Abstract
Background
Integration of Chinese medical drugs (CMD) and western medicine (WM) has been widely used in the treatment of Coronavirus Disease 2019 (COVID-19). This systematic review aimed to evaluate the efficacy and safety of CMD for COVID-19.
Method
A literature search was performed in six databases from injection to June 2020. Both randomized controlled trials (RCTs) and quasi-RCTs were considered as eligible. The quality of included RCTs were assessed by Cochrane Risk of Bias Tool, and Review Manager 5.3 software was used to do meta-analysis.
Result
Eleven studies with 1259 patients were included in this study. CMD included herbal decoction and Chinese patent medicine. The methodological quality was evaluated as generally unclear. The results of meta-analysis showed that the integration of CMD and WM had better efficacy than WM in number of patients turned to severe and critical type (RR = 0.47, 95% CI=[0.32, 0.69], P < 0.0001), length of hospital stay (MD= -7.95, 95% CI=[-14.66, -1.24], P = 0.02), defervescence time (MD= -1.20, 95% CI=[-2.03, -0.38], P = 0.004), cough resolution rate (RR = 1.37, 95% CI=[1.15, 1.64], P = 0.0004), fatigue resolution rate (RR = 1.37, 95% CI=[1.02, 1.83], P = 0.04), and tachypnea resolution rate (RR = 2.20, 95% CI=[1.11, 4.39], P = 0.02). As for safety, there was no significant difference between two groups.
Conclusion
CMD may bring potential benefit to patients suffered from COVID-19. However, the quality of included trials is not good enough. High quality study with core outcome set are still required.
Keywords: Coronavirus disease 2019, Integrative medicine, Chinese medical drug, Systematic review, Core outcome set, Meta analysis
Introduction
Coronavirus Disease 2019 (COVID-19) is a pandemic infection disease caused by 2019 novel coronavirus (2019-nCoV).1 The pathogenesis of COVID-19 is similar to pneumonia induced by other viruses. Specifically, 2019-nCoV infection can cause systemic inflammation, fever, hypoxia, electrolyte imbalance, acid-base disturbance and even shock.2, 3 Overaction of immune system lead to cytokine storm and excessive oxidative stress may be responsible for disease progression and even death.4, 5 COVID-19 can be classified into mild, ordinary, severe and critical types based on disease severity. The clinical features of mild and ordinary disease include fever, dry cough, fatigue, etc, or without prominent symptoms.6, 7, 8 On the other hand, severe and critical COVID-19 may develop acute respiratory distress syndrome, septic shock, refractory metabolic acidosis, coagulation dysfunction and multi -organ failure, and even death.9, 10, 11
In China, COVID-19 has been well controlled, with a total of 83,537 confirmed cases and 4634 deaths (July 2, 2020).12 Compared with the treatment strategies adopted by different countries, it was possible that integration of Chinese medical drugs (CMD) and western medicine (WM) helped the disease control in China.13 According to the data obtained from the National Administration of Traditional Chinese Medicine, 91.5% of confirmed cases were treated with CMD in China.14 Study showed CMD could relieve symptoms, prevent disease progression and reduce mortality rate.15
Core outcome set (COS) refers to an agreed-upon standard set of outcomes that should be measured and reported across different studies. The establishment of a COS helps to unify and compare results obtained from different clinical trials. It can improve the practicability, comparability, and transparency of the results.16, 17 In March 2020, Chinese Clinical Trials Core Outcome Set Research Center has published a COS for Clinical Trials on COVID-19 (COS-COVID), in order to regularize the clinical trial of COVID-19.18
In order to provide high-quality and practicable evidence for CMD in the treatment of COVID-19 and future research, based on COS -COVID, we conducted systematic review and meta-analysis on studies of CMD in treatment of COVID-19.
Methods
Study registration
The protocol of this systematic review was registered on PROSPERO (CRD42020176282), and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).19
Ethical statement
The ethical approval was waived considering this is a literature review article.
Criteria for study inclusion
-
1
Type of study: Randomized Controlled Trials (RCTs) and quasi- RCTs, published in English or Chinese.
-
2
Patients: Patients diagnosed as COVID-19 according to relevant diagnostic criteria (Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia Trial Version 5-720, 21, 22). No limitation of age, gender, nationality, birthplace and ethnicity.
-
3
Intervention and Comparison: CMD including herbal decoction and Chinese patent medicine were all analyzed as one entity in this study. The comparisons were set as: (1) CMD or CMD + WM compared with WM. (2) CMD or CMD + WM compared placebo. The type of WM used in each comparison remained the same. The difference of dosage was not powered to be detected in this study.
Outcome
-
1
Primary outcome: Composite events (number of patients turned to severe and critical type, all-cause death); Rate of 2019-nCoV reverse transcription- polymerase chain reaction (RT-PCR) turned to negativity; Length of hospital stay; Arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2); Duration of mechanical ventilation; Clinical symptoms score. The primary outcome were chosen from COS-COVID (Supplement 1).18
-
2
Secondary outcome: Defervescence time/rate; Cough resolution time/rate; Fatigue resolution time/rate; tachypnea resolution time/rate; Diarrhea resolution time/rate; Body pain resolution time/rate.
-
3
Adverse event: Allergy, digestive tract dysfunction; Blood, urine and stool tests abnormalities; Impaired heart, liver and kidney function.
Criteria for study exclusion
-
(1)
Incorrect or incomplete data.
-
(2)
Received integration of CMD and WM in control group.
-
(3)
Repeated publications, only the first was included.
Information source and search strategy
Six databases were searched to retrieve clinical trials, including SinoMed, China National Knowledge Infrastructure (CNKI), WanFang Database, PubMed, Embase and Cochrane Library about CMD in the treatment of COVID-19, from injection to June 5. 2020. The keywords applied in the search were: COVID-19, coronavirus disease 2019, novel coronavirus disease, SARS-CoV-2, novel coronavirus infection, integrative medicine, traditional Chinese medicine, herbal medicine, herbal injection, Chinese patent medicine. Taking PubMed as an example, the combination of MeSH terms and free words were used to develop literature search strategy (Supplement 2).
Study screening and data extraction
The study screening procedures were as follows: (1) Read title and abstract of the manuscripts and eliminated studies based on the inclusion and exclusion criteria. (2) The full text would be examined if additional information was required in the screening process. We designed a standardized data extraction sheet, the extract content included: title, author, study time, sample size, age and gender of patients, diagnosis, duration of disease, intervention measures, course of treatment and follow-ups, outcomes. When complete information could not be obtained, we contacted the author of the literature by email and excluded the study if failed. Study screening and data extraction were conducted by two researchers independently. Disagreement was resolved after consensus or consultant with the third researcher.
Quality assessment
The quality of RCTs were assessed by Cochrane Risk of Bias (ROB) assessment tool,23 which included six aspects: (1) Random sequence generation, (2) Allocation concealment, (3) Blinding of patients and personnel, (4) Incomplete outcome data, (5) Selective reporting, (6) Other bias. The assessment results were presented as “low risk”, “high risk” and “unclear risk”. The quality assessment was conducted by two researchers independently. Disagreement was resolved after consensus or consultant with the third researcher.
Statistical analysis
Review Manager 5.3 was used to do meta-analysis. Dichotomous data were presented as risk ratio (RR), continuous data were calculated as mean difference (MD), clinical symptoms score was calculated as Std mean difference (SMD), and all data was presented with 95% confidence intervals (CIs). Due to the variability between included studies, random effect model was selected to pool the data. The statistical heterogeneity was assessed by P value and I² test. If I²≤50% and P > 0.05, the heterogeneity among included studies was considered low. If I²>50% or P ≤ 0.05, the heterogeneity among included studies was considered high. If available data were enough, subgroup analysis or sensitivity analysis would be conducted. The publication bias was evaluated by funnel plot.
Certainty of evidence
The certainty of evidence was assessed by Grading of Recommendations Assessment, Development and Evaluation (GRADE), which considered five reasons: (1) Risk of bias, (2) Imprecision, (3) inconsistency, (4) Indirectness, (5) Publication bias. The main findings were presented by the Summary of Findings table. GRADE Pro GDT software (http://gradepro.org) was used to create the Summary of Findings table.
Result
Study screening
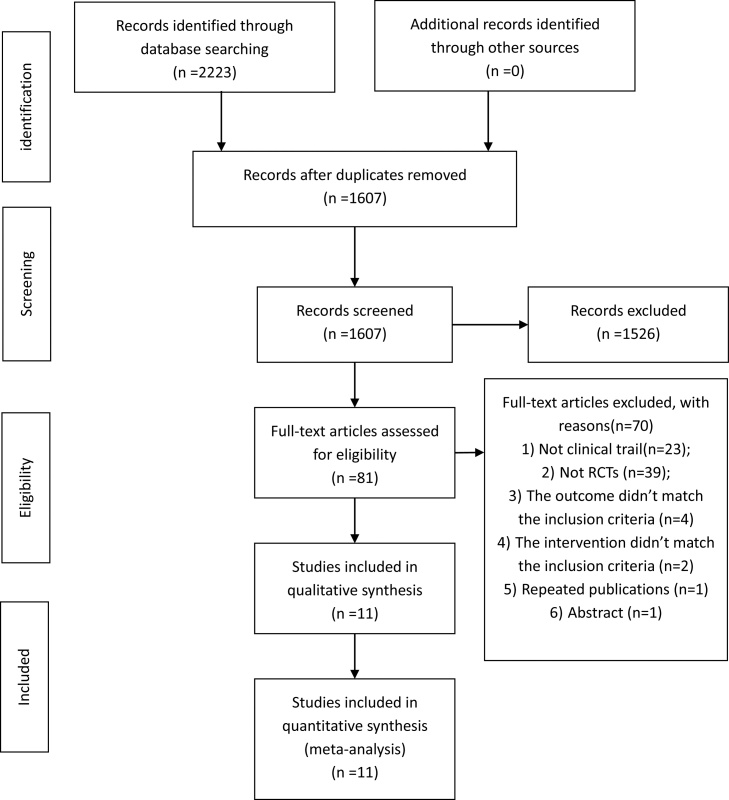
We extracted 2223 articles from 6 databases and excluded 616 due to redundancy. According to the screening criteria, 1526 articles were removed from our list based on their titles and abstracts. 81 articles were downloaded for further assessment, and 70 articles were excluded due to following reasons (1) Not clinical trial (n = 23); (2) Not RCTs (n = 39); (3) The outcome didn’t match the inclusion criteria (n = 4); (4) The intervention didn’t match the inclusion criteria (n = 2); (5) Repeated publications (n = 1); (6) Abstract (n = 1). As a result, eleven articles were included in this systematic review. The process of study screening is shown in Fig. 1.
Fig. 1.
Flow diagram of study screening.
Study characteristics
The details of study characteristics are summarized in Table 1. Eleven RCTs 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 enrolled a total of 1259 patients, 672 patients accepted integration of CMD and WM, 623 patients accepted WM alone. The CMD used in these studies including Chinese patent medicine and herbal decoction. Seven studies applied Chinese patent medicine (Lianhua qingwen granules, Jinhua qinggan granules, Toujie quwen granules),25, 26, 27, 28, 31, 32, 34 Four studies used herbal decoction (Qingfei paidu decoction, Qingfei touxie fuzheng decoction, Maxing xuanfei jiedu decoction,),24, 29, 30, 33 The outcomes that received the most attention were composite events (number of patients turned to the types of severe and critical; all-cause death), clinical symptoms (clinical symptoms score, defervescence, cough and fatigue resolution) and adverse event. Length of hospital stay was reported only twice and rate of 2019-nCoV RT-PCR turned to negativity was reported only once, no study reported PaO2/FiO2 and duration of mechanical ventilation.
Table 1.
Basic characteristics of the included studies.
| Authors [ref] | Sample Size (T/C) Type of patients | Age(years) T/ C | Course of disease (days)T/C | Intervention | Course of Treatment (days) | Outcome | Adverse events |
|---|---|---|---|---|---|---|---|
| Ding 24 | 100(51/49)mild ordinary severe | 54.7 ± 21.3/50.8 ± 23.5 | 5.3 ± 3.1/6.0 ± 3.7 | Qingfei touxie fuzheng decoction + WM* vs. WM | 10 | (1) Defervescence rate; (2) Cough resolution rate; (3) Tachypnea resolution rate; (4) Diarrhea resolution rate; (6) Adverse event | Abnormal liver function (T:2;C:3) |
| Duan 25 | 123(82/41)mild | 52.0 ± 13.9/50.3 ± 13.2 | 2.7 ± 1.6/2.5 ± 1.5 | Jinhua qinggan granules + WM vs. WM | 5 | (1) Clinical symptoms score; (2) Number of patients turned to the types of severe and critical; (3) Defervescence rate (4) Cough resolution rate; (5) Fatigue resolution rate; (6) Diarrhea resolution rate; (7) Body pain resolution rate; (8) Adverse event | Gastrointestinal reaction (T:27) |
| Fu_a26 | 73(37/36) ordinary |
45.3 ± 7.3/44.7 ± 7.5 | 7.6 ± 1.3/8.5 ± 1.4 | Toujie quwen granules + WM vs. WM | 15 | (1) Number of patients turned to the types of severe and critical; (2) Adverse event | NR in details |
| Fu_b27 | 65(32/33) mild ordinary | 43.3 ± 7.2/43.7 ± 6.5 | 7.56 ± 1.3/8.5 ± 1.6 | Toujie quwen granules + WM vs. WM | 10 | (1) Number of patients turned to the types of severe and critical; (2) Adverse event. | NR in details |
| Hu 28 | 284(142/142) mild ordinary | 50.4 ± 15.2/51.8 ± 14.8 | 9.5 ± 5.1/9.9 ± 5.9 | Lianhua qingwen granules + WM vs. WM | 14 | (1) Rate of 2019-nCoV RT-PCR turned to negativity; (2) Number of patients turned to the types of severe and critical; (3) Adverse event | Gastrointestinal reaction (T:24; C:33); Abnormal liver function (T:32;C:32); Renal dysfunction (T:8;C:11); headache (T:1;C:1) |
| Li 29 | 12(6/6) severe | 52.0 ± 6.6/50.0 ± 10.0 | NR/NR | Qingfei paidu decoction + WM vs. WM | 6 | (1) Length of hospital stay; (2) Adverse event | Pruritus (T:1) |
| Qiu 30 | 50(25/25) ordinary | 53.4 ± 18.4/51.3 ± 14.6 | 2.8 ± 0.8/3.2 ± 1.3 | Maxing Xuanfei Jiedu Decoction + WM vs. WM | 10 | (1) Clinical symptoms score; (2) Number of patients turned to the types of severe and critical; (3) Defervescence time (4) Cough resolution time | Not assessed |
| Sun 31 | 57(32/25) NR | 45.4 ± 14.1/42.0 ± 11.7 | 4.4 ± 2.5/6.0 ± 4.4 | Lianhua qingke granules + WM vs. WM | 14 | (1) Number of patients turned to the types of severe and critical; (2) Defervescence rate; (3) Cough resolution rate; (4) Fatigue resolution rate | Not assessed |
| Xiao 32 | 200(100/100) mild | 60.9 ± 8.7/62.2 ± 7.5 | 5.5 ± 2.09/6.4 ± 3.0 | Sufeng jiedu capsule + WM vs. WM | 14 | (1) Defervescence time; (2) Cough resolution time; (3) Fatigue resolution time; (4) Adverse event | Gastrointestinal reaction (T:2;C:1); Pruritus (T:1; C:2) |
| Ye33 | 42(28/14) severe | 65 (53.5–69)/59 (47–67) | NR/NR | Chinese herbal medicine + WM vs. WM | 7 | (1)Number of patients turned to the types of severe and critical; (2) All-cause death | Not assessed |
| Yu 34 | 295(147/148) mild ordinary | 48.3 ± 9.6/47.3 ± 8.7 | NR/NR | Lianhua qingwen granules + WM vs. WM | 7 | (1) Number of patients turned to the types of severe and critical; (2) All-cause death; (3) Adverse event | NR in details |
T, Treatment group/Experimental group; C, control group; NR, not reported; WM, western medicine; * Western medicine includes antibacterial, antiviral, hormone therapy, respiratory support, etc.
Quality assessment
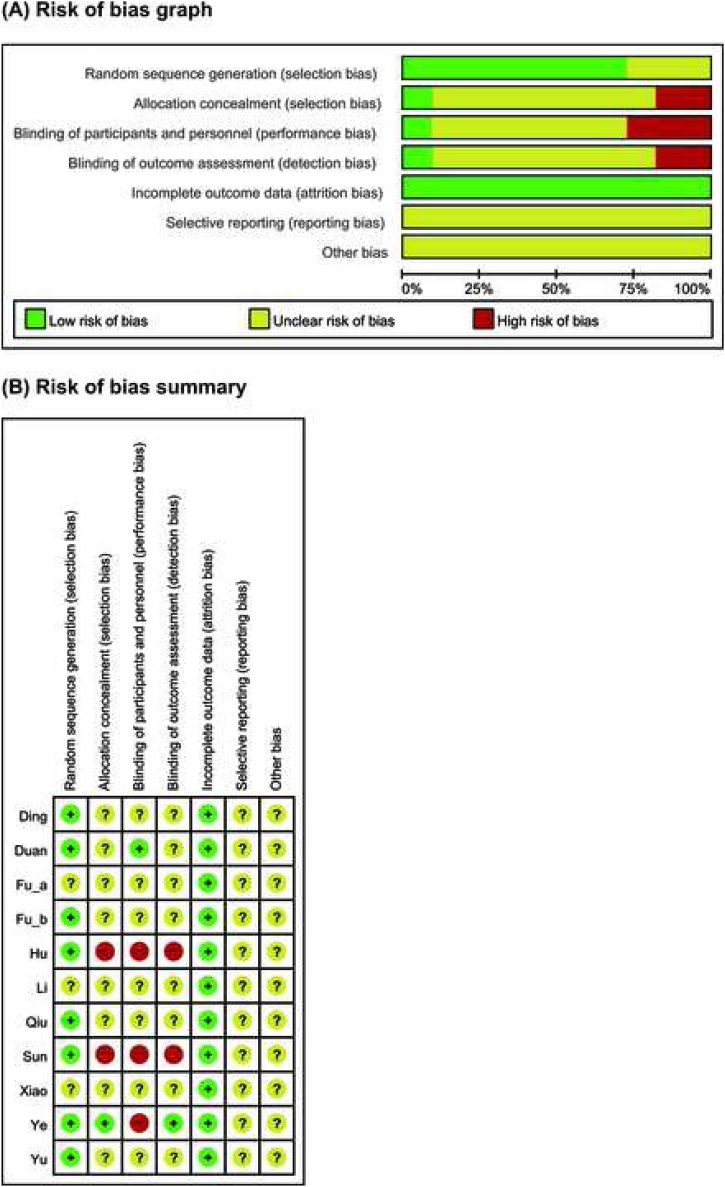
The quality of included RCTs were generally unclear. Eight studies reported adequate random sequence generation process were defined as low risk.24, 25, 27, 28,30, 31, 33, 34 One study reported allocation was concealed from laboratory personnel and outcome assessors, defined as low risk in selection bias and detection bias, high risk in performance bias.33 One study reported the blinding of patient, defined as low risk in performance bias.25 Two studies were not blinded due to emergency, defined as high risk in allocation concealment and blinding.28, 31 Incomplete outcome data had not been found in all studies, the risk of attrition bias were evaluated as low. Other parts of ROB were defined as unclear risk because of insufficient information (Fig. 2).
Fig. 2.
Risks of bias. (A) Risks of bias of the included studies. The authors reviewed each item’s risk of bias for each included study. (B) Risks of bias of individual studies. +: low risk of bias; −: high risk of bias;? : unclear risk of bias.
Meta-analysis
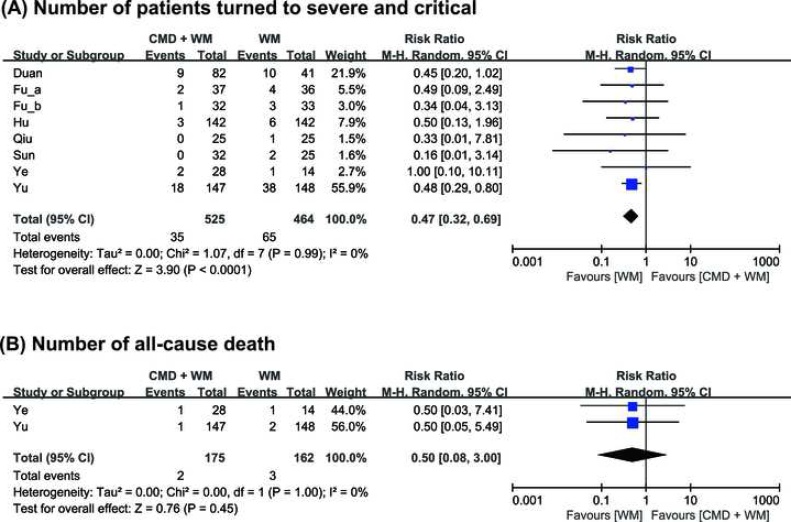
Eight studies (989 patients) reported composite events (number of patients turned to severe and critical type, all-cause death).25, 26, 27, 28, 30, 31, 33,34 All of eight studies reported number of patients turned to severe and critical type. No heterogeneity was identified between eight studies (I2 = 0%). The result of meta-analysis suggested that CMD can reduce the number of patients turned to severe and critical type (RR = 0.47, 95% CI=[0.32, 0.69], P < 0.0001) (Fig. 3A). Two studies (337 patients) reported number of all-cause death.33, 34 No heterogeneity was identified between two studies (I2 = 0%). From the result of meta-analysis, we did not observe significant difference between experimental and control group (RR = 0.50, 95% CI = [0.08, 3.00], P = 0.45) (Fig. 3B).
Fig. 3.
Comparison of Chinese medical drugs and Western medicine (CMD + WM) vs. Western medicine (WM) on (A) Number of patients turned to severe and critical type; (B) Number of all-cause death.
One study reported the length of hospital stay.29 It showed that CMD can reduce the length of hospital stay, (MD= -7.95, 95% CI=[-14.66, -1.24], P = 0.02). There was no statistically significant difference between two groups in the rate of 2019-nCov RT-PCR turned to negativity and the clinical symptom score. None of studies reported the PaO2/FiO2 and the duration of mechanical ventilation. Besides, the results of meta-analysis showed that integration of CMD and WM has better efficacy than WM in defervescence time (MD= -1.20, 95% CI=[-2.03, -0.38], P = 0.004), cough resolution rate (RR = 1.37, 95% CI=[1.15, 1.64], P = 0.0004), fatigue resolution rate (RR = 1.37, 95% CI=[1.02, 1.83], P = 0.04), and tachypnea resolution rate (RR = 2.20, 95% CI=[1.11, 4.39], P = 0.02). As for other outcomes, the results did not show statistically significant difference between two groups (Table 2).
Table 2.
The results of meta analysis of included studies.
| Outcome | Number of study | Sample Size(T/C) | Measures | Effect estimate (95 %CI) | Heterogeneity | P | Included studies[ref] |
|---|---|---|---|---|---|---|---|
| Primary outcomes | |||||||
| Length of hospital stay | 1 | 6/6 | MD (—) | −7.95 [-14.66, -1.24] | — | 0.02 | Li29 |
| Rate of 2019-nCov RT-PCR turned to negativity | 1 | 142/142 | RR (—) | 1.08 [0.94, 1.24] | — | 0.28 | Hu28 |
| Clinical symptom score | 2 | 125/125 | MD (Random) | −0.84 [-2.15, 0.47] | I2 = 92% | 0.21 | Duan,25 Qiu,30 |
| Arterial oxygen partial pressure /Fraction of inspired oxygen | 0 | — | — | — | — | — | — |
| Duration of mechanical ventilation | 0 | — | — | — | — | — | — |
| Secondary outcomes | |||||||
| Defervescence time | 2 | 125/125 | MD (Random) | −1.20 [-2.03, -0.38] | I2 = 77% | 0.004 | Qiu,30 Xiao,32 |
| Defervescence rate | 3 | 137/95 | RR (Random) | 1.18 [0.88, 1.60] | I2 = 69% | 0.27 | Ding,24 Duan,25Sun,31 |
| Cough resolution time | 2 | 125/125 | MD (Random) | −1.57 [-4.17, 1.03] | I2 = 94% | 0.24 | Qiu,30 Xiao,32 |
| Cough resolution rate | 3 | 157/107 | RR (Random) | 1.37 [1.15, 1.64] | I2 = 0% | 0.0004 | Ding,24] Duan,25 Sun,31 |
| Fatigue resolution time | 1 | 100/100 | MD (—) | −0.33 [-0.78, 0.12] | — | 0.15 | Xiao32 |
| Fatigue resolution rate | 2 | 96/51 | RR (Random) | 1.37 [1.02, 1.83] | I2 = 11% | 0.04 | Duan,,25Sun,31 |
| Tachypnea resolution rate | 1 | 18/17 | RR (—) | 2.20 [1.11, 4.39] | — | 0.02 | Ding,24 |
| Diarrhea resolution rate | 2 | 17/13 | RR (Random) | 0.32 [0.01, 15.49] | I2 = 87% | 0.56 | Ding,24 Duan,25 |
| Body pain resolution rate | 1 | 18/12 | RR (—) | 1.17 [0.73, 1.87] | — | 0.52 | Duan,25 |
| Adverse events | 8 | 597/555 | RD (Random) | 0.03 [-0.02, 0.08] | I2 = 83% | 0.31 | Ding,24 Duan,25 Fu_a,,26Fu_b,27 Hu,28Li,29Xiao,32Yu34 |
T, Treatment group/Experimental group; C, control group; MD, mean difference; RR, risk ratio;
Eight studies (1152 patients) reported the adverse events.24, 25, 26, 27, 28, 29, 32, 34 Three of them reported no adverse events,26, 27, 34 and five studies reported the occurrence of adverse events included gastrointestinal reaction (nausea, vomiting, diarrhea, loss of appetite), abnormal liver function, renal dysfunction, headache and pruritus.24, 25, 28, 29,32 As shown in Table 1, gastrointestinal reaction was the main adverse event in both experiment and control groups. The result of meta analysis indicated there was no statistically significant difference between two groups. So CMD may not increase the incidence of adverse events (Table 2).
Publication bias
Since the sample of included study was small, the assessment of publication bias was waived.
Certainty of evidence
The certainty of evidence was generally moderate and low. The results of number of patients turned to the types of severe and critical, and defervescence time were moderate level. The results of length of hospital stay and resolution rate of cough, fatigue and tachypnea were low level. The main reasons of downgrade were unclear risk of bias and small sample size (Table 3).
Table 3.
Summary of findings.
| Chinese medical drug and western medicine compared to western medicine for coronavirus disease 2019 | |||||
|---|---|---|---|---|---|
| Patient or population: Patients with coronavirus disease 2019 Intervention: CHM + WM Comparison: WM | |||||
| Outcomes | No. of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects |
|
| Risk with western medicine | Risk difference with Chinese medical drug and western medicine | ||||
| Number of patients turned to the types of severe and critical | 989 (8 RCTs) | ⨁⨁⨁◯ MODERATE a | RR 0.47 (0.32 to 0.69) | 140 per 1000 | 74 fewer per 1000 (95 fewer to 43 fewer) |
| Length of hospital stay | 12 (1 RCT) | ⨁⨁◯◯ LOW a,b | – | – | MD 7.95 lower (14.66 lower to 1.24 lower) |
| Defervescence time | 250 (2 RCTs) | ⨁⨁⨁◯ MODERATE a,c | – | – | MD 1.2 lower (2.03 lower to 0.38 lower) |
| Cough resolution rate | 264 (3 RCTs) | ⨁⨁◯◯ LOW a,b | RR 1.37 (1.15–1.64) | 523 per 1000 | 194 more per 1000 (79 more to 335 more) |
| Fatigue resolution rate | 147 (2 RCTs) | ⨁⨁◯◯ LOW a,b | RR 1.37 (1.02–1.83) | 431 per 1000 | 160 more per 1000 (9 more to 358 more) |
| Tachypnea resolution rate | 35 (1 RCT) | ⨁⨁◯◯ LOW a,b | RR 2.20 (1.11–4.39) | 353 per 1000 | 424 more per 1000 (39 more to 1196 more) |
GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference a. Unclear risk of bias b. Small sample size c. High heterogeneity.
Discussion
This systematic review evaluated the efficacy and safety of Chinese medical drugs for COVID-19 treatment. CMD has been widely used in China for the treatment of infectious disease, especially in the treatment of COVID-19. Many experimental studies and clinical trails have reported the anti-infection effects of CMD.35 The potential effect of CMD for COVID-19 mainly include: antivirus, anti-inflammation, immunoregulation, and target organs protection.36, 37
Summary of results
This systematic review included eleven RCTs investigated the efficacy and safety of CMD for the treatment of COVID-19. Meta-analyses showed that: (1) Less patients in CMD treatment group experienced disease progression; (2) CMD treatment was associated with the decreased length of hospital stay; (3) CMD treatment was associated with better efficacy in defervescence time, cough resolution rate, fatigue resolution rate, tachypnea resolution rate. (4) CMD treatment did not raise additional safe concerns compared with controls. The above discoveries suggested that CMD may increase the efficacy of WM in the treatment of COVID-19, which was consistent with the results of clinical observation and network pharmacology research.36, 38
FiO2/PaO2 ratio and ventilation time were not mentioned in these studies due to limited number of severe and/or critical cases enrolled. However, besides these two, all outcomes in the core outcome set were reported in different studies. It suggested that the core outcome set for COVID-19, i.e. COS-COVID, was well accepted by all related studies. However, it seemed that the outcomes like the number of patients turned to severe and critical type and clinical symptom resolution were more emphasized, especially among studies conducted early in the outbreak.
Overall completeness and applicability of the evidence
CMD may decreased the rate of disease progression, shorten the course of treatment, relief symptom and may not increase the incidence of adverse events. Although such results was promising, when referring to these results, the individual differences of patients should be paid attention to, especially the differences of TCM syndromes. And the specificity of COVID-19 across regions also needs to be considered.
Limitations of this review
There were several limitations of this systematic review. Firstly, we only included published clinical trials with limited sample size. Some trials which were not published were not included. There was potential publication bias. Secondly, most of COVID-19 clinical trials were carried out in real world practice leading to less frequent use of blind methods in the studies, and most of included studies did not report registration process. As a consequence, performance bias during clinical trials were not well controlled. Thirdly, the course of treatment in included studies were no more than 15 days and the follow-up was insufficient, which made the long-term efficacy of CMD for COVID-19 treatment could not be evaluated. Finally, this review evaluated the efficacy and safety of the integration therapy of CMD and WM in the treatment of COVID-19, which contained several different interventions and led to inter-study heterogeneity.
Comparison with prior systematic review
The results of this review are consistent with prior systematic reviews,39, 40, 41, 42 and substantially updated the evidence of CMD for COVID-19 treatment. Compared with the last published systematic review of RCTs,42 this review included four new RCTs,27, 28, 29, 30 and evaluated the efficacy focused on core outcomes. Compared with other three systematic reviews that included both RCTs and observational studies,39, 40, 41 this review excluded the observational studies and updated the data of RCTs.
Implications for practice and research
In this review, six kinds of Chinese medical drugs were evaluated, the results of meta- analyses showed CMDs had benefit effects. Thus CMD might be an option for better treatment of COVID-19. However, due to the limitation of evidence level and quality of included studies, we cannot make this recommendation with full confidence.
Rigorous RCTs on CMD for the treatment of COVID-19 are still urgently needed, especially for the severe and critical type. Study investigator should pay more attention to COS- COVID. The course of treatment and follow up should be extended, in order to evaluate the long term efficacy of CMD. The quality of RCTs should be paid attention to, especially for blinding and registration.
In conclusion, the results of meta- analysis suggested that CMD may bring benefit in the treatment of COVID-19 and may not increase the incidence of adverse events. However, the methodological quality of included studies was relatively unclear which might decrease the reliability of the results. Rigorous designed RCTs are still urgently needed.
Author contributions
Conceptualization: WP, ZL and JZ. Methodology: WP, ZL and JZ. Software: NL, YL and XJ. Validation: WP, ZL and JZ. Formal Analysis: WP and ZL. Investigation: WP and NL. Resources: WP and ZL. Data Curation: NL, YL and XJ. Writing – Original Draft: WP, ZL. Writing – Review & Editing: JZ, WZ, FY and BP. Visualization: WP, ZL and JZ. Supervision: JZ. Project Administration: WP and ZL. Funding Acquisition: JZ. All authors have read and approved the final manuscript.
Conflict of interest
The authors declared that there was no conflict of interest regarding the publication of this paper.
Funding
This work is supported by the COVID-19 Prevention and Treatment Drug Development Program (2020-CMKYGG-03).
Ethical statement
This work did not require an ethics approval as it did not involve any human or animal experiment.
Data availability
The authors confirm that the data supporting the findings of this study will be made available on request.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.imr.2020.100477.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao Y.Q. Therapeutic strategies for COVID-19 based on its pathophysiological mechanisms. Chin J Pathophysiol. 2020;36 568-572+576. [In Chinese, English abstract] [Google Scholar]
- 3.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H.J., Du S.H., Yue X., Chen C.X. Review and prospect of pathological features of corona virus disease. Fa Yi Xue Za Zhi. 2020;36:16–20. doi: 10.12116/j.issn.1004-5619.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J., Yuan S., Kok K., To K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Health Commission of the People’s Republic of China . National Health Commission of the People’s Republic of China; Beijing. China: 2020. Coronavirus disease 2019 (COVID-19) Situation Report - July 2, 2020. [Google Scholar]
- 13.Central People's Government of the People's Republic of China . Central People's Government of the People's Republic of China; Beijing. China: 2020. The deployment of further classification of effective prevention and control requires the optimization of diagnosis and treatment to speed up the scientific prevention and control of drugs. [Google Scholar]
- 14.State Council Information Office; Beijing. China: 2020. News Conference of the State Council Information Office: Yu Yanhong briefing the important role of TCM in COVID-19 containment and treatment. [Google Scholar]
- 15.Gao S., Ma Y., Yang F., Zhang J., Yu C. Zhang Boli: traditional Chinese medicine plays a role in the prevention and treatment on novel coronavirus pneumonia. Tianjin J Trad Chin Med. 2020;37:121–124. [In Chinese, English abstract] [Google Scholar]
- 16.Williamson P., Altman D., Blazeby J., Clarke M., Devane D., Gargon E. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:132. doi: 10.1186/1745-6215-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson P., Altman D., Bagley H., Barnes K., Blazeby J., Brookes S. The COMET handbook: Version 1.0. Trials. 2017;18:280. doi: 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin X., Pang B., Zhang J., Liu Q., Yang Z., Feng J. Core outcome set for clinical trials on coronavirus disease 2019 (COS-COVID) Engineering (Beijing. 2020 doi: 10.1016/j.eng.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 20.National Health Commission of the People’s Republic of China . National Health Commission of the People’s Republic of China; Beijing. China: 2020. Diagnosis and treatment protocol for novel coronavirus pneumonia trial version 5. [Google Scholar]
- 21.National Health Commission of the People’s Republic of China . National Health Commission of the People’s Republic of China; Beijing. China: 2020. Diagnosis and treatment protocol for novel coronavirus pneumonia trial version 6. [Google Scholar]
- 22.National Health Commission of the People’s Republic of China . National Health Commission of the People’s Republic of China; Beijing. China: 2020. . diagnosis and treatment protocol for novel coronavirus pneumonia trial version 7. [Google Scholar]
- 23.Higgins J., Green S.R. John Wiley & Sons; 2011. Cochrane handbook for systematic review of interventions. Version 5.1.0. [Google Scholar]
- 24.Ding X., Zhang Y., He D., Zhang M., Tan Y., Yu A. Clinical effect and mechanism of qingfei touxie fuzheng recipe in the treatment of novel coronavirus pneumonia. Herald Med. 2020:1–10. Avaliable from: http://kns.cnki.net/kcms/detail/42.1293.R.20200302.1615.002.html. Accessed April 30, 2020.[In Chinese, English abstract] [Google Scholar]
- 25.Duan C., Xia W.G., Zheng C.J. Clinical observation on the treatment of novel coronavirus pneumonia with Jinhua qinggan granules. J Tradit Chinese Med. 2020:1–5. Avaliable from: http://kns.cnki.net/kcms/detail/11.2166.R.20200323.0853.002.html. Accessed April 30, 2020.[In Chinese, English abstract] [Google Scholar]
- 26.Fu X., Lin L., Tan X. Clinical study on 37 case of COVID-19 treated with integrated traditional Chinese and Western Medicine. Trad Chin Drug Res Clin Pharmacol. 2020:1–9. Available from: http://kns.cnki.net/kcms/detail/44.1308.R.20200319.1644.002.html. Accessed April 30, 2020. [In Chinese, English abstract] [Google Scholar]
- 27.Fu X., Lin L., Tan X. Clinical study on treatment of cases of COVID-19 with toujie quwen granules. Chin J Experimen Trad Med Formul. 2020;26:44–48. [In Chinese, English abstract] [Google Scholar]
- 28.Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B. Efficacy and safety of Lianhuaqingwen Capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020:153242. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Zhang W. Evaluation on the clinical effect of traditional chinese medicine and western medicine regimens on COVID-19. Guangming J Chin Med. 2020;35:1273–1275. [In Chinese, English abstract] [Google Scholar]
- 30.Qiu M., Li Q., Zhu D., Wang C., Sun Q., Qian C. Efficacy observation of Maxing Xuanfei Jiedu decoction on common type of NCP. J Emerg Trad Chin Med. 2020:1–3. Available from: http://kns.cnki.net/kcms/detail/50.1102.R.20200506.0915.004.html. Accessed June 8, 2020. [In Chinese, English abstract] [Google Scholar]
- 31.Sun H., Xu F., Zhang L., Wei C., Chen J., Wang Q. Study on clinical efficacy of lianhua qingke granule in treatment of mild and ordinary COVID-19. Chin J Experim Trad Med Formul. 2020:1–8. doi: 10.13422/j.cnki.syfjx.20201438. [DOI] [Google Scholar]
- 32.Xiao Q., Jiang Y., Wu S., Wang Y., An J., Xu W. The value analysis of traditional Chinese medicine shufeng jiedu capsule combined with abidor in the treatment of mild COVID-19. J Emerg Trad Chin Med. 2020:1–3. Available from: http://kns.cnki.net/kcms/detail/50.1102.R.20200309.1528.004.html. Accessed April 30, 2020. [In Chinese, English abstract] [Google Scholar]
- 33.Ye Y.A., G-CHAMPS Collaborative Group Guideline-based Chinese herbal medicine treatment plus standard care for severe coronavirus disease 2019 (G-CHAMPS): evidence from China. Front Med (Lausanne) 2020;7:256. doi: 10.3389/fmed.2020.00256. Published 2020 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu P., Li Y., Wan S., Wang Y. Clinical observation of Lianhua Qingwen Granule combined with Abidor in the treatment of mild COVID-19. Chin Pharmaceut J. 2020:1–9. Available from: http://kns.cnki.net/kcms/detail/11.2162.R.20200422.1429.002.html. Accessed June 8, 2020. [In Chinese, English abstract] [Google Scholar]
- 35.Wang T., Han L., Wang Y., Miao L., Yang J., Zhang J. Recent advances in treatment of viral pneumonia using Chinese patent medicine. China J Chinese Matera Med. 2020;45:1509–1514. doi: 10.19540/j.cnki.cjcmm.20200312.502. [In Chinese, English abstract] [DOI] [PubMed] [Google Scholar]
- 36.Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19) Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren J., Zhang A., Wang X. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Administration of Traditional Chinese Medicine . National Administration of Traditional Chinese Medicine; Beijing. China: 2020. The efficacy of TCM against COVID-19 has been well tested by clinical data. [Google Scholar]
- 39.Gao C., Song C., Fu Y., Zhang J. The curative effect on treating COVID-19 by integrated medicine: a systematic review. J Shanxi Univ Chin Med. 2020:1–9. Available from: http://kns.cnki.net/kcms/detail/61.1501.r.20200528.1450.004.html. Accessed June 8, 2020. [In Chinese, English abstract] [Google Scholar]
- 40.Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J. Efficacy and safety of integrated traditional Chinese and Western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y., Zou L., Yu X., Sun D., Li S., Tang L. Clinical effects of integrated traditional Chinese and western medicine on COVID-19: a systematic review. Shanghai J Trad Chin Med. 2020:1–8. doi: 10.16305/j.1007-1334.2020.06.093. [DOI] [Google Scholar]
- 42.Ang L., Song E., Lee H.W., Lee M.S. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9:E1583. doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study will be made available on request.