Summary
Humoral responses in COVID-19 disease are often of limited durability, as seen with other human coronavirus epidemics. To address the underlying etiology, we examined postmortem thoracic lymph nodes and spleens in acute SARS-CoV-2 infection and observed the absence of germinal centers, a striking reduction in Bcl-6+ germinal center B cells but preservation of AID+ B cells. Absence of germinal centers correlated with an early specific block in Bcl-6+TFH cell differentiation together with an increase in T-bet+TH1 cells and aberrant extrafollicular TNF-α accumulation. Parallel peripheral blood studies revealed loss of transitional and follicular B cells in severe disease and accumulation of SARS-CoV-2-specific “disease-related” B cell populations. These data identify defective Bcl-6+TFH cell generation and dysregulated humoral immune induction early in COVID-19 disease, providing a mechanistic explanation for the limited durability of antibody responses in coronavirus infections and suggest that achieving herd immunity through natural infection may be difficult.
In Brief
In lymph nodes and spleens in acute COVID-19 there is a striking loss of germinal centers, depletion of Bcl-6+ B cells but preservation of AID+ B cells. A specific block in germinal center type Bcl-6+ T follicular helper cell differentiation explains the loss of germinal centers and the accumulation of non-germinal center derived activated B cells. These data provide a mechanism for the lower quality and lack of durability of humoral immune responses observed during natural infection with SARS-CoV-2 and have significant implications for expectations of herd immunity.
Graphical Abstract
Introduction
Adaptive immunity is initiated in secondary lymphoid organs and is influenced by the milieu generated by the initial activation of the innate immune system. Longitudinal studies on humoral immunity in COVID −19 as well as studies in convalescent subjects indicate that humoral immunity is often short lived and that most SARS-CoV-2 antibodies exhibit limited somatic hypermutation (Long et al., 2020, Robbiani et al., 2020). Understanding how the adaptive immune system is modulated in severe COVID-19 disease thus requires interrogation of secondary lymphoid organs in the acute phase of infection, where these responses are generated, but most studies to date have largely focused on peripheral blood samples.
SARS-CoV-2 infection results in a broad spectrum of clinical manifestations from asymptomatic to rapidly fatal, but the reasons for this heterogeneity are not known. Severely ill patients experience a life-threatening acute respiratory distress syndrome, and, even in an advanced care setting, some patients sustain severe lung damage and succumb early (Zhu et al. 2020; Zhou et al., 2020). Virus is found in the lungs and airways early in infection but not as the disease progresses (Schaefer et al., 2020). Damage-associated molecular patterns (DAMPs) released by infected pneumocytes likely combine with viral pathogen-associated molecular patterns (PAMPs) to activate innate immunity (Vardhana and Wolchok, 2020). The cytokine milieu thus generated would be predicted to influence the induction of lymphocyte activation by antigen conveyed directly in the lymph or by dendritic cells to draining lymph nodes. Viremia likely also leads to the initiation of immune responses in the spleen.
Many of the features of severe human coronavirus disease in COVID-19 and in SARS are strikingly similar. Progressive lymphopenia has been described in SARS-CoV-2 infection (Guan et al., 2020) and the degree of lymphopenia has been correlated with increases in circulating IL-6 and IL-8 (Zhang et al., 2020). Lymphopenia was also observed in SARS at the peak of active disease which was also characterized by cytokine storm and acute respiratory distress (Perlman and Dandekar, 2005). Autopsy studies in SARS showed atrophy of lymphoid organs including lymph nodes, spleen and Peyer’s patches and loss of germinal centers (Gu et al., 2005). Autopsy studies in COVID-19 have also identified splenic white pulp atrophy (Xu et al. 2020, Buja et al., 2020) and lymphocyte depletion in spleen and lymph nodes (Lax et al., 2020). However, numerous viral and non-viral infections do give rise to cytokine storm, acute respiratory distress and lymphopenia (Tisoncik et al., 2012). Splenic white pulp atrophy has also been histo-pathologically demonstrated in Ebola and Marburg disease (Martines et al., 2016, Rippey et. al., 1984) and in H5N1 influenza (Gao et al. 2010, Lu et al., 2008). These data, taken together, suggest that many different viral and infectious triggers can contribute to a similar constellation of immunological phenomena that may drive pathology.
In persons with COVID-19, the magnitude and durability of antibody responses are greater in those with more severe disease (Ju et al., 2020; Amanat et al., 2020) but are often of low magnitude (Robbiani et al., 2020) and appear to lack durability (Long et al., 2020). This may be similar to SARS and MERS where humoral responses were generally not durable except in a few who survived severe infections (Long et al., 2007, Mo et al., 2006, Zumla et al., 2015). Impaired infection-induced protective immunity has also been documented by repeated infections with the human coronaviruses CoV 229E, NL63, OC43 and HKU1 in patients with less severe respiratory tract infections (Galanti et al., 2018). Reinfection could be possibly attributed to viral strain subtypes, but the reason/s for the general lack of durable humoral immune responses to coronaviruses has never been established.
A better understanding of alterations to components of the humoral immune system, especially in secondary lymphoid organs, provides an opportunity to decipher why natural infections with coronaviruses often do not provide durable immunity. A granular analysis of B and T lymphocytes in draining lymph nodes and spleens of SARS or MERS patients was never reported, leaving the underlying basis for the lymphopenia and the general lack of durability of antibody responses in those diseases unresolved. Since COVID-19 disease most significantly affects the lungs, we undertook an analysis of thoracic lymph nodes examining lymphoid architecture and lymphocyte populations using multi-color immunofluorescence, multispectral imaging and cell-cell interaction analyses from the time of disease onset in persons with diverse disease outcomes. Given the viremia has been observed in this illness (Zheng et al., 2020, Lescure et al., 2020) we also interrogated spleens both in the acute and late disease settings and complemented these studies with examination of peripheral blood samples in a separate cohort wherein convalescence could also be studied. Our results identify a striking absence of lymph node and splenic germinal centers and Bcl-6 expressing B cells, defective Bcl-6+ T follicular helper cell generation and differentiation and dysregulated SARS-CoV-2 specific humoral immunity early in COVID-19 disease, providing a mechanistic explanation for the limited durability of humoral immunity and the less robust somatic hypermutation seen in this disease following natural infection.
Results
Absence of germinal centers, loss of Bcl-6+ germinal center B cells but preservation of AID+ B cells in lymph nodes early in COVID-19 disease
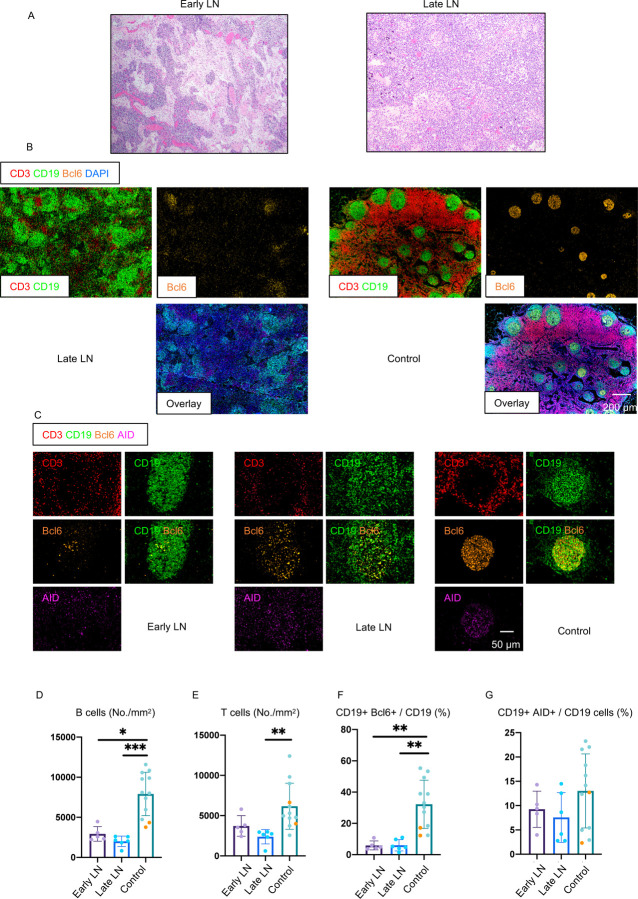
At the Ragon Institute, a human tissue imaging platform has been validated using quantitative high-resolution automated slide-scanning microscopy, using both regular and confocal approaches and multispectral imaging for approximately 110 validated human immune markers (using over 300 different antibodies), in order to interrogate human lymphoid and non-lymphoid organs at the single cell level. These approaches crucially preserve architecture over broad swaths of tissue. Thoracic lymph nodes in severely ill COVID-19 patients who succumbed in less than eight days after admission (the group designated “Early”; less than10 days from the onset of respiratory symptoms; Table S1), displayed a lack of germinal centers and these were also absent in those who succumbed later (15–36 days after admission, categorized as “Late”) (Fig. 1A, B). Quantitation revealed dramatic early loss of both B and T cells, absolute numbers declining to about a third of their non-COVID-19 controls, and this persisted in late disease (Fig.1 D, E), though distinct T and B cell zones could always be clearly discerned. Human control lymph nodes and spleens, even from apparently healthy accident victims, always contain germinal centers possibly because of ongoing adaptive immunity initiated by commensal antigens. The absence of germinal centers in the thoracic lymph nodes of acutely ill COVID-19 patients in whose lungs we have already described very high viral loads (Schaefer et al., 2020) was particularly surprising.
Figure 1. Early loss of germinal centers and Bcl-6 expressing B cells in COVID-19 thoracic lymph nodes.
(A) Hematoxylin–eosin staining of lymph nodes from early (left) and late (right) COVID-19 patients. (B) Low-power images of CD3 (red), CD19 (green), Bcl-6 (orange) and DAPI (blue) staining in a lymph node from a late COVID-19 patient (left) and control (right). (C) Representative multi-color immunofluorescence images of CD3 (red), CD19 (green), Bcl-6 (orange) and AID (purple) staining in lymph nodes from early (left) and late (middle) COVID-19 patients and controls (right). (D and E) Absolute numbers of CD19+ B cells (D) and CD3+ T cells (E) in lymph nodes from early (purple) (n = 5) and late (blue) (n = 6) COVID-19 patients and controls (green and orange) (n = 12). Controls include lymph nodes (n = 2) and tonsils (n = 10). Orange dots mark lymph nodes and green dots mark tonsils. (F and G) Relative proportion of Bcl6+ B cells (F) and AID+ B cells (G) among CD19+ B cells in lymph nodes from early (purple) (n = 5) and late (blue) (n = 6) COVID-19 patients and controls (green and orange) (n = 12). LN= Lymph node. Multiple comparisons are controlled for by Kruskal-Wallis test. Error bars represent mean ±SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
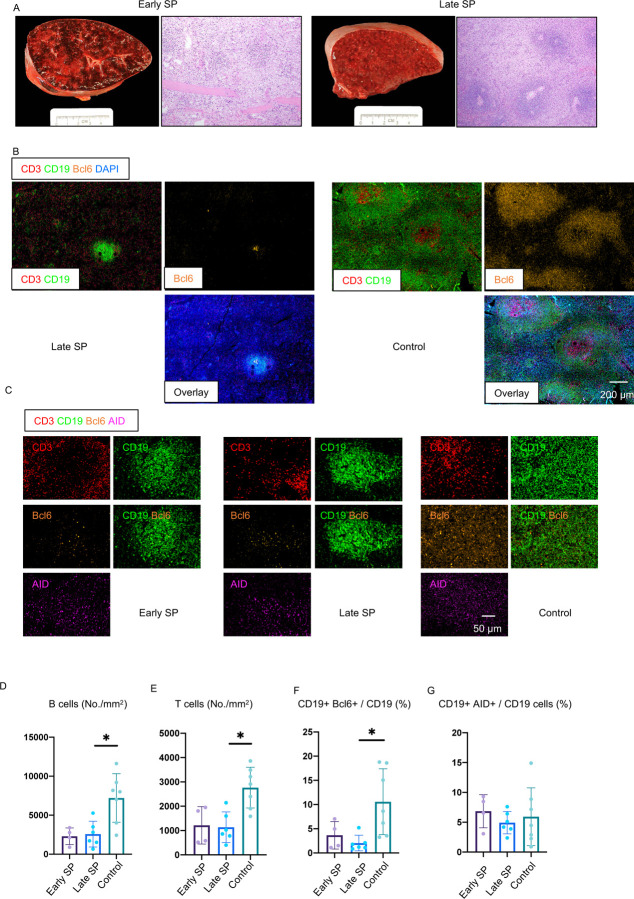
Bcl-6 expressing germinal center B cells were also markedly reduced in COVID-19 but there was a preservation of AID expressing B cells, although these were diffusely distributed compared to controls (Fig. 1C, F, G). Analysis of spleens in this same group of individuals also revealed a preponderance of red pulp and paucity of white pulp (Fig. 2A, B), a marked reduction in B and T cell numbers (Fig. 2B, D, E) and the absence of germinal centers with a marked reduction in Bcl-6+ B cells (Fig. 2C, F). There was, however, very clear and quantitative preservation of AID+ B cells in both early and late splenic tissue. Importantly follicular dendritic cells (FDC) were present in both lymph nodes and spleen in these patients, indicating that the lack of these cells was not contributing to the germinal center defect (Fig. S1). Together these data indicate that early in severe COVID-19 disease, even within ten days of the onset of respiratory symptoms, there is severe attrition in B and T cell numbers and a striking reduction in Bcl-6+ B cells and the loss of germinal centers. Interestingly AID+ B cells are preserved indicating that activated helper T cells are still likely to be in frequent contact with antigen specific B cells.
Figure 2. White pulp attrition, early loss of germinal centers and Bcl-6 expressing B cells in COVID-19 spleens.
(A) Cross-sectional view of whole spleen and hematoxylin–eosin staining from early (left) and late (right) COVID-19 patients. (B) Low-power images of CD3 (red), CD19 (green), Bcl-6 (orange) and DAPI (blue) staining in a spleen from late COVID-19 patient (left) and control (right). (C) Representative multi-color immunofluorescence image of CD3 (red), CD19 (green), Bcl-6 (orange) and AID (purple) staining in spleens from early (left) and late (middle) COVID-19 patients and controls (right). (D and E) Absolute numbers of CD19+ B cells (D) and CD3+ T cells (E) in spleens from early (purple) (n = 4) and late (blue) (n = 6) COVID-19 patients and controls (green) (n = 7). (F and G) Relative proportion of Bcl-6+ B cells (F) and AID+ B cells (G) among CD19+ B cells in spleens from early (purple) (n = 4) and late (blue) (n = 6) COVID-19 patients and controls (green) (n = 7). SP=Spleen. Multiple comparisons are controlled for by Kruskal-Wallis test. Error bars represent mean ±SEM. *p < 0.05.
COVID-related reduction in CD4+CXCR5+Bcl-6+ germinal center T follicular helper cells
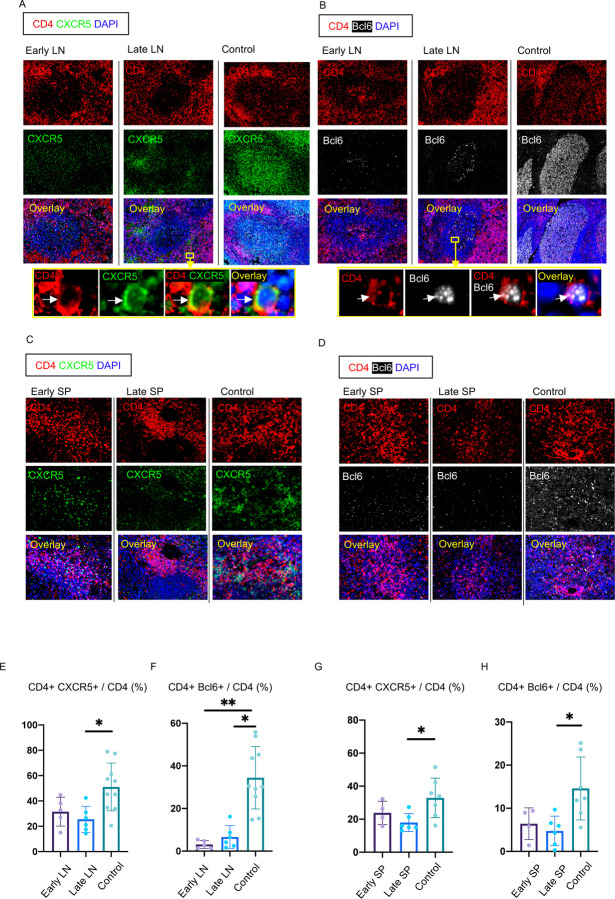
To better understand the absence of germinal centers in COVID-19, we explored the possibility that the tissue milieu might contribute to defective T follicular helper cell differentiation. In both the lymph nodes and spleen, in early as well as late disease, CD4+ CXCR5+ TFH cells were present but diminished in numbers (Fig. 3A, C, E and G) but the decrease in CD4+ Bcl-6+ germinal center type TFH (GC-TFH) cells was striking (Fig. 3B, D, F and H). Tissue quantitation confirmed significant differences for both early and late disease compared to controls. Because these changes were seen both in thoracic lymph nodes and in the spleen these data are consistent with the view that circulating factors in severely ill COVID-19 patients may impair GC-type TFH cell differentiation and thus abrogate the generation of germinal centers. Although in principle phenotypically defined CD4+Bcl-6+ T cells could include both TFH cells and T follicular regulatory cells, we stained cells simultaneously with CD4, CXCR5, FOXP3 and Bcl-6 among other markers and used multispectral imaging to establish that while there were FOXP3+T regs present, there was no overlap in Bcl-6 and FoxP3 expression in COVID-19 secondary lymphoid organs, indicating that there are very few if any T follicular regulatory cells in COVID-19 (Fig. S2A). Because germinal center loss has been described in the context of cytokine storm in mouse models and linked genetically to an abundance of TNF-α (Popescu et al., 2019), we also examined activated secondary lymphoid tissues from controls and COVID-19 lymph nodes for TNF-α expression. In this case we used tonsils from non-COVID infected patients as a control for activated lymphoid tissue. While TNF-α is expressed at low levels in the follicle in controls, in COVID-19 it is expressed very abundantly both inside and outside the follicle (Fig. S3). These data indicate that the differentiation of activated CD4+ T cells into GC-type Bcl-6+TFH cells is specifically blocked in COVID-19. Given the known information, validated by genetic deletion, that implicates excessive TNF-α production in the loss of germinal centers in infected mice, the aberrant and exuberant synthesis of TNF-α at the site of TFH differentiation in COVID-19 lymph nodes may contribute to the lack of germinal centers and the impaired quality and durability of the antibody response to SARS-CoV-2 in this disease.
Figure 3. Loss of germinal center type Bcl-6+ T follicular helper cells in COVID-19 lymph nodes and spleens.
(A) Representative multi-color immunofluorescence image of CD4 (red), CXCR5 (green) and DAPI (blue) staining in lymph nodes from early (left) and late (middle) COVID-19 patients and controls (right). Arrows indicate CD4+ CXCR5+ TFH cells. (B) Representative multi-color immunofluorescence images of CD4 (red), Bcl-6 (white) and DAPI (blue) staining in lymph nodes from early (left) and late (middle) COVID-19 patients and controls (right). Arrows indicate CD4+ Bcl-6+ GC-type TFH cells. (C) Representative multi-color immunofluorescence image of CD4 (red), CXCR5 (green) and DAPI (blue) staining in spleens from early (left) and late (middle) COVID-19 patients and controls (right). (D) Representative multi-color immunofluorescence image of CD4 (red), Bcl-6 (white) and DAPI (blue) staining in spleens from early (left) and late (middle) COVID-19 patients and controls (right). (E and F) Relative proportions of CD4+ CXCR5+ TFH cells (E) and CD4+ Bcl-6+ GC-type TFH cells among CD4+ T cells (F) in lymph nodes from early (purple) (n = 5) and late (blue) (n = 6) COVID-19 patients and controls (green) (n = 10). (G and H) Relative proportions of CD4+ CXCR5+ TFH cells (G) and CD4+ Bcl-6+ GC-type TFH (H) among CD4+ T cells in spleens from early (purple) (n = 4) and late (blue) (n = 6) COVID-19 patients and controls (green) (n = 7). Multiple comparisons are controlled for by Kruskal-Wallis test. Error bars represent mean ±SEM. *p < 0.05; **p < 0.01.
Increased frequency of secondary lymphoid organ TH1 cells in severe COVID-19
We hypothesized that the reduction in GC-TFH cell numbers likely reflects a block in differentiation, and next sought to determine if this reduction was specific to this particular CD4+ T cell subset. We quantitated CD4+ T cell subsets in the lymph nodes and spleens using the well-established transcription factors T-bet, GATA-3, RORγt and FOXP3 as key markers. In contrast to the reduced GC-TFH cell numbers, TH1 cells were consistently increased early and late in both the lymph nodes and spleen, whereas an increase in TH17 cells was more variable (Fig. 4, S4). In contrast, a consistent reduction in TH2 cells was observed (Fig. 4). Late in the disease FOXP3+Tregs made up a large part of the population of CD4+ T cells. Overall there was an increase in secondary lymphoid organ CD4+T cells relative to CD8+ T cells in COVID-19 secondary lymphoid organs though this was variable (Fig. S5). These data indicate that the defect in GC-type TFH cell differentiation is specific and suggest that this defect may indirectly be linked to the strong TH1 response seen in this disease.
Figure 4. TH1 cells are prominent among CD4+T cell subsets in thoracic lymph nodes and spleens.
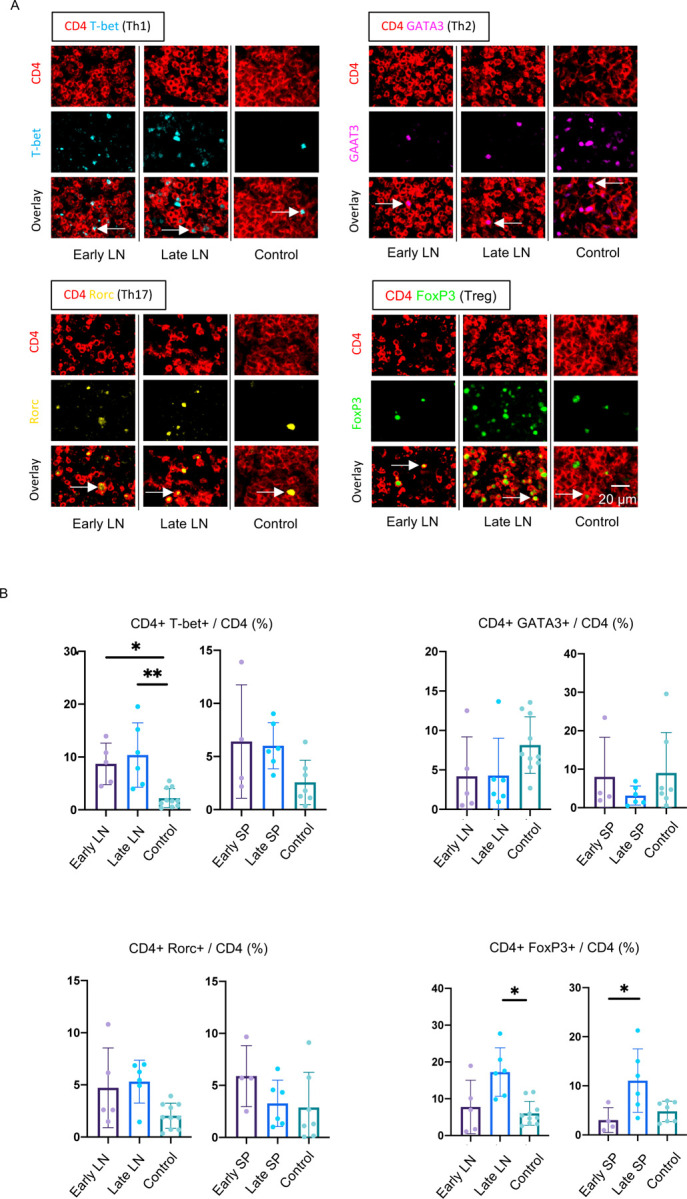
(A) Representative multi-color staining showing TH1, TH2, TH17 and Treg cells in lymph nodes form early (left) and late (middle) COVID-19 patients and control (right). [TH1: CD4+ (red) T-bet+ (light blue)] [TH2: CD4+ (red) GATA3+ (purple)] [TH17: CD4+ (red) RORγ+ (yellow)] [Treg: CD4+ (red) FoxP3+ (green)]. (B) Relative proportions of TH1 (upper left), TH2 (upper right), TH17 (lower left) and Treg (lower right) cells among CD4+ T cells in lymph nodes and spleens from early (purple) (n = 5) and late (blue) (n = 6) COVID-19 patients and controls (green) (n = 10). Multiple comparisons are controlled for by Kruskal-Wallis test. Error bars represent mean ±SEM. *p < 0.05; **p < 0.01.
Follicular and extra-follicular T-B conjugates and IgD−CD27− activated double negative B cells in COVID19 lymph nodes and spleens
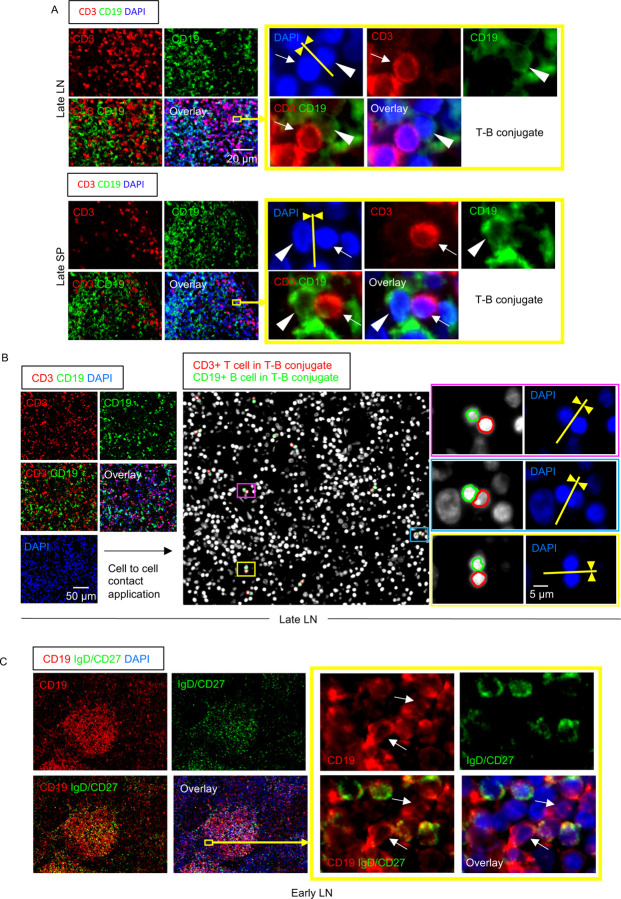
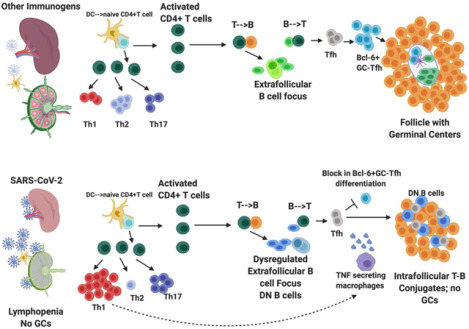
The preservation in COVID-19 of AID+ B cells and the relatively large proportions of CD4+ CXCR5+ TFH cells (that do not express Bcl-6) and TH1 cells, both known to express CD40L, led us to hypothesize that even though there were no germinal centers, there may be frequent T-B conjugates in COVID-19 within follicles as well as in extra-follicular locations. The absence of germinal centers (most germinal center B cells are IgD−CD27− Bcl-6+AID+CXCR5+ CD19+ cells) offered the opportunity to directly ask whether many of the scattered activated AID+ B cells inside and outside follicles in COVID-19 secondary lymphoid organs were also IgD−CD27− “double negative” B cells. These cells are often observed in chronic infectious contexts including in viral infections as well as in autoimmunity (Portugal et al., 2017; Jenks et al., 2019). They are considered to be “disease-related” cells and are generally described as “extra-follicular”, implying they are not derived from the germinal center reaction. At extra-follicular and follicular sites these double negative B cells may be less capable of inducing the differentiation of the Bcl-6+GC-TFH cells that are required to induce germinal centers, possibly because they lack the expression of ICOSL (HAC and SP unpublished observations).
By applying computational tools to tissue imaging, which systematically quantitate the area of cytoplasmic overlap between sets of two cells of two different cell types, and use predetermined cut-offs to assess true cell-cell interaction, we quantified the degree and intimacy of plasma membrane contacts between cells and observed the presence of numerous T-B conjugates in COVID-19 lymph nodes and spleens (Fig. 5A, B). The presence of IgD−CD27− double negative cells both within and outside follicles in COVID-19 lymph nodes and spleens was clearly evident (Fig. 5C). IgG expressing class- switched plasmablasts were prominent both within the follicular and extra-follicular areas (Fig. S2B). These data indicate that in the absence of germinal center formation in COVID-19 an “extra-follicular” type of class-switched B cell response, more typical of disease rather than of long-lasting protection, predominates in secondary lymphoid organs.
Figure 5. Follicular and extra-follicular T-B conjugates and IgD−CD27− B cells in COVID-19 lymph nodes.
(A) Immunofluorescence staining of CD3 (red), CD19 (green) and DAPI (blue) in a lymph node (top) and a spleen (bottom) in late COVID-19 patient. Arrows and arrowheads indicate CD3+ T cells and CD19+ B cells respectively. T cell and B cells formed close and extensive intercellular plasma membrane contacts. (B) Immunofluorescence staining (left panels) and visualization of T-B conjugates (middle and right panels) in a lymph node from a late COVID-19 patient using the Strata Quest cell-to-cell contact application. Masks of the nuclei based on DAPI staining establish the inner boundary of the cytoplasm and the software “looks” outwards towards the plasma membrane boundary. An overlap of at least 3 pixels of adjacent cell markers was required to establish each “contact” criterion. Details are in the Methods section. Nuclei surrounded by red and green lines respectively depict CD3+ T cells and CD19+ B cells in T-B conjugates. (C) Representative multi-color immunofluorescence image of CD19 (red), IgD and CD27 (both in green) and DAPI (blue) staining in a lymph node from an early COVID-19 patient. IgD−/CD27− double negative B cells (red staining with no green overlap) are abundant inside the follicle and also outside. Boxed area depicts some of these cells outside the follicle.
Decreases in early transitional and follicular B cells in severe COVID-19 patients correlate with systemic inflammation
While the interrogation of secondary lymphoid organs from autopsies helps provide snapshots regarding the sites of the induction of the immune response, we sought to obtain information from patients at different stages of the disease, including convalescence, by the use of extended flow cytometry panels for B cells and T cell subsets and by examining peripheral blood from patients. Given the observed accumulation of activated non-germinal center derived B cells such as IgD−CD27− double negative B cells in COVID-19 lymphoid organs, we examined whether similar activated B cell populations with binding specificity for SARS-CoV-2 could be found in the circulation in persons with disparate disease severity.
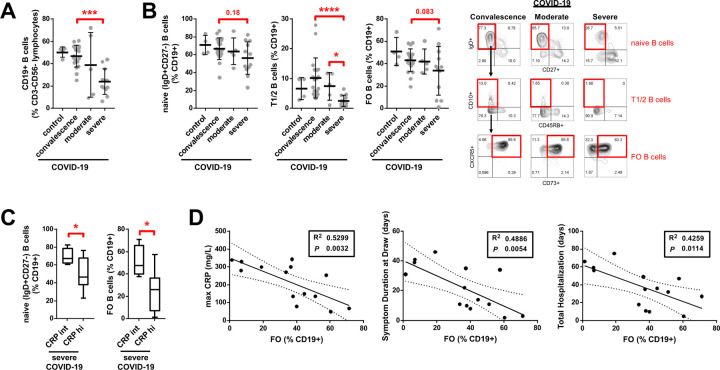
Patients were divided into gradations of severity that included asymptomatic convalescence, moderate clinical disease that did not require an intensive care unit (ICU) admission or mechanical ventilatory support, and severe clinical disease that did require an ICU admission and mechanical ventilatory support (Table S2). Studies on freshly isolated PBMCs revealed that total CD19+ B cells and early transitional T1 and T2 (IgD+CD27−CD10+) B cells were markedly reduced in severely ill as compared to convalescent patients (Fig. 6A, B). Further analysis of severe COVID-19 cases stratified based on clinical laboratory levels of inflammatory markers revealed a decreased frequency of CXCR5+ follicular B cells (IgD+CD27−CD10−CD73+; Farmer et al., 2019) in patients with maximum CRP levels > 200 mg/L (Fig. 6C). The loss of CXCR5+ CD73+ follicular B cells correlated with symptom duration at the time of blood draw and total days of hospital admission (Fig. 6D). These data suggest a direct association between these B cell phenotypic changes in blood and patient clinical morbidity from COVID-19. They indicate that in the milieu of severe COVID-19, B cells either develop very inefficiently in the bone marrow or are possibly generated but acquire an activated phenotype and are lost. This results in a marked reduction in follicular B cells and likely further compounds defects in humoral immunity.
Fig. 6. Decreased early transitional and follicular B cells in the peripheral blood of patients with severe COVID-19.
Quantitation of (A) total CD19+ B cells and (B) naïve, early transitional (T1/2), and follicular (FO) B cell subsets in the peripheral blood of patients with COVID-19 at states of convalescence (n=19), moderate disease (n=5), and severe disease (n=12) as defined by the clinical criteria listed in Table S2 as compared to healthy controls (n=4). Quantitation shown by B cell level for each individual patient with mean, standard deviation, and significance by one-way ANOVA of log % B cell value indicated (*P < 0.05, ***P < 0.001, ****P < 0.0001). Representative dot plots shown (B) with full B cell flow cytometry gating strategy outlined in Fig. S6. (C) Quantitation of naïve and FO B cell frequencies in the peripheral blood of patients with severe COVID-19 subdivided by intermediate (int, < 200 mg/L) versus high (hi, > 200 mg/L) maximum C-reactive protein (CRP) level and shown as box and whiskers plot with significance by Student’s t-test of log % B cell value indicated (*P < 0.05). (D) Association of FO B cell frequency in the peripheral blood of hospitalized COVID-19 patients (moderate and severe disease) with maximum CRP level, symptom duration at blood draw, and total length of hospital stay by linear regression with individual patients, 95% confidence bands, R2 and P values shown.
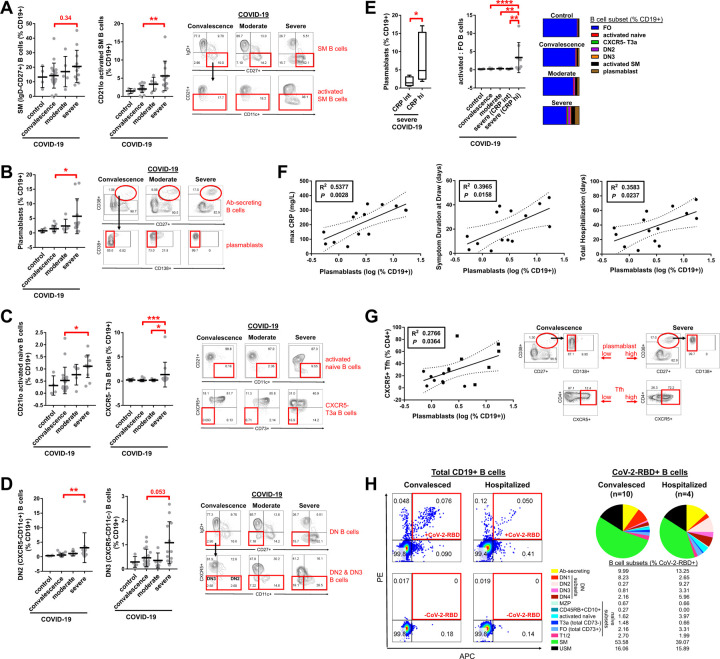
Increases in activated naïve B cells, IgD−CD27−CXCR5− B cells and plasmablasts in severely ill COVID-19 patients correlate with systemic inflammation and are specific for SARS-CoV-2
Within the CD27+ memory B cell compartment, there was a trend towards increased switched memory (IgD−) B cells; activated (CD21lo) total switched memory B cells and plasmablasts were significantly increased (as a proportion of all B cells) in severely ill as compared to convalescent COVID-19 patients (Fig. 7A, B). Within the IgD+CD27− compartment, severely ill patients with COVID-19 showed an increase in a number of disease-related presumed non-germinal center derived activated B cells as compared to convalescent patients. These include activated naïve B cells (IgD+CD27−CD21loCd11chi) (Kaminski et al., 2012) as well as atypical late transitional B cells (IgD+CD27−CD10−CD73−CXCR5−) (Fig.7C). Additionally, IgD−CD27−CXCR5− B cells that include populations described by Kaminski et al. based on the expression of CXCR5 and CD11c were expanded in severe COVID-19 as compared to convalescent patients (Fig.7D). Paralleling the trends observed with the loss of follicular B cells, the gain in circulating plasmablasts correlated with higher CRP levels and increased patient morbidity as measured by symptom duration and length of inpatient hospitalization (Fig. 7E, F). Plasmablast frequency additionally correlated with the numbers of circulating T follicular helper cells (Fig. 7G). We tested whether the activated B cell populations were specific for the SARS-CoV-2 Spike receptor binding domain (RBD) using recombinant RBD labeled separately with APC and PE fluorophores. Cells that stained with both labeled probes were considered authentic RBD-specific B cells. This probe contains a very small fraction of all the potential antibody epitopes in SARS-CoV-2 but is highly specific for this particular virus. All the activated and mainly disease-related populations of relevance in COVID-19 were SARS-CoV-2 specific (Fig. 7H), establishing that they were expanded as a result of an adaptive immune response to this virus. These data establish that the aberrant non-germinal center type activated B cells that accumulate in tissues also accumulate in the blood of severely ill and convalescent COVID-19 patients and these are virus-specific B cells. Given that they bear the hallmarks of not being from germinal centers, they are therefore unlikely to provide optimal or durable humoral immunity.
Fig. 7. Increased activated B cell subsets specific for CoV-2-RBD in the peripheral blood of patients with severe COVID-19.
Quantitation of (A) switched memory (SM) B cells, (B) plasmablasts, (C) activated naïve and CXCR5- late transitional (T3a) B cells, (D) double negative (DN) B cells, and (E, right) total (sum) activated to follicular B cell ratios in the peripheral blood of patients with COVID-19 at states of convalescence (n=19), moderate disease (n=5), and severe disease (n=12) as defined by the clinical criteria listed in Table S2 as compared to healthy controls (n=4). Quantitation shown by B cell level for each individual patient with mean, standard deviation, and significance by one-way ANOVA of log % B cell value indicated (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Representative dot plots shown with full B cell flow cytometry gating strategy outlined in Fig. S6. (E, left) Quantitation of plasmablast frequency in the peripheral blood of patients with severe COVID-19 subdivided by intermediate (int, < 200 mg/L) versus high (hi, > 200 mg/L) maximum C-reactive protein (CRP) level and shown as box and whiskers plot with significance by Student’s t-test of log % B cell value indicated (*p < 0.05). (F) Association of plasmablast frequency in the peripheral blood of hospitalized COVID-19 patients (moderate and severe disease) with maximum CRP level, symptom duration at blood draw, and total length of hospital stay by linear regression with individual patients, 95% confidence bands, R2 and P values shown. (G) Association of plasmablast frequency in the peripheral blood of COVID-19 patients with T follicular helper cell frequency by linear regression with individual patients (squares indicating severe disease), 95% confidence bands, R2 and P values shown. Representative dot plots shown. (H) Summation of all CD19+ B cells binding to CoV-2-RBD shown for n=10 convalesced and n=4 hospitalized COVID-19 patients with % CoV2-RBD reactivity by B cell subset indicated and representative dot plots shown. % CoV-2-RBD reactivity by B cell subset per individual patient further detailed in Fig. S7. DN2 and DN3 Double negative B cells are CXCR5 low, while DN1 and DN4 B cells are CXCR5hi.
Discussion
Long-lasting B cell memory and the highest affinity pathogen-specific antibodies are derived within germinal centers in secondary lymphoid organs. Germinal centers are anatomically structured to facilitate the selection of high affinity B cell with long life spans (Victora and Nussenzweig, 2012). Longevity of such responses exceeds decades for some infectious diseases and when a substantial portion of a population is infected, can contribute to herd immunity. In contrast, antibody responses to SARS-CoV-2 appear to be similar to other human coronaviruses in being short-lived (Long et al., 2020). Understanding the reasons for this decline in responses requires architectural studies of lymphoid organs from patients coupled with cell-cell interaction analyses, as well as approaches to reliably identify, quantify and physically locate the diverse immune cell types that contribute to antibody induction. Using quantitative multicolor immunofluorescence combined with multispectral imaging and cell-cell interaction analyses of autopsy specimens as well as analyses of peripheral blood samples in parallel cohorts with acute SARS-CoV-2 infection, we show evidence for dysregulated humoral immune induction early in COVID-19 including a striking absence of germinal centers in the earliest stages of infection, defective Bcl6+TFH cell generation and aberrant lymphoid TNF-α production.
The absence of Bcl-6+ T follicular helper cells (and the consequent absence of germinal centers) in COVID-19 secondary lymphoid organs provides an explanation for a phenomenon anecdotally observed in autopsies of many different severe viral infections. These findings also provide a mechanistic basis for the recent descriptions of non-durable humoral immune responses, impaired humoral immunity and the low levels of somatic hypermutation in antibodies from convalescent COVID-19 patients (Long et al., 2020, Robbiani et al., 2020). The alteration of the cytokine milieu in secondary lymphoid organs in this disease likely reflects a continuum across the spectrum of disease. While B cell activation, class switching and some low level somatic hypermutation do occur in COVID-19, the germinal center reaction is sub-optimal or totally absent and this will likely, in due course of time, be reflected in less durable class-switched antibody responses similar to those seen in SARS and MERS. These findings have some bearing on concepts such as herd immunity and immunity passports following natural infection with SARS-CoV-2. They strongly underscore the need and relevance of vaccination approaches to the prevention of COVID-19.
Severe infections by many different human viruses result in high levels of circulating cytokines and peripheral lymphopenia, but few studies have examined secondary lymphoid organs where immune responses are generated. Autopsy studies have revealed lymphoid depletion of the spleen and lymph nodes in Ebola, Marburg, and in H5N1 (Martines et al., 2015, Rippey et al., 1984., Lu et al., 2008, Gao et al., 2010). In SARS, lymphoid depletion and loss of germinal centers was also reported (Gu et al., 2005). Autopsy studies in these severe viral infections had not systematically examined lymphoid populations in secondary lymphoid organs. More detailed mechanistic studies of tissues have been undertaken in murine model systems. In a mouse model of severe malaria, germinal center responses were defective, and this was linked to a defect in T follicular helper cell differentiation (Ryg-Cornejo et al., 2016). Ehrlichia muris infection in mice has also been associated with the loss of germinal centers and accumulation of TNF-α, as seen in our studies, and the genetic deletion of TNF-α sufficed to restore germinal centers (Popescu et al, 2019). A mouse immunization model that involved prior generation of specific CD4+ T cell memory prior to infection with lymphocytic choriomeningitis virus generated a severe cytokine storm, splenic shrinkage, loss of germinal centers and bone marrow hypocellularity suggesting that lymphopenia and lymphoid organ abnormalities may be attributed to immune mechanisms rather than being a direct consequence of viral cytolysis (Penaloza-Macmaster, 2015). These studies in mice, taken together, suggest that significant elevation of secreted cytokines and chemokines seen in the context of protozoan, bacterial and viral infections can cause the loss of germinal centers.
The studies of Ryg-Cornejo et al. in murine malaria and our studies in COVID-19 suggest that the observed cytokine and chemokine dysregulation may block germinal center type T follicular helper cell differentiation. The contribution of TNF-α to germinal center formation as well as to the loss of germinal centers is complex and seemingly contradictory (Popescu et al., 2019). Local cytokine concentrations at the site of T follicular helper cell differentiation likely have important consequences for the germinal center reaction. The differentiation of CD4+Bcl-6−CXCR5+ pre-germinal center TFH cells into CD4+Bcl-6+CXCR5+ GC- TFH cells has never been spatially interrogated but most likely occurs extra-follicularly at the T-B interface (Crotty S, 2014; Vinuesa et al., 2016). Based on our findings, we suspect that the very high local levels of TNF-α and possibly other cytokines at this location in COVID-19 lymph nodes, possibly induced downstream of TH1 cell activation, block the final step in T follicular helper cell differentiation. Given that Bcl-6+ B cells, Bcl-6+ TFH cells and Bcl-6+ T follicular regulatory cells are all extremely sparse or absent in COVID-19 secondary lymphoid organs, the formal possibility that excessive TNF signaling (or excessive signaling by some combination of cytokines in the extra-follicular area) negatively impacts the expression of BCL6 either transcriptionally or post-transcriptionally also needs to be investigated. Deeper mechanistic studies can best be pursued in murine models.
Altered extra-follicular B cell activation could potentially also contribute to a defect in T follicular helper cell differentiation observed in SARS-CoV-2 infection. After the initial activation of naive CD4+ T cells by dendritic cells presenting the relevant MHC class II molecule and peptide, along with co-stimulation, these T cells activate antigen-specific B cells that present the same MHC-peptide complex and extra-follicular B cell foci are generated. It is in this vicinity that pre-germinal center T follicular helper cells are first generated, and we have shown in humans (Maehara et al., 2018) as others have in mice (Roco et al., 2019) that most isotype switching actually occurs at this location. In COVID −19 it is likely that some antibody generation occurs extra-follicularly, though we have identified IgG expressing plasmablasts both extra-follicularly as well as in the follicles bereft of germinal centers. Activated B cells expressing ICOSL provide additional differentiation signals to activated CD4+ T cells to acquire high levels of CXCR5, induce the expression of Bcl-6 and migrate into the follicles as fully differentiated germinal center type T follicular helper cells, that set up the germinal center reaction. Perhaps because cytokine alterations lead to the formation of dysfunctional B cells and plasmablasts outside the follicle, activated B cells in COVD-19 may be less capable of inducing cognate T cells to differentiate into germinal center type T follicular helper cells.
It is possible that our investigations may throw some light on the mechanisms of disease progression in severe COVID-19, although a deeper understanding will likely await the acquisition of more knowledge and the development of suitable animal models. The evasion of the anti-viral aspects of innate immunity and the overly aggressive activation of inflammation by the virus likely results in an altered milieu that contributes both to the relative attenuation of CD8+ T cell immunity and prevents the generation of Bcl-6+ T follicular helper cells. As a result of the latter, the early development of high affinity antibodies that could contribute to some attenuation of viral spread may be compromised. Whether the increased CD4+/CD8+ T cell ratio we observe in lymph nodes and spleens of COVID-19 patients reflects selective activation of CD4+T cells or a preferential depletion of CD8+ T cells is unclear. Why certain individuals are more prone to initial more aggressive and destructive immune responses also remains a very difficult question to address at this time. Based on our analyses, severe disease does not appear to be due to a paucity of regulatory T cells in secondary lymphoid organs at the earlier stages of the disease, though our approaches do not interrogate Treg function. The striking relative accumulation of FoxP3+ regulatory T cells that we observe late in disease could possibly reflect a homeostatic mechanism for the resolution of infection. More likely it reflects the reduced overall numbers of recirculating T cells entering lymphoid organs and the recurring activation of naive CD4+ T cells into activated subsets that egress these lymphoid organs while lymphoid organ-resident regulatory T cells perhaps do not leave these sites and therefore appear to accumulate in lymph nodes and the spleen.
In summary, even in acutely ill COVID-19 patients, at a time when they have extremely high viral loads and abundant virus has been demonstrated in their lungs, there is a striking absence of germinal centers associated with a marked reduction of germinal center B cells, but preservation of AID-expressing B cells, Thus though there is robust T cell mediated activation of B cells, germinal centers do not form. The robust activation of non-germinal center type B cell responses does not give rise to long-lived memory or high affinity B cells. The underlying basis for the loss of germinal centers is best explained by the striking failure of differentiation of Bcl-6+ T follicular helper cells likely because of dramatic changes in the extra-follicular cytokine milieu driven by TH1 cells and the aberrant local production of TNF-α in lymphoid organs. We believe these findings will be relevant to a range of human viral and non-viral diseases in which there is a cytokine storm, a better understanding of which will require a granular function-focused analysis of the architecture and composition of lymph nodes and spleens. We predict that a broadly applicable common mechanistic basis similar to what we describe here will be elucidated in future studies on MERS, H1N1, Ebola, Marburg and other viral diseases.
Supplementary Material
Highlights.
In lymph nodes and spleens in acute COVID-19, germinal centers, the sites at which a Darwinian genetic diversification and selection process normally generates high affinity and durable antibodies, are lost
Bcl-6+ GC B cells are very sparse but AID+B cells are preserved
A specific block in Bcl-6+ T follicular helper cell differentiation was observed
TH1 cells were predominant and aberrant TNF-α production was seen at the site of TFH cell differentiation in secondary lymphoid organs
T-B conjugates are common and non-germinal center derived activated disease-related B cells accumulate in COVID-19 lymphoid organs
Similar SARS-CoV-2 specific activated B cells accumulate in the blood of patients
Acknowledgments:
This work was supported by NIH U19 AI110495 to SP, NIH R01 AI146779 to AGS, NIH R01AI137057 and DP2DA042422 to DL, BMH was supported by NIGMS T32 GM007753, TMC was supported by T32 AI007245. Funding for these studies from the Massachusetts Consortium of Pathogen Readiness, the Mark and Lisa Schwartz Foundation and Enid Schwartz is also acknowledged.
REFERENCES
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., et al. (2020). A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buja L.M., Wolf D.A., Zhao B., Akkanti B., McDonald M., Lelenwa L., Reilly N., Ottaviani G., Elghetany M.T., Trujillo D.O., et al. (2020). The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol 48, 107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W.C., Liu W., Zhang P.H., Zhang F., and Richardus J.H. (2007). Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med 357, 1162–1163. [DOI] [PubMed] [Google Scholar]
- Crotty S. (2014). T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J.R., Allard-Chamard H., Sun N., Ahmad M., Bertocchi A., Mahajan V.S., Aicher T., Arnold J., Benson M.D., Morningstar J., et al. (2019). Induction of metabolic quiescence defines the transitional to follicular B cell switch. Sci Signal 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanti M., Birger R., Ud-Dean M., Filip I., Morita H., Comito D., Anthony S., Freyer G.A., Ibrahim S., Lane B., et al. (2019). Longitudinal active sampling for respiratory viral infections across age groups. Influenza Other Respir Viruses 13, 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Dong L., Dong J., Wen L., Zhang Y., Yu H., Feng Z., Chen M., Tan Y., Mo Z., et al. (2010). A systematic molecular pathology study of a laboratory confirmed H5N1 human case. PLoS One 5, e13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., et al. (2005). Multiple organ infection and the pathogenesis of SARS. J Exp Med 202, 415424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. (2020). Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks S.A., Cashman K.S., Woodruff M.C., Lee F.E., and Sanz I. (2019). Extrafollicular responses in humans and SLE. Immunol Rev 288, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., et al. (2020). Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. [DOI] [PubMed] [Google Scholar]
- Kaminski D.A., Wei C., Rosenberg A.F., Lee F.E., and Sanz I. (2012). Multiparameter flow cytometry and bioanalytics for B cell profiling in systemic lupus erythematosus. Methods Mol Biol 900, 109–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., Vander K., Bargfrieder U., and Trauner M. (2020). Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome: Results from a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., et al. (2020). Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis 20, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., et al. (2020). Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26, 845–848. [DOI] [PubMed] [Google Scholar]
- Lu M., Xie Z.G., Gao Z.C., Wang C., Li N., Li M., Shao H.Q., Wang Y.P., and Gao Z.F. (2008). [Histopathologic study of avian influenza H5N1 infection in humans]. Zhonghua Bing Li Xue Za Zhi 37, 145–149. [PubMed] [Google Scholar]
- Maehara T., Mattoo H., Mahajan V.S., Murphy S.J., Yuen G.J., Ishiguro N., Ohta M., Moriyama M., Saeki T., Yamamoto H., et al. (2018). The expansion in lymphoid organs of IL4(+) BATF(+) T follicular helper cells is linked to IgG4 class switching in vivo. Life Sci Alliance 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martines R.B., Ng D.L., Greer P.W., Rollin P.E., and Zaki S.R. (2015). Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol 235, 153–174. [DOI] [PubMed] [Google Scholar]
- Mo H., Zeng G., Ren X., Li H., Ke C., Tan Y., Cai C., Lai K., Chen R., Chan-Yeung M., et al. (2006). Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirology 11, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza-MacMaster P., Barber D.L., Wherry E.J., Provine N.M., Teigler J.E., Parenteau L., Blackmore S., Borducchi E.N., Larocca R.A., Yates K.B., et al. (2015). Vaccine-elicited CD4 T cells induce immunopathology after chronic LCMV infection. Science 347, 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., and Dandekar A.A. (2005). Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol 5, 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu M., Cabrera-Martinez B., and Winslow G.M. (2019). TNF-alpha Contributes to Lymphoid Tissue Disorganization and Germinal Center B Cell Suppression during Intracellular Bacterial Infection. J Immunol 203, 2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S., Obeng-Adjei N., Moir S., Crompton P.D., and Pierce S.K. (2017). Atypical memory B cells in human chronic infectious diseases: An interim report. Cell Immunol 321, 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippey J.J., Schepers N.J., and Gear J.H. (1984). The pathology of Marburg virus disease. S Afr Med J 66, 50–54. [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., et al. (2020). Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roco J.A., Mesin L., Binder S.C., Nefzger C., Gonzalez-Figueroa P., Canete P.F., Ellyard J., Shen Q., Robert P.A., Cappello J., et al. (2019). Class-Switch Recombination Occurs Infrequently in Germinal Centers. Immunity 51, 337–350 e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryg-Cornejo V., Ioannidis L.J., Ly A., Chiu C.Y., Tellier J., Hill D.L., Preston S.P., Pellegrini M., Yu D., Nutt S.L., et al. (2016). Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell Rep 14, 68–81. [DOI] [PubMed] [Google Scholar]
- Schaefer I.M., Padera R.F., Solomon I.H., Kanjilal S., Hammer M.M., Hornick J.L., and Sholl L.M. (2020). In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Njau M.N., Verhoeven D., Chandran S., Nadler S.G., Hasday J., and Farber D.L. (2009). Costimulation modulation uncouples protection from immunopathology in memory T cell responses to influenza virus. J Immunol 182, 6834–6843. [DOI] [PubMed] [Google Scholar]
- Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., and Katze M.G. (2012). Into the eye of the cytokine storm. Microbiol Mol Biol Rev 76, 16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora G.D., and Nussenzweig M.C. (2012). Germinal centers. Annu Rev Immunol 30, 429457. [DOI] [PubMed] [Google Scholar]
- Vinuesa C.G., Linterman M.A., Yu D., and MacLennan I.C. (2016). Follicular Helper T Cells. Annu Rev Immunol 34, 335–368. [DOI] [PubMed] [Google Scholar]
- Xu X., Chang X.N., Pan H.X., Su H., Huang B., Yang M., Luo D.J., Weng M.X., Ma L., and Nie X. (2020). [Pathological changes of the spleen in ten patients with new coronavirus infection by minimally invasive autopsies]. Zhonghua Bing Li Xue Za Zhi 49, E014. [DOI] [PubMed] [Google Scholar]
- Zhang X., Tan Y., Ling Y., Lu G., Liu F., Yi Z., Jia X., Wu M., Shi B., Xu S., et al. (2020). Viral and host factors related to the clinical outcome of COVID-19. Nature. [DOI] [PubMed] [Google Scholar]
- Zheng H.Y., Zhang M., Yang C.X., Zhang N., Wang X.C., Yang X.P., Dong X.Q., and Zheng Y.T. (2020). Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol 17, 541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., et al. (2020). Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 369, m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. (2020). A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 382, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Hui D.S., and Perlman S. (2015). Middle East respiratory syndrome. Lancet 386, 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.