Abstract
Objective
To investigate oncological outcomes in patients with bladder cancer who underwent minimally invasive radical cystectomy (MIRC) or open radical cystectomy (ORC).
Methods
We identified patients with bladder cancer who underwent radical cystectomy (RC) in 13 centers of the Chinese Bladder Cancer Consortium (CBCC). Perioperative outcomes were compared between MIRC and ORC. The influence of surgical approaches on overall survival (OS) and cancer-specific survival (CSS) in the entire study group and subgroups classified according to pathologic stage or lymph node (LN) status was assessed with the log-rank test. Multivariable Cox proportional hazard models were used to evaluate the association among OS, CSS and risk factors of interest.
Results
Of 2 098 patients who underwent RC, 1 243 patients underwent MIRC (1 087 laparoscopic RC and 156 robotic-assisted RC, respectively), while 855 patients underwent ORC. No significant differences were noted in positive surgical margin rate and 90-day postoperative mortality rate. MIRC was associated with less estimated blood loss, more LN yield, higher rate of neobladder diversion, longer operative time, and longer length of hospital stay. There was no significant difference in OS and CSS according to surgical approaches (p=0.653, and 0.816, respectively). Subgroup analysis revealed that OS and CSS were not significantly different regardless of the status of extravesical involvement or LN involvement. Multivariable Cox regression analyses showed that the surgical approach was not a significant predictor of OS and CSS.
Conclusions
Our study showed that MIRC was comparable to conventional ORC in terms of OS and CSS.
Keywords: Bladder cancer, Radical cystectomy, Minimally invasive surgery, Robotic surgery, Laparoscopy
1. Introduction
Radical cystectomy (RC) with pelvic lymph node dissection (PLND) is the standard treatment for non-metastatic high-risk bladder cancer [1]. Although open radical cystectomy (ORC) has been the gold standard procedure, this complicated procedure is associated with significant perioperative morbidity and mortality [2]. Minimally invasive approaches such as laparoscopic radical cystectomy (LRC) and robotic-assisted radical cystectomy (RARC) have been increasingly performed in order to minimize surgical morbidity and improve recovery. Many studies have documented the surgical feasibility and potential benefits of LRC and RARC for bladder cancer. Minimally invasive surgery appeared to offer advantages to patients in terms of overall lower perioperative complications, decreased pain, reduced scarring, and faster recovery [[3], [4], [5]]. However, oncologic outcomes following minimally invasive radical cystectomy (MIRC) are still controversial because of the lack of adequate comparative data and sufficient follow-up. In recent years, several small sample RCTs reported no major difference in short-term oncologic outcomes [[6], [7], [8]], while a retrospective study based on the Surveillance, Epidemiology, and End Results (SEER) database reported that RARC was associated with a benefit in 2-year overall survival (OS) compared with ORC [9]. Recently, two studies published in the New England Journal of Medicine found that minimally invasive radical hysterectomy was associated with shorter OS than open surgery among women with stage IA2 or IB1 cervical carcinoma [10,11]. Though the surgical procedures of cervical carcinoma were different from bladder cancer, these surprising results motivated us to reassess the value of minimally invasive surgery on cancer control.
Here, 13 centers of the Chinese Bladder Cancer Consortium (CBCC) [12] conducted a retrospective study to compare oncological outcomes in Asian patients with urothelial carcinoma of the bladder underwent MIRC or ORC.
2. Patients and methods
2.1. Patients selection
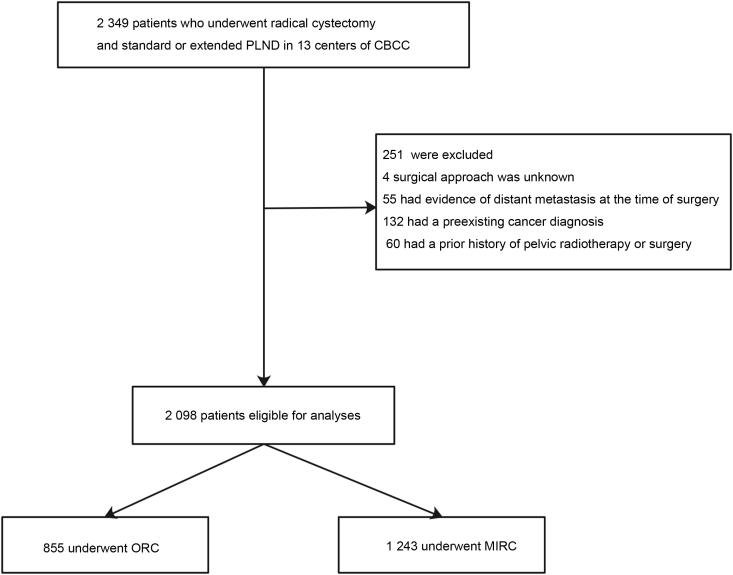
This study was approved by our institutional review board. Data of patients who were treated between 2007 and 2016 by experienced surgeons in 13 high-level centers of the CBCC were acquired from the CBCC database. We included patients with pathologic stage pT1-pT4a urothelial bladder cancer who had undergone RC and standard or extended PLND as primary treatment. The choice of surgical approach was mainly determined by the surgeon's preference and the patient's motivation. We excluded patients for whom the surgical approach was unknown, those who had evidence of distant metastasis at the time of surgery, those who had a preexisting cancer diagnosis, and those who had a prior history of pelvic radiotherapy or surgery. Following exclusions, 2 098 patients remained for further analyses (Fig. 1).
Figure 1.
Flowchart diagram depicting patient selection. PLND, pelvic lymph node dissection; CBCC, Chinese Bladder Cancer Consortium; MIRC, minimally invasive radical cystectomy; ORC, open radical cystectomy.
2.2. Measures
Cancer registrars documented the primary surgical approaches as open, laparoscopic, or robotic-assisted. Patients who had undergone LRC or RARC with PLND were categorized as having undergone MIRC, even when the urinary diversion was performed as open procedures or when conversion to open surgery occurred. Demographic, perioperative, pathologic, and follow-up data were recorded by cancer registrars and ascertained through the end of 2018. Perioperative outcomes included the type of PLND, type of urinary diversion, operative time, length of hospital stays (LOS), positive surgical margins (PSM), adjuvant chemotherapy, number of lymph node (LN) yield, estimated blood loss (EBL), 90-day postoperative mortality. Oncologic outcomes included OS and CSS. OS and CSS were defined as the time from RC to death due to any cause and bladder cancer, respectively.
2.3. Statistical analysis
Mean and standard deviation (SD) or median and interquartile range (IQR) were used for the descriptions of quantitative variables, and frequency and percentage were used for qualitative variables. Continuous variables were compared using the Student t-test or non-parametric tests, whereas categorical variables were compared using the Chi-square test. The influence of surgical approaches on OS and CSS in the entire study group and pathologic stage or LN status subgroups was analyzed using the Kaplan-Meier method, and differences were assessed with the log-rank test. Multivariable Cox proportional hazard models were used to evaluate the association among OS, CSS and risk factors of interest. A p-value <0.05 was considered statistically significant. Statistical analyses were conducted in Stata/SE 15.0 (Stata Corp LP, TX, USA).
3. Results
3.1. Patient and disease characteristics
A total of 2 098 patients were included in this study. Among these patients, 1 243 (59.2%) underwent minimally invasive surgery (1 087 LRC and 156 RARC, respectively), while 855 (40.8%) patients underwent open surgery. Patients and tumor characteristic were shown in Table 1. Quantity of surgery rapidly increased for both MIRC and ORC. Baseline characteristics including age, gender, body mass index (BMI), and ASA score were similar between MIRC and ORC (p=0.865, 0.917, 0.821, 0.147, respectively). There were no significant differences in pathologic stage, pathologic grade, and pathologic LN involvement rate between two treatment groups. The proportion of patients with extravesical involvement (≥pT3) in MIRC and ORC groups was 26.6% and 25.95%, respectively, while LN-positive rate was 15.5% and 14.0%, respectively.
Table 1.
Patient and tumor characteristics.
| Characteristics | Minimally invasive RC |
Open RC |
p-Value |
|---|---|---|---|
| n=1 243 | n=855 | ||
| Year of surgery, n (%) | |||
| 2007–2010 | 118 (9.5) | 137 (16.0) | <0.001 |
| 2011–2013 | 290 (23.3) | 306 (35.8) | |
| 2014–2016 | 835 (67.2) | 412 (48.2) | |
| Age, year | 63.4±10.1 | 63.5±10.2 | 0.865 |
| Male, n (%) | 1087 (87.5) | 749 (87.6) | 0.917 |
| BMI, kg/m2 | 22.8±2.4 | 22.9±2.2 | 0.821 |
| ASA score, n (%) | 0.147 | ||
| Ⅰ+Ⅱ | 1079 (86.8) | 713 (83.4) | |
| Ⅲ+Ⅳ | 164 (13.2) | 142 (16.6) | |
| Pathologic stage, n (%) | 0.587 | ||
| <T2 | 346 (27.8) | 244 (28.5) | |
| T2 | 566 (45.5) | 390 (45.6) | |
| T3 | 239 (19.2) | 148 (17.3) | |
| T4 | 92 (7.4) | 73 (8.5) | |
| Pathologic grade, n (%) | |||
| High grade | 1065 (85.7) | 745 (87.1) | 0.341 |
| Low grade | 178 (14.3) | 110 (12.9) | |
| Pathological nodal involvement, n (%) | 193 (15.5) | 120 (14.0) | 0.346 |
RC, radical cystectomy; BMI, body mass index; ASA, the American Society of Anesthesiologists.
3.2. Perioperative outcome
Perioperative variables were shown in Table 2. The vast majority of patients received standard PLND. There was no significant difference in the proportion of patients who underwent standard PLND (93.2% vs. 94.9%, p=0.130). The types of urinary diversion were different between MIRC and ORC. A higher proportion of patients underwent MIRC received a diversion of neobladder (45.9% vs. 23.6%, p<0.001). Conversion from minimally invasive surgery to open surgery was rare overall (2.2% of the cases). Minimally invasive surgery was associated with longer operative time and longer hospital stays. PSM occurred in 61 (4.9%) patients underwent MIRC and 43 (5.0%) patients underwent ORC (p=0.900). No difference was found in the percentage of patients who received adjuvant chemotherapy (p=0.212). MIRC yielded more LN than ORC with a mean of 16.2 versus 13.2 (p<0.001). ORC was associated with more estimate blood loss than MIRC. However, 90-day postoperative mortality was equivalent (1.1% vs. 1.2%, p=0.927).
Table 2.
Perioperative outcomes.
| Minimally invasive RC |
Open RC |
p-Value | |
|---|---|---|---|
| n=1 243 | n=855 | ||
| PLND, n (%) | |||
| Standard PLND | 1 159 (93.2) | 811 (94.9) | 0.130 |
| Extended PLND | 84 (6.8) | 44 (5.2) | |
| Urinary diversion, n (%) | <0.001 | ||
| Ileal conduit | 461 (37.1) | 339 (39.7) | |
| Neobladder | 571 (45.9) | 202 (23.6) | |
| Ureterocutaneostomy | 208 (16.7) | 311 (36.4) | |
| Other | 3 (0.2) | 3 (0.4) | |
| Open conversion, n (%) | 27 (2.2) | / | / |
| Operative time, min | 360.6±120.3 | 288.6±90.3 | <0.001 |
| Hospital stays, day | 28.0±9.9 | 25.0±10.0 | <0.001 |
| Positive surgical margin, n (%) | 61 (4.9) | 43 (5.0) | 0.900 |
| Adjuvant chemotherapy, n (%) | 251 (20.2) | 192 (22.5) | 0.212 |
| Lymph nodes yield | 16.2±7.9 | 13.2±5.0 | <0.001 |
| Estimate blood loss, mL | 402.7±517.8 | 623.1±692.9 | <0.001 |
| 90-day postoperative mortality, n (%) | 34 (2.7%) | 24 (2.8%) | 0.922 |
PLND, pelvic lymph node dissection; RC, radical cystectomy.
/, not required to provide.
3.3. Oncologic outcome
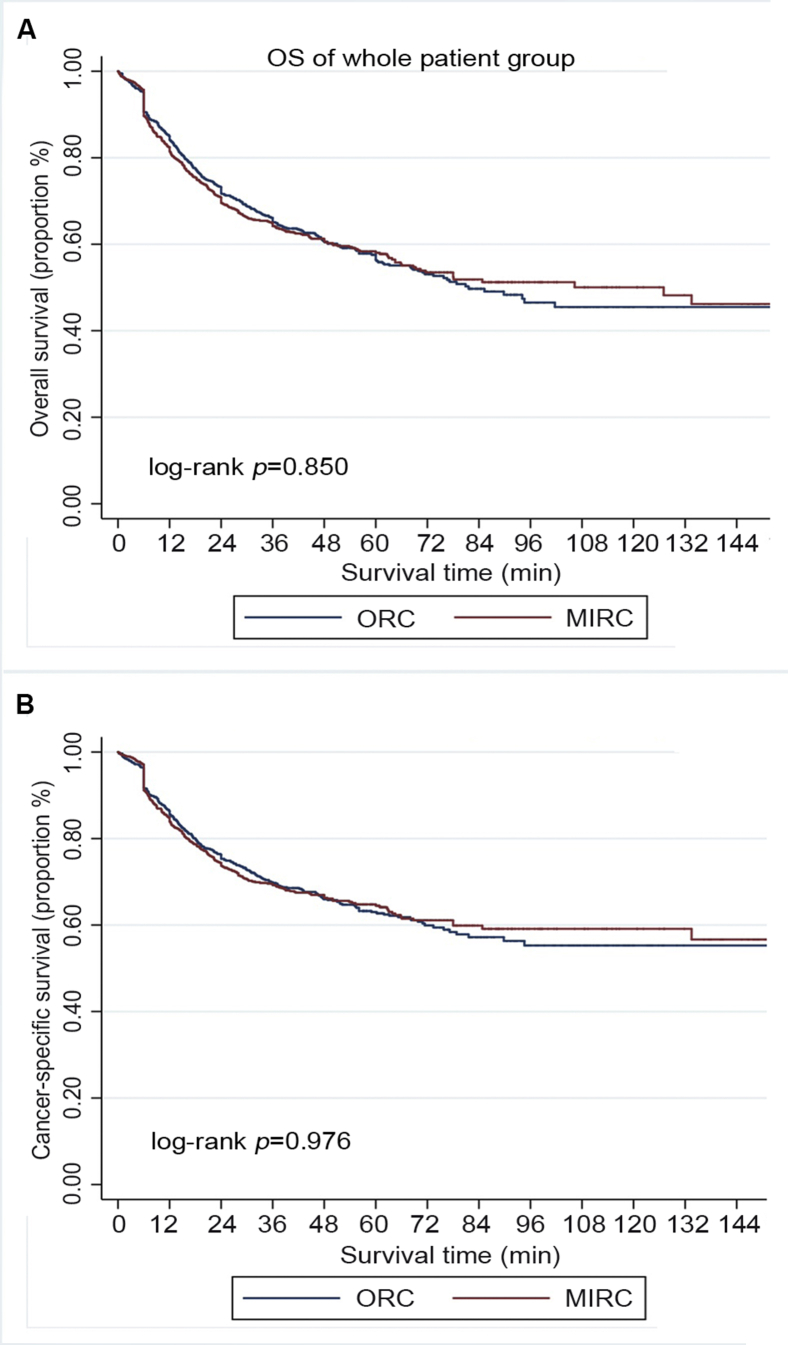
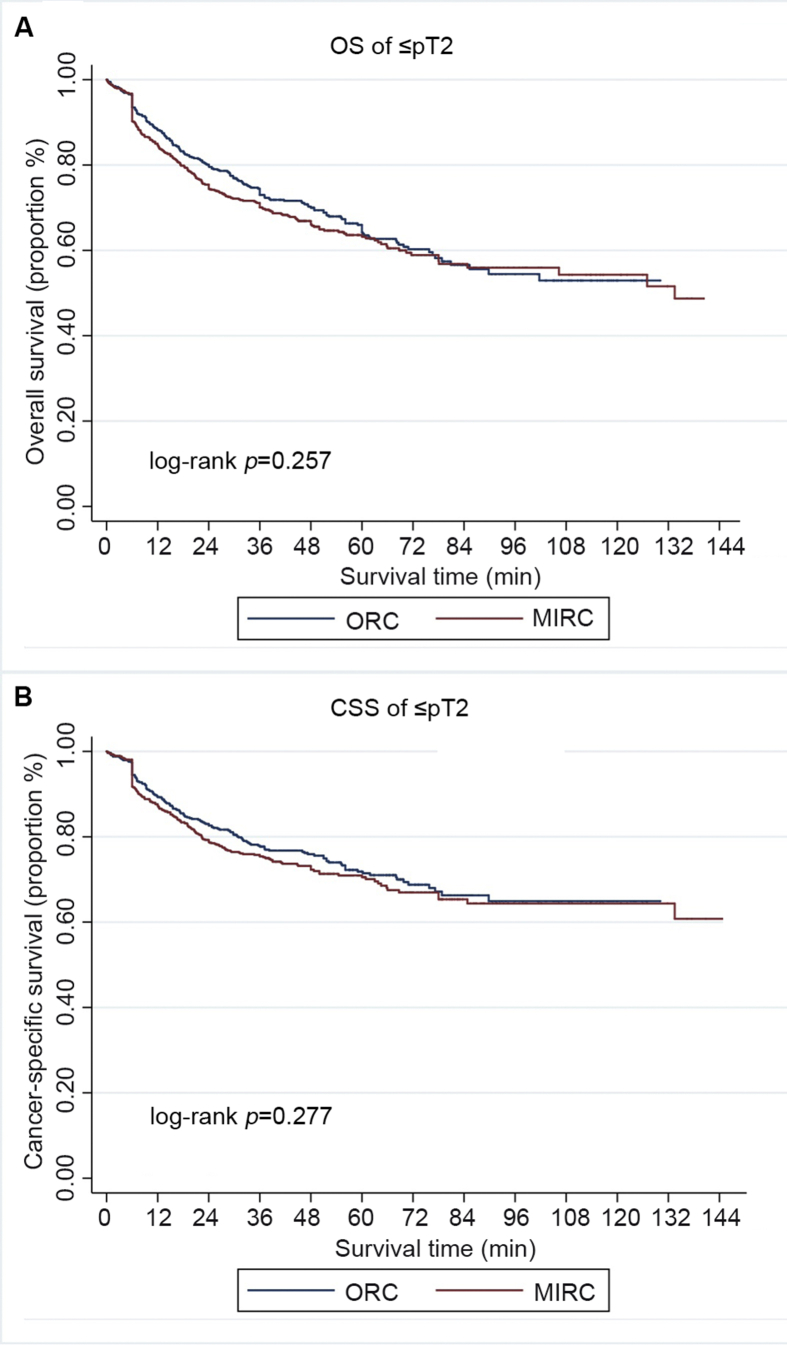
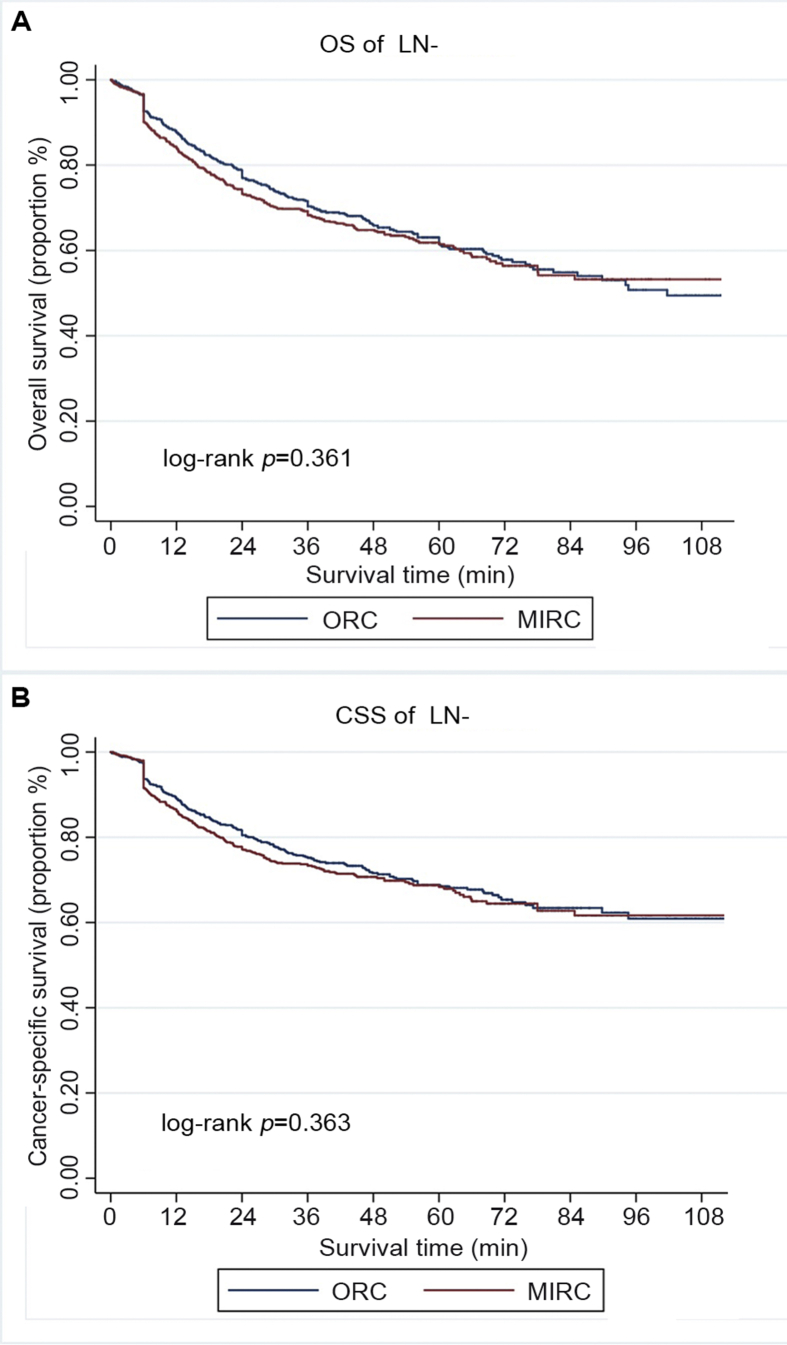
The median follow-up duration was 43.2 (IQR: 28.3–54.7) months and 60.3 (IQR: 38.2–76.9) months for MIRC and ORC, respectively (p<0.001). Patients underwent ORC had relatively longer follow-up duration. Overall, 476 (38.3%) patients in the MIRC group and 352 (41.2%) in the ORC group had died at the time of analysis, among whom 398 (32.0%), and 285 (33.3%), respectively, died from bladder cancer. There was no significant difference in OS and CSS according to surgical approaches (p=0.850, and 0.976, respectively, Fig. 2). Subgroup analysis by pathologic stage and LN status revealed that OS and CSS were similar for patients with organ-confined cancer (≤pT2) (p=0.257, and 0.277, respectively, Fig. 3), patients with extravesical involvement (≥pT3) (p=0.329, and 0.327, respectively Fig. 4), patients without LN involvement (LN negative) (p=0.361 and 0.363, respectively, Fig. 5), and patients with LN involvement (LN-positive) (p=0.175 and 0.119, respectively, Fig. 6). Multivariable Cox regression analyses revealed that pathologic tumor stage was independently associated with a significantly increased risk of death from all causes (Hazard ratio [HR]=1.84, p<0.001, Table 3) and death from bladder cancer (HR=2.41, p<0.001). Other predictive factors for all-cause death and cancer-cause death included LN involvement (HR=2.46, p<0.001, and HR=2.62, p<0.001, respectively), PSM (HR=1.71, p=0.004, and HR=1.78, p=0.005, respectively), and age (HR=1.03, p<0.001, and HR=1.03, p<0.001, respectively). ASA score ≥3 was a risk factor for all-cause death (HR=1.28, p=0.028) but not for cancer-cause death (HR=1.22, p=0.115). The surgical approach was not an independent predictive factor for neither all-cause death nor cancer-cause death.
Figure 2.
Survival of the whole patient group underwent radical cystectomy, stratified by surgical approach (MIRC or ORC). (A) Overall survival (OS); (B) Cancer-specific survival (CSS). MIRC, minimally invasive radical cystectomy; ORC, open radical cystectomy.
Figure 3.
Survival of patients with organ-confined cancer (≤pT2), stratified by surgical approach (MIRC or ORC). (A) Overall survival (OS); (B) Cancer-specific survival (CSS). MIRC, minimally invasive radical cystectomy; ORC, open radical cystectomy.
Figure 4.
Survival of patients with extravesical involvement (≥pT3), stratified by surgical approach (MIRC or ORC). (A) Overall survival (OS); (B) Cancer-specific survival (CSS). MIRC, minimally invasive radical cystectomy; ORC, open radical cystectomy.
Figure 5.
Survival of patients without lymph node involvement (LN-positive), stratified by surgical approach (MIRC or ORC). (A) Overall survival (OS); (B) Cancer-specific survival (CSS). MIRC, minimally invasive radical cystectomy; ORC, open radical cystectomy.
Figure 6.
Survival of patients with lymph node involvement (LN+), stratified by surgical approach (MIRC or ORC). (A) Overall survival (OS); (B) Cancer-specific survival (CSS). MIRC, minimally invasive radical cystectomy; ORC, open radical cystectomy.
Table 3.
Multivariable Cox proportional hazard regression analyses.
| characteristics | OS |
CSS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Year of surgery | ||||||
| 2007–2010 | Reference | |||||
| 2011–2013 | 1.03 | 0.88–1.21 | 0.703 | 1.01 | 0.85–1.16 | 0.889 |
| 2014–2016 | 0.93 | 0.76–1.08 | 0.232 | 0.96 | 0.81–1.15 | 0.686 |
| Age, year | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.01–1.04 | <0.001 |
| Gender | ||||||
| Male | Reference | |||||
| Female | 1.15 | 0.85–1.55 | 0.498 | 1.18 | 0.84–1.66 | 0.342 |
| BMI kg/m2 | 0.96 | 0.93–1.00 | 0.071 | 0.99 | 0.95–1.04 | 0.802 |
| ASA group | ||||||
| Ⅰ+Ⅱ | Reference | |||||
| Ⅲ+Ⅳ | 1.28 | 1.03–1.60 | 0.028 | 1.22 | 0.95–1.57 | 0.115 |
| Surgical approach | ||||||
| Open | Reference | |||||
| Minimally invasive | 0.95 | 0.77–1.17 | 0.653 | 0.97 | 0.77–1.23 | 0.816 |
| Pathologic stage | ||||||
| ≤T2 | Reference | |||||
| ≥T3 | 1.84 | 1.46–2.31 | <0.001 | 2.41 | 1.86–3.13 | <0.001 |
| Pathologic grade | ||||||
| LG | Reference | |||||
| HG | 1.26 | 0.88–1.80 | 0.209 | 1.07 | 0.72–1.59 | 0.749 |
| Pathological nodal involvement | ||||||
| No | Reference | |||||
| Yes | 2.46 | 1.93–3.13 | <0.001 | 2.62 | 2.00–3.42 | <0.001 |
| Positive surgical margin | ||||||
| No | Reference | |||||
| Yes | 1.71 | 1.19–2.46 | 0.004 | 1.78 | 1.19–2.66 | 0.005 |
| PLND | ||||||
| Standard PLND | Reference | |||||
| Extensive PLND | 1.26 | 0.93–1.72 | 0.135 | 1.30 | 0.92–1.83 | 0.139 |
BMI, body mass index; OS, overall survival; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval; ASA, the American Society of Anesthesiologists; PLND, pelvic lymph node dissection.
4. Discussion
Due to data limitations, LRC and RARC were still considered as investigational procedures for which no advantages in oncologic outcome could be shown as compared to ORC. Therefore, we conducted this multicenter study of minimally invasive surgery vs. open surgery, with a maximum follow-up of more than 10 years. A total of 2 098 patients who met the criterion were included. To our knowledge, this is the largest series studied oncologic outcome between MIRC and ORC in the Asian population. The baseline characteristics of the patients and tumor were well matched. All patients underwent RC and PLND by experienced surgeons from high-volume centers of the CBCC. Therefore, we minimized the bias introduced by patient selection and surgeon's experience and provided sound evidence comparing MIRC and ORC.
Consistent with other studies [13,14], we found that MIRC was associated with less EBL. The type of urinary diversion was determined by the preference of surgeons. Neobladder, which was a very complex procedure, was performed more often in MIRC than ORC (45.9% vs. 23.6%, p<0.001). Thus, it was reasonable that the operative time was significantly longer in MIRC compared with ORC. The LOS was determined by several factors, including recovery protocol and postoperative complications. Because the CBCC database does not contain enough information about postoperative complications, we cannot determine the main reason of longer LOS for patients who underwent MIRC. Previous study comparing cancer control in patients with neobladder and ileal conduit showed no difference in CSS between the two groups when adjusting for pathological stage [15]. Neobladder in male and female patients does not compromise the oncological outcome of cystectomy.
There were no significant differences in PSM rate between MIRC and ORC. PSM is an independent predictor of metastatic progression in patients undergoing radical cystectomy, which increased the risk of metastatic progression at 5 years from 32% to 74% [16]. The incidence of PSM ranged from 1% to 12.7% of the ORC cases, from 2% to 6% of the LRC cases, and from 0%–10.6% of the RARC cases [13,17]. In our trial, the PSM rate was 4.9% (61/1 263) in MIRC, and 5.0% (43/855) in ORC, respectively, which were similar to reported studies. Tumor infiltration may be the main limitation in achieving a negative margin [7,13]. PSM was associated with extravesical involvement tumor but not surgical approaches.
PLND is essential for both accurate staging and adequate local and regional control [18]. Several studies have taken LN yield as an indicator of the surgical quality of RC [4,19]. Although extensive PLND may yield more LN, it meanwhile associated with higher morbidity. It was still controversial for the optimal range of PLND [20,21]. It was widely accepted that qualified RC should include at least standard PLND. We conducted standard PLND as routine and extensive PLND if necessary, for example, when patients were diagnosed with non-organ-confined or LN-positive cancer preoperatively or intraoperatively. The proportion of extensive PLND was similar between MIRC and ORC. Our result showed that MIRC yielded more LN than ORC, with a mean of 16.2 versus 13.2. Previous study [9] reported that RARC was associated with higher LN count than ORC, with a median of 17 versus 12, which was consistent with our study. Herr et al. [22] proposed that a PSM rate of less than 10% with a median of 10–14 LNs examined is a reasonable overall standard for RC. PSM rates for MIRC and ORC in this study were 4.9% and 5.0%, respectively (p=0.633). Median LN yields were significantly lower in ORC than in MIRC (p<0.001), but all gave adequate yields of >10 LNs. We consider that on the basis of qualified PLND, LN yielded was not associated with better survival.
Long-term survival is the final touchstone for the oncologic efficiency of minimally invasive RC. A European LRC multicenter study [23] reported that actuarial recurrence-free survival (RFS), CSS and OS rates were 66%, 75% and 62% at 5 years and 62%, 55%, 38% at 10 years with a median follow-up of 50 months, which was comparable to our previously reported Asian LRC single-surgeon study [19]. Ten-year oncologic outcomes of RARC based on the International Robotic Cystectomy Consortium (IRCC) database seemed comparable to previously reported open series [24]. However, few studies have reported a comparison of survival between MIRC and ORC. In the present study, we noted that CSS and OS were not affected by surgical technique with a mean follow-up of 50.2 months, which was consistent with previous studies. Lin et al. [7] found no significant difference in RFS and OS between LRC and ORC with a median follow-up of 26 months. Recently, two RCTs also reported the oncologic outcome of RARC vs. ORC [6,8]. The RAZOR trial [8] revealed that RARC was non-inferior to ORC for 2-year progression-free survival (PFS). Its update still showed no difference in recurrence, 3-year PFS and 3-year OS between RARC and ORC [25]. Bochner et al. [6] demonstrated no difference in RFS, OS, or CSS between RARC and ORC with a median follow-up of 4.9 years. Retrospective comparative studies also found that MIRC and ORC provide similar oncologic outcomes [[26], [27], [28]]. Most of the previous studies showed that MIRC was equivalent to ORC on cancer control. However, Hanna et al. [9] reported that RARC was associated with a 7.7% higher OS rate at 2 years based on the SEER database. In contrast to other studies, we additionally performed subgroup analysis according to pathologic stage and LN status, confirmed that there was no significant difference between minimally invasive and open approach with regard to OS and CSS.
The present study showed that pathologic tumor stage, LN status, and presences of PSM predicted OS and CSS by multivariable cox regression. Age was also a predictive factor associated with OS and CSS because elderly patients were more likely to have high-risk tumor and inactive follow-up. Consistent with our study, several previous studies [16,19,29] also demonstrated that these prognostic factors were associated with oncologic outcomes in patients who underwent RC. However, the type of surgical approach for RC was not a significant predictor of OS or CSS.
This study has several limitations. Foremost, the retrospective nature of this study should be considered. There might have been a selection bias and surgeon bias. The type of surgical approach is mainly determined by surgeon's preference and patient's characteristic. The uneven distribution among ORC and MIRC between centers could have affected the observed differences. The study period is long, and it remains unclear whether this factor would influence the outcomes. Moreover, the database does not provide enough information about postoperative complications, recovery protocol, extracorporeal or intracorporeal diversion, so we cannot provide a complete depiction of some perioperative outcomes. Additionally, although most of these patients received regular follow-up and physical check, we still cannot accurately document the date of recurrence and metastasis of some patients, so RFS and cancer-free survival were not examined in our study.
It is so far the study with the longest follow-up period and the largest sample-size comparing minimally invasive and open radical cystectomy in the Asian population. All the surgeons are from Chinese high-level urological centers and have extensive experience in minimally invasive and open surgery. Although our results showed no difference in the survival of bladder cancer between these two groups, it does not mean that minimally surgery is generally equivalent to open surgery. It requires more skill, more training, and a longer learning curve. Therefore, the interpretation of the results must be more careful.
5. Conclusion
Minimally invasive cystectomy, such as RARC or LRC, was comparable to ORC with regard to OS and CSS. MIRC can be performed as an alternative to ORC. Future large sample size RCTs with longer follow-up are needed to provide more convincing survival outcomes.
Author contributions
Study concept and design: Tianxin Lin, Jian Huang.
Data acquisition: Weibin Xie, Junming Bi, Qiang Wei, Ping Han, Dongkui Song, Lei Shi, Dingwei Ye, Yijun Shen, Xin Gou, Weiyang He, Shaogang Wang, Zheng Liu, Jinhai Fan, Kaijie Wu, Zhiwen Chen, Xiaozhou Zhou, Chuize Kong, Yang Liu, Chunxiao Liu, Abai Xu, Baiye Jin, Guanghou Fu, Wei Xue, Haige Chen, Tiejun Pan, Zhong Tu.
Data analysis: Weibin Xie, Junming Bi.
Drafting of manuscript: Weibin Xie.
Critical revision of the manuscript: Tianxin Lin, Jian Huang.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No.81825016, 81772719, 81772728, 81572514), the Key Areas Research and Development Program of Guangdong (Grant No. 2018B010109006), and Medical Scientific Research Foundation of Guangdong Province (Grant No. A2018388).
Footnotes
Peer review under responsibility of Second Military Medical University.
Contributor Information
Tianxin Lin, Email: lintx@mail.sysu.edu.cn.
Jian Huang, Email: urolhj@sina.com.
References
- 1.Milowsky M.I., Rumble R.B., Booth C.M., Gilligan T., Eapen L.J., Hauke R.J. Guideline on muscle-invasive and metastatic bladder cancer (European association of urology guideline): American society of clinical oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34:1945–1952. doi: 10.1200/JCO.2015.65.9797. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance W.T., Rumohr J.A., Chang S.S., Clark P.E., Smith J.A., Jr., Cookson M.S. Contemporary open radical cystectomy: analysis of perioperative outcomes. J Urol. 2008;179:1313–1318. doi: 10.1016/j.juro.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 3.Modi P.K., Hollenbeck B.K., Oerline M., Weizer A.Z., Montgomery J.S., Kaffenberger S.D. Real-world impact of minimally invasive versus open radical cystectomy on perioperative outcomes and spending. Urology. 2019;125:86–91. doi: 10.1016/j.urology.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nix J., Smith A., Kurpad R., Nielsen M.E., Wallen E.M., Pruthi R.S. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010;57:196–201. doi: 10.1016/j.eururo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Rai B.P., Bondad J., Vasdev N., Adshead J., Lane T., Ahmed K. Robotic versus open radical. cystectomy for bladder cancer in adults. Cochrane DB Syst Rev. 2019;4 doi: 10.1002/14651858.CD011903.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochner B.H., Dalbagni G., Marzouk K.H., Sjoberg D.D., Lee J., Donat S.M. Randomized trial comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: oncologic outcomes. Eur Urol. 2018;74:465–471. doi: 10.1016/j.eururo.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin T., Fan X., Zhang C., Xu K., Liu H., Zhang J. A prospective randomised controlled trial of laparoscopic vs open radical cystectomy for bladder cancer: perioperative and oncologic outcomes with 5-year follow-up. Br J Canc. 2014;110:842–849. doi: 10.1038/bjc.2013.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parekh D.J., Reis I.M., Castle E.P., Gonzalgo M.L., Woods M.E., Svatek R.S. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018;391:2525–2536. doi: 10.1016/S0140-6736(18)30996-6. [DOI] [PubMed] [Google Scholar]
- 9.Hanna N., Leow J.J., Sun M., Friedlander D.F., Seisen T., Abdollah F. Comparative effectiveness of robot-assisted vs. open radical cystectomy. Urol Oncol. 2018;36:88.e81–88.e89. doi: 10.1016/j.urolonc.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Melamed A., Margul D.J., Chen L., Keating N.L., Del Carmen M.G., Yang J. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905–1914. doi: 10.1056/NEJMoa1804923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez P.T., Frumovitz M., Pareja R., Lopez A., Vieira M., Ribeiro R. Minimally Invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 12.Li K., Lin T., Xue W., Mu X., Xu E., Yang X. Current status of diagnosis and treatment of bladder cancer in China --analyses of Chinese Bladder Cancer Consortium database. Asian J Urol. 2015;2:63–69. doi: 10.1016/j.ajur.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwata T., Kimura S., Foerster B., Fossati N., Briganti A., Karakiewicz P.I. Oncologic outcomes after robot-assisted versus open radical cystectomy: a systematic review and meta-analysis. World J Urol. 2019;37:1557–1570. doi: 10.1007/s00345-019-02708-8. [DOI] [PubMed] [Google Scholar]
- 14.Tang K., Li H., Xia D., Hu Z., Zhuang Q., Liu J. Laparoscopic versus open radical cystectomy in bladder cancer: a systematic review and meta-analysis of comparative studies. PloS One. 2014;9 doi: 10.1371/journal.pone.0095667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yossepowitch O., Dalbagni G., Golijanin D., Donat S.M., Bochner B.H., Herr H.W. Orthotopic urinary diversion after cystectomy for bladder cancer: implications for cancer control and patterns of disease recurrence. J Urol. 2003;169:177–181. doi: 10.1016/S0022-5347(05)64062-1. [DOI] [PubMed] [Google Scholar]
- 16.Dotan Z.A., Kavanagh K., Yossepowitch O., Kaag M., Olgac S., Donat M. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol. 2007;178:2308–2313. doi: 10.1016/j.juro.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Chade D.C., Laudone V.P., Bochner B.H., Parra R.O. Oncological outcomes after radical cystectomy for bladder cancer: open versus minimally invasive approaches. J Urol. 2010;183:862–869. doi: 10.1016/j.juro.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Abol-Enein H., El-Baz M., Abd El-Hameed M.A., Abdel-Latif M., Ghoneim M.A. Lymph node involvement in patients with bladder cancer treated with radical cystectomy: a patho-anatomical study-a single center experience. J Urol. 2004;172:1818–1821. doi: 10.1097/01.ju.0000140457.83695.a7. [DOI] [PubMed] [Google Scholar]
- 19.Huang J., Lin T., Liu H., Xu K., Zhang C., Jiang C. Laparoscopic radical cystectomy with orthotopic ileal neobladder for bladder cancer: oncologic results of 171 cases with a median 3-year follow-up. Eur Urol. 2010;58:442–449. doi: 10.1016/j.eururo.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Bruins H.M., Veskimae E., Hernandez V., Imamura M., Neuberger M.M., Dahm P. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: a systematic review. Eur Urol. 2014;66:1065–1077. doi: 10.1016/j.eururo.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Gschwend J.E., Heck M.M., Lehmann J., Rubben H., Albers P., Wolff J.M. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur Urol. 2018;75:604–611. doi: 10.1016/j.eururo.2018.09.047. [DOI] [PubMed] [Google Scholar]
- 22.Herr H., Lee C., Chang S., Lerner S. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: a collaborative group report. J Urol. 2004;171:1823–1828. doi: 10.1097/01.ju.0000120289.78049.0e. [DOI] [PubMed] [Google Scholar]
- 23.Albisinni S., Rassweiler J., Abbou C.C., Cathelineau X., Chlosta P., Fossion L. Long-term analysis of oncological outcomes after laparoscopic radical cystectomy in Europe: results from a multicentre study by the European Association of Urology (EAU) section of Uro-technology. BJU Int. 2015;115:937–945. doi: 10.1111/bju.12947. [DOI] [PubMed] [Google Scholar]
- 24.Hussein A.A., Elsayed A.S., Aldhaam N.A., Jing Z., Osei J., Kaouk J. Comparison of long-term oncologic outcomes among historical open and minimally invasive retrospective studies. J Urol. 2019;202:927–935. [Google Scholar]
- 25.Venkatramani V., Reis I.M., Castle E.P., Gonzalgo M.L., Woods M.E., Svatek R.S. Predictors of recurrence, progression-free and overall survival following open versus robotic radical cystectomy: analysis from the RAZOR Trial with a 3-year follow-up. J Urol. 2020;203:522–529. doi: 10.1097/JU.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandaglia G., Karl A., Novara G., de Groote R., Buchner A., D'Hondt F. Perioperative and oncologic outcomes of robot-assisted vs. open radical cystectomy in bladder cancer patients: a comparison of two high-volume referral centers. Eur J Surg Oncol. 2016;42:1736–1743. doi: 10.1016/j.ejso.2016.02.254. [DOI] [PubMed] [Google Scholar]
- 27.Kim T.H., Sung H.H., Jeon H.G., Seo S.I., Jeon S.S., Lee H.M. Oncological outcomes in patients treated with radical cystectomy for bladder cancer: comparison between open, laparoscopic, and robot-assisted approaches. J Endourol. 2016;30:783–791. doi: 10.1089/end.2015.0652. [DOI] [PubMed] [Google Scholar]
- 28.Faraj K.S., Abdul-Muhsin H.M., Rose K.M., Navaratnam A.K., Patton M.W., Eversman S. Robot assisted radical cystectomy vs open radical cystectomy: over 10 years of the Mayo clinic experience. Urol Oncol. 2019;37:862–869. doi: 10.1016/j.urolonc.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L., Wu B., Zha Z., Qu W., Zhao H., Yuan J. Clinicopathological factors in bladder cancer for cancer-specific survival outcomes following radical cystectomy: a systematic review and meta-analysis. BMC Cancer. 2019;19:716. doi: 10.1186/s12885-019-5924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]