Abstract
Background
Sarcoidosis is a systemic inflammatory disorder and can often affect any other organs beyond the heart. Whole-body 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) is used to detect not only cardiac but also extra-cardiac involvement of sarcoidosis. However, the features and clinical impact of extra-cardiac lesions have not yet been fully elucidated. Therefore, this study aimed to clarify these using FDG-PET.
Methods and results
We enrolled 120 consecutive patients with abnormal findings clinically suggesting cardiac sarcoidosis who underwent whole-body FDG-PET. In this study, a patient with suspected cardiac sarcoidosis was defined as one having both clinically suspected findings and FDG-PET positive cardiac uptake. Subsequently, a total of 36 patients with suspected cardiac sarcoidosis were found and analyzed. Extra-cardiac involvement was detected in 35 lesions of 14 patients (39% per patient). In particular, the extra-cardiac lesions were widely distributed throughout the body, and mediastinal/hilar lymph node involvement was most commonly observed. In most of the patients (93% per patient, 13/14), the extra-cardiac lesions were localized in the regions that were considered more accessible with less risk of complication compared with endomyocardial biopsy (EMB). Based on the FDG-PET findings, 8 patients underwent extra-cardiac biopsy without complication, and its diagnostic sensitivity for histological sarcoidosis was high (75%, 6/8). Moreover, FDG-PET-guided extra-cardiac biopsy could confirm histological sarcoidosis in 4 lesions that EMB failed to prove.
Conclusions
Extra-cardiac involvement in patients with suspected cardiac sarcoidosis was relatively high. FDG-PET-guided extra-cardiac biopsy may be safe and useful for the imaging based diagnosis of cardiac sarcoidosis.
Keywords: Cardiac sarcoidosis, Positron emission tomography, Extra-cardiac lesion, Endomyocardial biopsy
1. Introduction
Sarcoidosis is a systemic inflammatory disease of unknown etiology [1], [2]. Because cardiac involvement is considered to be a major contributing factor related to prognosis, its diagnosis has clinical impact [3]. However, the histological confirmation of cardiac sarcoidosis is still challenging because of the limited diagnostic sensitivity of endomyocardial biopsy (EMB) [2]. Whole-body 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) is known to be a useful imaging modality for the detection of not only cardiac but also extra-cardiac involvement of sarcoidosis [4]. A recent study showed that FDG-PET-guided sampling of the mediastinal lymph nodes could be helpful to confirm histological cardiac sarcoidosis [5]. However, the features and clinical impact of the other lymph nodes or extra-cardiac organ involvement detected by FDG-PET remain unknown. Thus, the present study aimed to investigate these using whole-body FDG-PET in patients with suspected cardiac sarcoidosis.
2. Methods
2.1. Patient selection
A total of 120 consecutive patients with clinically suggestive cardiac sarcoidosis who underwent whole-body FDG-PET imaging in Ehime University Hospital from November 2010 to August 2016 were retrospectively studied. A patient with clinically suggestive cardiac sarcoidosis was defined as one having either (1) advanced atrioventricular block or ventricular tachycardia on electrocardiogram, (2) basal thinning of the interventricular septum or ventricular aneurysm by echocardiography, or (3) depressed ejection fraction of the left ventricle (<50%) of unknown etiology. Suspected cardiac sarcoidosis in this study was defined both these clinically suggestive findings and FDG-PET positive cardiac uptake. Patients were excluded if they had the following: (1) positive non-cardiac biopsy for sarcoidosis, (2) treatment with steroid for sarcoidosis or another disease, (3) aim for the evaluation of disease activity, or (4) physiological uptake of FDG in their heart. The investigation conforms with the principles outlined in the Declaration of Helsinki, and was approved by the Institutional Review Board of Ehime University Graduate School of Medicine (approval number: 1611009).
2.2. Image acquisition and analysis
A multi-slice scanner (Discovery PET/CT 600, GE Healthcare, Milwaukee, WI, USA) was used to perform FDG-PET examinations. PET-CT scans were obtained 60 min after the intravenous administration of 3.0–3.7 MBq of FDG per kilogram of body weight. Before the examinations, all patients ate a low-carbohydrate (<5 g) dinner and were asked to refrain from consuming anything except non-caloric beverages to avoid physiological FDG uptake in the heart. In addition, overnight fasting (>18 h) was required until the PET examinations [6]. Whole-body PET studies were evaluated by two experienced cardiologists (HH and CI). According to the previous report, the cardiac involvement of sarcoidosis was visually classified into four patterns: none, diffuse, focal, and focal on diffuse [7]. The FDG uptake lesions obviously unrelated to sarcoidosis (e.g., pneumonia, cancer, and dental caries) were excluded even though it had significant FDG uptake in this study. The presence of late gadolinium enhancement by cardiac magnetic resonance imaging (MRI) was defined as any hyperenhancement in the myocardium. Regarding a diagnostic approach, the Japanese Ministry of Health and Welfare criteria were described based on a previous report [8]. Furthermore, in this study, all EMBs were performed from the right interventricular septum.
2.3. Statistical analysis
Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [9]. Categorical variables were presented as n (%), and continuous variables were displayed as median and interquartile range. Fisher’s exact test and the Mann–Whitney U test were used for categorical and continuous variables, respectively. P values < 0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
A flowchart of patient selection is shown in Fig. 1. Of the 120 patients with clinically suggestive cardiac sarcoidosis who underwent FDG-PET, 29 were excluded due to the exclusion criteria described in the Methods section. Thus, in total, 91 patients were included in this study, and 36 patients with cardiac uptake by FDG-PET were suspected of cardiac sarcoidosis. Of the 36 patients, 14 (39%) had a lesion having extra-cardiac uptake, and the patient and lesion characteristics with and without extra-cardiac involvement were compared (Table 1). Most of the patients enrolled in this study suspected of cardiac sarcoidosis had late gadolinium enhancement by cardiac MRI. Although no significant difference was noted, the extra-cardiac involvement tended to be more common in female, particularly in younger female when divided study population into 2 groups by the median value of age, 61 (Supplemental Figure). The frequency of bilateral hilar lymphadenopathy was significantly higher in patients with extra-cardiac involvement compared with that in those without extra-cardiac uptake. Moreover, the focal-on-diffuse cardiac uptake pattern by FDG-PET was commonly observed in patients with extra-cardiac involvement, while no diffuse uptake pattern was noted. Furthermore, 71% (10/14) of patients with extra-cardiac involvement met the Japan Ministry of Health and Welfare criteria, and EMB confirmed cardiac sarcoidosis in 17% (2/12) histologically, despite that the patients without extra-cardiac involvement failed to prove both clinical and histological cardiac sarcoidosis.
Fig. 1.
Flowchart of patient selection. FDG, fluorodeoxyglucose; PET, positron emission tomography.
Table 1.
Patient and lesion characteristics with or without extra-cardiac uptake.
| Variable | Cardiac uptake, positive |
||
|---|---|---|---|
| Extra-cardiac uptake (+) n = 14 |
(−) n = 22 |
P-value | |
| Age (years) | 59 [56,61] | 67 [55,76] | 0.21 |
| Female | 64% (9/14) | 32% (7/22) | 0.09 |
| Body mass index (kg/m2) | 21 [20,26] | 24 [20,26] | 0.43 |
| Bilateral hilar lymphadenopathy | 29% (4/14) | 0% (0/22) | 0.02 |
| Electrocardiographic findings | |||
| Advanced atrioventricular block | 36% (5/14) | 9% (2/22) | 0.08 |
| History of sustained ventricular tachycardia or ventricular-fibrillation | 36% (5/14) | 36% (8/22) | 1.00 |
| Echocardiographic findings | |||
| Left ventricular ejection fraction (LVEF), (%) | 53 [42,67] | 46 [41,52] | 0.14 |
| Decreased LVEF < 50% | 50% (7/14) | 68% (15/22) | 0.31 |
| Basal thinning of interventricular septum | 57% (8/14) | 50% (11/22) | 0.74 |
| LV morphological abnormality (aneurysm or wall thickening) | 79% (11/14) | 64% (14/22) | 0.47 |
| Cardiac magnetic resonance imaging | |||
| Late gadolinium enhancement, positive | 92% (11/12) | 95% (19/20) | 1.00 |
| 18F-Fluorodeoxyglucose positron emission tomography:cardiac uptake pattern | |||
| Focal | 36% (5/14) | 50% (11/22) | 0.50 |
| Focal on diffuse | 64% (9/14) | 23% (5/22) | 0.02 |
| Diffuse | 0% (0/14) | 27% (6/22) | 0.06 |
| Japan Ministry of Health and Welfare criteria, positive | 71% (10/14) | 0% (0/22) | <0.01 |
| Endomyocardial biopsy, positive | 17% (2/12) | 0% (0/17) | 0.16 |
| Extra-cardiac lesion biopsy, positive | 75% (6/8) | – | – |
Values are expressed as median [IQR] or percentage (number of observation/total number of patients).
3.2. Extra-cardiac lesions of sarcoidosis
The localization of the extra-cardiac lesions is shown in Fig. 2. In total, 35 extra-cardiac lesions in 14 patients (median, 2.5 lesions per patient) were detected by FDG-PET. Although the extra-cardiac lesions were widely distributed, the mediastinal/hilar lymph nodes were the most common sites (71% per patient, 10/14). In 13 patients, 21 extra-cardiac lesions (10 mediastinal/hilar lymph nodes, 7 subcutaneous lymph nodes, 3 muscles, and 1 skin) were considered safer and more accessible compared with EMB. Extra-cardiac lesion biopsy was performed in 8 lesions of 8 patients, and a majority of the lesions (75%, 6/8 lesions) were confirmed to be histological sarcoidosis (Table 1). No biopsy-related complications in the extra-cardiac lesions were observed. Notably, extra-cardiac biopsy could confirm histological sarcoidosis in the 4 lesions that EMB failed to prove.
Fig. 2.
Localization of FDG-PET positive extra-cardiac lesion. The values are expressed as percentage (number of observation/total number of patients: 14).
3.3. Representative case
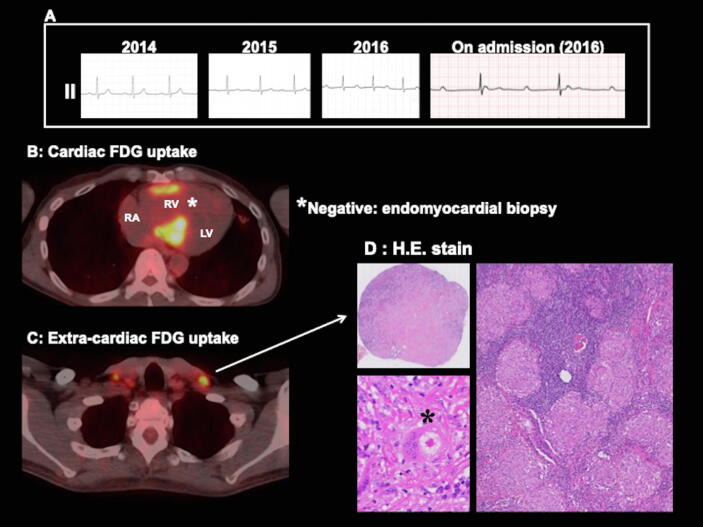
A representative case is shown in Fig. 3, and the detailed information of extra-cardiac biopsy is summarized in Fig. 4. In the representative case (case 1 in Fig. 4), FDG-PET showed cardiac focal uptake with right ventricular involvement and the septum. EMB was performed from the right interventricular septum. Although 10 specimens were sampled and stained, the histological evidence of sarcoidosis could not be found. However, FDG-PET-guided extra-cardiac lesion biopsy from the subcutaneous (cervical) lymph nodes successfully confirmed histological sarcoidosis without complication. Based on the histological confirmation, steroid therapy was started in this patient.
Fig. 3.
Representative case of histological cardiac sarcoidosis confirmed by extra-cardiac biopsy. A: ECG changes showing the gradual prolongation of PR interval. ECG on admission showed complete atrioventricular block. B: FDG-PET showing cardiac uptake with right ventricular involvement. Endomyocardial biopsy from the right ventricular septum failed to prove sarcoidosis. C: FDG-PET showing extra-cardiac uptake in the cervical lymph nodes. D: Extra-cardiac biopsy from the cervical lymph nodes identified the presence of noncaseating granulomas (asterisk = giant cell with asteroid body). RA, right atrium; RV, right ventricule; LV, left ventricule.
Fig. 4.
Summary of all extra-cardiac lesion biopsies and their relation to the result of endomyocardial biopsy.
4. Discussion
The main findings in the present study were as follows: (1) the prevalence of extra-cardiac involvement in suspected cardiac sarcoidosis was 39%, (2) the extra-cardiac lesions on whole-body FDG-PET were widely distributed, (3) most of the patients with extra-cardiac involvement had regions that were suitable for safe and accessible biopsy, and (4) the diagnostic sensitivity of extra-cardiac lesion biopsy was 75%.
EMB is still the gold standard to confirm histological cardiac sarcoidosis. In particular, the clinical significance of EMB increases in the confirmation of isolated cardiac sarcoidosis. However, EMB has two major problems: biopsy-related complication and low diagnostic sensitivity. It has been reported that the complication rate of EMB is very low, especially in EMB from the right ventricular septum [10]. However, every effort should be made to prevent complications because major complications induced by EMB (i.e., cardiac tamponade or complete atrioventricular block) are potentially life-threatening. Sarcoidosis is a systemic disease that can widely involve extra-cardiac organs and lymph nodes. Several reports showed that mediastinal lymph node involvement is most frequently observed [2], [5]. Our data confirm these previous reports. Simonen et al. demonstrated the usefulness of FDG-PET-guided extra-cardiac biopsy from the mediastinal lymph nodes [5]. In the present study, except for 10 mediastinal lymph nodes, 11 extra-cardiac lesions (7 subcutaneous lymph nodes, 3 muscles, and 1 skin) were found to be suitable for biopsy. These lesions have a potential to be superior to the mediastinal lymph nodes in the safety and accessibility of biopsy. Indeed, in this study, 3 of 4 biopsies from the subcutaneous lymph nodes and 1 of 1 biopsy each from the muscle or skin successfully confirmed histological sarcoidosis without complication. Thus, the diagnostic sensitivity of extra-cardiac lesion biopsy was high compared with that of conventional EMB. The reported diagnostic rate by EMB is low, ranging from 1% to 23% [2]. This is because of the patchy and heterogeneous involvement of cardiac sarcoidosis. These results of our study indicate that the detection of extra-cardiac lesion by whole-body FDG-PET may be a promising strategy for its diagnosis. However, in this study, 61% of suspected cardiac sarcoidosis had no extra-cardiac lesion. Recent studies reported the usefulness of imaging- or electrogram-guided biopsy for the diagnosis of cardiac sarcoidosis [11], [12]. Further investigation to improve the diagnostic accuracy of EMB is needed.
In this study, isolated cardiac sarcoidosis may be largely included in 22 patients with suspected cardiac sarcoidosis without extra-cardiac involvement. Therefore, our data of these patients shown in Table 1 may have the possibility to describe the features of isolated cardiac sarcoidosis that EMB failed to prove histologically. These cases did not meet the Japan Ministry of Health and Welfare criteria because the criteria cannot confirm cardiac sarcoidosis in cases without extra-cardiac involvement or positive EMB. An autopsy study revealed the frequency of isolated cardiac sarcoidosis is 40% of patients dying suddenly from cardiac sarcoidosis [13]. However, by the limited of diagnostic sensitivity of EMB, the investigation of isolated cardiac sarcoidosis in the clinical setting is still challenging. A previous report indicated that gender, race, and age may have an influence in each organ involvement [14]. Our data in Asia suggested extra-cardiac involvement was more common in younger-aged female. Contrary to our result, a previous study demonstrated that isolated cardiac sarcoidosis without extra-cardiac involvement is common in female [15]. However, the following concerns should be considered: (1) small number of study population, (2) race difference, and (3) different diagnostic method of extra-cardiac lesion. To clarify this issue, a large-scale study using whole-body FDG-PET is needed.
Due to the difficulty of histological confirmation by EMB, cardiac MRI and FDG-PET are emerging modalities for the diagnosis of cardiac sarcoidosis. In fact, recent studies revealed high diagnostic sensitivity of cardiac sarcoidosis in both modalities [16], [17]. Therefore, these modalities are included in the 2014 Heart Rhythm Society criteria for the clinical diagnosis of cardiac sarcoidosis [3]. However, in the detection of extra-cardiac involvement related to sarcoidosis, whole-body FDG-PET has high superiority compared with MRI because of the limited evaluable range of cardiac MRI. Baughman RP et al. previously reported that almost half of 736 patients had multiple organ involvement in sarcoidosis [14]. One problem is that multiple organ involvement in sarcoidosis is often asymptomatic. Therefore, to detect the disease extent of clinically silent sarcoidosis, assessment with imaging modalities such as whole-body FDG-PET may be required. FDG-PET is useful for not only the diagnosis or extent but also assessment of disease activity contributing to treatment strategy with corticosteroid [4]. Therefore, it is considered that the investigation using FDG-PET in suspected cardiac sarcoidosis has clinical impact.
4.1. Limitations
This study has several limitations that warrant mention. First, FDG uptake does not mean equal the lesion of sarcoidosis. Because of methodological limitations, the lesion of sarcoidosis cannot be distinguished completely from the other inflammatory lesions or malignancies. Second, this study had a relatively small number of participants and was conducted in a single institution. Third, the study design was retrospective. Our findings need to be confirmed in other larger prospective studies.
5. Conclusions
In conclusion, the evaluation of extra-cardiac involvement with whole-body FDG-PET may provide reliable information for the histological confirmation of cardiac sarcoidosis with safer and higher diagnostic sensitivity compared with conventional EMB. We may consider FDG-PET-guided extra-cardiac biopsy prior to EMB in patients with suspected cardiac sarcoidosis, if feasible.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Haruhiko Higashi: Conceptualization, Investigation, Writing - original draft. Shinji Inaba: Methodology, Supervision. Chiharuko Iio: Investigation, Visualization. Katsuji Inoue: Resources. Akiyoshi Ogimoto: Resources. Masao Miyagawa: Methodology, Supervision. Teruhito Mochizuki: Supervision. Shuntaro Ikeda: Supervision, Writing - review & editing. Osamu Yamaguchi: Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100587.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.

Supplemental Figure: Gender distribution and its relation to age with or without extra-cardiac involvement. We divided the patients with suspected cardiac sarcoidosis into 2 groups by the median value of the age (61 years old). The patient having extra-card.
References
- 1.Valeyre D., Prasse A., Nunes H., Uzunhan Y., Brillet P.Y., Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–1167. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 2.Ungprasert P., Ryu J.H., Matteson E.L. Clinical manifestations, diagnosis, and treatment of sarcoidosis. Mayo Clin. Proc. Innov. Qual. Outcomes. 2019;3:358–375. doi: 10.1016/j.mayocpiqo.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnie D.H., Sauer W.H., Bogun F., Cooper J.M., Culver D.A., Duvernoy C.S., Judson M.A., Kron J., Mehta D., Cosedis Nielsen J., Patel A.R., Ohe T., Raatikainen P., Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 4.Akaike G., Itani M., Shah H., Ahuja J., Yilmaz Gunes B., Assaker R., Behnia F. PET/CT in the diagnosis and workup of sarcoidosis: focus on atypical manifestations. Radiographics. 2018;38:1536–1549. doi: 10.1148/rg.2018180053. [DOI] [PubMed] [Google Scholar]
- 5.Simonen P., Lehtonen J., Kandolin R., Schildt J., Marjasuo S., Miettinen H., Airaksinen J., Vihinen T., Tuohinen S., Haataja P., Kupari M. F-18-fluorodeoxyglucose positron emission tomography-guided sampling of mediastinal lymph nodes in the diagnosis of cardiac sarcoidosis. Am. J. Cardiol. 2015;116:1581–1585. doi: 10.1016/j.amjcard.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama R., Miyagawa M., Okayama H., Inoue T., Miki H., Ogimoto A., Higaki J., Mochizuki T. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int. J. Cardiol. 2015;195:180–187. doi: 10.1016/j.ijcard.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 7.Ishimaru S., Tsujino I., Takei T., Tsukamoto E., Sakaue S., Kamigaki M., Ito N., Ohira H., Ikeda D., Tamaki N., Nishimura M. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur. Heart J. 2005;26:1538–1543. doi: 10.1093/eurheartj/ehi180. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis and Granulomatous Disorders [in Japanese] 2007;27:89-102.
- 9.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzmann M., Nicko A., Kühl U., Noutsias M., Poller W., Hoffmann W., Morguet A., Witzenbichler B., Tschöpe C., Schultheiss H.P., Pauschinger M. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation. 2008;118:1722–1728. doi: 10.1161/CIRCULATIONAHA.107.743427. [DOI] [PubMed] [Google Scholar]
- 11.Rogers T., Ratnayaka K., Karmarkar P., Campbell-Washburn A.E., Schenke W.H., Mazal J.R., Kocaturk O., Faranesh A.Z., Lederman R.J. Real-time magnetic resonance imaging guidance improves the diagnostic yield of endomyocardial biopsy. J. Am. Coll. Cardiol. Basic Trans. Sci. 2016;1:376–383. doi: 10.1016/j.jacbts.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang J.J., Hebl V.B., DeSimone C.V., Madhavan M., Nanda S., Kapa S., Maleszewski J.J., Edwards W.D., Reeder G., Cooper L.T., Asirvatham S.J. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. J. Am. Coll. Cardiol. HF. 2014;2:466–473. doi: 10.1016/j.jchf.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavora F., Cresswell N., Li L., Ripple M., Solomon C., Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am. J. Cardiol. 2009;104:571–577. doi: 10.1016/j.amjcard.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 14.Baughman R.P., Teirstein A.S., Judson M.A., Rossman M.D., Yeager H., Jr, Bresnitz E.A., DePalo L., Hunninghake G., Iannuzzi M.C., Johns C.J., McLennan G., Moller D.R., Newman L.S., Rabin D.L., Rose C., Rybicki B., Weinberger S.E., Terrin M.L., Knatterud G.L., Cherniak R. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am. J. Respir. Crit. Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 15.Kandolin R., Lehtonen J., Airaksinen J., Vihinen T., Miettinen H., Ylitalo K., Kaikkonen K., Tuohinen S., Haataja P., Kerola T., Kokkonen J., Pelkonen M., Pietilä-Effati P., Utrianen S., Kupari M. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 16.Smedema J.P., Snoep G., van Kroonenburgh M.P., van Geuns R.J., Dassen W.R., Gorgels A.P., Crijns H.J. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J. Am. Coll. Cardiol. 2005;45:1683–1690. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 17.Youssef G., Leung E., Mylonas I., Nery P., Williams K., Wisenberg G., Gulenchyn K.Y., Dekemp R.A., Dasilva J., Birnie D., Wells G.A., Beanlands R.S. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J. Nucl. Med. 2012;53:241–248. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]