Google Scholar]
- 17. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225–231. [DOI] [PubMed] [Google Scholar]
- 19. Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Linares IM, Zuardi AW, Pereira LC, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Rev Bras Psiquiatr. 2019;41(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zuardi AW, Rodrigues NP, Silva AL, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol. 2017;8:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linge R, Jimenez-Sanchez L, Campa L, et al. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology. 2016;103:16–26. [DOI] [PubMed] [Google Scholar]
- 23. Zanelati TV, Biojone C, Moreira FA, Guimarães FS, Joca SR. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmacol. 2010;159(1):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shoval G, Shbiro L, Hershkovitz L, et al. Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology. 2016;73(2):123–129. [DOI] [PubMed] [Google Scholar]
- 25. Sales AJ, Fogaca M V, Sartim AG, et al. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol Neurobiol. 2019;56(2):1070–1081. [DOI] [PubMed] [Google Scholar]
- 26. Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;(168):299–325. [DOI] [PubMed] [Google Scholar]
- 27. Ramirez SH, Hasko J, Skuba A, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32(12):4004–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walter L, Franklin A, Witting A, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23(4):1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16(10):579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donvito G, Nass SR, Wilkerson JL, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43(1):52–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65(7):772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishiguro H, Horiuchi Y, Ishikawa M, et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry. 2010;67(10):974–982. [DOI] [PubMed] [Google Scholar]
- 33. Renteria ME, Schmaal L, Hibar DP, et al. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry. 2017;7(5):e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA bipolar disorder working group. Mol Psychiatry. 2018;23(4):932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hibar DP, Westlye LT, van Erp TGM, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016;21(12):1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haarman BC, Burger H, Doorduin J, et al. Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder—a combined magnetic resonance imaging and positron emission tomography study. Brain Behav Immun. 2016;56:21–33. [DOI] [PubMed] [Google Scholar]
- 37. Haarman BC, Riemersma-Van der Lek RF, de Groot JC, et al. Neuroinflammation in bipolar disorder—A [(11)C]-(R)-PK11195 positron emission tomography study. Brain Behav Immun. 2014;40:219–225. [DOI] [PubMed] [Google Scholar]
- 38. Pinto JV, Moulin TC, Amaral OB. On the transdiagnostic nature of peripheral biomarkers in major psychiatric disorders: a systematic review. Neurosci Biobehav Rev. 2017;83:97–108. [DOI] [PubMed] [Google Scholar]
- 39. Goldsmith D, Rapaport M, Miller B. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Morais H, Chaves YC, Waltrick APF, et al. Sub-chronic treatment with cannabidiol but not with URB597 induced a mild antidepressant-like effect in diabetic rats. Neurosci Lett. 2018;682:62–68. [DOI] [PubMed] [Google Scholar]
- 41. Sales AJ, Crestani CC, Guimaraes FS, Joca SRL. Antidepressant-like effect induced by cannabidiol is dependent on brain serotonin levels. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:255–261. [DOI] [PubMed] [Google Scholar]
- 42. Shbiro L, Hen-Shoval D, Hazut N, et al. Effects of cannabidiol in males and females in two different rat models of depression. Physiol Behav. 2019;201:59–63. [DOI] [PubMed] [Google Scholar]
- 43. Reus GZ, Stringari RB, Ribeiro KF, et al. Administration of cannabidiol and imipramine induces antidepressant-like effects in the forced swimming test and increases brain-derived neurotrophic factor levels in the rat amygdala. Acta Neuropsychiatr. 2011;23(5):241–248. [DOI] [PubMed] [Google Scholar]
- 44. Valvassori SS, Elias G, de Souza B, et al. Effects of cannabidiol on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychopharmacol. 2011;25(2):274–280. [DOI] [PubMed] [Google Scholar]
- 45. Sartim AG, Guimaraes FS, Joca SRL. Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex—Possible involvement of 5-HT1A and CB1 receptors. Behav Brain Res. 2016;303:218–227. [DOI] [PubMed] [Google Scholar]
- 46. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [accessed 2019 May 9] http://www.ohri.ca/programs/clinical_epidemiol-ogy/oxford.asp.
- 48. Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3(4):479–486. [DOI] [PubMed] [Google Scholar]
- 49. Spielberger C. Manual of the State-Trait Anxiety Inventory. Palo Alto, CA: Psychologists Press; 1983. [Google Scholar]
- 50. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 51. Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12(2):245–258. [DOI] [PubMed] [Google Scholar]
- 52. Martin-Santos R, Crippa JA, Batalla A, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18(32):4966–4979. [DOI] [PubMed] [Google Scholar]
- 53. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. [DOI] [PubMed] [Google Scholar]
- 54. Jadoon KA, Ratcliffe SH, Barrett DA, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care. 2016;39(10):1777–1786. [DOI] [PubMed] [Google Scholar]
- 55. Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47(3):211–218. [Google Scholar]
- 56. Morgan CJA, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38(9):2433–2436. [DOI] [PubMed] [Google Scholar]
- 57. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 58. Stefanis NC, Hanssen M, Smirnis NK, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32(2):347–358. [DOI] [PubMed] [Google Scholar]
- 59. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. text rev Washington, DC: American; Psychiatric Association; 2000. [Google Scholar]
- 60. Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed Oxford, England: Oxford University Press; 2004. [Google Scholar]
- 61. Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. [DOI] [PubMed] [Google Scholar]
- 62. Solowij N, Broyd SJ, Beale C, et al. Therapeutic effects of prolonged cannabidiol treatment on psychological symptoms and cognitive function in regular cannabis users: a pragmatic open-label clinical trial. Cannabis cannabinoid Res. 2018;3(1):21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Devinsky O, Vickrey BG, Cramer J, et al. Development of the quality of life in epilepsy inventory. Epilepsia. 1995;36(11):1089–1104. [DOI] [PubMed] [Google Scholar]
- 64. Gaston TE, Szaflarski M, Hansen B, Bebin EM, Szaflarski JP; UAB CBD Program Quality of life in adults enrolled in an open-label study of cannabidiol (CBD) for treatment-resistant epilepsy. Epilepsy Behav. 2019;95:10–17. [DOI] [PubMed] [Google Scholar]
- 65. McNair DM, Lorr M, Droppleman L. Profile of mood states. San Diego, CA: Education and Industrial Testing Service; 1971. [Google Scholar]
- 66. Griffith NM, Szaflarski JP, Szaflarski M, et al. Measuring depressive symptoms among treatment-resistant seizure disorder patients: POMS Depression scale as an alternative to the BDI-II. Epilepsy Behav. 2005;7(2):266–272. [DOI] [PubMed] [Google Scholar]
- 67. Zuardi A, Crippa J, Dursun S, et al. Cannabidiol was ineffective for manic episode of bipolar affective disorder. J Psychopharmacol. 2010;24(1):135–137. [DOI] [PubMed] [Google Scholar]
- 68. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 69. Flemenbaum A, Zimmermann RL. Inter- and intra-rater reliability of the Brief Psychiatric Rating Scale. Psychol Rep. 1973;32(3):783–792. [DOI] [PubMed] [Google Scholar]
- 70. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. [DOI] [PubMed] [Google Scholar]
- 71. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. [DOI] [PubMed] [Google Scholar]
- 72. Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58(10):917–924. [DOI] [PubMed] [Google Scholar]
- 73. Crippa JAS, Hallak JEC, Machado-de-Sousa JP, et al. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J Clin Pharm Ther. 2013;38(2):162–164. [DOI] [PubMed] [Google Scholar]
- 74. Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The cannabis withdrawal scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119(1-2):123–129. [DOI] [PubMed] [Google Scholar]
- 75. Lovibond S, Lovibond P. Manual for the depression anxiety stress scales. Sydney, Australia: Psychology Foundation; 1995. [Google Scholar]
- 76. Arnau RC, Broman-Fulks JJ, Green BA, Berman ME. The Anxiety Sensitivity Index–Revised: confirmatory factor analyses, structural invariance in Caucasian and African American samples, and score reliability and validity. Assessment. 2009;16(2):165–180. [DOI] [PubMed] [Google Scholar]
- 77. Allsop DJ, Copeland J, Lintzeris N, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71(3):281–291. [DOI] [PubMed] [Google Scholar]
- 78. Rabins P V, Brooks BR. Emotional disturbance in multiple sclerosis patients: validity of the general health questionnaire (GHQ). Psychol Med. 1981;11(2):425–427. [DOI] [PubMed] [Google Scholar]
- 79. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 “N of 1” studies. Anaesthesia. 2004;59(5):440–452. [DOI] [PubMed] [Google Scholar]
- 80. Aragona M, Onesti E, Tomassini V, et al. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clin Neuropharmacol. 2009;32(1):41--47. [DOI] [PubMed] [Google Scholar]
- 81. Prinz U, Nutzinger DO, Schulz H, Petermann F, Braukhaus C, Andreas S. Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry. 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10(4):434–441. [DOI] [PubMed] [Google Scholar]
- 83. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438–449. [DOI] [PubMed] [Google Scholar]
- 84. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 85. Corroon J, Phillips JA. A cross-sectional study of cannabidiol users. Cannabis cannabinoid Res. 2018;3(1):152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Morgan CJA, Gardener C, Schafer G, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med. 2012;42(2):391–400. [DOI] [PubMed] [Google Scholar]
- 87. Schubart CD, Sommer IEC, van Gastel WA, Goetgebuer RL, Kahn RS, Boks MP. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res. 2011;130(1-3):216–221. [DOI] [PubMed] [Google Scholar]
- 88. Cuttler C, Spradlin A, McLaughlin RJ. A naturalistic examination of the perceived effects of cannabis on negative affect. J Affect Disord. 2018;235:198–205. [DOI] [PubMed] [Google Scholar]
- 89. Bluett RJ, Baldi R, Haymer A, et al. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat Commun. 2017;8:14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Agrawal A, Nelson EC, Littlefield AK, et al. Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Arch Gen Psychiatry. 2012;69(7):732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bossong MG, van Hell HH, Jager G, Kahn RS, Ramsey NF, Jansma JM. The endocannabinoid system and emotional processing: a pharmacological fMRI study with 9-tetrahydrocannabinol. Eur Neuropsychopharmacol. 2013;23(12):1687–1697. [DOI] [PubMed] [Google Scholar]
- 92. Kranaster L, Hoyer C, Aksay SS, et al. Electroconvulsive therapy enhances endocannabinoids in the cerebrospinal fluid of patients with major depression: a preliminary prospective study. Eur Arch Psychiatry Clin Neurosci. 2017;267(8):781–786. [DOI] [PubMed] [Google Scholar]
- 93. Murray RM, Englund A, Abi-Dargham A, et al. Cannabis-associated psychosis: neural substrate and clinical impact. Neuropharmacology. 2017;124:89–104. [DOI] [PubMed] [Google Scholar]
- 94. Sami MB, Bhattacharyya S. Are cannabis-using and non-using patients different groups? Towards understanding the neurobiology of cannabis use in psychotic disorders. J Psychopharmacology. 2018;32(8):825–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rasic D, Weerasinghe S, Asbridge M, Langille DB. Longitudinal associations of cannabis and illicit drug use with depression, suicidal ideation and suicidal attempts among Nova Scotia high school students. Drug Alcohol Depend. 2013;129(1-2):49–53. [DOI] [PubMed] [Google Scholar]
- 96. Esmaeelzadeh S, Moraros J, Thorpe L, Bird Y. Examining the association and directionality between mental health disorders and substance use among adolescents and young adults in the U.S. and Canada—a systematic review and meta-analysis. J Clin Med. 2018;7(12):pii: E543. doi: 10.3390/jcm7120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schoeler T, Theobald D, Pingault JB, Farrington DP, Coid JW, Bhattacharyya S. Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: findings from 40 years of follow-up. Psychol Med. 2018;48(13):2169–2176. [DOI] [PubMed] [Google Scholar]
- 98. Agrawal A, Nelson EC, Bucholz KK, et al. Major depressive disorder, suicidal thoughts and behaviours, and cannabis involvement in discordant twins: a retrospective cohort study. Lancet Psychiatry. 2017;4(9):706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Leadbeater BJ, Ames ME, Linden-Carmichael AN. Age-varying effects of cannabis use frequency and disorder on symptoms of psychosis, depression, and anxiety in adolescents and adults. Addiction. 2019;114(2):278–293. doi: 10.1111/add.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gobbi G, Atkin T, Zytynski T, et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: a systematic review and meta-analysis. JAMA Psychiatry. 2019;76(4):426–434. doi: 10.1001/jamapsychiatry.2018.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Marwaha S, Winsper C, Bebbington P, Smith D. Cannabis use and hypomania in young people: a prospective analysis. Schizophr Bull. 2018;44(6):1267–1274. doi: 10.1093/schbul/sbx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mammen G, Rueda S, Roerecke M, Bonato S, Lev-Ran S, Rehm J. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79(4):pii: 17r11839. doi: 10.4088/JCP.17r11839. [DOI] [PubMed] [Google Scholar]
- 103. Gibbs M, Winsper C, Marwaha S, Gilbert E, Broome M, Singh SP. Cannabis use and mania symptoms: a systematic review and meta-analysis. J Affect Disord. 2015;171:39–47. [DOI] [PubMed] [Google Scholar]
- 104. Pinto JV, Medeiros LS, Santana da Rosa G, et al. The prevalence and clinical correlates of cannabis use and cannabis use disorder among patients with bipolar disorder: A systematic review with meta-analysis and meta-regression. Neurosci Biobehav Rev. 2019;101:78–84. [DOI] [PubMed] [Google Scholar]
- 105. Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Johnston KM, Powell LC, Anderson IM, Szabo S, Cline S. The burden of treatment-resistant depression: A systematic review of the economic and quality of life literature. J Affect Disord. 2019;242:195–210. [DOI] [PubMed] [Google Scholar]
- 107. Golden JC, Goethe JW, Woolley SB. Complex psychotropic polypharmacy in bipolar disorder across varying mood polarities: a prospective cohort study of 2712 inpatients. J Affect Disord. 2017;221:6–10. [DOI] [PubMed] [Google Scholar]
- 108. Fung VC, Overhage LN, Sylvia LG, et al. Complex polypharmacy in bipolar disorder: Side effect burden, adherence, and response predictors. J Affect Disord. 2019;257:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Barata L, Arruza L, Rodriguez MJ, et al. Neuroprotection by cannabidiol and hypothermia in a piglet model of newborn hypoxic-ischemic brain damage. Neuropharmacology. 2019;146:1–11. [DOI] [PubMed] [Google Scholar]
- 110. Mori MA, Meyer E, Soares LM, Milani H, Guimarães FS, de Oliveira RMW. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog Neuropsychopharmacol Biol Psychiatry. 2017;75:94–105. [DOI] [PubMed] [Google Scholar]
- 111. Mohammed N, Ceprian M, Jimenez L, Pazos MR, Martínez-Orgado J. Neuroprotective effects of cannabidiol in hypoxic ischemic insult. The therapeutic window in newborn mice. CNS Neurol Disord Drug Targets. 2017;16(1):102–108. [DOI] [PubMed] [Google Scholar]
- 112. Campos AC, Fogaca MV, Sonego AB, Guimarães FS. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–127. [DOI] [PubMed] [Google Scholar]
- 113. Kozicky JM, McGirr A, Bond DJ, et al. Neuroprogression and episode recurrence in bipolar I disorder: A study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18(6):511–519. [DOI] [PubMed] [Google Scholar]
- 114. Silveira LE, Bond DJ, MacMillan EL, et al. Hippocampal neurochemical markers in bipolar disorder patients following the first-manic episode: A prospective 12-month proton magnetic resonance spectroscopy study. Aust N Z J Psychiatry. 2017;51(1):65–74. [DOI] [PubMed] [Google Scholar]
- 115. Peres FF, Diana MC, Levin R, et al. Cannabidiol administered during peri-adolescence prevents behavioral abnormalities in an animal model of schizophrenia. Front Pharmacol. 2018;9:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wilson R, Bossong MG, Appiah-Kusi E, et al. Cannabidiol attenuates insular dysfunction during motivational salience processing in subjects at clinical high risk for psychosis. Transl Psychiatry. 2019;9(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66(1):95–105. [DOI] [PubMed] [Google Scholar]
- 119. Crippa JA de S, Zuardi AW, Garrido GEJ, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29(2):417–426. [DOI] [PubMed] [Google Scholar]
- 120. Crippa JAS, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25(1):121–130. [DOI] [PubMed] [Google Scholar]
- 121. Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176(11):911–922. doi: 10.1176/appiajp201918101191. [DOI] [PubMed] [Google Scholar]
- 122. Pavlova B, Perlis RH, Mantere O, et al. Prevalence of current anxiety disorders in people with bipolar disorder during euthymia: a meta-analysis. Psychol Med. 2017;47(6):1107–1115. [DOI] [PubMed] [Google Scholar]
- 123. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Arvilommi P, Suominen K, Mantere O, Valtonen H, Leppämäki S, Isometsä E. Predictors of long-term work disability among patients with type I and II bipolar disorder: a prospective 18-month follow-up study. Bipolar Disord. 2015;17(8):821–835. [DOI] [PubMed] [Google Scholar]
- 125. Kim SW, Berk L, Kulkarni J, et al. Impact of comorbid anxiety disorders and obsessive-compulsive disorder on 24-month clinical outcomes of bipolar I disorder. J Affect Disord. 2014;166:243–248. [DOI] [PubMed] [Google Scholar]
- 126. Hung CI, Liu CY, Yang CH, Gan ST. Comorbidity with more anxiety disorders associated with a poorer prognosis persisting at the 10-year follow-up among patients with major depressive disorder. J Affect Disord. 2019;260:97–104. [DOI] [PubMed] [Google Scholar]
- 127. Correa-Ghisays P, Balanza-Martinez V, Selva-Vera G, et al. Manual motor speed dysfunction as a neurocognitive endophenotype in euthymic bipolar disorder patients and their healthy relatives. Evidence from a 5-year follow-up study. J Affect Disord. 2017;215:156–162. [DOI] [PubMed] [Google Scholar]
- 128. Baandrup L, Fagerlund B, Glenthoj B. Neurocognitive performance, subjective well-being, and psychosocial functioning after benzodiazepine withdrawal in patients with schizophrenia or bipolar disorder: a randomized clinical trial of add-on melatonin versus placebo. Eur Arch Psychiatry Clin Neurosci. 2017;267(2):163–171. [DOI] [PubMed] [Google Scholar]
- 129. He Q, Chen X, Wu T, Li L, Fei X. Risk of dementia in long-term benzodiazepine users: evidence from a meta-analysis of observational studies. J Clin Neurol. 2019;15(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Richardson K, Mattishent K, Loke YK, et al. History of benzodiazepine prescriptions and risk of dementia: possible bias due to prevalent users and covariate measurement timing in a nested case-control study. Am J Epidemiol. 2019;188(7):1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fornaro M, Anastasia A, Novello S, et al. Incidence, prevalence and clinical correlates of antidepressant-emergent mania in bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2018;20(3):195–227. [DOI] [PubMed] [Google Scholar]
- 132. Liu B, Zhang Y, Fang H, Liu J, Liu T, Li L. Efficacy and safety of long-term antidepressant treatment for bipolar disorders—a meta-analysis of randomized controlled trials. J Affect Disord. 2017;223:41–48. [DOI] [PubMed] [Google Scholar]
- 133. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological treatments. Can J Psychiatry. 2016;61(9):540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP; UAB CBD Program. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586–1592. [DOI] [PubMed] [Google Scholar]
- 136. Morrison G, Crockett J, Blakey G, Sommerville K. A phase 1, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol drug Dev. 2019;8(8):1009–1031. doi: 10.1002/cpdd.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Freeman D, Dunn G, Murray RM, et al. How cannabis causes paranoia: using the intravenous administration of 9-tetrahydrocannabinol (THC) to identify key cognitive mechanisms leading to paranoia. Schizophr Bull. 2015;41(2):391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sulak D, Saneto R, Goldstein B. The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 2017;70(Pt B):328–333. [DOI] [PubMed] [Google Scholar]
- 139. Crippa JAS, Crippa ACS, Hallak JEC, Martín-Santos R, Zuardi AW. Delta9-THC intoxication by cannabidiol-enriched cannabis extract in two children with refractory epilepsy: full remission after switching to purified cannabidiol. Front Pharmacol. 2016;7:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Porcari GS, Fu C, Doll ED, Carter EG, Carson RP. Efficacy of artisanal preparations of cannabidiol for the treatment of epilepsy: practical experiences in a tertiary medical center. Epilepsy Behav. 2018;80:240–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, 19039-d-CJP-2019-218-SR.R1-_abstract_for_translation for Cannabidiol as a Treatment for Mood Disorders: A Systematic Review by Jairo Vinícius Pinto, Gayatri Saraf, Christian Frysch, Daniel Vigo, Kamyar Keramatian, Trisha Chakrabarty, Raymond W. Lam, Márcia Kauer-Sant’Anna and Lakshmi N. Yatham in The Canadian Journal of Psychiatry