Highlights
-
•
Aminocoumarins can be used to treat acute pulmonary melioidosis in a murine model.
-
•
Formulation with l-tyrosine or dl-tryptophan enhances the bioactivity of aminocoumarins.
-
•
Utility of Galleria mellonella larvae for in vivo drug efficacy screening.
Keywords: Aminocoumarin, Novobiocin, Clorobiocin, Coumermycin, Burkholderia pseudomallei, Melioidosis
Abstract
Burkholderia pseudomallei causes melioidosis, a potentially lethal disease that can establish both chronic and acute infections in humans. It is inherently recalcitrant to many antibiotics, there is a paucity of effective treatment options and there is no vaccine. In the present study, the efficacies of selected aminocoumarin compounds, DNA gyrase inhibitors that were discovered in the 1950s but are not in clinical use for the treatment of melioidosis were investigated. Clorobiocin and coumermycin were shown to be particularly effective in treating B. pseudomallei infection in vivo. A novel formulation with dl-tryptophan or l-tyrosine was shown to further enhance aminocoumarin potency in vivo. It was demonstrated that coumermycin has superior pharmacokinetic properties compared with novobiocin, and the coumermycin in l-tyrosine formulation can be used as an effective treatment for acute respiratory melioidosis in a murine model. Repurposing of existing approved antibiotics offers new resources in a challenging era of drug development and antimicrobial resistance.
1. Introduction
Burkholderia pseudomallei, a Gram-negative, soil-dwelling bacterium, is the causative agent of the potentially fatal disease, melioidosis. Reports of B. pseudomallei being isolated from soil are now commonplace in subtropical climates, including Southeast Asia, Africa and South America, leading to concerns that the incidence of melioidosis will increase in the future [1,2].
Burkholderia pseudomallei can infect humans via two main routes of entry: (i) inhalation of contaminated particles; and (ii) wound site infection. It can then establish itself as either a chronic infection, potentially lasting for many years [3], or as an acute illness owing to bacteraemia. It is also able to disseminate in the host via the circulatory and lymphatic systems. Melioidosis is difficult to treat because B. pseudomallei has inherent resistance to many commonly used antibiotics, notably β-lactam compounds, fluoroquinolones and ciprofloxacin [4]. There is currently no effective vaccine that is safe for use in humans and it is estimated that melioidosis was responsible for more than 89 000 deaths in 2016 [5]. Treatment of the acute infection involves intravenous administration of the frontline antibiotic ceftazidime for up to 4 weeks, followed by secondary treatment with oral trimethoprim/sulfamethoxazole (co-trimoxazole) for a further 3 months to eradicate persistent intracellular bacteria [6].
Antibiotic resistance in B. pseudomallei is mediated by a formidable array of factors encoded by a large genome spread over two chromosomes. The thick polysaccharide capsule can resist complement, aminoglycosides and antimicrobial peptide stress [6]; efflux pumps can reduce susceptibility to chloramphenicol and macrolides; and overproduction of β-lactamases and deletion of penicillin-binding proteins can confer resistance to ceftazidime [6]. A recent study of the related species Burkholderia cepacia found that only around one-quarter of >2500 isolates from cystic fibrosis patients were susceptible to any treatment whatsoever, despite testing 23 different antibiotic combinations [7].
Novel, repurposed or reformulated antibiotics are required to provide more effective treatment options for Burkholderia infections. The current state of drug development is that no new classes of antibiotic effective against Gram-negative bacteria have been approved since 1962 [8]. Compounding this, many pharmaceutical companies are withdrawing from antibiotic development, in part due to unfavourable economic incentives [9]. In light of this, making better use of antibiotics that are already in clinical use represents a possible cost-effective and expeditious solution.
Aminocoumarins, which include novobiocin, coumermycin and clorobiocin, are a class of antibiotics that target the ATPase activity of the GyrB subunit of DNA gyrase and the ParE subunit of topoisomerase IV, disrupting DNA replication and transcription. They bind gyrase with greater affinity than quinolones, which target the GyrA subunit, and they additionally prevent ATP binding [10].
Novobiocin, also known as albamycin, was discovered in the 1950s derived from Streptomyces niveus and was shown to be active against B. pseudomallei in vitro, particularly in synergy with tetracycline [11]. Despite this early promise, there is a paucity of data on the use of aminocoumarins to treat melioidosis. Commonly cited reasons include their poor solubility as well as concerns over the potential emergence of drug resistance [12]. Efforts to improve the pharmacokinetics of these compounds have sometimes been accompanied by a loss of affinity for the bacterial target site, and such programmes have since been discontinued (reviewed by Bisacchi and Manchester [13]).
Given the high mortality associated with acute melioidosis (>70% without appropriate antibiotic treatment [5]), the difficulties with existing treatment options and the unrealised potential shown by aminocoumarins, we sought to revisit this class of compounds as a potential therapeutic agent against B. pseudomallei infection using two different in vivo models. In the current study, the efficacies of commercially available aminocoumarins were assessed and the Galleria mellonella (wax moth) larvae model was used to screen for effective formulations that could improve in vivo activity in a murine model. These findings were applied to demonstrate that coumermycin in equimolar solution with l-tyrosine significantly improves survival in a murine infection model of acute melioidosis.
2. Methods
2.1. Galleria mellonella infection assay
Different formulations were investigated to improve the bioavailability and efficacy of poorly soluble compounds, using novobiocin as a representative aminocoumarin. The compounds clorobiocin, coumermycin (A1) and novobiocin (all from Sigma-Aldrich, Gillingham, UK) were solubilised in dimethyl sulfoxide (DMSO) carrier at a stock concentration of 10 mg/mL. For injection into G. mellonella larvae, the stock solution was diluted in sterile saline to a final concentration of 1 mg/mL. Saline solutions were prepared with either sodium alginate, povidone or methylcellulose at different concentrations ranging from 0–1% [w/v sterile phosphate-buffered saline (PBS)]. Alternatively, and also in combination, the amino acids l-tyrosine, dl-tryptophan or casamino acids (Sigma-Aldrich) were added at various molar ratios relative to the antibiotic. Casamino acids contain all amino acids except tryptophan. For infection, five Galleria larvae per group of matching size were injected with ~150 CFU of B. pseudomallei in a 10 μL volume in the uppermost right footpad. Galleria larvae were then incubated at 37 °C for 1 h. At this time, Galleria were injected in the uppermost left footpad with 10 μL of antibiotic solution or saline control. Galleria larvae were then incubated for 24 h at 37 °C. To enumerate the bacterial load, haemolymph was drained into a sterile 1.5 mL Eppendorf tube. Then, 10 μL of haemolymph was serially diluted in sterile PBS with 0.1% Tween 80 and was plated onto Luria–Bertani (LB) agar for CFU enumeration of ex vivo isolated B. pseudomallei.
2.2. Murine infection
Female BALB/c mice (Charles Rivers Laboratories International, Inc., Margate, UK) aged 6–8 weeks were used. Mice were housed under specific pathogen-free (SPF) conditions with free access to food and water. Animal work was performed in accordance with the Animals (Scientific Procedures) Act of 1986 and the local Ethical Review Committee, under animal biohazard Containment Level 3 conditions. All units were infected intranasally with a total of 500 CFU of B. pseudomallei K96243 by pipetting 25 μL into each nostril. Treatment began at 6 h post-infection, followed by treatment once every 24 h for 4 consecutive days thereafter. Treatment comprised either sterile saline (negative control), 1200 mg/kg ceftazidime (intraperitoneal), 30 mg/kg coumermycin (subcutaneous) or 30 mg/kg coumermycin in solution with equimolar l-tyrosine (subcutaneous). In all cases, mice were checked at least daily for signs of illness and, if determined to have reached the humane endpoint specified in the Project Licence, were euthanised. The experiment was concluded at 30 days post-infection.
2.3. Pharmacokinetics
Pharmacokinetic studies were performed by Domainex (Saffron Walden, UK). Three BALB/c units per group were injected intraperitoneally with 15 mg/kg novobiocin or coumermycin ± 0.7 mg/mL dl-tryptophan, formulated in 5% DMSO in 95% KleptoseⓇ (8% w/v in PBS). Animals were housed in pre-assigned cages until sampling. Appropriate samples were taken at the defined time points and were stored immediately at –20 °C. Protein was precipitated with acetonitrile and then ultra-high performance liquid chromatography time-of-flight mass spectrometry (UHPLC-TOF/MS) using electrospray ionisation was used to quantify the compound concentration in plasma.
2.4. Statistical analysis
All data analysis was performed using GraphPad Prism 8.1.2 (GraphPad Software Inc., La Jolla, CA, USA). For comparison of the effect of different treatment conditions on the bacterial load in Galleria haemolymph, one-way analysis of variance (ANOVA) was performed with Dunnett's multiple comparisons test compared with the untreated condition or unformulated novobiocin as indicated. For murine survival studies, log-rank (Mantel–Cox) test was performed for each treatment condition versus the saline control group.
3. Results and discussion
3.1. The aminocoumarin formulation significantly alters its in vivo activity
Historically, only limited assessment of aminocoumarin activity against B. pseudomallei infection in vivo has been performed. In the current study, two different in vivo models were chosen to assess the efficacy of a group of aminocoumarin compounds, initially using G. mellonella larvae to screen the effect of different drug formulations before proceeding to an experimental model of acute melioidosis using a murine infection model. Use of G. mellonella larvae is an increasingly popular method for host–pathogen virulence studies as well as for screening drug efficacy and safety. They represent a more complex model than any single cell line since they possess multiple different cell types, tissue differentiation and serum factors including host defence peptides and complement-like proteins [14,15]. They are particularly valuable not as a complete replacement of murine studies but in reducing the size of those experiments by defining experimental conditions and testing hypotheses a priori.
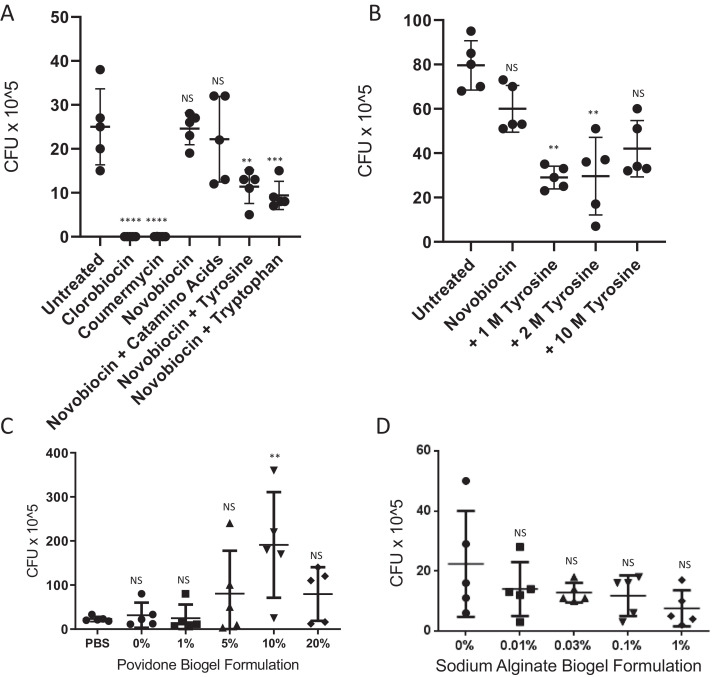
To assess compound activity in vivo, G. mellonella larvae were treated 1 h after infection with ~150 CFU of B. pseudomallei. Novobiocin, whilst highly active in vitro [minimum inhibitory concentration (MIC) <10 μg/mL], showed no significant killing activity in vivo, whereas clorobiocin and coumermycin significantly reduced the bacterial load (Fig. 1A). Clorobiocin is reportedly a more potent inhibitor of gyrase and topoisomerase IV than novobiocin, although it is difficult to source commercially and is not in clinical use [16]. Coumermycin has a structure similar to a dimer of novobiocin and, like clorobiocin, proved to be more potent than novobiocin in vivo at the same dose. Both Clorobiocin and coumermycin are reported to have improved membrane permeability compared with novobiocin [13], which may explain these findings. With these two aminocoumarins, all Galleria larvae survived and showed no signs of disease after 24 h of infection, whereas no larvae treated with novobiocin survived longer than 24 h post-infection. Haemolymph from clorobiocin- and coumermycin-treated larvae was also clear from significant amounts of B. pseudomallei, and in some cases (100% and 60%, respectively) was sterile of any bacteria.
Fig. 1.
The novobiocin formulation modifies in vivo potency in Galleria mellonella larvae. Galleria larvae (n = 5 per group) were infected with Burkholderia pseudomallei K96243 (110–178 CFU) for 1 h at 37 °C and were then treated with different aminocoumarins (10 μL of 1 mg/mL stock solution) or novobiocin in different formulations as indicated. One-way analysis of variance (ANOVA) was performed with Dunnett's multiple comparisons test versus (A,B) untreated larvae or (C,D) novobiocin formulation in phosphate-buffered saline (PBS). Representative figures from at least three independent biological replicates are shown. Error bars represent the standard deviation from the mean. ** P < 0.01; *** P < 0.001; **** P < 0.0001; NS, not significant.
Use of certain amino acids as co-amorphous partners has been reported to increase the solubility of poorly water-soluble drugs [17,18]. We therefore investigated the effect of addition of either l-tyrosine, dl-tryptophan or casamino acids at various molar ratios on the in vivo efficacy of novobiocin (Fig. 1A,B). Tryptophan and tyrosine, but not casamino acids, improved the activity of novobiocin at equimolar ratios, showing reduced bacterial burden in Galleria larvae. Since casamino acids, which contain a mixture of amino acids minus tryptophan, were not effective at enhancing activity, we suggest that a unique property of tyrosine and tryptophan—potentially their non-polar aromatic carbon ring—is responsible for the improved novobiocin activity. The fact that the molar ratio of amino acid to novobiocin influenced synergy suggests that specific stoichiometry is important for optimal interaction.
To investigate the synergy with amino acid formulation further, novobiocin was chemically conjugated to a fluorescent reporter compound (Supplementary methods; Supplementary Fig. S1A) and its tissue distribution was imaged in histological samples of treated, non-infected Galleria larvae (Supplementary Fig. S1C). No significant effect on drug accumulation in adipose or haemolymphatic tissue was observed. The mechanism of in vivo synergy between dl-tryptophan, l-tyrosine and aminocoumarins remains to be established.
Novobiocin exists in solution as an equilibrium between two forms, its less bioactive crystalline form and its more bioactive amorphous form. Unlike the crystalline form, the amorphous form is readily absorbed in vivo via the gastrointestinal tract leading to high blood concentrations [19]. Gelling agents have been previously described to suppress crystallisation of similar compounds, thus in the current study different formulations of povidone and sodium alginate with novobiocin were tested. Whilst povidone significantly increased bacterial CFU during Galleria infection, sodium alginate formulations appeared to reduce the bacterial burden, although this was not statistically significant (Fig. 1C,D). Gelling agents can be problematic due to the associated changes in solution viscosity. Techniques such as nanosuspension and prodrug formulation with phosphate esters have been explored by pharmaceutical companies with mixed success. Alternative methods such as polymersome encapsulation [20,21] or microgel formulation may enhance novobiocin efficacy in vivo and remain to be tested.
3.2. Coumermycin shows improved pharmacokinetics in vivo compared with novobiocin
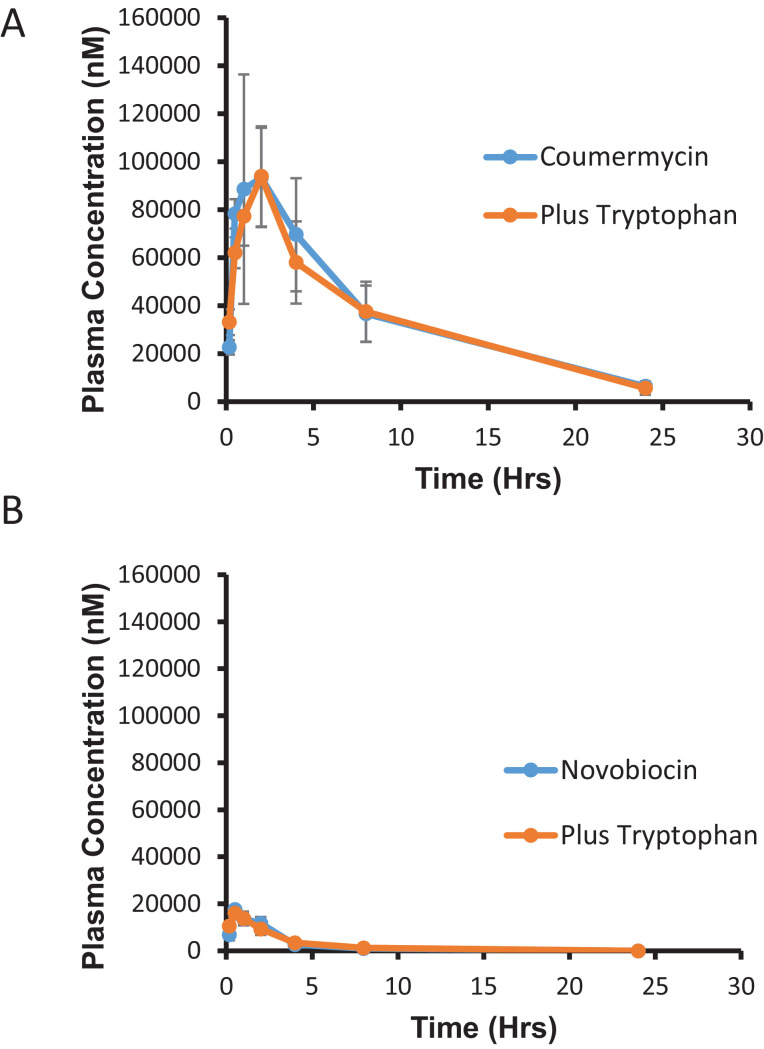
The G. mellonella data suggested that while both compounds are effective against B. pseudomallei in vitro, coumermycin retains activity in vivo, in contrast to novobiocin. We sought to characterise the pharmacokinetic profile of both compounds in a murine model by injection of either compound into the intraperitoneal cavity and quantification of its absorption into peripheral blood plasma (Fig. 2). Whilst some mild toxicity including skin rash and nausea has been reported with the use of novobiocin, other reports suggest that it is in fact generally well tolerated and that impurities in the formulation may instead be responsible for such side effects [10]. In the present study, no toxicity was observed at the dosage of coumermycin used in the murine experiments.
Fig. 2.
Pharmacokinetics of novobiocin and coumermycin. Female BALB/c mice (n = 3 per group) were intraperitoneally administered (A) 15 mg/kg coumermycin or (B) 15 mg/kg novobiocin with or without formulation in an equimolar concentration of dl-tryptophan (dose volume 10 mL/kg). Error bars represent the standard deviation from the mean. Plasma concentrations of the compound were assessed by mass spectrometry at different time points as indicated.
It was found that while novobiocin reached a peak concentration of 10 739 nM, coumermycin peaked at 102 767 nM and also had a significantly longer half-life in plasma (5.9 h vs. 2.8 h) (Table 1). The half-life values are similar to those reported by others [10,22], whilst the plasma concentrations for coumermycin are substantially higher than in studies that used a subcutaneous rather than intraperitoneal injection route [23], even accounting for differences in treatment dose. The relatively low plasma concentration of novobiocin may explain its loss of activity in vivo since the unbound (free) drug available is likely to be less than the threshold required to inhibit topoisomerase and gyrase [13]. Interestingly, addition of dl-tryptophan, which improved antibiotic activity in Galleria, did not influence the pharmacokinetics for either drug in the murine model.
Table 1.
Summary of mean pharmacokinetic parameters.
| Parameter | Coumermycin |
Novobiocin |
||
|---|---|---|---|---|
| Alone | + dl-tryptophan | Alone | + dl-tryptophan | |
| t1/2 (h) | 6 | 6 | 3 | 3 |
| Tmax (h) | 2 | 2 | 1 | 1 |
| Cmax (nM) | 102 767 | 103 297 | 10 739 | 10 739 |
| AUClast (h•nM) | 957 128 | 909 773 | 33 198 | 33 198 |
| AUCall (h•nM) | 957 128 | 909 773 | 33 198 | 33 198 |
| AUCinf (h•nM) | 1 016 517 | 963 025 | 33 240 | 33 240 |
t1/2, elimination half-life; Tmax, time to maximum concentration; Cmax, maximum concentration; AUC, area under the concentration–time curve (AUClast, AUC from 0 h to last measurable concentration; AUCall, AUC for all values; AUCinf, AUC from 0 h to infinity).
3.3. Treatment with coumermycin protects against acute melioidosis in a murine model
Since coumermycin was highly active in the Galleria screen and showed enhanced pharmacokinetics over novobiocin, this compound was selected as the test aminocoumarin for treatment of melioidosis in an acute murine model of infection [24]. Novobiocin has previously shown potential in the treatment of sepsis caused by Streptococcus pneumoniae [10]. Since formulation with l-tyrosine enhanced the in vivo activity of novobiocin, we reasoned that it may similarly enhance the potency of coumermycin. Ceftazidime, the currently recommended antibiotic for treating melioidosis, was used as a comparative control.
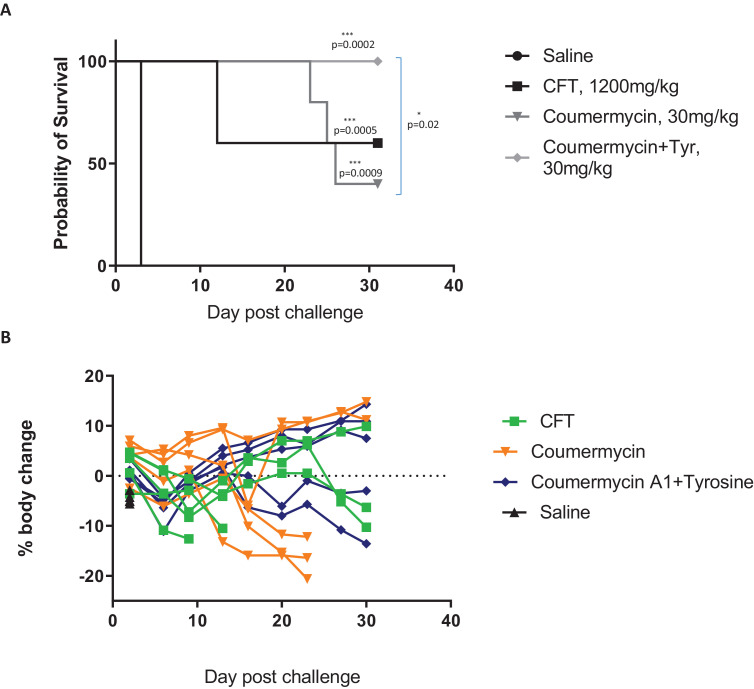
All treatment groups had a significantly improved survival rate until the end of the experiment compared with the saline-treated control group (Fig. 3A). In agreement with the Galleria data, the efficacy of coumermycin was enhanced by formulation with a 1:1 molar ratio l-tyrosine, with 100% survival at 30 days post-infection compared with 60% survival with ceftazidime and 40% survival with coumermycin alone. In general, the coumermycin treatment groups scored better for clinical symptoms of disease than the ceftazidime group and recovered a greater percentage of body weight (Fig. 3B).
Fig. 3.
Coumermycin treatment significantly improved survival in a murine model of melioidosis. Female BALB/c mice were infected intranasally with 500 CFU of Burkholderia pseudomallei K96243 (n = 5 per group). Ceftazidime (CFT) (1200 mg/kg, intraperitoneal) or coumermycin ± l-tyrosine (Tyr) (30 mg/kg, subcutaneous) was administered at 6 h post-infection, with subsequent treatments every 24 h for 4 days thereafter. (A) Survival and (B) body weight was assessed until the end of the experiment at 30 days post-infection. Statistical comparison of survival curves was performed by log-rank (Mantel–Cox) test.
It is notable that successful treatment with coumermycin was achieved with one-fortieth the dose of ceftazidime. Of the surviving units at the termination of the experiment, homogenised lung and spleen tissue were examined for pathology and bacterial burden. All surviving ceftazidime-treated mice showed splenomegaly containing >1 × 107 CFU bacteria, and two of three also had B. pseudomallei bacilli present in their lungs. By comparison, 40% of surviving units from the coumermycin plus l-tyrosine group demonstrated splenomegaly, with no bacteria in their lung. At the end of the experiment, 40% of units from the coumermycin treatment group and 60% from the coumermycin plus l-tyrosine group showed complete organ sterility.
The only previous report of coumermycin being tested against B. pseudomallei in vivo was from a 1970 study that used pig mucin to deplete host immunity [25]. Six doses of coumermycin over 72 h showed significant activity against multiple strains of B. pseudomallei using an intraperitoneal infection model. In the present work, a mode of infection via the aerosol route was simulated, which more closely reflects the clinical situation in endemic countries.
Antimicrobial resistance among many pathogens is a legitimate concern. Success in this regard has been reported by utilising novobiocin combined with rifampicin in a dual-therapy approach that mitigates drug resistance [10]. Novel related antibiotics such as kibdelomycin are also effective against strains of bacteria that are otherwise resistant to aminocoumarins or quinolones [26]. There is also promise in the rational design of novel aminocoumarins through metabolic engineering of the producer strains of Streptomyces sp. [27].
4. Conclusions
Taken together, these data suggest that aminocoumarins deserve renewed attention for their potential to treat melioidosis and potentially other diseases. Some doubts over their use have focussed on their pharmacokinetic properties, potential side effects and the potential for the development of resistance. In the present work, we demonstrate innovative methods for improving their bioactive potential. Given the lack of development of new antibiotics, we cannot afford to ignore the potential benefits that aminocoumarins may have to offer.
Acknowledgment
The authors acknowledge the practical advice and support from Domainex (Saffron Walden, UK) for the pharmacology study.
Funding: This study was funded by the Medical Research Council, UK and the BBSRC.
Competing interests: None declared.
Ethical approval: Animal work was performed in accordance with the Animals (Scientific Procedures) Act of 1986 and the local Ethical Review Committee.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2020.106002.
Appendix. Supplementary materials
References
- 1.Steinmetz I, Wagner GE, Kanyala E, Sawadogo M, Soumeya H, Teferi M. Melioidosis in Africa: time to uncover the true disease load. Trop Med Infect Dis. 2018;3 doi: 10.3390/tropicalmed3020062. pii: E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolim DB, Vilar DC, Sousa AQ, Miralles IS, de Oliveira DC, Harnett G. Melioidosis, northeastern Brazil. Emerg Infect Dis. 2005;11:1458–1460. doi: 10.3201/eid1109.050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melioidosis Currie BJ. an important cause of pneumonia in residents of and travellers returned from endemic regions. Eur Respir J. 2003;22:542–550. doi: 10.1183/09031936.03.00006203. [DOI] [PubMed] [Google Scholar]
- 4.Thibault FM, Hernandez E, Vidal DR, Girardet M, Cavallo JD. Antibiotic susceptibility of 65 isolates of Burkholderia pseudomallei and Burkholderia mallei to 35 antimicrobial agents. J Antimicrob Chemother. 2004;54:1134–1138. doi: 10.1093/jac/dkh471. [DOI] [PubMed] [Google Scholar]
- 5.Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 6.Schweizer HP. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol. 2012;7:1389–1399. doi: 10.2217/fmb.12.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Chen Y, Tabibi S, Alba L, Garber E, Saiman L. Antimicrobial susceptibility and synergy studies of Burkholderia cepacia complex isolated from patients with cystic fibrosis. Antimicrob Agents Chemother. 2007;51:1085–1088. doi: 10.1128/AAC.00954-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates AR, Halls G, Hu Y. Novel classes of antibiotics or more of the same? Br J Pharmacol. 2011;163:184–194. doi: 10.1111/j.1476-5381.2011.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciarretta K, Rottingen JA, Opalska A, Van Hengel AJ, Larsen J. Economic incentives for antibacterial drug development: literature review and considerations from the Transatlantic Task Force on Antimicrobial Resistance. Clin Infect Dis. 2016;63:1470–1474. doi: 10.1093/cid/ciw593. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Cerrato V, Del Prado G, Huelves L, Naves P, Ruiz V, Garcia E. Comparative efficacy of novobiocin and amoxicillin in experimental sepsis caused by β-lactam-susceptible and highly resistant pneumococci. Int J Antimicrob Agents. 2010;35:544–549. doi: 10.1016/j.ijantimicag.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Calabi O. Bactericidal synergism of novobiocin and tetracycline against Pseudomonas pseudomallei. J Med Microbiol. 1973;6:293–306. doi: 10.1099/00222615-6-3-293. [DOI] [PubMed] [Google Scholar]
- 12.Schmutz E, Muhlenweg A, Li SM, Heide L. Resistance genes of aminocoumarin producers: two type II topoisomerase genes confer resistance against coumermycin A1 and clorobiocin. Antimicrob Agents Chemother. 2003;47:869–877. doi: 10.1128/AAC.47.3.869-877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisacchi GS, Manchester JI. A new-class antibacterial—almost. Lessons in drug discovery and development: a critical analysis of more than 50 years of effort toward ATPase inhibitors of DNA gyrase and topoisomerase IV. ACS Infect Dis. 2015;1:4–41. doi: 10.1021/id500013t. [DOI] [PubMed] [Google Scholar]
- 14.Cutuli MA, Petronio Petronio G, Vergalito F, Magnifico I, Pietrangelo L, Venditti N. Galleria mellonella as a consolidated in vivo model hosts: new developments in antibacterial strategies and novel drug testing. Virulence. 2019;10:527–541. doi: 10.1080/21505594.2019.1621649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira TC, de Barros PP, Fugisaki LRO, Rossoni RD, Ribeiro FC, de Menezes RT. Recent advances in the use of Galleria mellonella model to study immune responses against human pathogens. J Fungi (Basel) 2018;4 doi: 10.3390/jof4040128. pii: E128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flatman RH, Eustaquio A, Li SM, Heide L, Maxwell A. Structure–activity relationships of aminocoumarin-type gyrase and topoisomerase IV inhibitors obtained by combinatorial biosynthesis. Antimicrob Agents Chemother. 2006;50:1136–1142. doi: 10.1128/AAC.50.4.1136-1142.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasten G, Lobmann K, Grohganz H, Rades T. Co-former selection for co-amorphous drug–amino acid formulations. Int J Pharm. 2019;557:366–373. doi: 10.1016/j.ijpharm.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Lobmann K, Grohganz H, Laitinen R, Strachan C, Rades T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs—Part 1: preparation, stability and dissolution enhancement. Eur J Pharm Biopharm. 2013;85:873–881. doi: 10.1016/j.ejpb.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Mullins JD, Macek TJ. Some pharmaceutical properties of novobiocin. J Am Pharm Assoc Am Pharm Assoc. 1960;49:245–248. [PubMed] [Google Scholar]
- 20.Wayakanon K, Thornhill MH, Douglas CW, Lewis AL, Warren NJ, Pinnock A. Polymersome-mediated intracellular delivery of antibiotics to treat Porphyromonas gingivalis-infected oral epithelial cells. FASEB J. 2013;27:4455–4465. doi: 10.1096/fj.12-225219. [DOI] [PubMed] [Google Scholar]
- 21.Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–375. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- 22.Farrar MA, Olson SH, Perlmutter RM. Coumermycin-induced dimerization of GyrB-containing fusion proteins. Methods Enzymol. 2000;327:421–429. doi: 10.1016/s0076-6879(00)27293-5. [DOI] [PubMed] [Google Scholar]
- 23.Hirschl AM, Georgopoulos A, Stanek G, Breyer S, Rotter ML. Efficacy of coumermycin, ofloxacin and vancomycin against methicillin-resistant Staphylococcus aureus in vitro and in experimental infections of mice. Zentralbl Bakteriol Mikrobiol Hyg A. 1988;267:541–548. doi: 10.1016/s0176-6724(88)80038-5. [DOI] [PubMed] [Google Scholar]
- 24.Tan GY, Liu Y, Sivalingam SP, Sim SH, Wang D, Paucod JC. Burkholderia pseudomallei aerosol infection results in differential inflammatory responses in BALB/c and C57Bl/6 mice. J Med Microbiol. 2008;57:508–515. doi: 10.1099/jmm.0.47596-0. [DOI] [PubMed] [Google Scholar]
- 25.Grunberg E, Beskid G, Delorenzo WF, Titsworth E. Activity of selected antimicrobial agents against the Pseudomonas pseudomallei infection of mice. Am Rev Respir Dis. 1970;101:623–626. doi: 10.1164/arrd.1970.101.4.623. [DOI] [PubMed] [Google Scholar]
- 26.Singh SB, Dayananth P, Balibar CJ, Garlisi CG, Lu J, Kishii R. Kibdelomycin is a bactericidal broad-spectrum aerobic antibacterial agent. Antimicrob Agents Chemother. 2015;59:3474–3481. doi: 10.1128/AAC.00382-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderle C, Hennig S, Kammerer B, Li SM, Wessjohann L, Gust B. Improved mutasynthetic approaches for the production of modified aminocoumarin antibiotics. Chem Biol. 2007;14:955–967. doi: 10.1016/j.chembiol.2007.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.