Highlights
-
•
Peripheral endothelial dysfunction (PED) predicts adverse outcomes in patients with stage B HF.
-
•
PED was independently associated with stage B HF, even after adjusting for co-variables.
-
•
PED was strongly associated with progression from stage B to overt stage C HF.
-
•
Detecting PED may stratify the risk of HF progression.
Keywords: Peripheral endothelial dysfunction, Heart Failure, Systolic dysfunction, Novel risk factor
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CV, cardiovascular; EF, ejection fraction; HF, heart failure; LV, left ventricular; RH-PAT, reactive hyperemia-peripheral arterial tonometry
Abstract
Background
ACC/AHA guidelines recognize the progressive nature of heart failure (HF). Patients with risk factors (Stage A) are at risk for developing asymptomatic cardiac dysfunction (Stage B), which may then lead to symptomatic HF (Stage C). As such, therapies targeting abnormalities in stages A and B may protect against development of symptomatic HF. peripheral endothelial dysfunction (PED) is an independent predictor of adverse outcomes in patients with stage C HF. The aim of the current study was to evaluate whether PED might be associated with Stage B HF, where therapeutic interventions to prevent progression might be more efficacious.
Methods
We performed a retrospective cross-sectional analysis of patients who were referred for routine cardiovascular evaluation that included an assessment of peripheral endothelial function with reactive hyperemia-peripheral arterial tonometry. Individuals in this study underwent routine clinically indicated echocardiography within 2 months of testing for PED. Patients with clinical HF were excluded.
Results
The study included 355 patients (mean age 51.5 ± 14.6 years, 231 (65.1%) female). There was a significant association between PED and Stage B HF (Odds Ratio (OR) 6.38; P < 0.0001) that persisted after stratifying by sex. In multivariate analyses PED was significantly associated with Stage B HF (OR 5.33; P = 0.0038), and was also associated with progression to overt stage C HF (OR 4.63; P = 0.033).
Conclusion
Peripheral endothelial dysfunction is risk factor for Stage B HF, even in low risk individuals. Further study is warranted to better understand the mechanistic basis of PED in HF to reduce the risk of symptomatic progression.
1. Introduction
Heart failure (HF) is a debilitating syndrome that may is characterized by stages progressing temporally from A (asymptomatic with HF risk factors) to B (asymptomatic with cardiac structural or functional remodeling) to C and D (symptomatic HF). Reduction in left ventricular ejection fraction (EF) without symptoms is an important constituent of Stage B HF. Therapies targeted to abnormalities present in Stage B, such as ACE-inhibitors, reduce the risk of progression to stage C [1]. Identification of other novel modifiable risk factors that promote progression is of great importance to reduce the overall burden of HF.
Endothelial dysfunction precedes atherosclerosis and independently leads to adverse cardiovascular (CV) events [2]. Peripheral endothelial function can be assessed noninvasively by measuring the increase in digital blood flow following upper arm cuff occlusion (reactive hyperemia index, RHI; EndoPAT) [3], [4], [5]. Peripheral endothelial dysfunction (PED) has been shown to be associated with stage C HF [6], [7], where it is also a prognostic marker and potential therapeutic target [8], [9]. We previously demonstrated that coronary microvascular endothelial dysfunction is present in patients with stage B HF [10]. However, it is unknown whether PED is a risk factor for stage B HF. Impaired endothelial-dependent vasodilation, measured using forearm blood flow in response to intra-arterial methacholine, has been shown to be present in individuals with mild HF (NYHA class I and II), suggesting that endothelial dysfunction may be an early finding in HF [11]. We therefore hypothesized that the presence of PED, as detected using RHI with EndoPAT, would be independently associated with Stage B HF in a broad spectrum of patients, and that this would be associated with progression to symptomatic HF.
2. Methods
2.1. Study population
The current study is a retrospective cross-sectional study. Patients were referred by their primary physicians between January 17, 2006 and February 14, 2014 to Mayo Clinic for evaluation of chest pain and/or CV risk that included an assessment of microvascular PED. The decision to undergo testing for PED was at the clinical discretion of the evaluating physician. Only the first test for each patient was included in the final analysis.
Patients who underwent routine, clinically indicated echocardiographic evaluation for suspected cardiac disease, and who did not have evidence of acute and/or chronic HF were included from this study. Stage B HF was defined as an EF < 55% with no prior clinical diagnosis of symptomatic HF [12]. The study was approved by Mayo Clinic International Review Board.
2.2. Endothelial function assessment
Peripheral microvascular endothelial function was assessed using RH-PAT in a designated quiet, temperature controlled and uniformly lit room. Subjects were instructed to fast for 4-hours prior to the study and to abstain from coffee or tobacco on the day of the examination. All vasoactive medications were discontinued for 24-hours prior to testing. A fitted blood pressure cuff was placed on one arm, and the finger cuffs of the Endo-PAT 2000 device (designed by Itamar Medical Ltd, Caesarea, Israel) was placed on the middle finger of each hand [13]. The EndoPAT 2000 is a Food and Drug Administration–approved noninvasive device allows continuous recording of the Peripheral Arterial Tone signal measured from the fingertip by recording finger arterial pulsatile volume changes. The interpretation is operator independent.
The reactive hyperemia protocol consists of the following sequence. A five-minute baseline measurement, after which a blood pressure cuff on the test arm is inflated to 60 mmHg above baseline systolic blood pressure, or at least 200 mmHg for five minutes (occlusion of pulsatile arterial flow confirmed by the reduction of the PAT tracing to zero) after which the cuff is deflated, and the post-deflation PAT tracing is recorded for an additional six minutes. The ratio of the PAT signal after cuff release to baseline is calculated through a computer algorithm automatically normalizing for baseline signal, and indexed to the contralateral arm. A calculated RH-PAT index ≤ 2.0 is a clinically used cut-off value for diagnosis of PED at Mayo Clinic and we have previously demonstrated that RH-PAT index ≤ 2.0 correlated with reduced exercise capacity and greater dyspneic symptoms [14]. Several studies have shown a good association between PED assessment using EndoPAT and other methods, such as intra-arterial acetylcholine infusion and brachial artery Doppler ultrasound following reactive hyperemia [15], [16], [17].
2.3. Patient information
Data was collected on demographic factors (sex and age), and traditional CV risk factors, including hypertension, defined as a known history of or treated hypertension; diabetes mellitus, defined as a known history of or treated diabetes; dyslipidemia, defined as a diagnosis of hyperlipidemia, treatment with lipid lowering therapy, elevated low density lipoprotein (LDL) cholesterol, high triglycerides level or low high density lipoprotein (HDL) cholesterol [18], smoking status and body mass index (BMI) using BMI ≥ 30 kg/m2 as the threshold to define obesity [19].
Because coronary artery disease (CAD) is the leading cause of HF, its presence was categorized according to the following scheme: i) no-known CAD, ii) non-obstructive CAD defined as stenosis of <50% of any coronary artery, and iii) obstructive CAD defined as history of percutaneous coronary intervention or coronary artery bypass graft surgery, or coronary artery stenosis of ≥50% in at least one coronary artery on coronary angiogram or coronary computed tomography angiography. The Framingham score was calculated in all patients (n = 300) who had the required lab data available (total cholesterol and high-density lipoprotein cholesterol levels) and who were in the acceptable age range (19–74 years). Individuals were defined as having a low risk for coronary disease events if the Framingham score was <10 [20]. Echocardiography findings were collected including left ventricular (LV) EF, and right ventricular systolic pressure, where available. Data on laboratory information was also collected including fasting glucose, hemoglobin A1C, lipid profile, and creatinine with which estimated glomerular filtration rate was calculated using the MDRD equation [21]. Information was also collected on medication use, including aspirin, statins, anti-diabetic therapy and anti-hypertensive therapy.
Data for conversion to clinically overt stage C HF was collected in patients who continued to be followed up at Mayo Clinic. The duration of follow-up was the period of time between testing for PED and either the first description of clinically overt HF in the medical chart or the last documented follow-up if the patient did not develop HF.
2.4. Statistical analysis
Patients were categorized based on the presence of normal peripheral endothelial function versus PED. The mean LVEF as well as the frequency of Stage B HF (defined as a LVEF < 55%) was then compared between groups among all patients and after stratifying by sex, CAD status and Framingham based risk. Continuous variables are presented as a mean (standard deviation) where data is normally distributed and as a median (quartile 1, quartile 3) for skewed data. Categorical variables are presented as frequencies (percentages). Differences between groups were analyzed using Student’s t-test for continuous variables and chi-squared test for proportions. A multivariate logistic regression analysis assessing the association between PED and Stage B HF was undertaken after adjusting for age, sex, BMI, Framingham based risk score and CAD status. A sensitivity analysis using LVEF threshold of <40% was also undertaken but did not constitute the primary analysis due to small sample sizes.
The frequency of conversion to clinically overt stage C HF was compared between patients with versus without PED. We undertook a multivariate logistic regression analysis for the association between PED and conversion to clinically overt stage C HF after adjusting for sex, age, BMI, and the presence of CAD. Area under the receiver operator curves were fitted for the relationship between baseline PED and baseline stage B HF, and between baseline PED and progression to clinically overt stage C HF in all patients and after stratifying by sex. A Kaplan-Meier curve evaluating survival without clinically overt stage C HF in patients with versus without PED was analyzed, and the log-rank test was used to test for a significant difference between groups. P-values of less than 0.05 were accepted as significant, and all tests were two-sided. All statistical analyses were performed using JMP 9 software (SAS Institute, Inc., Cary, NC, USA).
3. Results
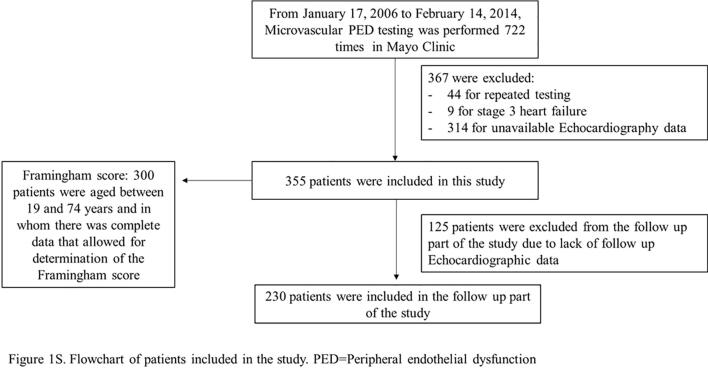
Microvascular PED testing was performed 722 times. After excluding patients for repeated testing (n = 44), those with clinically overt stage C HF (n = 9), and patients for whom echocardiography was unavailable (n = 314), 355 patients were included in this study. Supplemental Fig. 1 shows the flowchart of the patients in the study. In the final study cohort, 231 (65.1%) were female, and the mean age was 51.5 ± 14.6 years.
Two hundred fourteen (71.3%) patients had a Framingham score < 10, out of 300 patients who were aged between 19 and 74 years and in whom there was complete data that allowed for determination of the Framingham score; 169 (84.9%) out of 199 women compared to 45 (44.6%) out of 101 men (P < 0.0001). Patients’ baseline characteristics are summarized in Table 1.
Table1.
Patient characteristics.
| Characteristic | Without PED (N = 195) | With PED (N = 160) | P value |
|---|---|---|---|
| Age - mean ± SD (yr) | 50.9 ± 14.5 | 52.2 ± 14.7 | 0.403 |
| Male sex - no./total no. (%) | 59/195 (30.3) | 65/160 (40.6) | 0.042 |
| Body mass index - mean ± SD | 27.4 ± 5.8 | 29.3 ± 6.4 | 0.004 |
| CAD status - no./total no. (%) | |||
| No known CAD | 136/195 (69.7) | 85/160 (53.1) | 0.001 |
| Non obstructive | 19/195 (9.7) | 20/160 (12.5) | 0.409 |
| Obstructive CAD | 40/195 (20.5) | 55/160 (34.4) | 0.003 |
| Framingham score‡<10 | |||
| All the individuals - no./total no. (%) | 121/162 (74.7) | 93/138 (67.4) | 0.163 |
| Men - no./total no. (%) | 23/47 (48.9) | 22/54 (40.7) | 0.409 |
| Women - no./total no. (%) | 98/115 (85.2) | 71/84 (84.5) | 0.893 |
| Smoking (past or current) - no./total no. (%) | 67/195 (34.4) | 61/160 (38.1) | 0.462 |
| Obesity (BMI ≥ 30 kg/m2) - no./total no. (%) | 54/195 (27.7) | 71/160 (44.4) | 0.001 |
| Dyslipidemia - no./total no. (%) | 126/195 (64.6) | 120/160 (75.0) | 0.035 |
| Type 2 diabetes - no./total no. (%) | 13/195 (6.7) | 20/160 (12.5) | 0.06 |
| Hypertension - no./total no. (%) | 90/195 (46.2) | 78/160 (48.8) | 0.626 |
| Echocardiography findings | |||
| LVEF (%), median (IQR) | 64.0 (60.0–66.00) | 61.5 (58.8–65.0) | 0.001 |
| LVEF range | 40–75 | 35–75 | |
| LVEF < 55% - no./total no. (%) | 5/195 (2.6) | 23/160 (14.4) | <0.0001 |
| Diastolic dysfunction - no./total no. ∫ (%) | 40/189 (21.2) | 42/152 (27.6) | 0.212 |
| RVSP - no./total no. (%), mean ± SD | 27.0 ± 5.1 | 27.4 ± 6.2 | 0.576 |
| Medications - no./total no. (%) | |||
| Aspirin | 88/195 (45.1) | 89/160 (55.6) | 0.049 |
| Statins | 75/195 (38.5) | 74/160 (46.3) | 0.139 |
| Metformin | 9/195 (4.6) | 15/160 (9.4) | 0.076 |
| Sulfonylurea | 2/195 (1.0) | 4/160 (2.5) | 0.284 |
| Insulin | 4/195 (2.1) | 3/160 (1.9) | 0.905 |
| DPP4 inhibitors | 1/195 (0.5) | 1/160 (0.6) | 0.885 |
| Pioglitazone | 1/195 (0.5) | 3/160 (1.9) | 0.226 |
| ACE inhibitors/ARB's | 49/195 (25.1) | 49/160 (30.6) | 0.249 |
| Beta blockers | 68/195 (34.9) | 70/160 (43.8) | 0.088 |
| CCB | 37/195 (19.0) | 44/160 (27.5) | 0.057 |
| Any diuretics | 32/195 (16.4) | 35/160 (21.9) | 0.198 |
| Lab data- no./total no. (%), mean ± SD | |||
| NT-Pro BNP (pg/mL) | 33 /195 (16.9), 70.0 (30.2–163.3) | 45/160 (26.6), 81.5 (43.0–151.5) | 0.246 |
| FPG (mg/dL) | 180/195 (92.3), 99.4 ± 22.0 | 143/160 (89.4), 101.0 ± 19.3 | 0.474 |
| HBA1C (%) | 52/195 (26.7), 5.7 ± 0.9 | 66/160 (41.3), 5.7 ± 0.8 | 0.928 |
| Total cholesterol (mg/dL) | 169/195 (86.7), 191.3 ± 51.4 | 146/160 (91.3), 186.0 ± 45.8 | 0.338 |
| LDL cholesterol (mg/dL) | 166/195 (85.1), 107.6 ± 43.5 | 144/160 (90.0), 104.5 ± 38.6 | 0.5 |
| HDL cholesterol (mg/dL) | 169/195 (86.7), 58.2 ± 18.2 | 147/160 (91.9), 54.5 ± 18.0 | 0.069 |
| Non HDL cholesterol (mg/dL) | 169/195 (86.7), 133.1 ± 51.2 | 146/160 (91.3), 131.6 ± 45.9 | 0.783 |
| Triglycerides (mg/dL) | 169/195 (86.7), 102 (75–150) | 147/160 (91.9), 114 (75–182) | 0.130 |
| Creatinine (mg/dL) in women | 125/195 (64.1), 0.85 ± 0.19 | 89/160 (55.6), 0.84 ± 0.17 | 0.692 |
| Creatinine (mg/dL) in men | 55/195 (28.2), 1.02 ± 0.18 | 59/160 (36.9), 1.08 ± 0.28 | 0.171 |
| eGFR (mL/min/1.73 m2) | 181/195 (92.8), 76.4 ± 18.1 | 147/160 (91.9), 78.4 ± 20.3 | 0.349 |
‡ Framingham score was calculated in individuals with all the required lab data (total cholesterol and high density lipoprotein cholesterol levels) and who were in the acceptable age range (19–74 years).
∫ Total number of patients in whom diastolic function was evaluated.
Abbr eviations: PED - peripheral endothelial dysfunction, SD - Standard deviation, CAD - Coronary artery disease, DLP - Dyslipidemia, DPP4- Dipeptidyl peptidase4, ACE- Angiotensin converting enzyme, ARB- Angiotensin receptor agonist, CCB - Calcium channel blockers, NT-Pro BNP - N-terminal pro brain natriuretic peptide, FPG - Fasting plasma glucose HBA1C- glycated hemoglobin, LDL- low density lipoprotein, HDL - High density lipoprotein eGFR- estimated glomerular filtration rate (based on MDRD equation [21]).
Patients with PED (N = 160, 45.1%) had a higher BMI, percentage of males, and percentage of combined non-obstructive and obstructive CAD, compared to patients without PED. Mean LVEF was also lower in patients with PED compared to those without PED (60.5 ± 6.8% vs 62.8 ± 5.2%; P = 0.0005). There was no significant difference in RVSP and percentage of individuals with diastolic dysfunction, as documented in the echocardiogram report, between groups. Comparison of baseline characteristics between patients with and without echocardiographic assessment is summarized in Supplemental Table 1.
Patients who underwent echocardiography had more significant coronary artery disease and hypertension. NT-proBNP at baseline was higher in patients who underwent echocardiography than patients who didn’t undergo echocardiography.
3.1. The association between PED and HF
Reactive hyperemic index was significantly correlated with LVEF (r = 0.19, P = 0.0004). In a univariate analysis, there was a significant association between PED, categorized as a binary variable, and Stage B HF (Odds Ratio (OR): 6.38; P < 0.001). This relationship persisted in all groups after stratifying by sex and the presence of CAD. After stratifying by Framingham risk score, the association only persisted in individuals with a low Framingham score (<10) (Supplemental Table 2a). Consistent with these findings, PED categorized as a continuous variable using reactive hyperemic index was also significantly associated with Stage B HF (Supplemental Table 2b). If an LVEF < 40% was used as part of the diagnostic criteria for ALVSD, only 2 out of 355 (0.6%) individuals displayed Stage B HF, so further evaluation was not possible due to sample size.
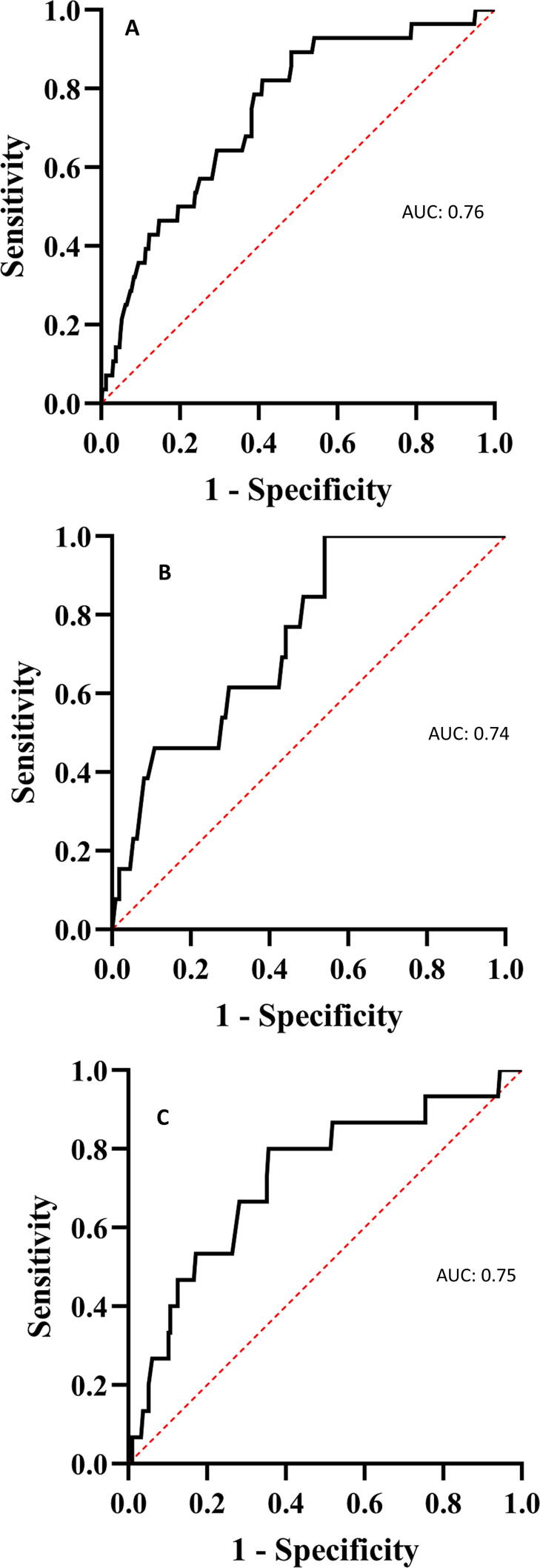
The area under the receiver operator curve for an RH-PAT index ≤ 2.0 in discriminating Stage B HF with an LVEF < 55% in all patients was 0.76 (sensitivity 0.58, specificity 0.82). In men, the area under the curve was 0.74 (sensitivity 0.51, specificity 0.85), and in women the area under the curve was 0.75 (sensitivity 0.61, specificity 0.80) (Fig. 1A–C).
Fig. 1.
Area under the receiver operator curve for the relationship between peripheral endothelial dysfunction and stage B heart failure at baseline. Area under the receiver operator curve for the relationship between peripheral endothelial dysfunction defined as an Reactive-Hyperemia Peripheral Arterial Tonometry index ≤ 2.0 and stage B heart failure at baseline in all patients (A), in men (B), and in women (C).
Supplemental Table 3a shows the results of a multivariate analysis for the association between PED and Stage B HF adjusted for age, sex, BMI, Framingham based risk score (<10 vs. others) and presence of any documented CAD. The variables selected included: age, sex, BMI and CAD status. These variables were selected as each could influence progression to stage C HF. Given the small number in the study sample, and the limitations that this would lead to in the number of variables we could select, we chose only the most relevant clinical factors and CAD status rather than each CV risk factors. Stage B HF was significantly associated with PED in all patients (OR 5.33; P = 0.0038). With the same multivariate analysis after stratifying by sex, this association was found to be significant only among women. Consistent with these findings, PED categorized as a continuous variable using reactive hyperemic index was also significantly associated with Stage B HF in a multivariate analysis adjusting for age, sex, BMI, Framingham based risk score (<10 vs. others) and presence of any documented CAD (Supplemental Table 3b).
3.2. The association between PED and progression of HF
Documented follow-up, including a clinically indicated echocardiogram, was not available in 125 patients (35.2%). Patients without follow-up were significantly younger, had a lower frequency of hypertension, diabetes mellitus and coronary artery disease, had lower values of NT-proBNP, and better renal function compared to those who were underwent follow-up (Supplemental Table 4). There were no significant differences in the percentage of patients without follow-up or in the duration of follow-up between those with versus without Stage B HF. Among those who were followed-up, 15 (6.5%) developed stage C HF. Of these patients, 3 out of 15 had an RH-PAT index > 2.0, and the remaining 12 had an RH-PAT index ≤ 2.0 at baseline.
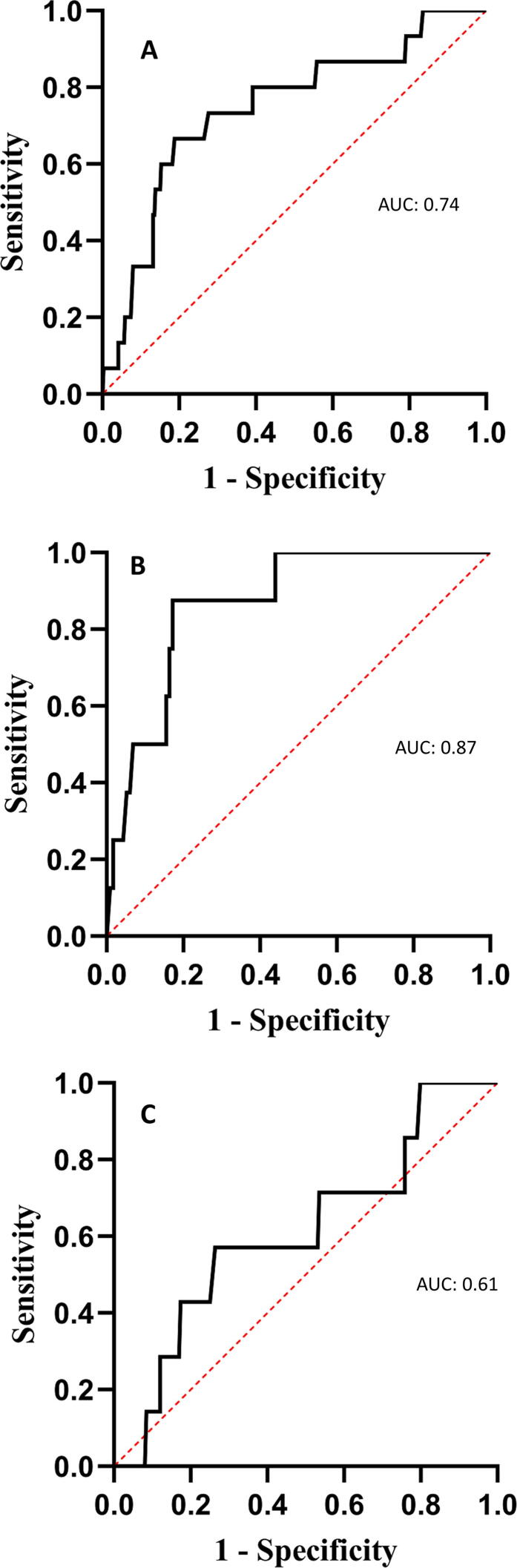
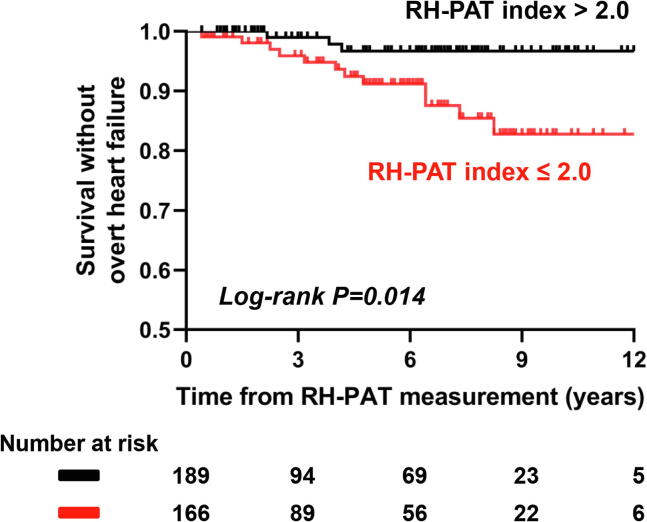
The presence of PED was significantly associated with progression to overt stage C HF in all individuals (OR 5.19; 95% CI 1.44–18.72; P = 0.006). This association remained significant after adjusting for age, sex, BMI and CAD status (OR 4.63; 95% CI 1.13–18.97; P = 0.033). The area under the receiver operator curve for an RH-PAT index < 2.0 in discriminating overt stage C HF at follow-up was 0.74 (sensitivity 0.80, specificity 0.56). In men, the area under the curve was 0.87 (sensitivity 1.00, specificity 0.50), and in women the area under the curve was 0.61 (sensitivity 0.57, specificity 0.58) (Fig. 2A–C). There was a significant difference in survival without overt stage C HF in patients with versus without PED (log rank test P = 0.014) shown with Kaplan-Meier curves in Fig. 3.
Fig. 2.
Area under the receiver operator curve for the relationship between peripheral endothelial dysfunction and clinically overt stage C heart failure at follow-up in all patients. Area under the receiver operator curve for the relationship between peripheral endothelial dysfunction defined as an Reactive-Hyperemia Peripheral Arterial Tonometry index ≤ 2.0 and clinically overt stage C heart failure at follow-up in all patients (A), in men (B), and in women (C).
Fig. 3.
Kaplan-Meier curve showing survival without overt stage C heart failure in all patients with versus without peripheral endothelial dysfunction. Patients with RH-PAT index ≤ 2.0 showed significantly decreased survival without overt stage C heart failure compared to patients with RH-PAT index > 2.0 (log-rank P = 0.014).
4. Discussion
This study demonstrates for the first time that PED is a risk factor for stage B HF. Even after adjusting for confounding variables, there was a significant association between PED and stage B HF. Importantly, the presence of PED was significantly associated with progression from stage B to overt symptomatic (stage C) HF. Further study is warranted to determine the mechanisms by which endothelial dysfunction may leads to ventricular dysfunction and symptomatic HF and to explore how it might be treated to prevent or mitigate this progression.
4.1. The association between HF and PED
The association between PED and overt (Stage C) HF has been shown in several studies. In one study [6] consisting of 362 patients with HFrEF who were followed for 3 years, a significant association was found between PED and HF-related events including the composite of CV death and HF hospitalization. In another study [7], baseline PED predicted HF-related hospitalization in patients who had implanted cardiac resynchronization therapy for HF. In patients with stage C HF with preserved EF (HFpEF), reduced RH-PAT index was also shown to be an independent predictor of CV events [8].
Importantly, the association between PED and stage B HF has not been previously reported. The current study is the first to evaluate this relationship in a large sample of individuals at low risk of cardiac disease. We show that PED is associated with stage B HF, and its presence discriminates stage B HF well in both sexes, highlighting a potential link between peripheral endothelial function and the detection and prognostication of the early stages of HF.
4.2. The mechanism of the association between HF and PED
Stage C HF, irrespective of the presence of CAD, is associated with endothelial dysfunction due to reduced levels of synthesis, release and/or response to nitric oxide [22], [23], [24]. Impaired nitric oxide-mediated vasodilatory reserve contributes to exercise intolerance by increasing left ventricular afterload and abnormal skeletal muscle signaling [14], [25]. Lack of endothelium-derived nitric oxide signaling may be associated with reduced capillary density in cardiac muscle through insufficient activation of vascular endothelial growth factor leading to systolic dysfunction [26]. Impaired endothelium-mediated vasodilation in HF is a generalized abnormality that occurs both in the peripheral and coronary circulation [27].
We have previously demonstrated that endothelial dysfunction in the coronary microvasculature is present in patients with stage B HF [10]. Thus, given the central role for nitric oxide bioavailability and activity in the assessment of PED using indices such as RH-PAT for example [28], the association shown in the current study between PED and stage B HF could be related to the potential role of nitric oxide to the pathophysiology of LV systolic dysfunction and the progression of HF from its early stages. The mechanism underpinning the observed relationship between PED and stage B HF remains uncertain. Nevertheless, the current study supports the concept that a reduced EF, even in the absence of symptomatic or clinically overt HF, is linked to impaired nitric oxide function that can be identified in clinical practice as a reduced RH-PAT suggestive of PED. These patients may benefit from the initiation of therapy targeted at the nitric oxide pathway to address endothelial dysfunction and in turn potentially mitigate the progression to overt HF, though further study is needed to explore this possibility.
4.3. The importance of early diagnosis of depressed LVEF
In the Cardiovascular Health Study, echocardiography was performed in 5,649 subjects [12], 7.3% of which had stage B HF defined by low LVEF without symptoms. Compared to a normal LVEF, those with stage B HF displayed an increased risk of incident HF, CV mortality, and all-cause mortality, albeit with a lower risk compared to individuals with symptomatic LV systolic dysfunction. The risk was highest in patients with the greatest severity of LV dysfunction [29]. In the MESA study, individuals with asymptomatic reduction in LVEF were 8-times more likely to develop Stage C HF and mortality was 2-fold greater [30]. In the SOLVD trial, individuals with reduced LVEF randomized to placebo progressed developed stage C HF at an annual rate of 9.7% [1]. Treatment with enalapril reduced the rate to 6.7%. This finding underlines the value of proactive treatments targeted to the pathophysiology that drives progression toward symptomatic HF, and forms the basis for the recommendation from the ACC/AHA consensus guidelines to treat patients with depressed LVEF with ACE-inhibitor (or ARB) and beta blockers.
The current strategy is to also identify and mitigate other cardiotoxic exposures that could drive HF progression, such as alcohol, illicit drugs, or oncologic treatments. The current data reveal a new and potentially important role for identifying and treating PED, which may also hold promise to help reduce the burden of HF progression. The current data call for additional studies to better understand the progression and the potential role of novel treatments targeting PED in this cohort.
4.4. Study limitations
To our knowledge, this is the first study investigating the association between PED and stage B HF in a large sample of low-risk individuals undergoing evaluation for chest pain and/or CV risk. However, the current study has several limitations, including its retrospective nature and the definitions used for symptomatic and asymptomatic HF. Nevertheless, we did review all clinical reports for each patient included in this study to confirm that the patients were not deemed to have any HF symptoms either at the first evaluation, or after echocardiography. All reports were summarized by supervising senior physicians in the cardiovascular division in Mayo Clinic. Further limitations include the fact that an evaluation for microvascular PED was performed at the discretion of the evaluating physician and not according to prospective study protocol, and limited follow-up in a significant proportion of patients although there were no significant differences in the duration of follow-up between those with and those without Stage B HF. Further study is required to confirm these findings with prospective evaluation and pre-specified follow up visits.
5. Conclusions
The current retrospective study demonstrates that PED is independently associated with Stage B HF defined by asymptomatic reduction in LVEF, and that endothelial dysfunction also predicts progression to overt stage C HF. The clinical and potential therapeutic implications of these findings need to be clarified with larger studies.
CRediT authorship contribution statement
Riad Taher: Conceptualization, Methodology, Formal analysis, Writing - original draft. Jaskanwal D. Sara: Conceptualization, Methodology, Formal analysis, Writing - original draft. Takumi Toya: Formal analysis, Writing - review & editing. Barry A. Borlaug: Writing - review & editing. Lilach O. Lerman: Writing - review & editing. Amir Lerman: Supervision, Writing - review & editing.
Acknowledgements
Nil
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100584.
Contributor Information
Riad Taher, Email: riad.nrh@gmail.com.
Jaskanwal D. Sara, Email: sara.jaskanwal@mayo.edu.
Takumi Toya, Email: toya.takumi@mayo.edu.
Barry A. Borlaug, Email: borlaug.barry@mayo.edu.
Lilach O. Lerman, Email: lerman.lilach@mayo.edu.
Amir Lerman, Email: r_mahamid@rambam.health.gov.il.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Investigators S., Yusuf S., Pitt B., Davis C.E., Hood W.B., Jr., Cohn J.N. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N. Engl. J. Med. 1992;327(10):685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 2.Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 3.Hammadah M., Al Mheid I., Wilmot K., Ramadan R., Alkhoder A., Obideen M., Abdelhadi N., Fang S., Ibeanu I., Pimple P., Mohamed Kelli H., Shah A.J., Pearce B., Sun Y., Garcia E.V., Kutner M., Long Q., Ward L., Bremner J.D., Esteves F., Raggi P., Sheps D., Vaccarino V., Quyyumi A.A. Association Between High-Sensitivity Cardiac Troponin Levels and Myocardial Ischemia During Mental Stress and Conventional Stress. JACC Cardiovasc Imaging. 2018;11(4):603–611. doi: 10.1016/j.jcmg.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P.K., Hermel M., Nelson M.D., Cook-Wiens G., Martin E.A., Alkhoder A.A., Wei J., Minissian M., Shufelt C.L., Marpuri S., Hermel D., Shah A., Irwin M.R., Krantz D.S., Lerman A., Noel Bairey Merz C. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction: Results from the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) study. Int. J. Cardiol. 2018;251:8–13. doi: 10.1016/j.ijcard.2017.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin E.A., Prasad A., Rihal C.S., Lerman L.O., Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J. Am. Coll. Cardiol. 2010;56(22):1840–1846. doi: 10.1016/j.jacc.2010.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujisue K., Sugiyama S., Matsuzawa Y., Akiyama E., Sugamura K., Matsubara J., Kurokawa H., Maeda H., Hirata Y., Kusaka H., Yamamoto E., Iwashita S., Sumida H., Sakamoto K., Tsujita K., Kaikita K., Hokimoto S., Matsui K., Ogawa H. Prognostic Significance of Peripheral Microvascular Endothelial Dysfunction in Heart Failure With Reduced Left Ventricular Ejection Fraction. Circ. J. 2015;79(12):2623–2631. doi: 10.1253/circj.CJ-15-0671. [DOI] [PubMed] [Google Scholar]
- 7.Yufu K., Shinohara T., Ebata Y., Ayabe R., Fukui A., Okada N., Nakagawa M., Takahashi N. Endothelial Function Predicts New Hospitalization due to Heart Failure Following Cardiac Resynchronization Therapy. Pacing Clin. Electrophysiol. 2015;38(11):1260–1266. doi: 10.1111/pace.12698. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama E., Sugiyama S., Matsuzawa Y., Konishi M., Suzuki H., Nozaki T., Ohba K., Matsubara J., Maeda H., Horibata Y., Sakamoto K., Sugamura K., Yamamuro M., Sumida H., Kaikita K., Iwashita S., Matsui K., Kimura K., Umemura S., Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J. Am. Coll. Cardiol. 2012;60(18):1778–1786. doi: 10.1016/j.jacc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Marti C.N., Gheorghiade M., Kalogeropoulos A.P., Georgiopoulou V.V., Quyyumi A.A., Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 2012;60(16):1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 10.Prasad A., Higano S.T., Al Suwaidi J., Holmes D.R., Jr., Mathew V., Pumper G., Lennon R.J., Lerman A. Abnormal coronary microvascular endothelial function in humans with asymptomatic left ventricular dysfunction. Am. Heart J. 2003;146(3):549–554. doi: 10.1016/S0002-8703(03)00364-8. [DOI] [PubMed] [Google Scholar]
- 11.Bank A.J., Lee P.C., Kubo S.H. Endothelial dysfunction in patients with heart failure: relationship to disease severity. J Card Fail. 2000;6(1):29–36. doi: 10.1016/s1071-9164(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 12.Fried L.P., Borhani N.O., Enright P., Furberg C.D., Gardin J.M., Kronmal R.A., Kuller L.H., Manolio T.A., Mittelmark M.B., Newman A. The Cardiovascular Health Study: design and rationale. Ann. Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 13.Martin E.A., Tan S.L., MacBride L.R., Lavi S., Lerman L.O., Lerman A. Sex differences in vascular and endothelial responses to acute mental stress. Clin. Auton. Res. 2008;18(6):339–345. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borlaug B.A., Olson T.P., Lam C.S., Flood K.S., Lerman A., Johnson B.D., Redfield M.M. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2010;56(11):845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonetti P.O., Pumper G.M., Higano S.T., Holmes D.R., Jr., Kuvin J.T., Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Kuvin J.T., Mammen A., Mooney P., Alsheikh-Ali A.A., Karas R.H. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12(1):13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 17.Kuvin J.T., Patel A.R., Sliney K.A., Pandian N.G., Sheffy J., Schnall R.P., Karas R.H., Udelson J.E. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am. Heart J. 2003;146(1):168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 18.Jellinger P.S., Handelsman Y., Rosenblit P.D., Bloomgarden Z.T., Fonseca V.A., Garber A.J., Grunberger G., Guerin C.K., Bell D.S.H., Mechanick J.I., Pessah-Pollack R., Wyne K., Smith D., Brinton E.A., Fazio S., Davidson M. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract. 2017;23(Suppl 2):1–87. doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 19.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res 1998;6 Suppl 2:51S-209S. [PubMed]
- 20.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143-421. [PubMed]
- 21.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kubo S.H., Rector T.S., Bank A.J., Williams R.E., Heifetz S.M. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84(4):1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 23.Katz S.D., Krum H., Khan T., Knecht M. Exercise-induced vasodilation in forearm circulation of normal subjects and patients with congestive heart failure: role of endothelium-derived nitric oxide. J. Am. Coll. Cardiol. 1996;28(3):585–590. doi: 10.1016/0735-1097(96)00204-5. [DOI] [PubMed] [Google Scholar]
- 24.Katz S.D., Khan T., Zeballos G.A., Mathew L., Potharlanka P., Knecht M., Whelan J. Decreased activity of the L-arginine-nitric oxide metabolic pathway in patients with congestive heart failure. Circulation. 1999;99(16):2113–2117. doi: 10.1161/01.cir.99.16.2113. [DOI] [PubMed] [Google Scholar]
- 25.Guazzi M., Samaja M., Arena R., Vicenzi M., Guazzi M.D. Long-term use of sildenafil in the therapeutic management of heart failure. J. Am. Coll. Cardiol. 2007;50(22):2136–2144. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 26.Giordano F.J., Gerber H.P., Williams S.P., VanBruggen N., Bunting S., Ruiz-Lozano P., Gu Y., Nath A.K., Huang Y., Hickey R., Dalton N., Peterson K.L., Ross J., Jr., Chien K.R., Ferrara N. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98(10):5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornig B., Maier V., Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93(2):210–214. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- 28.Nohria A., Gerhard-Herman M., Creager M.A., Hurley S., Mitra D., Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985) 2006;101(2):545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 29.Pandhi J., Gottdiener J.S., Bartz T.M., Kop W.J., Mehra M.R. Comparison of characteristics and outcomes of asymptomatic versus symptomatic left ventricular dysfunction in subjects 65 years old or older (from the Cardiovascular Health Study) Am. J. Cardiol. 2011;107(11):1667–1674. doi: 10.1016/j.amjcard.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeboah J., Rodriguez C.J., Stacey B., Lima J.A., Liu S., Carr J.J., Hundley W.G., Herrington D.M. Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the multi-ethnic study of atherosclerosis (MESA) Circulation. 2012;126(23):2713–2719. doi: 10.1161/CIRCULATIONAHA.112.112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.