Abstract
Prostate cancer is an androgen-dependent cancer with unique metabolic features compared to many other solid tumors, and typically does not exhibit the “Warburg effect”. During malignant transformation, an early metabolic switch diverts the dependence of normal prostate cells on aerobic glycolysis for the synthesis of and secretion of citrate towards a more energetically favorable metabolic phenotype, whereby citrate is actively oxidised for energy and biosynthetic processes (i.e. de novo lipogenesis). It is now clear that lipid metabolism is one of the key androgen-regulated processes in prostate cells and alterations in lipid metabolism are a hallmark of prostate cancer, whereby increased de novo lipogenesis accompanied by overexpression of lipid metabolic genes are characteristic of primary and advanced disease. Despite recent advances in our understanding of altered lipid metabolism in prostate tumorigenesis and cancer progression, the intermediary metabolism of the normal prostate and its relationship to androgen signaling remains poorly understood. In this review, we discuss the fundamental metabolic relationships that are distinctive in normal versus malignant prostate tissues, and the role of androgens in the regulation of lipid metabolism at different stages of prostate tumorigenesis.
Keywords: Androgen receptor, Fatty acids, Metabolism, Phospholipids, Prostate gland
1. Introduction
The prostate gland is exquisitely sensitive to androgenic hormones, and this dependence has been exploited to treat metastatic prostate cancer (PCa) since the 1930s. Whereas the interplay between androgen action and the unique lipid metabolic phenotype of PCa cells has been extensively studied [[1], [2], [3], [4], [5], [6], [7], [8]], our knowledge of normal metabolism in the prostate lags considerably behind that of other tissues such as liver or heart. Understanding lipid metabolism in the normal prostate is essential to reveal the intermediary processes that mediate cell transformation, and in turn provide new insights into PCa detection, preventive and/or therapeutic strategies that are not evident from current experimental cancer models. This review article aims to provide a contemporary perspective on androgens and their impact on different aspects of lipid metabolism in normal and malignant prostate tissues, and highlight some recently identified metabolic vulnerabilities related to androgen action.
2. Normal prostate metabolism and androgens
The main function of the prostate is to produce and secrete prostatic fluid, which constitutes approximately one-third of the total volume of semen. Prostatic fluid is comprised of enzymes including prostate-specific antigen (PSA) that are responsible for liquefying semen, zinc, lipids and citrate. High citrate production and secretion are unique characteristics of the prostate; citrate concentration in prostate cells is about 1.0–3.0 mmol/L compared with only 0.1–0.5 mmol/L in other mammalian cells. Accordingly, citrate concentration in the prostatic fluid is about 24–130 mmol/L, which is 240–1 300-fold greater than that in blood plasma [[9], [10], [11]]. Typically, citrate is either retained and oxidized in the mitochondria to generate energy as an essential intermediate in the citric acid cycle, or is exported into the cytoplasm where it is cleaved by citrate lyase (ACLY) to generate acetyl-CoA, which is used for fatty acid synthesis. The complete oxidation of citrate via the citric acid cycle fuels the cell with 12 mol ATP/mol citrate and 24 mol ATP/mol glucose oxidized. However, as described above, the prostate secretes enormous quantities of citrate, depriving prostate cells of a major pool of energy. Although the function of citrate within the prostatic fluid is not fully understood, it presumably contributes to the seminal fluid buffering capacity, chelation of calcium, zinc and other cations, and as an energy source to maintain sperm viability and/or for capacitation processes [[12], [13], [14], [15], [16], [17], [18]].
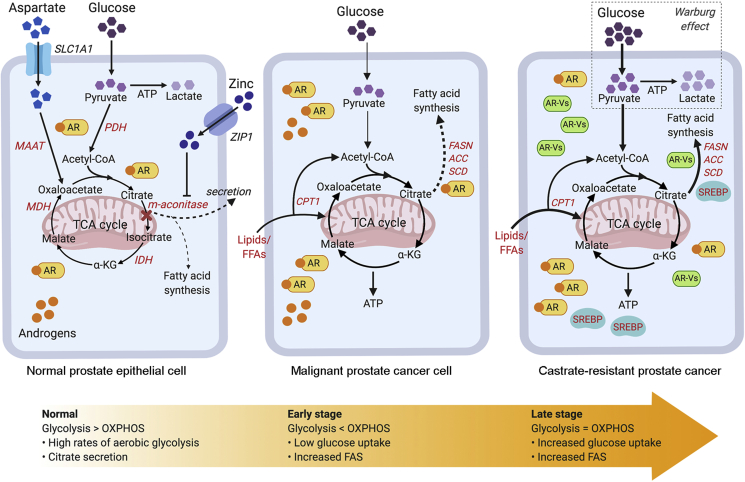
Studies on human and rat ventral prostate tissues or isolated prostate epithelial cells have revealed the dependence of prostate cells on aerobic glycolysis [[19], [20], [21], [22], [23], [24]]. The aerobic glycolysis metabolic phenotype in normal prostate tissues is accompanied by another phenotype of secretion of high citrate concentrations within the prostatic fluid. However, PCa cells exhibit a decrease in citrate concentration, which suggests a metabolic transformation from citrate producing cells into citrate oxidizing cells [11]. In support of this, it was recently reported that significantly lower levels of citrate were detected in PCa tissues compared with normal prostate tissues as evaluated using desorption electrospray ionization mass spectrometry (DESI-MSI) [25]. This switch in citrate fate is suggested to take place by inhibition of the m-aconitase enzyme by zinc (Fig. 1). M-aconitase is responsible for the stereo-specific isomerization of citrate to isocitrate. The citrate/isocitrate ratio in most mammalian tissues is 10:1 compared with 30:1 in prostate cells, suggesting an inhibition of this key enzymatic activity [26]. In addition to producing and secreting large concentrations of citrate, normal prostate tissues are further characterized by the ability to uptake and accumulate large concentrations of zinc [[27], [28], [29]]. Zinc concentration is 10-fold greater in normal prostate tissues compared with other soft tissues, and its concentration in the prostatic fluid is 500-fold greater than in blood plasma [30]. Zinc inhibits m-aconitase activity, paving the way for prostate cells to halt citrate oxidation [31,32]. In addition to the secretion of citrate, the prostate is also a source of cholesterol, which comprises a major proportion of the seminal fluid [33].
Figure 1.
Metabolic landscape of prostate cancer progression (from normal prostate to malignant to metastatic/CRPC). Normal prostate epithelial cells exhibit high rates of aerobic glycolysis and low rates of oxidative phosphorylation. Glucose is used for citrate production and secretion, resulting in an impaired TCA cycle; this process is facilitated by zinc and aspartate through inhibition of m-aconitase and supply of metabolic precursors (i.e. oxaloacetate). Alternatively, citrate may be used for lipid biosynthesis through androgen-mediated activation of lipogenic enzymes. During malignant transformation, prostate cancer cells exhibit increased oxidative phosphorylation and hence, reactivate the TCA cycle to oxidize citrate for energy production. Instead of glucose, FFAs are the dominant bioenergetic substrates that feed into the TCA cycle for energy production. More importantly, de novo lipogenesis is enhanced at this stage of disease through the up-regulation of AR-regulated lipogenic enzymes. Despite initial positive responses to androgen-deprivation therapy, patients eventually progress to CRPC. AR signalling is maintained in CRPC; AR resistant mechanisms such as the AR-variants (AR-Vs) and/or indirect activation of alternative metabolic pathways (i.e. SREBP) play a role in driving the androgen-mediated lipogenic phenotype that may contribute to prostate cancer progression and treatment resistance. Notably, increased aerobic glycolysis or the Warburg effect is observed in advanced stages of the disease/CRPC. Words highlighted in red: AR-regulated genes. Thin/dotted lines: Baseline level; thick lines: Up-regulated. TCA, tricarboxylic acid cycle; PDH, pyruvate dehydrogenase; MAAT, aspartate aminotransferase; FASN, fatty acid biosynthesis; ACC, acetyl-CoA-carboxylase; SCD, stearoyl-CoA-desaturase; OXPHOS, oxidative phosphorylation; CRPC, castrate-resistant prostate cancer; AR, androgen receptor; FFAs, lipids or free fatty acids; MDH, malate dehydrogenase; α-KG, alpha ketoglutarate; AR-Vs, androgen receptor variants; CPT1, carnitine palmitoyltransferase 1; FAS, fatty acid synthesis. Created with BioRender.com.
Few studies have focused on the impact of androgen receptor (AR) signaling in normal prostate cell metabolism [[34], [35], [36]], and have largely focused on the role of androgens in increasing the intracellular citrate pool and lipid biosynthesis. The former is accomplished by increasing citrate production via enhanced availability of citrate precursors, acetyl-CoA and oxaloacetic acid, and/or decreased citrate consumption. Androgens were found to induce pyruvate oxidation by increasing the expression of pyruvate dehydrogenase E1 component subunit alpha (PDHE1α) [37], resulting in an increase in acetyl-CoA. Androgens were also reported to induce aspartate uptake and transamination processes by inducing expression of the aspartate transporter (EAAC1) and aspartate aminotransferase (mAAT) genes [[38], [39], [40], [41]], providing cells with oxaloacetic acid. Moreover, androgens can induce zinc uptake by the prostate [42,43], inhibiting citrate oxidation (Fig. 1). 5α-dihydrotestosterone (DHT) increased mAAT expression in PC3 cells expressing AR [44], and in isolated pig prostate epithelial cells [45]. Testosterone increases the levels of m-aconitase mRNA and the transcription rates of m-aconitase in rat ventral prostate cells, pig prostate cells, and human malignant prostate cells (LNCaP and AR-PC-3) [36]. Testosterone up-regulated EAAC1 expression in rat ventral prostate epithelial cells [46]. PDHE1α expression was stimulated in response to testosterone in ventral prostate epithelial cells, rat ventral prostate cells, and in PC3 cells transfected with AR [37,47]. In contrast with this induction of citrate accumulation, androgens were found to induce m-aconitase activities in normal and malignant prostate cells [[34], [35], [36],48]. Using castrated mature monkey models, androgens were found to control enzymes involved in the citric acid cycle, such as isocitrate dehydrogenase (IDH) and malate dehydrogenase (MDH) [49]. It is plausible that this induction is not enough to counteract other processes that increase citrate levels in prostate cells. Of note, the effects of testosterone in rodents were lobe-specific; testosterone induced m-aconitase mRNA expression in rat ventral prostate lobes but not in the dorsal lobes and decreased m-aconitase mRNA expression in the lateral lobes [50]. There is no consensus as to which of these rodent lobes is the most similar to the human prostate peripheral zone, where most prostate tumours arise [24,51] and although there are some anatomical resemblances between the rodent dorsolateral lobe and the human peripheral zone, molecular comparison studies are warranted. In contrast to humans, rodents rarely develop spontaneous PCa [41], suggesting that rodent models should be supplemented with better human-mimic models.
Other studies on androgens and normal prostate tissue metabolism have focused on lipogenesis. Androgens were found to be essential in the prostate for lipid biosynthesis. Castration of the rat or monkey results in repression of lipogenic enzymes including ATP citrate lyase (ACLY), fatty acid synthase (FASN) and acetyl-CoA-carboxylase (ACC), and decreased total prostatic lipid content. Conversely, androgen administration after castration restored these lipogenic enzyme activities and lipid levels to baseline [49,[52], [53], [54], [55]].
The metabolism of other nutrients such as fatty acids or amino acids in normal prostate tissues have not been examined thoroughly yet. It is surprising that our understanding of androgen regulation on prostate tissues is limited to relatively few processes and genes, given that androgens are the main orchestrator of prostate functions and physiology. Whereas prostate epithelial androgen receptor knockout (PEARKO) mouse models exist, to date there has been no investigation on the role of AR on prostate cell metabolism using these models. Similarly, little is known about the altered metabolic pathways in prostate cells during oncogenic transformation, and whether these alterations are driving malignancy or reflect changes to other genes or signaling pathways. One obvious manifestation of PCa is a striking decrease in citrate secretion into the prostatic fluid [11]. Redirecting citrate into the mitochondria allows cell to use other nutrients to generate energy, including fatty acids. Fatty acid and lipid metabolism, with a focus on the androgen-regulated facets, will be discussed in the following sections.
3. Androgens and lipid metabolism in PCa
In the 1920s, Warburg [56,57] observed that rapidly proliferating tumor cells consume glucose at significantly higher rates compared to normal cells. Interestingly, most of the glucose-derived carbon was secreted as lactate rather than undergoing complete oxidation. This phenomenon is known as the “Warburg effect”. This provides proliferating cancer cells several advantages including (1) rapid and abundant ATP/energy production and (2) essential intermediates/precursors needed for biosynthetic pathways [58,59]. More importantly, the Warburg effect has become the basis for the development of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) as a staging tool for diverse types of cancers.
The Warburg effect is a classic example of metabolic reprogramming in cancer. However, unlike most solid tumors, PCa typically does not exhibit the “glycolytic switch” (or Warburg effect) and is poorly detected with FDG-PET due to its weak avidity for glucose [60]. Given that rapid cell proliferation in malignant tumors requires increased energy supply, alternative sources of energy likely fuel PCa growth. Early studies revealed that primary PCa is associated with alterations in lipid metabolism (see below). Banerjee et al. [25] observed an increase in the glucose to citrate ratio in PCa tissues compared with benign prostate tissues using DESI-MSI. This difference was largely driven by the marked decrease in citrate levels in PCa while the increase in glucose signal intensity was less pronounced. The authors subsequently detected higher levels of lipids in PCa compared with normal prostate tissues and proposed the shunting of citrate into lipogenesis and oxidation in PCa [25]. Conversely, an increased aerobic glycolysis phenotype has been reported in advanced stages of PCa, while other studies have reported a spectrum in the degree of glycolysis between androgen-dependent and androgen-independent PCa [61,62]. Moreover, increased glucose utilization as indicated by higher FDG-PET avidity in PCa patients was able to predict biochemical recurrence and PCa progression to castrate-resistant PCa (CRPC) [63]. More recently, it was found that AR-dependent CRPC exhibited higher aerobic glycolysis in vivo using patient-derived xenograft models [64]. In addition, Bok et al. [65] demonstrated using transgenic mouse prostate models that an increased Warburg effect was essential to support tumour progression and metastasis, particularly in hypoxic conditions. Taken together, these findings suggest that the Warburg effect is still applicable in PCa and the binary notion that PCa is non-glycolytic should be reconsidered.
3.1. Role of the AR in lipid metabolism
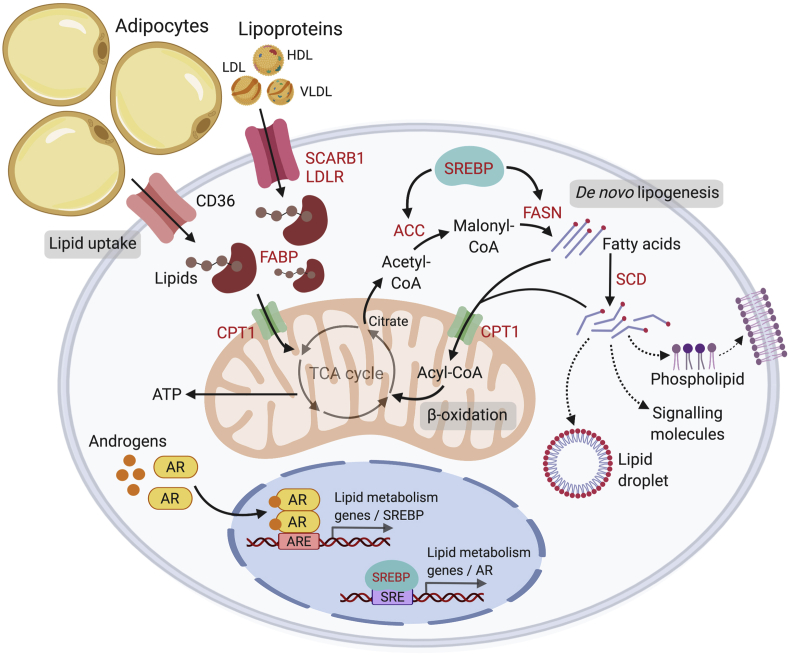
The AR modulates multiple metabolic gene networks in PCa. In support of this, combined analysis of direct AR binding targets and androgen regulated genes revealed a significant enrichment of metabolic targets in LNCaP and VCaP PCa cells [66]. Interestingly, lipid metabolism has been identified as one of the key cellular processes affected by androgens. We previously reported that exposure of LNCaP cells to androgens induced a marked accumulation of lipids such as cholesteryl esters and triacylglycerols. Further analysis of the origins of the accumulated lipids revealed that the observed lipid accumulation was due to androgen stimulated de novo lipogenesis, rather than uptake from extracellular sources [67]. More importantly, this lipid accumulation was abrogated by treatment with an AR-antagonist and was not observed in AR-negative PC3 and DU-145 PCa cells. Subsequently, numerous studies have reported androgen stimulation of the expression of diverse lipogenic enzymes, including several additional enzymes involved in lipid binding, uptake, metabolism and transport [2,3] (Fig. 2). Androgenic regulation of these genes has also been validated in several cell line RNA microarray and sequencing studies [66,68,69]. Overall, these findings highlighted an anabolic program downstream of the AR signaling axis to facilitate PCa development and progression.
Figure 2.
Overview of lipid metabolism in prostate cancer. Androgens regulate a number of lipid metabolic pathways in prostate cancer cells, including lipid uptake, biosynthesis and degradation. The AR directly (via binding of AR to the ARE) or indirectly (via activation of SREBP) regulates key lipid metabolic genes. Lipids can be derived from lipolysis of adipocytes or from circulating lipoproteins (LDL, HDL, VLDL). Alternatively, lipids can be synthesized endogenously by the cells via enzymes ACC and FASN. Androgens up-regulate lipid transporters that transport lipids or free fatty acids to the mitochondria for fatty acid oxidation (β-oxidation) or de novo lipogenesis. Free fatty acids are shuttled into the mitochondria as Acyl-CoAs by coupling with CPT1 (a mitochondrial transporter); a cyclical series of reactions result in the shortening of Acyl-CoA and the production of energy in the form of ATP via the TCA cycle. Citrate produced from the TCA cycle may also be cleaved to produce Acetyl-CoA, which is a precursor for de novo lipogenesis in the cytosol. Synthesized fatty acids may be further modified (i.e. degree of saturation via SCD) to generate a variety of lipids such as phospholipids that compose the plasma membranes, to promote lipid droplet production or to act as oncogenic signalling molecules to drive disease progression. Words in red = androgen-regulated genes. ARE, androgen response element; SRE, SREBP response element; AR, androgen receptor; LDL, low density lipoprotein; HDL, high density lipoprotein; VLDL, very low density lipoprotein; FASN, fatty acid biosynthesis; TCA, tricarboxylic acid cycle; SCD, stearoyl-CoA-desaturase; SREBP, sterol response element binding protein. Created with BioRender.com.
3.2. Androgens and lipid biosynthesis
Increased de novo lipogenesis is an early hallmark of PCa tumorigenesis and is significantly associated with PCa progression and poor disease survival outcomes [[70], [71], [72], [73]]. More than half a century ago, it was demonstrated that cancer cells shunted glucose-derived carbons from the mitochondrial Kreb's cycle to the cytosol for fatty acid synthesis (FAS) despite an abundant supply of extracellular lipids [74]. In rapidly proliferating cells, citrate generated from glucose and glutamine metabolism is essential for the production of acetyl-CoA which is the building block for de novo lipogenesis. In line with this observation, candidate-gene expression studies have reported tumor-related overexpression of key enzymes involved in lipogenesis that are essential for development and progression of PCa [75]. Notably, androgens have been reported to regulate the expression of lipogenic and lipid-modifying enzymes [76,77], such as FASN, ACC and steroyl-CoA-desaturase (SCD), to meet the growing demands of PCa cells for lipids, which serve as building blocks for membrane production, energy supply and storage, and act as mediators of intracellular signaling [78]. FASN forms a fatty acid by catalysing successive condensation reactions from malonyl-CoA and acetyl-CoA substrates. Overexpression of FASN occurs at the early stages of PCa pathogenesis and has been correlated to poor prognosis and reduced disease-free survival [71,79]. Moreover, elevated expression of FASN has been associated with aggressive PCa and metastasis to the bone [70]. ACC catalyses the rate-limiting step in FAS by converting acetyl-CoA to malonyl-CoA. Two isoforms, ACC1 and ACC2 exist: (1) ACC1 primarily produces malonyl-CoA to serve as a substrate for FAS; (2) ACC2 produces malonyl-CoA primarily to inhibit fatty acid β-oxidation via allosteric inhibition of carnitine palmitoyltransferase 1 (CPT1) [80]. It has been demonstrated that depletion of ACC1 expression by siRNA inhibited cell proliferation and induced caspase-mediated apoptosis in PCa cells, but non-malignant cells were unaffected [81]. Similar results were observed through chemical inhibition of ACC1 and ACC2 by Soraphen A in both androgen-dependent and androgen-independent PCa cells [82]. Generally, fatty acids are composed of a terminal carboxyl group and an alkyl chain which can undergo further modifications performed by desaturases or elongases. The rate-limiting enzyme SCD1 catalyses the biosynthesis of monounsaturated fatty acids (MUFA) from saturated fatty acids (SFA) by introducing a cis-double bond to the acyl chain, specifically palmitate and stearate to yield palmitoleate and oleate. It has been reported that PCa cells upregulate lipid biosynthesis to generate SFA and MUFA-rich phospholipids that partition into detergent-resistant lipid rafts to markedly alter signal transduction cascades, vesicular trafficking and cell migration [83,84]. Alterations in the desaturation index (MUFA to SFA ratio) affect phospholipid composition and hence, cancer cell behaviour [85]. One of the potential consequences of this altered desaturation ratio is changes to membrane fluidity [86]. Of note, Fritz et al. [87] reported an increase in MUFA/SFA ratio in human PCa patient tissues compared with normal prostate tissues. Consistent with this observation, the authors found enhanced expression of SCD1 in androgen-sensitive and resistant PCa cell lines and patient tissues. More importantly, SCD1 inhibition was shown to repress proliferation of androgen-dependent LNCaP and androgen-independent C4-2 PCa cells in vitro and in prostate tumor xenografts [87]. This was attributed to the inhibition of the AKT signaling pathway due to a decrease in the generation of phosphatidylinositols (precursors of a known AKT activator) following pharmacological inhibition of SCD1 (BZ36).
3.3. Androgens and fatty acid oxidation (FAO)
FAO is the dominant bioenergetic pathway in PCa [88,89]. The early “metabolic switch” towards citrate oxidation during PCa development coupled with the low glucose avidity of PCa (see above) diverts the focus away from glucose catabolism as the major source of energy/ATP to fatty acids. Mounting evidence suggests that FAO plays a crucial role in (1) sensitizing cancer cells to apoptosis induction by pro-apoptotic agents [90], (2) impairing NADPH production resulting in oxidative stress-induced cell death [91], and (3) increasing resistance to radiation and chemotherapeutic agents [92]. Moreover, it was proposed that FAO could play a role in driving specific oncogenic pathways (i.e. Src signaling) associated with tumor progression and metastasis [93]. Altogether, these findings suggest that cancer cells depend on FAO for their survival and disease progression, particularly in cancers such as PCa which rely on FAO as a key energy source. In line with this observation, a recent study showed that knockdown of a FAO enzyme, enoyl-CoA-isomerase 2 (ECI2) inhibited PCa cell proliferation and activated cell death in LNCaP and VCaP PCa cells [4]. This study demonstrated that using ChIP-qPCR androgen stimulation resulted in a 6-fold increase in AR binding to the putative AR binding site of ECI2 and enhanced ECI2 mRNA expression [4]. While androgenic regulation of de novo lipogenesis has gained considerable attention, this suggests that androgens may also stimulate FAO for growth and survival. A previous study found that androgens promoted the complete oxidation of fatty acids in LNCaP and VCaP PCa cells, and inhibition of FAO via treatment with Etomoxir (inhibitor of CPT1) suppressed androgen-mediated PCa cell growth [94]. Overexpression of CPT1 (the rate-limiting enzyme of mitochondrial FAO) has been reported in several cancers, including PCa [88,95,96], and Etomoxir causes toxic lipid accumulation and, subsequently, the development of endoplasmic reticulum stress leading to PCa cell apoptosis [96]. Interestingly, this study also showed a reduction in expression AR-regulated genes (PSA, NKX3.1) following a significant decrease in AR as a result of FAO inhibition, suggesting a feed-forward loop to sustain PCa cell growth [96]. Overall, FAO is an important component of cancer metabolism that remains underexplored in PCa, and further study of this process may also inform new mechanisms of clinical resistance to androgen targeting therapies.
3.4. Androgens and lipid uptake
In light of enhanced de novo lipogenesis as a hallmark of PCa, and the fact that fatty acids are the dominant metabolic substrate for the development and progression of PCa, it was recently reported that exogenous lipids are the major contributor to lipid biosynthesis and oxidation in PCa [97]. Exogenous lipids can be derived from adipose tissue lipolysis or the breakdown of triglycerides contained in circulating lipoproteins. Moreover, the prostate is surrounded by peri-prostatic adipose tissue which has the capacity to directly supply substantial quantities of fatty acids [98,99]. In line with this, recent studies have demonstrated that increased lipid uptake in PCa cells can drive proliferation and potentially invasiveness [98,100]. Furthermore, PCa cells co-cultured with peri-prostatic derived adipocytes from obese mice showed enhanced migratory properties compared with lean mice [101]. Mechanistically, androgens have previously been reported as stimulating lipid uptake in androgen-responsive PCa cells through increased expression of FABPpm (a transporter protein) [102]. More recently, it was reported that androgens significantly increased cellular lipid uptake (including fatty acids, cholesterol and lipoproteins) in AR-positive PCa cells (LNCaP, VCaP, C4-2B and DuCaP) via direct AR-mediated induction of several lipid transporters [100]. This effect was abrogated when cells were co-treated with the AR antagonist enzalutamide. Notably, it was demonstrated that androgen-enhanced lipid uptake was maintained in LNCaP PCa cells under cell cycle arrest. This suggests that AR regulated lipid uptake is independent of cell cycle progression and proliferation, and is not indirectly caused by lipid biomass demand as a requirement for cell division [100]. Moreover, this study also confirmed the association of these lipid transporters with PCa progression from localized disease to bone metastatic disease [100]. In view of the critical roles for fatty acid uptake in PCa, CD36, a major transporter for exogenous fatty acids into cells, has been reported to play critical roles in cancer progression, metastasis and epithelial-to-mesenchymal transition in several tumour types [[103], [104], [105]]. In the context of PCa, it was shown that DHT significantly increased CD36 expression, and this effect was abrogated by enzalutamide treatment [100]. In addition, it was shown that CD36 was expressed at a higher level in metastatic disease (although there was no evidence for increased CD36 protein expression in tumor regions of human PCa tissues relative to benign regions, stromal and epithelial cells) and that high expression of CD36 was associated with reduced relapse-free survival in PCa [98]. It was demonstrated that CD36 blockade reduces fatty acid uptake, resulting in a reduction of cancer-mediated lipid biosynthesis from fatty acid precursors and accumulation of oncogenic lipid signaling molecules, ultimately attenuating PCa growth and progression in cultured cells (LNCaP and PC3) and patient-derived xenograft (PDX)-derived organoids [98]. Given that anti-tumor effects of inhibiting lipogenesis can be rescued by the addition of exogenous fatty acids, lipid uptake poses an important mechanism of treatment resistance to inhibitors of de novo lipogenesis. Accordingly, this study demonstrated in PDX-derived human organoids that CD36 blockade enhanced the efficacy of de novo lipogenesis inhibition [98].
3.5. Cross-talk between lipid metabolic processes in PCa
Taken together, the above findings suggest that androgens actively regulate different aspects of lipid metabolism—biosynthesis, degradation and uptake—that are essential for driving PCa tumorigenesis and disease progression. Intriguingly, androgens stimulate both FAO and FAS in PCa cells suggesting that an intricate balance may exist between these processes to provide both biomass generation and energy required to support PCa cell growth and survival [78]. It was previously considered that simultaneous mitochondrial FAO and cytosolic FAS are incompatible due to the inhibition of CPT1 by malonyl-CoA. However, studies have shown that genetic manipulation of ACC1 or ACC2 in cancer cells may exert different outcomes in terms of FAO and FAS [106]. ACC2-generated malonyl-CoA can inhibit CPT1, blocking fatty acid transport to the mitochondria and its subsequent β-oxidation, while malonyl-CoA generated by ACC1 serves as an intermediate for FAS and exert no suppressive effects on CPT1 [107,108]. In addition, FAO can contribute to the accumulation of acetyl-CoA in the cytoplasm that is needed to initiate FAS, thereby supporting each other. On the basis of this idea, not only do the levels of acetyl-CoA and malonyl-CoA determine which metabolic pathways are activated, but also the ACC1 and ACC2 localization-dependent compartmentalization of these metabolites that allow FAO and FAS to occur simultaneously and independently from each other [95,107,109]. Alternatively, FAO may also play a protective role during conditions of metabolic stress such as hypoxia or glucose deprivation. Schlaepfer et al. [110] reported that LNCaP, C4-2B and DU-145 PCa cells were significantly enriched in triglycerides due to induction of lipogenic genes in response to hypoxic stress, and proposed that the accumulated lipids could support growth following reoxygenation. Accordingly, the inhibition of lipid utilization via treatment with etomoxir and shRNA-mediated CPT1 knockdown significantly compromised hypoxic PCa cell proliferation following reoxygenation. Altogether, these findings imply the importance of both FAO and FAS to promote tumour survival. In line with this observation, it has been shown that simultaneously targeting FAS and FAO significantly decreased PCa cell viability and AR expression [96]. Further research is needed to elucidate the precise mechanism of AR-mediated metabolic switching between de novo lipogenesis and FAO.
One possible explanation could be the cross-talk between AR and other metabolic or oncogenic pathways. A recent study reported that androgens induce mTOR relocalization to the nucleus to drive androgen-mediated metabolic reprogramming of PCa cells [111]. It was subsequently demonstrated that SREBF1, which acts as a downstream effector of the AR/mTOR signalling axis, helps regulate the balance between citrate consumption for mitochondrial energy production and as a carbon source for FAS in the cytoplasm [112]. These findings suggest that AR coordinates its actions with other transcription factors or oncogenes to establish or fine tune specific metabolic programs in PCa cells. Correspondingly, other transcriptional co-activators and signalling pathways that interplay with the AR signalling axis such as PGC-1a and estrogen related receptor gamma (ERRy) have also been described [94,113]. However, it remains to be determined how these metabolic pathways can be most optimally targeted in a setting of PCa.
3.6. Direct versus indirect regulation of lipid metabolic genes by AR
A major transcriptional regulator of lipogenic enzymes is the sterol response element binding protein 1 (SREBP1) transcription factor. Previous studies have shown that SREBP1 is androgen-regulated and that androgens activate SREBP1 expression via elevated expressions of the SREBP1 cleavage activating protein (SCAP) [3,114]. In line with this observation, androgen-induced lipogenic genes such as FASN harbor SREBP1-binding sites. Furthermore, SREBP1 induces fatty acid and lipid droplet formation and accumulation in AR-positive PCa cells. In contrast, several studies have shown that inhibition of SREBP1, achieved by shRNA knockdown or synthetic inhibitors (i.e. fatostatin) repressed expression of key lipogenic enzymes and suppressed growth of tumor xenografts [75,115,116]. Intriguingly, it was also shown that the expression of AR was, in turn, regulated by SREBP1 via binding of SREBP1 upstream of the AR gene [117]. This creates a positive feedback loop for the expression of lipogenic enzymes as well as AR expression and function. Collectively, these findings suggest that androgens can exert a coordinate control over lipogenesis that is regulated by the reciprocal relationship between AR and SREBP1 in support of PCa growth and survival (Fig. 2). While the androgenic regulation of SREBP1 has largely been reported in the context of PCa, little is known about the relationship between the AR and SREBP1 signaling, and the various roles of SREBP1 signaling in normal prostate cells. Elevated SREBP1 protein levels were reported in clinical human prostate tumours compared with normal prostate tissue or benign prostatic hypertrophy tissue [118]. Previous studies have shown that castration decreases expression of lipogenic enzymes in rat ventral prostate tissues, and that the effects were restored upon androgen treatment [52]. Given that these enzymes (i.e. FASN, ACLY, ACC) are known to be regulated by SREBP and that they harbour SREBP-binding sites, it is plausible that SREBPs may be involved in the coordinated activation of lipogenic gene expression by androgens in the normal prostate. Intriguingly, this study also demonstrated that androgen deprivation resulted in a marked decrease of mature nuclear-SREBP1 (nSREBP1) in the normal prostate in vivo; in contrast, readministration of testosterone completely restored nSREBP1 protein levels [52]. Nevertheless, the precise mechanism underlying the coordinated activation of the lipogenic phenotype by androgens and SREBP1 in the context of normal prostate cells remains to be elucidated.
Genome-wide analyses of AR binding and activity also suggest a distinct role for the AR in direct transcriptional regulation of lipogenic genes. Based on their proximity to AR binding sites [66,119] and their transcriptional regulation by androgens, genes involved in lipid metabolism and other key cellular metabolic processes have the potential to be directly regulated by the AR in malignant and non-malignant PCa cells [3]. This suggests that in addition to the effects of androgens on transcriptional activators such as SREBP1, androgens may also directly stimulate a coordinated metabolic network to regulate energy production and biosynthesis in PCa.
4. Effects of ADT on lipid metabolism
4.1. Metabolic effects of ADT
The current standard treatment for advanced and metastatic PCa is ADT [120]. This targets androgen signaling in PCa by reducing circulating levels of androgen or by blocking binding of androgens to the AR. Despite initial positive responses to ADT, the majority of patients eventually proceed to become resistant to ADT while maintaining functional AR signaling. This stage of cancer is termed CRPC. Several mechanisms of resistance to ADT including AR-dependent mechanisms (i.e. AR overexpression, AR point mutations and constitutively active AR splice variants) and AR-independent mechanisms (i.e. glucocorticoid receptor [GR] activity) have been described. Accordingly, multiple clinical attempts to further suppress AR activity have been made. In spite of recent approval of novel AR-targeted therapies (e.g. enzalutamide), metastatic CRPC (mCRPC) remains incurable, with patients having a median survival of less than 2 years.
While there is abundant evidence that ADT has profound effects on systemic metabolism, few studies have examined the effects of ADT on lipid metabolism in PCa cells. An earlier study by Ettinger et al. [118] observed that androgen-regulated genes (i.e. PSA) including SREBP1 were significantly decreased following androgen deprivation in prostate tumours. Given that SREBP1 is a known transcriptional activator of genes involved in lipogenesis and cholesterol synthesis, androgen withdrawal resulted in a decrease in several genes involved in fatty acid biosynthesis (FASN), energy production (ACBP) and cholesterol synthesis (FDPS). Intriguingly, another study linked Siah2 (an E3 ubiquitin ligase) to regulation of AR target genes associated with sterol and lipid metabolism [121]. This was attributed to the finding that SREBF1 was a Siah2-dependent androgen-responsive gene, consistent with their downregulation in Siah2 knockdown PCa cells. Of note, Siah2 expression was significantly reduced after ADT and is required for PCa cell growth under androgen-deprivation conditions in vitro and in vivo, suggesting that Siah2-regulated lipid metabolism may contribute to CRPC [121]. More recently, Alsannawi et al. [122] investigated the role of SLCO-encoded transporters (proteins that transport bile acids, xenobiotics, steroid conjugates and drugs) in response of primary PCa to ADT. Despite the small sample sizes, the authors observed clear differences in SLCO expression levels suggesting that ADT-induced changes in PCa SLCO transporters may influence steroid uptake and response to ADT, including uptake and response to PCa therapies (i.e. abiraterone). Altogether, these studies have begun to shed light into the connection between ADT and lipid metabolism in PCa cells, but much more work is needed to distinguish between the systemic effects of ADT on patient metabolism that may also impact on PCa cell properties.
4.2. Resistance to ADT: The role of lipid metabolism
Overexpression of key lipid biosynthetic enzymes in PCa is characteristic of both primary and advanced disease [123]. In view of the relationship between androgens and lipid metabolism, several studies have reported that genes mediating the lipogenesis pathway (i.e. FASN, SREBP1) that were initially suppressed following castration were subsequently up-regulated or re-activated during the emergence of CRPC [70,118,124]. Indeed, AR-mediated lipogenesis is a highly-conserved metabolic feature of CRPC (Fig. 1), suggesting the potential of lipid metabolism as a key survival pathway that promotes resistance [124].
As AR signaling is initially repressed during ADT, it is plausible that other genes or pathways critical to survival (including metabolic adaptations) are activated to help facilitate progression to CRPC. This includes, but is not limited to, lipid metabolism; FAS and cholesterol synthesis provide CRPC cells with fatty acids for energy and membrane synthesis, and cholesterol allows intratumoral de novo synthesis of androgens. Previous studies have reported overexpression of a key enzyme in cholesterol synthesis, 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) in enzalutamide-resistant PCa cells [125]. Notably, a combination of simvastin and enzalutamide significantly inhibited the growth of enzalutamide-resistant PCa cells in vitro and in vivo [125]. Another study demonstrated that silencing the expression of ELOVL7 (a fatty acid elongase enzyme) reduced growth of CRPC xenograft tumours in mice [124]. Furthermore, Flaig et al. [126] showed that targeting FAO via inhibition of CPT1A enhanced sensitivity of both androgen-dependent PCa (LNCaP) and CRPC (22Rv1) cells to enzalutamide, and that co-targeting the AR axis and FAO significantly inhibited PCa cell growth in vitro and in tumours. Altogether, these studies highlight the potential importance of lipid metabolism in CRPC. However, further research is required to understand the interactions of AR with lipid metabolism in driving treatment resistance and CRPC progression, particularly in more complex tumour environments containing multiple cell types.
One potential mechanism for the re-activation of lipid metabolic genes is the emergence of constitutively active AR splice variants (AR-Vs), such as AR-V7. AR-V7 lacks the C-terminal ligand-binding domain of the full-length AR and functions as a constitutively active, ligand-independent transcription factor that drives the growth of metastatic CRPC (mCRPC) cells in vitro and in vivo [127]. In support of this, AR-V7 was shown to be increased in patients resistant to the anti-androgen enzalutamide and abiraterone [128]. A recent study reported that pharmacological inhibition of FASN activity (IPI-9119) in CRPC not only suppressed fatty acid synthesis, but also inhibited full-length AR and AR-V7 expression in androgen-dependent and androgen-independent PCa cells [127]. Given that AR-V7 has the potential to re-activate lipid biosynthesis and is associated with treatment resistance, this suggests that AR-V7-mediated reactivation of lipid biosynthesis (Fig. 1) may drive disease recurrence and that targeting lipid metabolism pathways in combination with ADT and AR-directed therapies may be an effective therapeutic approach in CRPC (particularly for patients with high AR-V expression) or delay disease relapse.
Intriguingly, an AR blockade “bypass” mechanism was recently proposed as a prevalent mechanism of resistance. Upregulation of the GR was a common feature of drug-resistant tumours as evidenced from two preclinical models (LNCaP & VCaP) and was supported by correlative data showing increased GR expression in patients with enzalutamide resistance [129]. Importantly, comparative AR and GR transcriptome and ChIP-Seq studies have reported that a large majority of genes robustly regulated by GR are also regulated by AR [129,130]. Furthermore, a recent study revealed metabolic regulation of GR activation to stimulate enzalutamide resistance in PCa [131], which in turn, suggests a role for perturbed metabolic regulation in promoting tumour progression through activation of alternative steroid receptors. Finally, studies in non-prostatic tissues have reported that the GR directly activates several genes in lipid metabolism (i.e. FASN, ACC, SCD1) [132]. In view of these findings, it is plausible that the upregulation of GR permits the re-expression of AR-responsive genes, including genes involved in lipid metabolism, to promote PCa progression or CRPC.
Likewise, SREBP1-mediated activation of lipid biosynthesis pathways is critical to survival and facilitates progression to androgen-independence or CRPC [50,118]. Previous study showed that overexpression of SREBP1 is sufficient to increase tumorigenicity and invasion of PCa in vitro and its activation promoted CRPC progression in vivo [116]. Given the reciprocal relationship between the AR and SREBP1, SREBP1 may provide a further level of complexity through its interactions with upstream effectors or oncogenic signalling pathways in conjunction with AR signaling to enable an SREBP1-dependent lipogenic program to drive CRPC [50,116,133]. Having said all the above, although these studies have enhanced our understanding of how several distinct mechanisms of AR resistance may drive PCa progression to CRPC, the opportunity now is to determine whether a coordinated signaling network exists between these previously unlinked mechanisms of AR resistance (i.e. AR-V7, SREBP1 and GR).
5. Conclusion and new opportunities
The landscape of PCa progression is intimately linked to androgen signaling and alterations in lipid metabolism. Androgens regulate a large repertoire of genes involved in lipid uptake, biosynthesis, remodeling and degradation to drive prostate cell transformation and tumor development. Accordingly, numerous groups have exploited this facet of metabolic reprogramming in PCa as a therapeutic vulnerability by co-targeting lipid metabolism pathways with ADT or anti-androgens (i.e. enzalutamide). In linking these observations, it is crucial that we understand the AR-driven metabolic changes (including, but not limited to, other lipid metabolism processes such as fatty acid elongation and desaturation) that occur during the malignant transformation of prostate epithelial cells in order to target the key processes hijacked by tumor cells to survive. Likewise, it is important to recognise that PCa is a highly heterogenous and multifocal disease and that major discrepancies exist between patient tissues and cell line models (particularly for metabolism) which may have significant impact on translational applications. Cancer metabolic reprogramming is profoundly impacted by spatial heterogeneity, a fundamental feature of the tumour microenvironment [134]. While the vast majority of research has been undertaken in the epithelial cells of the normal and malignant prostate, recent insights into the importance of metabolic reprogramming in the TME underscore the need to broaden our focus to different cell types in the prostate such as the stromal fibroblasts and infiltrating immune cells. These supporting players in the microenvironment can be co-opted or modified by cancer cells to enhance the proliferation and invasion of the tumor, and provide exciting new targeting strategies to improve clinical outcomes for patients with PCa. For this reason, there is an increasing need to integrate information on lipid metabolism with spatial information on gene expression and lipid abundance. Recent technologies such as spatial transcriptomics have enabled transcriptomic profiling of hundreds of locations within tissue sections with high spatial resolution to dissect spatial metabolic heterogeneity in the tumour microenvironment and uncover novel tumour specific metabolic vulnerabilities [135]. However, this still poses a challenge due to the complex metabolic landscape of cancer. Mass spectrometry imaging (MSI), on the other hand, has the potential to distinguish tumour regions of different grade within the same tissue specimen, and characterize histologically benign tissue adjacent to the tumour to assess field cancerization [[136], [137], [138]]. Accordingly, a study demonstrated using a high-resolution matrix-assisted laser desorption/ionization time-of-flight MSI (MALDI MSI) method for metabolomic/lipidomic analysis and imaging of PCa tissues to characterize metabolic signatures associated with different Gleason scores (GS) [138]. In addition, Morse et al. [139], used desorption electrospray ionization coupled to MSI (DESI-MSI) to identify metabolites with differential abundances between regions of benign and PCa tissues which also validated with pre-established metabolic pathways highlighting an importance in altered lipid metabolism in PCa. In future studies, the incorporation of patient-derived explants or xenograft models and, more recently, human PCa organoids offers the opportunity to elucidate the interactions between PCa and the tumour microenvironment in systems that more closely recapitulate the disease heterogeneity and complexity of the clinical tissue microenvironment to provide a more robust mechanistic basis by which lipid metabolism may drive PCa disease progression and treatment resistance [99].
Author contributions
Study concept and design: Chui Yan Mah, Zeyad D. Nassar, Johannes V. Swinnen, Lisa M. Butler.
Drafting of manuscript: Chui Yan Mah, Zeyad D. Nassar.
Critical revision of the manuscript: Johannes V. Swinnen, Lisa M. Butler.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
This work was supported by grants from the Movember Foundation and Prostate Cancer Foundation of Australia (MRTA3 to Lisa M. Butler, Johannes V. Swinnen), Prostate Cancer Foundation of Australia (NDDA2711 to Lisa M. Butler, Johannes V. Swinnen), the Research Foundation—Flanders (FWO G.0841.15 to Johannes V. Swinnen), the Stichting tegen Kanker (to Johannes V. Swinnen), KU Leuven (C16/15/073 and C32/17/052 to Johannes V. Swinnen), Interreg V-A (EMR23 “EURLIPIDS” to Johannes V. Swinnen). Chui Yan Mah is supported by a Master of Philosophy International Scholarship and a Top-Up Scholarship from the Freemasons Foundation Centre for Men's Health. Zeyad D. Nassar is supported by an Early Career Fellowship from the National Health and Medical Research Council of Australia (1138648), a John Mills Young Investigator Award from the Prostate Cancer Foundation of Australia (YI 1417) and the Cure Cancer Australia Priority-driven Collaborative Cancer Research Scheme (1164798). Lisa M. Butler was supported by an ARC Future Fellowship (130101004) and Beat Cancer SA Beat Cancer Project Principal Cancer Research Fellowship (PRF1117).
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Gonthier K., Poluri R.T.K., Audet-Walsh É. Functional genomic studies reveal the androgen receptor as a master regulator of cellular energy metabolism in prostate cancer. J Steroid Biochem Mol Biol. 2019;191:105367. doi: 10.1016/j.jsbmb.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Barfeld S.J., Itkonen H.M., Urbanucci A., Mills I.G. Androgen-regulated metabolism and biosynthesis in prostate cancer. Endocr Relat Cancer. 2014;21:T57–T66. doi: 10.1530/ERC-13-0515. [DOI] [PubMed] [Google Scholar]
- 3.Butler L.M., Centenera M.M., Swinnen J.V. Androgen control of lipid metabolism in prostate cancer: novel insights and future applications. Endocr Relat Cancer. 2016;23:R219–R227. doi: 10.1530/ERC-15-0556. [DOI] [PubMed] [Google Scholar]
- 4.Itkonen H.M., Brown M., Urbanucci A., Tredwell G., Ho Lau C., Barfeld S. Lipid degradation promotes prostate cancer cell survival. Oncotarget. 2017;8:38264–38275. doi: 10.18632/oncotarget.16123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura K., Makino A., Hullin-Matsuda F., Kobayashi T., Furihata M., Chung S. Novel lipogenic enzyme ELOVL7 is involved in prostate cancer growth through saturated long-chain fatty acid metabolism. Cancer Res. 2009;69:8133–8140. doi: 10.1158/0008-5472.CAN-09-0775. [DOI] [PubMed] [Google Scholar]
- 6.Lamont K.R., Tindall D.J. Androgen regulation of gene expression. Adv Cancer Res. 2010;107:137–162. doi: 10.1016/S0065-230X(10)07005-3. [DOI] [PubMed] [Google Scholar]
- 7.Swinnen J.V., Heemers H., van de Sande T., de Schrijver E., Brusselmans K., Heyns W. Androgens, lipogenesis and prostate cancer. J Steroid Biochem Mol Biol. 2004;92:273–279. doi: 10.1016/j.jsbmb.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Swinnen J.V., Verhoeven G. Androgens and the control of lipid metabolism in human prostate cancer cells. J Steroid Biochem Mol Biol. 1998;65:191–198. doi: 10.1016/s0960-0760(97)00187-8. [DOI] [PubMed] [Google Scholar]
- 9.Costello L.C., Franklin R.B. Concepts of citrate production and secretion by prostate. 1. Metabolic relationships. Prostate. 1991;18:25–46. doi: 10.1002/pros.2990180104. [DOI] [PubMed] [Google Scholar]
- 10.Kavanagh J.P. Sodium, potassium, calcium, magnesium, zinc, citrate and chloride content of human prostatic and seminal fluid. J Reprod Fertil. 1985;75:35–41. doi: 10.1530/jrf.0.0750035. [DOI] [PubMed] [Google Scholar]
- 11.Costello L.C., Franklin R.B. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000;59:269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicks J.J., Martínez-Manautou J., Pedron N., Rosado A. Metabolic changes in human spermatozoa related to capacitation. Fertil Steril. 1972;23:172–179. [PubMed] [Google Scholar]
- 13.Arver S. Zinc and zinc ligands in human seminal plasma. III. The principal low molecular weight zinc ligand in prostatic secretion and seminal plasma. Acta Physiol Scand. 1982;116:67–73. doi: 10.1111/j.1748-1716.1982.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 14.Tomlins A.M., Foxall P.J., Lynch M.J., Parkinson J., Everett J.R., Nicholson J.K. High resolution 1H NMR spectroscopic studies on dynamic biochemical processes in incubated human seminal fluid samples. Biochim Biophys Acta. 1998;1379:367–380. doi: 10.1016/s0304-4165(97)00116-5. [DOI] [PubMed] [Google Scholar]
- 15.Searcy R.L., Simms N.M. A practical approach for acid-base characterization of human semen. Int J Fertil. 1967;12:329–334. [PubMed] [Google Scholar]
- 16.Ford W.C., Harrison A. The role of citrate in determining the activity of calcium ions in human semen. Int J Androl. 1984;7:198–202. doi: 10.1111/j.1365-2605.1984.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 17.Hori T., Masuda T., Kobayashi M., Kawakami E. Role of prostatic fluid in cooled canine epididymal sperm. Reprod Domest Anim. 2017;52:655–660. doi: 10.1111/rda.12963. [DOI] [PubMed] [Google Scholar]
- 18.Medrano A., Fernández-Novell J.M., Ramió L., Alvarez J., Goldberg E., Montserrat Rivera M. Utilization of citrate and lactate through a lactate dehydrogenase and ATP-regulated pathway in boar spermatozoa. Mol Reprod Dev. 2006;73:369–378. doi: 10.1002/mrd.20414. [DOI] [PubMed] [Google Scholar]
- 19.Härkönen P. Androgenic control of glycolysis, the pentose cycle and pyruvate dehydrogenase in the rat ventral prostate. J Steroid Biochem. 1981;14:1075–1084. doi: 10.1016/0022-4731(81)90219-3. [DOI] [PubMed] [Google Scholar]
- 20.Müntzing J., Varkarakis M.J., Saroff J., Murphy G.P. Comparison and significance of respiration and glycolysis of prostatic tissue from various species. J Med Primatol. 1975;4:245–251. doi: 10.1159/000459860. [DOI] [PubMed] [Google Scholar]
- 21.Farnsworth W.E. Testosterone stimulation of citric acid synthesis in the rat prostate. Biochim Biophys Acta. 1966;117:247–254. doi: 10.1016/0304-4165(66)90172-3. [DOI] [PubMed] [Google Scholar]
- 22.Härkönen P., Isotalo A., Santti R. Studies on the mechanism of testosterone action on glucose metabolism in the rat ventral prostate. J Steroid Biochem. 1975;6:1405–1413. doi: 10.1016/0022-4731(75)90077-1. [DOI] [PubMed] [Google Scholar]
- 23.Santti R.S., Villee C.A. Hormonal control of hexokinase in male sex accessory glands. Endocrinology. 1971;89:1162–1170. doi: 10.1210/endo-89-5-1162. [DOI] [PubMed] [Google Scholar]
- 24.Costello L.C., Akuffo V., Franklin R.B. Net citrate production by isolated prostate epithelial cells. Enzyme. 1988;39:125–133. doi: 10.1159/000469108. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee S., Zare R.N., Tibshirani R.J., Kunder C.A., Nolley R., Fan R. Diagnosis of prostate cancer by desorption electrospray ionization mass spectrometric imaging of small metabolites and lipids. Proc Natl Acad Sci USA. 2017;114:3334–3339. doi: 10.1073/pnas.1700677114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costello L.C., Franklin R., Stacey R. Mitochondrial isocitrate dehydrogenase and isocitrate oxidation of rat ventral prostate. Enzyme. 1976;21:495–506. doi: 10.1159/000458902. [DOI] [PubMed] [Google Scholar]
- 27.Costello L.C., Franklin R.B., Narayan P. Citrate in the diagnosis of prostate cancer. Prostate. 1999;38:237–245. doi: 10.1002/(sici)1097-0045(19990215)38:3<237::aid-pros8>3.0.co;2-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello L.C., Franklin R.B. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate. 1998;35:285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Costello L.C., Feng P., Milon B., Tan M., Franklin R.B. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7:111–117. doi: 10.1038/sj.pcan.4500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franklin R.B., Milon B., Feng P., Costello L.C. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci. 2005;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costello L.C., Franklin R.B., Liu Y., Kennedy M.C. Zinc causes a shift toward citrate at equilibrium of the m-aconitase reaction of prostate mitochondria. J Inorg Biochem. 2000;78:161–165. doi: 10.1016/s0162-0134(99)00225-1. [DOI] [PubMed] [Google Scholar]
- 32.Costello L.C., Liu Y., Franklin R.B., Kennedy M.C. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 33.Eliasson R. Cholesterol in human semen. Biochem J. 1966;98:242–243. doi: 10.1042/bj0980242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costello L.C., Liu Y., Franklin R.B. Testosterone stimulates the biosynthesis of m-aconitase and citrate oxidation in prostate epithelial cells. Mol Cell Endocrinol. 1995;112:45–51. doi: 10.1016/0303-7207(95)03582-r. [DOI] [PubMed] [Google Scholar]
- 35.Costello L.C., Liu Y., Franklin R.B. Testosterone and prolactin stimulation of mitochondrial aconitase in pig prostate epithelial cells. Urology. 1996;48:654–659. doi: 10.1016/S0090-4295(96)00217-8. [DOI] [PubMed] [Google Scholar]
- 36.Costello L.C., Liu Y., Zou J., Franklin R.B. Mitochondrial aconitase gene expression is regulated by testosterone and prolactin in prostate epithelial cells. Prostate. 2000;42:196–202. doi: 10.1002/(sici)1097-0045(20000215)42:3<196::aid-pros5>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costello L.C., Liu Y., Zou J., Franklin R.B. The pyruvate dehydrogenase E1 alpha gene is testosterone and prolactin regulated in prostate epithelial cells. Endocr Res. 2000;26:23–39. doi: 10.1080/07435800009040143. [DOI] [PubMed] [Google Scholar]
- 38.Franklin R.B., Brandly R.L., Costello L.C. Mitochondrial aspartate aminotransferase and the effect of testosterone on citrate production in rat ventral prostate. J Urol. 1982;127:798–802. doi: 10.1016/s0022-5347(17)54052-5. [DOI] [PubMed] [Google Scholar]
- 39.Franklin R.B., Brandly R.L., Costello L.C. Effect of inhibitors of RNA and protein synthesis on mitochondrial aspartate aminotransferase response to testosterone in rat ventral prostate. Prostate. 1982;3:637–642. doi: 10.1002/pros.2990030614. [DOI] [PubMed] [Google Scholar]
- 40.Franklin R.B., Kahng M.W., Akuffo V., Costello L.C. The effect of testosterone on citrate synthesis and citrate oxidation and a proposed mechanism for regulation of net citrate production in prostate. Horm Metab Res. 1986;18:177–181. doi: 10.1055/s-2007-1012264. [DOI] [PubMed] [Google Scholar]
- 41.Costello L.C., Akuffo V., Franklin R.B. Testosterone stimulates net citrate production from aspartate by prostate epithelial cells. Horm Metab Res. 1988;20:252–253. doi: 10.1055/s-2007-1010807. [DOI] [PubMed] [Google Scholar]
- 42.Costello L.C., Liu Y., Zou J., Franklin R.B. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J Biol Chem. 1999;274:17499–17504. doi: 10.1074/jbc.274.25.17499. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Franklin R.B., Costello L.C. Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. Prostate. 1997;30:26–32. doi: 10.1002/(sici)1097-0045(19970101)30:1<26::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 44.Juang H.H., Costello L.C., Franklin R.B. Androgen modulation of multiple transcription start sites of the mitochondrial aspartate aminotransferase gene in rat prostate. J Biol Chem. 1995;270:12629–12634. doi: 10.1074/jbc.270.21.12629. [DOI] [PubMed] [Google Scholar]
- 45.Qian K., Franklin R.B., Costello L.C. Testosterone regulates mitochondrial aspartate aminotransferase gene expression and mRNA stability in prostate. J Steroid Biochem Mol Biol. 1993;44:13–19. doi: 10.1016/0960-0760(93)90146-n. [DOI] [PubMed] [Google Scholar]
- 46.Franklin R.B., Zou J., Yu Z., Costello L.C. EAAC1 is expressed in rat and human prostate epithelial cells; functions as a high-affinity L-aspartate transporter; and is regulated by prolactin and testosterone. BMC Biochem. 2006;7:10. doi: 10.1186/1471-2091-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costello L.C., Liu Y., Franklin R.B. Prolactin specifically increases pyruvate dehydrogenase E1 alpha in rat lateral prostate epithelial cells. Prostate. 1995;26:189–193. doi: 10.1002/pros.2990260404. [DOI] [PubMed] [Google Scholar]
- 48.Franklin R.B., Juang H.H., Zou J., Costello L.C. Regulation of citrate metabolism by androgen in the LNCaP human prostate carcinoma cell line. Endocrine. 1995;3:603–607. doi: 10.1007/BF02953026. [DOI] [PubMed] [Google Scholar]
- 49.Arunakaran J., Balasubramanian K., Srinivasan N., Aruldhas M.M., Govindarajulu P. Interaction of androgens and prolactin on prostatic enzymes of the pyruvate-malate cycle involved in lipogenesis in castrated mature monkey, Macaca radiata. Cytobios. 1992;70:33–40. [PubMed] [Google Scholar]
- 50.Chen M., Zhang J., Sampieri K., Clohessy J.G., Mendez L., Gonzalez-Billalabeitia E. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat Genet. 2018;50:206–218. doi: 10.1038/s41588-017-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi S.M., Kam S.C. Metabolic effects of androgen deprivation therapy. Korean J Urol. 2015;56:12–18. doi: 10.4111/kju.2015.56.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heemers H., Vanderhoydonc F., Roskams T., Shechter I., Heyns W., Verhoeven G. Androgens stimulate coordinated lipogenic gene expression in normal target tissues in vivo. Mol Cell Endocrinol. 2003;205:21–31. doi: 10.1016/s0303-7207(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 53.Arunakaran J., Aruldhas M.M., Govindarajulu P. Effects of prolactin and androgens on the prostatic lipids of castrated mature bonnet monkeys. Prostate. 1990;17:247–260. doi: 10.1002/pros.2990170309. [DOI] [PubMed] [Google Scholar]
- 54.Arunakaran J., Aruldhas M.M., Govindarajulu P. Influence of castration and testosterone propionate on prostatic and seminal vesicular lipids in mature monkeys. Indian J Physiol Pharmacol. 1987;31:184–189. [PubMed] [Google Scholar]
- 55.Nyden S.J., Williams-Ashman H.G. Influence of androgens on synthetic reactions in ventral prostate tissue. Am J Physiol. 1953;172:588–600. doi: 10.1152/ajplegacy.1953.172.3.588. [DOI] [PubMed] [Google Scholar]
- 56.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 57.Warburg O. Über den stoffwechsel der carcinomzelle. Naturwissenschaften. 1924;12:1131–1137. [Google Scholar]
- 58.DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci Adv. 2016;2 doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeBerardinis R.J., Lum J.J., Hatzivassiliou G., Thompson C.B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metabol. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–89. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li W., Cohen A., Sun Y., Squires J., Braas D., Graeber T.G. The role of CD44 in glucose metabolism in prostatic small cell neuroendocrine carcinoma. Mol Cancer Res. 2016;14:344–353. doi: 10.1158/1541-7786.MCR-15-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaz C.V., Alves M.G., Marques R., Moreira P.I., Oliveira P.F., Maia C.J. Androgen-responsive and nonresponsive prostate cancer cells present a distinct glycolytic metabolism profile. Int J Biochem Cell Biol. 2012;44:2077–2084. doi: 10.1016/j.biocel.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Lavallée E., Bergeron M., Buteau F.A., Blouin A.C., Duchesnay N., Dujardin T. Increased prostate cancer glucose metabolism detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in localised gleason 8–10 prostate cancers identifies very high-risk patients for early recurrence and resistance to castration. Eur Urol Focus. 2019;5:998–1006. doi: 10.1016/j.euf.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Zacharias N., Lee J., Ramachandran S., Shanmugavelandy S., McHenry J., Dutta P. Androgen receptor signaling in castration-resistant prostate cancer alters hyperpolarized pyruvate to lactate conversion and lactate levels in vivo. Mol Imaging Biol. 2019;21:86–94. doi: 10.1007/s11307-018-1199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bok R., Lee J., Sriram R., Keshari K., Sukumar S., Daneshmandi S. The role of lactate metabolism in prostate cancer progression and metastases revealed by dual-agent hyperpolarized 13C MRSI. Cancers (Basel) 2019;11:E257. doi: 10.3390/cancers11020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Massie C.E., Lynch A., Ramos-Montoya A., Boren J., Stark R., Fazli L. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011;30:2719–2733. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swinnen J.V., Van Veldhoven P.P., Esquenet M., Heyns W., Verhoeven G. Androgens markedly stimulate the accumulation of neutral lipids in the human prostatic adenocarcinoma cell line LNCaP. Endocrinology. 1996;137:4468–4474. doi: 10.1210/endo.137.10.8828509. [DOI] [PubMed] [Google Scholar]
- 68.Nelson P.S., Clegg N., Arnold H., Ferguson C., Bonham M., White J. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ngan S., Stronach E.A., Photiou A., Waxman J., Ali S., Buluwela L. Microarray coupled to quantitative RT-PCR analysis of androgen-regulated genes in human LNCaP prostate cancer cells. Oncogene. 2009;28:2051–2063. doi: 10.1038/onc.2009.68. [DOI] [PubMed] [Google Scholar]
- 70.Rossi S., Graner E., Febbo P., Weinstein L., Bhattacharya N., Onody T. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;1:707–715. [PubMed] [Google Scholar]
- 71.Swinnen J.V., Roskams T., Joniau S., Van Poppel H., Oyen R., Baert L. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98:19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 72.Swinnen J.V., Vanderhoydonc F., Elgamal A.A., Eelen M., Vercaeren I., Joniau S. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int J Cancer. 2000;88:176–179. doi: 10.1002/1097-0215(20001015)88:2<176::aid-ijc5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 73.Kuhajda F.P., Jenner K., Wood F.D., Hennigar R.A., Jacobs L.B., Dick J.D. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci USA. 1994;91:6379–6383. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Medes G., Thomas A., Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953;13:27–29. [PubMed] [Google Scholar]
- 75.Zadra G., Photopoulos C., Loda M. The fat side of prostate cancer. Biochim Biophys Acta. 2013;1831:1518–1532. doi: 10.1016/j.bbalip.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santos C.R., Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 77.Swinnen J.V., Brusselmans K., Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 78.Zadra G., Loda M. Metabolic vulnerabilities of prostate cancer: diagnostic and therapeutic opportunities. Cold Spring Harb Perspect Med. 2018;8:a030569. doi: 10.1101/cshperspect.a030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shurbaji M.S., Kalbfleisch J.H., Thurmond T.S. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol. 1996;27:917–921. doi: 10.1016/s0046-8177(96)90218-x. [DOI] [PubMed] [Google Scholar]
- 80.Luo X., Cheng C., Tan Z., Li N., Tang M., Yang L. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16:76. doi: 10.1186/s12943-017-0646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brusselmans K., De Schrijver E., Verhoeven G., Swinnen J.V. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–6725. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- 82.Beckers A., Organe S., Timmermans L., Scheys K., Peeters A., Brusselmans K. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67:8180–8187. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- 83.Staubach S., Hanisch F.G. Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics. 2011;8:263–277. doi: 10.1586/epr.11.2. [DOI] [PubMed] [Google Scholar]
- 84.Swinnen J.V., Van Veldhoven P.P., Timmermans L., De Schrijver E., Brusselmans K., Vanderhoydonc F. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302:898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 85.Deep G., Schlaepfer I.R. Aberrant lipid metabolism promotes prostate cancer: role in cell survival under hypoxia and extracellular vesicles biogenesis. Int J Mol Sci. 2016;17:E1061. doi: 10.3390/ijms17071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barelli H., Antonny B. Lipid unsaturation and organelle dynamics. Curr Opin Cell Biol. 2016;41:25–32. doi: 10.1016/j.ceb.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 87.Fritz V., Benfodda Z., Rodier G., Henriquet C., Iborra F., Avancès C. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol Cancer Ther. 2010;9:1740–1754. doi: 10.1158/1535-7163.MCT-09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y., Zuckier L., Ghesani N. Fatty acid rather than glucose metabolism is the dominant bioenergetic pathway in prostate cancer. J Nucl Med. 2008;49(Suppl 1):104P. [Google Scholar]
- 90.Samudio I., Harmancey R., Fiegl M., Kantarjian H., Konopleva M., Korchin B. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Investig. 2010;120:142–156. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pike L.S., Smift A.L., Croteau N.J., Ferrick D.A., Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807:726–734. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 92.Harper M.E., Antoniou A., Villalobos-Menuey E., Russo A., Trauger R., Vendemelio M. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. FASEB J. 2002;16:1550–1557. doi: 10.1096/fj.02-0541com. [DOI] [PubMed] [Google Scholar]
- 93.Park J.H., Vithayathil S., Kumar S., Sung P.L., Dobrolecki L.E., Putluri V. Fatty acid oxidation-driven Src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep. 2016;14:2154–2165. doi: 10.1016/j.celrep.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tennakoon J.B., Shi Y., Han J.J., Tsouko E., White M.A., Burns A.R. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1α-mediated metabolic switch. Oncogene. 2014;33:5251–5261. doi: 10.1038/onc.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qu Q., Zeng F., Liu X., Wang Q.J., Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016;7:e2226. doi: 10.1038/cddis.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schlaepfer I.R., Rider L., Rodrigues L.U., Gijón M.A., Pac C.T., Romero L. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol Cancer Ther. 2014;13:2361–2371. doi: 10.1158/1535-7163.MCT-14-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Balaban S., Nassar Z.D., Zhang A.Y., Hosseini-Beheshti E., Centenera M.M., Schreuder M. Extracellular fatty acids are the major contributor to lipid synthesis in prostate cancer. Mol Cancer Res. 2019;17:949–962. doi: 10.1158/1541-7786.MCR-18-0347. [DOI] [PubMed] [Google Scholar]
- 98.Watt M.J., Clark A.K., Selth L.A., Haynes V.R., Lister N., Rebello R. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau5758. [DOI] [PubMed] [Google Scholar]
- 99.Nassar Z.D., Aref A.T., Miladinovic D., Mah C.Y., Raj G.V., Hoy A.J. Peri-prostatic adipose tissue: the metabolic microenvironment of prostate cancer. BJU Int. 2018;121(Suppl 3):9–21. doi: 10.1111/bju.14173. [DOI] [PubMed] [Google Scholar]
- 100.Tousignant K.D., Rockstroh A., Taherian Fard A., Lehman M.L., Wang C., McPherson S.J. Lipid uptake is an androgen-enhanced lipid supply pathway associated with prostate cancer disease progression and bone metastasis. Mol Cancer Res. 2019;17:1166–1179. doi: 10.1158/1541-7786.MCR-18-1147. [DOI] [PubMed] [Google Scholar]
- 101.Laurent V., Guérard A., Mazerolles C., Le Gonidec S., Toulet A., Nieto L. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pinthus J.H., Lu J.P., Bidaisee L.A., Lin H., Bryskine I., Gupta R.S. Androgen-dependent regulation of medium and long chain fatty acids uptake in prostate cancer. Prostate. 2007;67:1330–1338. doi: 10.1002/pros.20609. [DOI] [PubMed] [Google Scholar]
- 103.Nath A., Li I., Roberts L.R., Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752. doi: 10.1038/srep14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pascual G., Avgustinova A., Mejetta S., Martín M., Castellanos A., Attolini C.S. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 105.Hale J.S., Otvos B., Sinyuk M., Alvarado A.G., Hitomi M., Stoltz K. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells. 2014;32:1746–1758. doi: 10.1002/stem.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeon S.-M., Chandel N.S., Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abu-Elheiga L., Matzuk M.M., Abo-Hashema K.A., Wakil S.J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 108.Wakil S.J., Abu-Elheiga L.A. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50(Suppl):S138–S143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carracedo A., Cantley L.C., Pandolfi P.P. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schlaepfer I.R., Nambiar D.K., Ramteke A., Kumar R., Dhar D., Agarwal C. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget. 2015;6:22836–22856. doi: 10.18632/oncotarget.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Audet-Walsh É., Dufour C.R., Yee T., Zouanat F.Z., Yan M., Kalloghlian G. Nuclear mTOR acts as a transcriptional integrator of the androgen signaling pathway in prostate cancer. Genes Dev. 2017;31:1228–1242. doi: 10.1101/gad.299958.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Audet-Walsh É., Vernier M., Yee T., Laflamme C., Li S., Chen Y. SREBF1 activity is regulated by an ar/mtor nuclear axis in prostate cancer. Mol Cancer Res. 2018;16:1396–1405. doi: 10.1158/1541-7786.MCR-17-0410. [DOI] [PubMed] [Google Scholar]
- 113.Audet-Walsh É., Yee T., McGuirk S., Vernier M., Ouellet C., St-Pierre J. Androgen-dependent repression of ERRγ reprograms metabolism in prostate cancer. Cancer Res. 2017;77:378–389. doi: 10.1158/0008-5472.CAN-16-1204. [DOI] [PubMed] [Google Scholar]
- 114.Heemers H.V., Verhoeven G., Swinnen J.V. Androgen activation of the sterol regulatory element-binding protein pathway: current insights. Mol Endocrinol. 2006;20:2265–2277. doi: 10.1210/me.2005-0479. [DOI] [PubMed] [Google Scholar]
- 115.Li X., Chen Y.T., Hu P., Huang W.C. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol Cancer Ther. 2014;13:855–866. doi: 10.1158/1535-7163.MCT-13-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang W.C., Li X., Liu J., Lin J., Chung L.W.K. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res. 2012;10:133–142. doi: 10.1158/1541-7786.MCR-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang W.C., Zhau H.E., Chung L.W.K. Androgen receptor survival signaling is blocked by anti-beta2-microglobulin monoclonal antibody via a MAPK/lipogenic pathway in human prostate cancer cells. J Biol Chem. 2010;285:7947–7956. doi: 10.1074/jbc.M109.092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ettinger S.L., Sobel R., Whitmore T.G., Akbari M., Bradley D.R., Gleave M.E. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64:2212–2221. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- 119.Sharma N.L., Massie C.E., Ramos-Montoya A., Zecchini V., Scott H.E., Lamb A.D. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell. 2013;23:35–47. doi: 10.1016/j.ccr.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 120.Pullar B., Shah N. Prostate cancer. Surgery (Oxford) 2016;34:505–511. [Google Scholar]
- 121.Qi J., Tripathi M., Mishra R., Sahgal N., Fazli L., Ettinger S. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell. 2013;23:332–346. doi: 10.1016/j.ccr.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alsinnawi M., Zhang A., Bianchi-Frias D., Burns J., Cho E., Zhang X. Association of prostate cancer SLCO gene expression with Gleason grade and alterations following androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2019;22:560–568. doi: 10.1038/s41391-019-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shafi A.A., Putluri V., Arnold J.M., Tsouko E., Maity S., Roberts J.M. Differential regulation of metabolic pathways by androgen receptor (AR) and its constitutively active splice variant, AR-V7, in prostate cancer cells. Oncotarget. 2015;6 doi: 10.18632/oncotarget.5585. 31997–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Han W., Gao S., Barrett D., Ahmed M., Han D., Macoska J.A. Reactivation of androgen receptor-regulated lipid biosynthesis drives the progression of castration-resistant prostate cancer. Oncogene. 2018;37:710–721. doi: 10.1038/onc.2017.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kong Y., Cheng L., Mao F., Zhang Z., Zhang Y., Farah E. Inhibition of cholesterol biosynthesis overcomes enzalutamide resistance in castration-resistant prostate cancer (CRPC) J Biol Chem. 2018;293:14328–14341. doi: 10.1074/jbc.RA118.004442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flaig T.W., Salzmann-Sullivan M., Su L.J., Zhang Z., Joshi M., Gijón M.A. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget. 2017;8:56051–56065. doi: 10.18632/oncotarget.17359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zadra G., Ribeiro C.F., Chetta P., Ho Y., Cacciatore S., Gao X. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2019;116:631–640. doi: 10.1073/pnas.1808834116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Antonarakis E.S., Lu C., Wang H., Luber B., Nakazawa M., Roeser J.C. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arora V.K., Schenkein E., Murali R., Subudhi S.K., Wongvipat J., Balbas M.D. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sahu B., Laakso M., Pihlajamaa P., Ovaska K., Sinielnikov I., Hautaniemi S. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013;73:1570–1580. doi: 10.1158/0008-5472.CAN-12-2350. [DOI] [PubMed] [Google Scholar]
- 131.Li J., Alyamani M., Zhang A., Chang K.H., Berk M., Li Z. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. Elife. 2017;6 doi: 10.7554/eLife.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J.C., Gray N.E., Kuo T., Harris C.A. Regulation of triglyceride metabolism by glucocorticoid receptor. Cell Biosci. 2012;2:19. doi: 10.1186/2045-3701-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Z., Hou X., Shao C., Li J., Cheng J.X., Kuang S. Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer. Cancer Res. 2014;74:6635–6647. doi: 10.1158/0008-5472.CAN-14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yuan Y. Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb Perspect Med. 2016;6:a026583. doi: 10.1101/cshperspect.a026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Berglund E., Maaskola J., Schultz N., Friedrich S., Marklund M., Bergenstråhle J. Spatial maps of prostate cancer transcriptomes reveal an unexplored landscape of heterogeneity. Nat Commun. 2018;9:2419. doi: 10.1038/s41467-018-04724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Curtius K., Wright N.A., Graham T.A. An evolutionary perspective on field cancerization. Nat Rev Cancer. 2018;18:19–32. doi: 10.1038/nrc.2017.102. [DOI] [PubMed] [Google Scholar]
- 137.De Vivar A.D., Sayeeduddin M., Rowley D., Cubilla A., Miles B., Kadmon D. Histologic features of stromogenic carcinoma of the prostate (carcinomas with reactive stroma grade 3) Hum Pathol. 2017;63:202–211. doi: 10.1016/j.humpath.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 138.Randall E.C., Zadra G., Chetta P., Lopez B.G.C., Syamala S., Basu S.S. Molecular characterization of prostate cancer with associated Gleason Score using mass spectrometry imaging. Mol Cancer Res. 2019;17:1155–1165. doi: 10.1158/1541-7786.MCR-18-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morse N., Jamaspishvili T., Simon D., Patel P.G., Ren K.Y.M., Wang J. Reliable identification of prostate cancer using mass spectrometry metabolomic imaging in needle core biopsies. Lab Investig. 2019;99:1561–1571. doi: 10.1038/s41374-019-0265-2. [DOI] [PubMed] [Google Scholar]