Abstract
The phrB gene encoding a putative cold-adapted DNA photolyase was cloned from the bacterial genomic DNA of Colwellia psychrerythraea 34H, a psychrophilic bacterium. Recombinant DNA photolyase, rCpPL, was overexpressed and purified from three different vectors. rCpPL binds its DNA substrate by flipping a cyclobutane pyrimidine dimer (CPD) into its active site and repairs CPD-containing DNA in vitro. rCpPL contains one catalytic flavin adenine dinucleotide (FAD) cofactor, but displays promiscuity in cofactor binding, in which either a flavin mononucleotide (FMN) or a methenyltetrahydrofolate (MTHF) molecule is bound as an antenna molecule and found in sub-stoichiometric amounts. The UV/Vis spectrum of oxidized rCpPL shows that the FADoxabsorption maximum is the most red-shifted reported for a PL, suggesting a unique cavity electrostatic environment. Modest FAD vibronic structure suggests that the binding pocket is more flexible than warmer PLs, corroborating the hypothesis that psychrophilic proteins must be highly flexible to function at low temperatures. Fluorescence excitation data show that the freshly purified flavin cofactor is in its fully reduced state (FADĦ). A homology analysis of PL protein structures spanning 70 °C in growth temperature supports the data that the structure of CpPL is quite diffrent from its warmer cousions.
Keywords: Photolyase, DNA repair, Cyclobutane pyrimidine dimer, Psychrophilic
Introduction
One of the signature characteristics of life is the capture, transduction, and use of energy for cellular processes. The abundance of solar energy incident on Earth has driven the evolution of sophisticated photobiochemical systems that harness and utilize light energy directly for, among other things, DNA repair. One such system is the blue-light-mediated repair of UV-damaged DNA by DNA photolyase, a flavoprotein (Sancar and Sancar 1984).
DNA photolyase is a member of the photolyase/cryptochrome (PL/CRY) superfamily of proteins (Kiontke et al. 2011). Members of this superfamily share a conserved catalytic FAD-binding site, and a second light-harvesting antenna cofactor (which varies depending on species, but serves the same function), but PL and CRY have different functions. Photolyase (PL) cleaves UV-damaged DNA via photoexcitation of the catalytic hydroquinone anionic flavin, resulting in ultrafast electron transfer to the UV lesion and subsequent cleavage (Kay et al. 2006). Cyclobutane dimer (CPD) and 6–4 photodimers are repaired by distinct PLs found in all three domains, but non-placental mammals lack PL repair. Cryptochromes (Ozturk et al. 2014) are also blue-light driven, but excitation of the oxidized flavin leads to photoreduction to its semiquinone state, resulting in a conformational change of a C-terminal domain that PL lacks. This is the signaling state results in photomorphogenesis, circadian rhythm regulation, photo-magnetoreception, and other blue-light sensory functions found in eukaryotes. Unlike PLs, CRYs are found in higher mammals (Losi and Gartner 2012).
The mechanism of E. coli DNA photolyase has been studied in detail, and kinetic models have been developed that demonstrate that blue-light absorption by the conserved reduced flavin adenine dinucleotide (FADĦ) cofactor results in an ultrafast electron transfer reaction, driving repair of the bound UV-DNA lesion (Clivio and Fourrey 1996; Kao et al. 2005; Liu et al. 2011; MacFarlane IV and Stanley 2003; Thiagarajan et al. 2011). Most studies on biologically important photoinduced ET reactions have focused on proteins from mesophilic organisms that have optimum growth temperatures around 20–45 °C (Willey et al. 2008). However, the study extremophiles raises interesting questions about what adaptation evolution has provided, to optimize temperature-sensitive chemical reactions, under conditions that would denature mesophilic enzymes (enzymes derived from mesophilic organisms).
Basic biological processes such as protein electron transfer, substrate binding, and product formation must be adapted to environments, where the reaction rates for these processes might differ by several orders magnitude. Take the temperature dependence of a general Arrhe-nius process as a simple example, where k = Ae-E†/RT. For an activation barrier of E† = 40 kJ/mol and a frequency factor of A = 1013/s, the rate constant for a first-order reaction in going from 373 to 273 K decreases by more than 100-fold. Indeed, it was shown that the second step in CPD splitting by PL, having a time constant of several hundred picoseconds, is accompanied by a barrier of this magnitude (Langenbacher et al. 1997; MacFarlane IV and Stanley 2003). Competing side reactions, such as charge recombination, should affect the repair efficiency of PL, and hence, the survivability of the organism changes in temperature. Thus, reactions that were optimized in one environment may have to be adapted to function properly if the organism finds itself faced with slow, but inexorable changes in environmental conditions.
As genomic sequencing of organisms has become widely available, in vitro comparative studies of the properties of proteins from organisms inhabiting a variety of ecosystems have become possible (Bae and Phillips 2004; Cipolla et al. 2012; Sheridan et al. 2000). Earlier, we reported on the cloning and characterization of a hyper-thermophilic PL from S. solfataricus (Gindt et al. 2016). Psychrophilic (cold-adapted) proteins are much less stable under ambient laboratory conditions than their thermophilic (hot-adapted) counterparts and consequently more difficult to study. Yields of recombinant psychrophile enzymes can be comparatively low, and even finding stable buffering systems for these enzymes can be a considerable challenge.
Here, we report on the cloning, overexpression, purification, and characterization of a cold-adapted DNA photolyase from the psychrophile Colwellia psychrerythraea 34H (CpPL), whose genome was sequenced by Methe et al. (2005). This photolyase, like all others, binds a catalytic flavin adenine dinucleotide (FAD) cofactor non-covalently. Recombinant CpPL (rCpPL) was also found to bind two different second cofactor molecules, flavin mononucleotide (FMN) when overexpressed and purified from E. coli BL21 (DE3) inclusion bodies, and a folate (possibly MTHF) when overexpressed and purified from E. coli Arctic Express (DE3) cells as a 6×-HisTag construct or in BL21-DE3 cells as a maltose binding protein (MBP) fusion. This suggests that CpPL might be somewhat promiscuous in antenna cofactor binding. CpPL is shown to be fully competent to bind and base flip CPDs, and to repair them when exposed to blue light. UV/Vis and fluorescence spectroscopy reveals that the FAD-binding site in this psychrophilic protein is unique compared to meso/thermophilic PLs. Finally, we present a comparison of structural parameters potentially involved in the thermal adaptation of various PLs that suggest that this psychrophilic protein is very different structurally from meso/thermophilic counterparts. This difference is discussed in the context of the hypothesis that protein flexibility is a requirement for cold-adapted organisms (Hoyoux et al. 2004).
Materials and methods
FAD and FMN were purchased from Tokyo Chemical Industries, Co. Ltd. (TCI) and used as received. All other reagents were of the highest quality available. HPLC-purified DNA oligonucleotides were obtained from IDT, Inc.
Cloning, overexpression, and purification of rCpPL
The detailed procedure for the cloning of the phrB gene from the genomic DNA of C. psychrerythraea 34H into pET14b expression vector, to form the CpphrB-pET14b construct, is described in the SI. Detailed procedures for the overexpression and purification of rCpPL, using the CpphrB-pET14b construct, from solubilized inclusion bodies [rCpPL(IB)] and from the soluble fraction [rCpPL(S)], are also described in the SI. In addition, procedures for the cloning, overexpression, and purification of the rCpPL-MBP fusion protein are described in detail in the SI.
HPLC analysis of rCpPL(IB) cofactors
An aliquot of purified rCpPL(IB) in Buffer T was desiccated in a Savant SpeedVac® SC110. 100 μl of chloroform was added to the dry sample to release the cofactors. The pellet was resuspended in chloroform by gently pipetting the solution. The sample was then desiccated until all of the chloroform was removed. This pellet was resuspended in HPLC-grade water and centrifuged at 15,000×g for 10 min at 4 °C. After centrifugation, the supernatant was carefully recovered and filtered through a 3 kDa MWCO centrifugal filter (Vivaspin). The filtered cofactors in the flow-through were stored in the dark at −20 °C until further use. Flavin controls for the identification of the cofactors in rCpPL(IB) were prepared and processed in the same way as the cofactor sample. The cofactors extracted from rCpPL(IB) and the flavin controls were run on an Agilent 1100 HPLC using a 150 × 4.6 mm C18J-sphere ODS-H80 column (YMC Co.). The HPLC method was modified from Light et al. (1980). The mobile phases were 5 mM ammonium acetate (Fisher Scientific), pH 6.0 (solvent A), and methanol (Sigma-Aldrich) (solvent B). An isocratic flow at 1 mL/min of 83% solvent A and 17% solvent B was used. Absorbances at 264 and 450 nm were measured using a photodiode array detector running under ChemStation (version A.10.01).
Irradiation and purification of DNA oligonucleotides
An HPLC-purified 11-mer oligonucleotide containing two adjacent thymidines, T1V1: 5'-GCAAGTTGGAG-3', was used to make the CPD substrate. The irradiation and purification of the CPD substrate (CPD–T1V1) have been described in detail elsewhere (Yang et al. 2007). Either CPD substrate or un-irradiated T1V1 was annealed with its complement 5'-CTC CAA CTTGC-3' (NP11) to make double-stranded DNA by heating mixtures of 1:1.2 (e.g., CPD–T1V1/NP11) at ~80 μM in 40 μL water to 75°C for 10 min. The mixture was allowed to cool on the bench to room temperature for at least 2 h.
HPLC photo-repair assay
50 μL of each aliquot of the photo-repair assay reactions (described in detail in the SI) was manually injected into an Agilent 1100 HPLC equipped with a 150 mm × 4.6 mm Hypersil ODS M-80 column. The separation was achieved in 0.1 M TEAA with a gradient of ACN from 8 to 12%. The C18 column was heated to 50 °C to obtain the separate ssDNA oligos, as indicated by the control chromatogram. Detailed procedures for the photoreduction of rCpPL, photo-repair assay conditions, and HPLC-based repair assay are described in the SI.
Steady-state fluorescence spectroscopy
Fluorescence spectra were measured using a Photon Technology Inc. QM3 fluorimeter. Unless otherwise indicated, all fluorescence spectra were measured in the dark, at 4 °C in a 4 mm × 10 mm quartz cuvette, and were buffer-corrected.
Thermal denaturation determination
The thermal denaturation temperature of the flavoprotein was determined by monitoring the emission quantum yield (ΦF) of the FAD cofactor. The degree of base stacking between the isoalloxazine and adenine rings determines ΦF. As shown below, ΦF(free FAD) > ΦF(CpPL-FAD). Thus, as the protein denatures and releases its FAD cofactor, the emission intensity of the FAD increases (Nishimoto et al. 2006). Emission spectra were measured from 480 to 700 nm with 460 nm excitation. A temperature controlled cuvette holder (QNW Flash 300) was ramped from 2 to 40 °C at 2 °C increments with an equilibration time of 2 min at each temperature point. Relative quantum yields were estimated using FMN and FAD as secondary standards (ΦF = 0.26 and 0.033, relative to quinine sul-fate, respectively) (Islam et al. 2003a) run under the same protocol.
Base-flipping assay
Base flipping of the CPD in duplex DNA was performed using the 2-aminopurine reporter base-flipping assay developed in our laboratory (Christine et al. 2002). Base stacking around a 2Ap adenosine analog, incorporated opposing the target CPD, is lost upon base-flipping by PL, resulting in a ~3-fold increase in its emission quantum yield. For this purpose, 0.5 μM rCpPL(IB) and 2.5 μM of the 5'-2Ap/CPD 11-mer DNA duplex in buffer T were used, where 5'-2Ap is 5'-CTCCAACTTGC-3' (A is the 2Ap adenine analog and CPD is the complementary strand containing a CPD). The fluorimeter was set to λex = 310 nm with a bandpass of 2 nm. The emission monochromator was scanned from 330 to 600 nm with a bandwidth of 2 nm.
Homology modeling of CpPL
The three-dimensional structure of CpPL was modeled using the Swiss Model homology modeling server (Schwede et al. 2003) using CPD-PL from Sulfolobus tokodaii (StPL, 2E0I. PDB, chain A) as a template. Although this protein had only 26.6% sequence identity with CpPL, it was automatically selected by HHSEARCH (v. 1.5.01) (Soding 2005) using an E value cutoff of 0.0001. It may be interesting to note that CpPL has a 42% sequence identity with the mesophilic, bacterial E. coli PL (EcPL). Structural analysis and visualization of models were done using UCSF Chimera v.1.6.2 (Pettersen et al. 2004). Chimera’s built-in functions were used to assess different structural parameters and for multiple sequence alignments. Other tools used for specific types of analyses are described below.
Results
Cloning, overexpression, and purification of three variants of rCpPL—from inclusion bodies [rCpPL(IB)], and from the soluble fraction [rCpPL(S) and rCpPL-MBP fusion].
The phrB gene from C. psychrerythraea 34H was initially cloned into a pET-14b expression vector (CpphrB-pET-14b construct, see SI for details). Sequencing of the phrB gene (Genewiz, data not shown) suggested that rCpPL consists of 470 amino acid residues. In case of rCpPL(IB), the CpphrB-pET-14b construct was used to transform BL21 (DE3) host cells for protein overexpression. SDS-PAGE analysis showed that CpPL was overexpressed to approximately 7% of the total cellular protein (SI, lanes 2 and 3, after and before induction, respectively). Based on the molecular weight markers, the molar mass of rCpPL was 60 ± 1 kDa. This can be compared to a molar mass of 57.1 kDa derived from the amino acid sequence, including 6×-His tag and thrombin cleavage sites, using the Compute pI/Mw tool in ExPASy (Artimo et al. 2012). However, analysis of the soluble and insoluble fractions after cell lysis revealed that rCpPL was locked in inclusion bodies in the cell lysate pellet.
Although denaturant-based methods for the solubilization of IBs and subsequent refolding procedures are well established (Parrilli et al. 2010), given the inherently sensitive nature of our target protein, these approaches proved to be too harsh. In addition, without knowing the number and type of cofactors associated with recombinant CpPL, a milder IB solubilization technique was used (Parrilli et al. 2010). Using N-lauroylsarcosine (NLS), a mild anionic detergent, gave variable results, but solubilization with IB bu?er containing 0.2% NLS (w/v) at a ratio of approximately 40:1 (v/v; buffer:IB material) gave the best protein yield (~1 μg/g cells). After 24 h of incubation with gentle stirring in IB buffer at 4 °C, the solubilized IB material contained rCpPL(IB) in relatively pure form (SI, lane 4). Residual NLS was removed from the protein sample by progressive dilution using NLS-free buffer and ultrafiltration. However, this led to major losses due to aggregation of the protein during the concentration procedure and/or loss of the bound cofactor. Purification of rCpPL(IB) from the solubilized IB material by affinity chromatography using Blue Sepharose™ also resulted in a considerable loss of the active protein. However, the resulting rCpPL(IB) was purified to 70% homogeneity (SI, lane 5). Eluted fractions were analyzed spectrophotometrically and those displaying protein-bound oxidized flavin, the only redox state evident, were pooled together.
To enhance the amount of rCpPL in the soluble fraction, we tried two different approaches. The first approach was overexpression in the Arctic Express (DE3) cell line using the CpphrB-pET14b construct. The Arctic Express (DE3) cell line co-expresses two cold-adapted chaperonins, Cpn10 and Cpn60, which are optimized for low-temperature folding and solubilization of recombinant proteins in E. coli. SDS-PAGE analysis of the soluble and insoluble fractions after cell lysis indicated that about 14% of the soluble fraction consisted of rCpPL(S). However, purification by nickel-affinity chromotography resulted in co-elution of Cpn60 along with rCpPL(S) (SI Fig. 2). In the most concentrated elutions, rCpPL(S) was typically purified to ~30% homogeneity, with the contaminant Cpn60 being present as about 60% of the total soluble protein [ε280(Cpn60) ~6000/M/cm, based on a single Trp residue] The UV–Vis spectrum of the partially purified rCpPL(S) sample is shown in SI Fig. 3 suggesting that PL is in the reduced form. A shoulder at around 425 nm could be taken for MTHF (see below).
The second approach was to clone the phrB gene into a pMAL-c5x vector to produce a rCpPL-MBP fusion protein. Although the rCpPL-MBP fusion protein was purified to >90% homogeneity (SI Fig. 4), it was recalcitrant to cleavage by Factor Xa (SI Fig. 6). The UV–Vis spectrum of rCpPL-MBP fusion (SI Fig. 5) was similar to that obtained for rCpPL(S), suggesting reduced flavin and an absorption band ~425 nm indicating protein-bound MTHF. Details of the experimental procedures, including attempts to cleave the fusion protein and remove the Cpn60 contaminant in case of rCpPL(S), are described in the SI.
Cofactor analysis of rCpPL(IB) by absorption and emission spectroscopies
All photolyases characterized to date have a conserved catalytic FAD cofactor and a second cofactor that functions as a light-gathering antenna (Aubert et al. 1999), with only one exception (Selby and Sancar 2012). The visible absorption spectrum of purified CpPL(IB) showed only oxidized flavin (Fig. 1). The ratio of apo- to holo-PL was estimated from the ratio of the 280 nm absorption of PL (with cofactors) to the ca. 450 nm absorption of the FAD. It should be noted that EcPL and CpPL (based on its codon usage) both contain 14 tryptophan residues. In the absence of the second cofactor this ratio for pure holo-PL is A280/A444 ~11 for EcPL, based on an extinction coefficient of 100, 100/M/cm at 280 nm for apo-PL (Jorns et al. 1990; Wang and Jorns 1989). A higher ratio typically indicates the presence of apoprotein. The absorbance ratio is A280/A450 ~27 for this preparation. Some PLs contain an FMN (Klar et al. 2006) or FAD (Fujihashi et al. 2007) as the second cofactor. To address this, HPLC analysis was used (Fig. 1, inset). rCpPL(IB) bound 0.8 equivalents of FMN for every equivalent of FAD (SI Table 1). On this basis, we (initially) assumed that FMN was the authentic antenna cofactor of CpPL. Taken together, the A280/A454 ~27 indicates that the CpPL(IB) is 63% apoprotein and 37% holoprotein.
Fig. 1.
Absorption spectrum of purified rCpPL(IB). Inset HPLC analysis of cofactors isolated from rCpPL(IB) and FAD and FMN controls. Asterisks indicate flavin impurities in the FMN sample, including riboflavin (Rbf) at 49 min
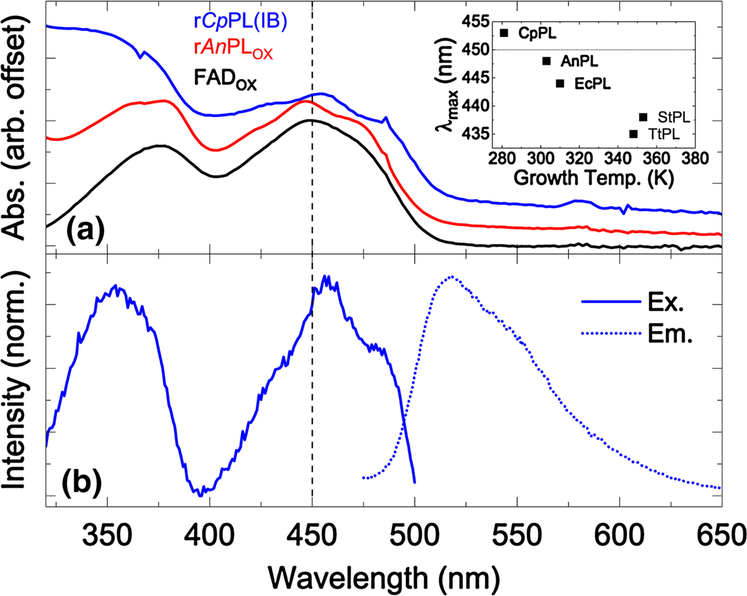
As discussed below, our results in overexpressing rCpPL revealed differences in flavin oxidation states depending on expression vector and purification schemes. The absorption spectrum of rCpPL(IB) showed the signature spectrum of oxidized flavin (Fig. 2a), but one that is quite red-shifted compared to all other known photolyases (Biernat et al. 2012; Eker et al. 1994; Fujihashi et al. 2007; Kato et al. 1997; Kiener et al. 1989; Kim et al. 1996; Kiontke et al. 2011; Klar et al. 2006; Sancar 1990; Yasui et al. 1994) and is indicative of an important difference in the FAD-binding site of this cold-adapted DNA repair enzyme.
Fig. 2.
a Absorption spectra of Blue Sepharose™-purified rCpPL, purified apo-AnPL reconstituted with FAD, and FAD in solution plotted for comparison. The AnPL spectrum shown here was taken from a previous study. Note the blue shift of the protein-bound FADOX in rAnPL and the red-shift in case of rCpPL relative to FADaq. The inset is the wavelength of the absorption maximum for the S01 transition for five PLs spanning ~70 °C. b Normalized fluorescence excitation (λEM = 520 nm) and emission spectra of rCpPL(IB) (λEX = 460 nm)
Oxidized FAD in solution has an absorption maximum at 448 nm for its lowest energy optical transition (S0→ S1, or S01, see black spectrum and dashed line at 448 nm in Fig. 2a) (Stanley 2001). In AnPL, a mesophilic PL from the cyanobacterium Anacystis nidulans, the S01 transition for oxidized FAD is blue-shifted to 446 nm (red, offset for clarity), with highly resolved vibronic structure indicating a tightly bound FAD (MacFarlane IV and Stanley 2001; Sancar 2003).
The S01 transition in rCpPL(IB) (blue, offset for clar-ity) is red-shifted to 454 nm and shows attenuated vibronic structure. This result was verified from three different pro-preparations. The spectral red-shift suggests that the FAD-binding pocket has greater solvent accessibility with a potentially altered network of water-mediated hydrogen bonds (Anderson et al. 2005; Gauden et al. 2006). The reduced vibronic structure in the absorption of the psychrophilic photolyase also suggests weaker binding of the FAD cofactor. This is supported by the fact that the cofactors were easily lost during the purification procedure. Weak binding of the catalytic cofactor may indicate a flexible active site geometry in CpPL. This result is consistent with other cold-adapted enzymes, which also display a high degree of conformational flexibility, especially around the active site (Altermark et al. 2007; Bae and Phillips 2004; Kim et al. 1999; Leiros et al. 2007).
As a caveat, the relatively weak vibronic structure might be due, in part, to the spectral overlap weakly-bound antenna FMNOX. This would have the effect of smearing out this vibronic structure. However, the well red-shifted absorption maximum can only be due to a unique FAD-binding cavity. Indeed, the overlapping FMN spectrum, centered at 446 nm, suggests that the true absorption maximum of the FAD S01 band is further red-shifted than reported here.
There is a remarkable apparent linear relationship between λmax of the lowest energy S01 transition in FADOX and the growth temperatures from which that PL was derived. λmax undergoes a blue shift as the growth temperature increases (Fig. 2a, inset, λmax for FAD in water is shown as a dotted horizontal line for reference). The growth temperature-dependent band shift of the flavin appears to be related to thermal adaptation in proteins, since the shift correlates with solvent accessibility (see below). This suggests a special role for cavity electrostatics in photolyases that might promote efficient repair as a function of temparature.
The excitation and emission spectra are shown in Fig. 2b. The red-shift of S01 to 456 nm in the excitation spectrum of CpPL(IB) (λEM = 520 nm) coincides with the absorption red-shift. Interestingly, the S02 transition has maximum intensity around 353 nm, well blue-shifted from the transition for FADaq. Since the difference dipoles of the states have quite different directions (Hopkins and Stanley 2003; Kodali et al. 2009; Stanley and Siddiqui 2001), we expect that the cavity electric field for CpPL is quite different from other PLs. The emission spectrum has a maximum at 518 nm for λEX = 460 nm. This is within experimental error of the result for EcPL reconstituted with FADOX (Jorns et al. 1990). A red-shifted excitation spectrum with an un-shifted emission spectrum is difficult to interpret without time-resolved emission studies.
The fluorescence quantum yield, ΦF, of oxidized rCpPL(IB) was measured using FMN as a standard(Islam et al. 2003b). At 4 °C ΦF = 0.028, as compared to free FAD which has ΦF = 0.033, rCpPL(IB) was used as it lacks overlapping folate emission. Interestingly, ΦFCp ≈ 7ΦFEc for reconstituted EcPL-FADOX, where ΦFEc = 0.004, as estimated by Jorns et al. (1990). Since the degree of quenching is indicative of the distance between the excited isoalloxazine ring and the potential quenchers (e.g., Ade, Trp, etc.), the brighter rCpPL emission suggests less dense packing around the FAD.
Evidence for promiscuity in rCpPL cofactor binding
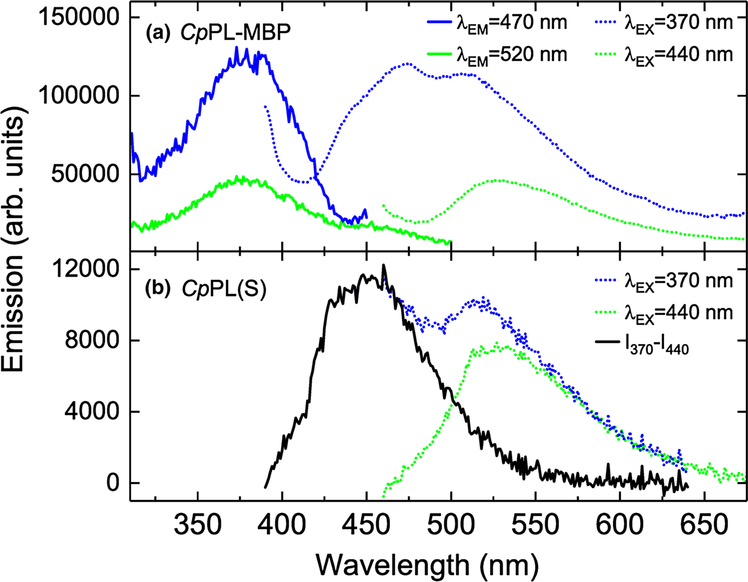
As noted above, rCpPL(IB) was isolated with the catalytic FAD and an FMN antenna cofactor. However, when purified from the soluble fraction, both rCpPL(S) and the rCpPL-MBP fusion contained a folate molecule, possibly MTHF (Johnson et al. 1988; Wang and Jorns 1989), as determined by fluorescence spectroscopy (Fig. 3; SI Figs. 3, 5). This result is similar to that observed for a recently characterized CPD photolyase from the extremophile Acinetobacter sp. Ver3 (Albarracin et al. 2014).
Fig. 3.
Fluorescence spectra of rCpPL-MBP. a Excitation (straight line) and emission (dotted line) spectra of freshly eluted protein taken at two sets of excitation wavelengths. b Emission spectra of cofactors from denatured rCpPL(S) taken at different excitation wave-show MTHF emission (dotted line)
With excitation at 370 nm, the majority emission peaked at about 472 nm with a shoulder at ~506 nm (Fig. 3a). The excitation spectrum of freshly prepared rCpPL-MBP has a maximum at ~380 nm, where MTHF emission is observed in EcPL (Johnson et al. 1988). FADH− is not observed, probably because of its low ΦF relative to MTHF (ΦFMTHF ~ 0.4) (Lipman and Jorns 1992). Evidence for the reduced flavin is given by the emission peak at 505 nm (Jorns et al. 1990). rCpPL(S) also appears to incorporate MTHF, as evidenced upon denaturation of the protein and isolation of the cofactors. The emission spectra of denatured rCpPL(S) at 370 and 440 nm were obtained, as shown in Fig. 3b. By subtracting the λEX = 440 nm emission spectrum from the λEX = 370 nm emission untill a zero baseline was achieved (black line; Fig. 3b), the characteristic blue-shifted emission of the folate released from rCpPL(S) is consistent with that observed for EcPL(25–27).
To help explain these results, it is useful to examine the sequence homology between CpPL and other PLs. Using ClustalW (Chenna et al. 2003), the amino acid sequence homologies of a variety of PLs were correlated. They revealed that the amino acid residues that contact MTHF within 5 Å in EcPL are conserved in CpPL (SI Fig. 8, red). This suggests that MTHF is the true antenna cofactor of CpPL. Whether the difference in antenna cofactors bound by rCpPL is a result of PL being partitioned into the periplasm, as in case of rCpPL(IB), or remaining in the cytoplasm, where there is access to MTHF, as in case of rCpPL-MBP and rCpPL(S), is not clear. What these results do suggest is that rCpPL antenna cofactor promiscuity is the result of a flexible cofactor binding site.
rCpPL recognizes and flips out a CPD into its active site
Biochemical (Berg and Sancar 1998; Christine et al. 2002) and X-ray crystallography (Mees et al. 2004; Park et al. 1995) studies have shown that CPD photolyases flip the target DNA lesion into the active site. Flipping of the CPD substrate into the active site of photolyase is particularly important for repair, since the CPD needs to be close enough to the FADĦ for efficient electron transfer (Kim et al. 1991; Park et al. 1995). We developed an assay using the fluorescent base analog 2-aminopurine (2Ap) as a probe of base flipping upon binding of the ds-substrate to the protein (Christine et al. 2002; Yang and Stanley 2006). Since base flipping of the CPD by photolyase is accompanied by a large distortion of the local structure of the DNA duplex around the lesion, including the loss of DNA base stacking, it was shown that the fluorescence quantum yield of a 2Ap probe incorporated opposite a CPD in a duplex DNA strand increased by more than a factor of 3 upon binding of photolyase to the substrate, as compared to the probe/CPD duplex in the absence of PL (Yang and Stanley 2006).
The 2-Ap base-flipping assay results are shown in Fig. 4. In panel a, the addition of oxidized rCpPL(IB) to a 5× excess of 2Ap-containing duplex CPD substrate increases the 2Ap emission by a factor of about 2.6, indicating that the CPD opposite the 2Ap probe was flipped out, resulting in destacking of the bases and loss of quenching of the 2Ap fluorescence. In addition, the emission maximum of 2Ap in the enzyme:substrate complex was blue-shifted by about 18 nm compared to the duplex alone. Data taken on the EcPL system are shown in panel b for comparison (Christine et al. 2002; Yang and Stanley 2006). Here, the emission increase is similar (~3.3×), but CPD binding by the mesophilic enzyme produces only a 7 nm blue shift.
Fig. 4.
CPD binding and repair assays. a CPD base flipping in dsDNA by rCpPL(IB) detected by the change in fluorescence emission of 2-aminopurine (2Ap) in the complementary strand (details in text). Note the ca. ×2.6 increase in 2Ap emission and the 18 nm blue shift of the emission maximum upon addition of rCpPL(IB) to the 2Ap/CPD duplex. b Same assay using mesophilic EcPL. A smaller 7 nm blue shift is observed, with ×3.3 emission increase upon binding the target duplex
In terms of solvatochromism (Liptay 1969), a blue shift is expected if a chromophore goes from a more polar to a less polar environment. For the 2Ap in the DNA duplex complementary to the CPD bound to photolyase, this would be the case if it was (a) less solvent exposed, (b) closer to adjacent non-polar bases for stacking interactions, or (c) due to specific interactions with residues in the protein. In the crystal structure of the AnPL:CPD complex (Mees et al. 2004), the duplex DNA strand is bent by 50° with the CPD flipped into the active site, but the major contacts are made with the DNA phosphates. The complementary adenosines only contact the protein through P402 (Mees et al. 2004), which is also conserved in EcPL (P395) and CpPL (P399). A comparison of the preceding residue, A394 and Q398, respectively, does not shed much light as to the magnitude of the blue shift in CpPL. For CpPL, it is reasonable to imagine that its greater flexibility would affect the degree of base-stacking interactions in the complementary 2Ap-containing strand, thereby modulating its emission properties.
CPD repair activity of rCpPL
To verify the CPD repair activity of rCpPL, a qualitative in vitro repair assay was performed. The reactions were run at ~0 °C because of the thermal instability of the protein. Aliquots collected during photo-repair at different timepoints were analyzed by HPLC. The chromatography indicated that, following exposure to 365 nm light, rCpPL repaired thymine dimers in CPD-containing ssDNA (T1V1–CPD) to yield native DNA, verifying its repair activity (SI Fig. 7). A comparison with mesophilic and thermophilic photolyases under nearly identical conditions (4 °C) suggests that rCpPL repairs CPDs significantly more slowly (manuscript in preparation). While this could be due to structural or enzymatic differences in rCpPL related to cold adaptation, it could also be a result of sub-optimal buffer/environmental conditions used in the assay.
Although some photolyases or cryptochromes (Cry-DASH) are known to repair only ss-CPD DNA (Selby and Sancar 2006), we have evidence to believe that CpPL is indeed a true photolyase and not a Cry-DASH. First, as shown in SI Fig. 9, the amino acids which contact the MTHF cofactor in EcPL (a true photolyase) are identically conserved in CpPL, but not in any of the Cry-DASH proteins, which have their own set of conserved MTHF binding residues (Huang et al. 2006) that are not conserved in PLs. Only E110 in EcPL, which is known to be critical for MTHF binding, is conserved in both PLs and Cry-DASH.
Second, MTHF is known to bind very strongly to Cry-DASH proteins (as evidenced by the large number of amino acid contacts) and is found to be present in stoichiometric amounts in purified Cry-DASH proteins (Huang et al. 2006). However, the MTHF in PLs is loosely bound to the protein due to fewer amino acid contacts and is found in sub-stoichiometric amounts in purified PLs, such as EcPL (42). As discussed above, CpPL was also found to contain a loosely bound MTHF and displayed promiscuity in cofactors binding to the MTHF site, thus lending further support to our claim that CpPL is indeed a true photolyase.
rCpPL denaturation temperature (TM)
The emission from oxidized flavin in flavoproteins is often quenched relative to flavin in solution. This quantum yield change has been used to measure the thermal denaturation temperature (apparent TM) (Nishimoto et al. 2006). The emission quantum yield of rCpPL(IB) from 2 to 40 °C decreases until about 26 °C and then turns towards higher intensity, suggesting that the protein is releasing the FAD cofactor, whose quantum yield is much higher in solution, as shown in Fig. 5. The reversibility of the transition was not checked due to aggregation of the protein. Thus, we estimate the apparent melting temperature (apparent TM) of the protein to be 26 (±4) °C.
Fig. 5.
Thermal denaturation of rCpPL(IB) as measured by the temperature dependence of the fluorescence quantum yield of rCpPL(IB) and FAD (relative to FMN). The temperature at the inflection point of the two linear regions (~26 °C) is assumed to be the apparent melting temperature (apparent TM) of rCpPL(IB)
Homology model of CpPL
Lacking a crystal structure, a homology model of CpPL was constructed. Model quality was estimated using the QMEAN server (Benkert et al. 2009) which gave a reasonably good QMEANnorm score of 0.625. This score ranges from 0 to 1, where unity indicates the most reliable model. The absolute quality is given by the QMEAN Z score = −1.69, where the Z score is a normalized score relative to scores obtained for experimental structures of similar size in the PDB. This further reinforced the reliability of the model (Benkert et al. 2011).
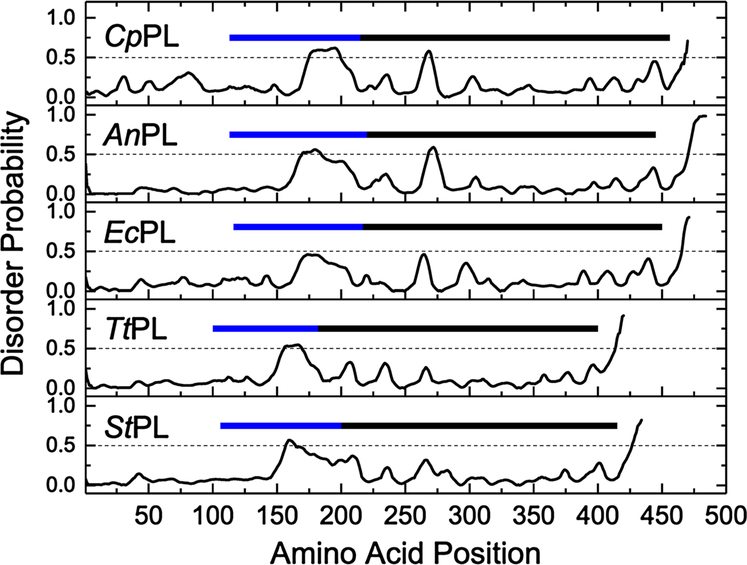
For the CpPL model, the local QMEAN scores for individual residues indicate a large number of disordered regions making unfavorable energy contributions. Interestingly, the majority of these disordered regions were found in the numerous flexible loops in the protein structure, giving rise to an overall disordered structure. As shown in Fig. 6, CpPL contains an unusually high proportion of disordering residues in the region that forms the active site.
Fig. 6.
Comparison of the relative disorder probabilities of residues making up the sequence of five photolyases: psychrophilic CpPL, mesophiles AnPL and EcPL, thermophilic TtPL, and hyper-thermophilic StPL. The average disorder probability of 0.5 is represented by the dashed black line in each panel. The blue bar represents regions in the primary sequence that forms the flexible inter-domain linker region connecting the N and C-terminal domains. The black bar represents the region in the primary sequence that forms the active site and the DNA binding groove
In comparison, the corresponding regions in the mesophilic and thermophilic photolyases indicate a gradual reduction in disorder with increasing thermostability (also see SI Figs. 10 and 11). The integrated disorder probabilities for each PL are 0.82, 0.74, 0.70, 0.57, and 0.62 for CpPL, AnPL, EcPL, TtPL [Thermus thermophilus PL (Kato et al. 1997)], and StPL, respectively. Thus, CpPL has about 35% greater disorder than the thermophile proteins.
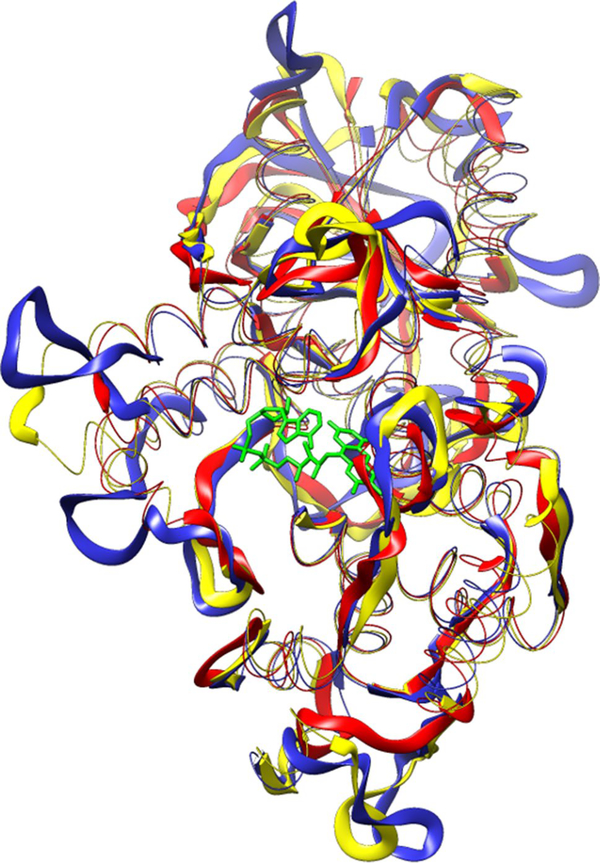
The model of CpPL displays the same N-terminal α/β and C-terminal α-helical domains found in mesophilic and thermophilic DNA photolyases (Fujihashi et al. 2007; Kiontke et al. 2011; Klar et al. 2006; Komori et al. 2002; Mees et al. 2004; Park et al. 1995; Tamada et al. 1997). As shown in Fig. 7, CpPL (blue) shares conserved 2° and 3° structural elements with mesophilic EcPL (yellow) and thermophilic StPL (red) proteins. The structure of the FAD-binding pocket of the CpPL model was predicted using the CASTp web server (Dundas et al. 2006). To check the accuracy of the prediction, the FAD-binding pockets of photolyases with experimentally determined X-ray structures were predicted using CASTp and the position and identity of each predicted residue were cross-checked for its proximity to the FAD molecule with the crystal structure of the respective photolyase (data not shown). SI Fig. 8 shows a ClustalW (Chenna et al. 2003) multiple sequence alignment of the photolyases. Residues in contact with FAD are highlighted in green. These residues are highly conserved, as are the Trp residues (grey) that constitute the “Trp triad” that is critical for photoreduction of the FAD (Aubert et al. 1999).
Fig. 7.
Comparison of the predicted 3D structure of CpPL (blue) with X-ray crystal structures of the mesophilic EcPL (1DNP, yellow) and the thermophilic StPL (2E0I, red). The looped regions in all three structures have been exaggerated in comparison with other secondary structural elements to highlight the differences. The catalytic FAD molecule of EcPL is shown in green as a stick figure in the foreground. The folate, seen on the right side of the protein, is loosely bound and easily lost upon purification
Structural features potentially contributing to temperature adaptation
A genomic analysis of C. psychrerythraea 34H revealed a general trend towards increased polar residues (particularly serine) and a decreased surface charge in proteins from this psychrophilic bacterium (Methe et al. 2005). Recent studies suggest a direct correlation between the number of surface ion pairs and temperature adaptation (Bae and Phillips 2004; Cipolla et al. 2012; Kumar and Nussinov 2004). To probe this further, we have used Prot Param (Gasteiger et al. 2003), ESBRI (Costantini et al. 2008), and the Protein Interactions Calculator (Tina et al. 2007) to analyze trends in amino acid composition, salt bridge, and surface ion pair numbers across a number of PLs. Table 1 shows these results, where < > refers to an averaged value followed by its standard deviation.
Table 1.
A comparison of structural parameters for PLs adapted to various thermal environments
| Cp | An | Ec | Tt | St | (Meso) | (Thermo) | |
|---|---|---|---|---|---|---|---|
| # of amino acidsa | 470.0 | 484.0 | 472.0 | 420.0 | 432.0 | 478 ± 6 | 426 ± 6 |
| Proline content (%) | 4.0 | 7.2 | 5.5 | 10.0 | 4.4 | 6.4 ± 0.9 | 7 ± 3 |
| Arginine content (%) | 4.7 | 6.4 | 7.2 | 11.7 | 7.6 | 6.8 ± 0.4 | 10 ± 2 |
| Non-polar residues (%)a | 53.4 | 57.4 | 55.1 | 62.8 | 40.7 | 56.3 ± 1.2 | 52 ± 11 |
| Polar residues (%)a | 46.6 | 42.5 | 44.9 | 37.1 | 28.9 | 43.7 ± 1.2 | 33 ± 4 |
| Charged residues (%)a | 25.9 | 21.9 | 25.2 | 30.2 | 30.3 | 23.6 ± 1.7 | 30.3 ± 0.1 |
| # of salt bridgesb | 4.3 | 8.3 | 6.8 | 9.3 | 10.6 | 7.6 ± 0.8 | 10.0 ± 0.7 |
| # of surface ion pairsc | 6.4 | 4.5 | 6.6 | 10.5 | 11.6 | 5.6 ± 1.1 | 11.1 ± 0.6 |
| TSASAa (Å2) | 22,833 | 20,372 | 20,306 | 18,555 | 18,305 | 20,339 ± 33 | 18,430 ± 125 |
| TSASA/residue | 48.6 | 42.1 | 43.0 | 44.2 | 42.4 | 42.6 ± 0.5 | 43.3 ± 0.9 |
| CHASAb (Å2) | 10,159 | 7587 | 7302 | 7220 | 7083 | 7445 ± 143 | 7152 ± 69 |
| CHASA/residue | 21.6 | 15.7 | 15.5 | 17.2 | 16.4 | 15.6 ± 0.1 | 16.8 ± 0.4 |
| % CHASA/TSASA | 44.5 | 37.2 | 36.0 | 38.9 | 38.7 | 36.6 ± 0.6 | 38.8 ± 0.1 |
| % per residue | 2.3 | 2.7 | 2.8 | 2.6 | 2.6 | 2.8 ± 0.1 | 2.6 |
TSASA total solvent accessible surface area, CHASA conditional hydrophobic accessible surface area. See text for referencing
ProtParam tool
ESBRI
Protein interactions calculator
With 40% fewer Arg residues than mesophilic PLs, CpPL cannot form as many surface ion pairs, salt bridges, or hydrogen bonding networks as the hotter PLs, suggesting that it is less rigid with a lower TM. There is also a prominent dfference between CpPL and hotter PLs in the number, length, and composition of flexible loops connecting individual α-helices and β-sheets, especially in the FAD-binding region. Structure-constraining residues such as Pro, Ile, or Tyr were replaced by smaller residues (e.g., Gly, Ser, Ala), while the opposite is true for thermophilic photolyases (SI Fig. 8; Table 1). Although this kind of residue replacement is seen mostly in looped regions, FAD-binding residues such as F249 in AnPL and L218 in TtPL are replaced by A246 in CpPL.
Hydrophobic interactions
The hydrophobic effect is the primary determinant of the folding of globular protein chains. Most proteins proceed through the folding process by the entropically-favorable burial of solvent-exposed hydrophobic groups to form the core of the molten globule intermediate. The free energy of solvation, however, is dependent on the conditional hydrophobic surface area (CHASA and CHASA/residue) of a protein (Dundas et al. 2006). Our model suggests that CpPL has a lower proportion of buried hydrophobic residues while exposing a higher number of hydrophobic residues to the solvent. Since the %CHASA/TSASA, the hydrophobic accessible surface area divided by the total solvent-accessible surface area, is largest for CpPL, it is expected that the change in entropy between the folded and unfolded states of CpPL would be smaller than warmer PLs.
In addition, the low enthalpic compensation upon folding due to fewer intramolecular interactions would make the folded state less stable. Thermodynamically, the apparent lack of rigidity of the chains forming the active site would impart a high degree of conformational entropy to the active site of CpPL (Altermark et al. 2007; Sheridan et al. 2000; Siddiqui and Cavicchioli 2006; Thomas and Cavicchioli 1998).
Discussion
Experimental characterization and computed 3D structure of CpPL: a protein governed by disorder?
This study of CpPL shows that the psychrophilic DNA repair enzyme has all the hallmarks of a highly flexible protein structure. The facile loss of cofactors upon purification, weak vibronic structure of the flavin spectrum, along with the surprisingly red-shifted absorption spectrum and the low apparent denaturation temperature all suggest that the FAD-binding site in CpPL has fewer contacts and greater solvent exposure compared to PLs isolated from organisms with higher growth temperatures.
The current view on cold-adapted enzymes is that the major selective pressure for cold adaptation is enhanced catalytic activity at low temperatures (Cipolla et al. 2012; Gerday et al. 1997). One of the major consequences of this is a conformationally flexible but rather unstable structure. Thus, the optimum temperature and buffer conditions for maximum catalytic activity are not necessarily the same for maximum structural stability of the protein (Sheridan et al. 2000).
An interesting comparison can be drawn between this study and the comparative crystal structure study of three adenylate kinases (AK, ~217 amino acids) from homologically similar psychrophilic, mesophilic, and thermophilic bacteria (genus Bacillus) (Bae and Phillips 2004). Bae and Phillips showed that the psychrophilic AK had a higher exposed non-polar surface area than its warmer counterparts, in agreement with our result for CpPL (Table 1). However, psychrophilic AK showed a modest 4% increase in non-polar solvent-exposed surface in going from the meso- to the psychrophile, compared with a 37% increase for CpPL relative to AnPL. This large dfference may be due to issues of cold adaptation, but the lower homology between PL proteins in our study may also be skewing this result. Cold-adapted and mesophile photolyases also had fewer salt bridges and surface ion pairs than the thermophile, in rough agreement with the AK results. An interesting difference is that there are proportionally fewer salt bridges and surface ion pairs for psychrophilic PL compared to AK. Since surface charged residues are important in substrate binding in PL, it could be that fewer salt bridges/ion pairs provide cold adaptation for greater substrate flexibility.
With regard to the importance of flexible loops for the function of CpPL as a psychrophilic protein, an interesting comparison can be made with the comparative study of the psychrophilic β-glucosidase, BgIU, with its mesophilic and thermophilic counterparts (Miao et al. 2016). Miao et al. (2016) shortened loop L3 in the psychrophilic protein by ten amino acids to mimic its mesophilic and thermophilic counterparts. This resulted in a reduction of the low-temperature catalytic activity of the cold-adapted protein which was attributed to the importance of the long loop in providing structural flexibility, increasing the solvent-accessible area and contributing to substrate recognition by the psychrophilic enzyme. Based on our results, a similar role could be assigned to the flexible loops around the active site of CpPL. It is interesting to note that promiscuity of antenna cofactors was also found in TtPL (Kato et al. 1997). Essen and coworkers determined that an FMN molecule constituted the second cofactor (Klar et al. 2006). The ratio of FAD:FMN was 1.0:0.7. This ratio was obtained, in part, due to loss of bound FMN from TtPL when purified using Blue Sepharose™ gel. It is worth noting that the antenna cofactor site is on the periphery of the N-terminal domain in PL. In EcPL, MTHF is found in sub-stoichiometric amounts when purified using Blue Sepharose™ (Hamm-Alvarez et al. 1989). Since we employed, the same gel our result is consistent with this observation. However, TtPL was also capable of binding 8-HDF in a promiscuous manner (Klar et al. 2006), suggesting that the second cofactor binding site flexibility is a general property of all PLs, and not due to adaptive pressures exerted by changes in the environment.
Finally, it can be argued that photolyase is an ideal enzyme with which to explore adaptation to extreme environments. Since catalytic efficiency is the ratio of the cata-rate constant to the Michaelis constant, keff = kcat/KM, the light-independent nature of substrate binding with the light-dependent nature of kcat(~kET) in PL affords a complete discrimination of these kinetic parameters as a function of thermal adaptation.
Supplementary Material
Acknowledgements
We wish to thank Mark Olsen and Cephalon for the donation of the Agilent HPLC. We also want to acknowledge Dr. Georges Feller, Dr. Aurora Martinez, Dr. Yvonne Gindt, and Dr. Peter S. Kessler for helpful discussions. The AnPL expression plasmid was a generous gift of Prof. A. Sancar, UNC Chapel Hill. S.M. and R.J.S. acknowledge support from NASA Exobiology Grant NNX13AH33G. This research was supported in part by the NSF Grant CHE-0847855. A.R. received support from NSF-REU supplement to CHE-0847855.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00792-017-0953-z) contains supplementary material, which is available to authorized users.
Communicated by L. Huang.
References
- Albarracin VH et al. (2014) First characterisation of a CPD-class I photolyase from a UV-resistant extremophile isolated from high-altitude Andean Lakes. Photochem Photobiol Sci 13:739–750. 10.1039/c3pp50399b [DOI] [PubMed] [Google Scholar]
- Altermark B, Niiranen L, Willassen NP, Smalas AO, Moe E (2007) Comparative studies of endonuclease I from cold-adapted Vibrio salmonicida and mesophilic Vibrio cholerae. FEBS J 274:252–263. 10.1111/j.1742-4658.2006.05580.x [DOI] [PubMed] [Google Scholar]
- Anderson S, Dragnea V, Masuda S, Ybe J, Moffat K, Bauer C (2005) Structure of a novel photoreceptor, the BLUF domain of AppA from Rhodobacter sphaeroides. Biochemistry 44:7998–8005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artimo P et al. (2012) ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res 40:W597–W603. 10.1093/nar/gks400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert C, Mathis P, Eker AP, Brettel K (1999) Intraprotein electron transfer between tyrosine and tryptophan in DNA photolyase from Anacystis nidulans. Proc Natl Acad Sci USA 96:5423–5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae E, Phillips GN (2004) Structures and analysis of highly homologous psychrophilic, mesophilic, and thermophilic adenylate kinases. J Biol Chem 279:28202–28208. 10.1074/jbc.M401865200 [DOI] [PubMed] [Google Scholar]
- Benkert P, Kunzli M, Schwede T (2009) QMEAN server for protein model quality estimation. Nucleic Acids Res 37:W510–W514. 10.1093/nar/gkp322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350. 10.1093/bioinformatics/btq662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg BJV, Sancar GB (1998) Evidence for dinucleotide flipping by DNA photolyase. J Biol Chem 273:20276–20284 [DOI] [PubMed] [Google Scholar]
- Biernat MA, Eker APM, van Oers MM, Vlak JM, van der Horst GTJ, Chaves I (2012) A baculovirus photolyase with DNA repair activity and circadian clock regulatory function. J Biol Rhythms 27:3–11. 10.1177/0748730411429665 [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500. 10.1093/nar/gkg500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christine KS, MacFarlane AW, Yang K, Stanley RJ (2002) Cyclobutylpyrimidine dimer base flipping by DNA photolyase. J Biol Chem 277:38339–38344 [DOI] [PubMed] [Google Scholar]
- Cipolla A, Delbrassine F, Da Lage JL, Feller G (2012) Temperature adaptations in psychrophilic, mesophilic and thermophilic chloride-dependent alpha-amylases. Biochimie 94:1943–1950. 10.1016/j.biochi.2012.05.013 [DOI] [PubMed] [Google Scholar]
- Clivio P, Fourrey JL (1996) (6–4) Photoproduct DNA photolyase mechanistic studies using s5-(6–4) photoproducts. Chem Com-mun 18:2203–2204 [Google Scholar]
- Costantini S, Colonna G, Facchiano AM (2008) ESBRI: a web server for evaluating salt bridges in proteins. Bioinformation 3:137–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J (2006) CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res 34:W116–W118. 10.1093/nar/gkl282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eker APM, Yajima H, Yasui A (1994) DNA photolyase from the fungus Neurospora crassa. Purification, characterization and comparison with other photolyases. Photochem Photobiol 60:125–133 [DOI] [PubMed] [Google Scholar]
- Fujihashi M et al. (2007) Crystal structure of archaeal photolyase from Sulfolobus tokodaii with two FAD molecules: implication of a novel light-harvesting cofactor. J Mol Biol 365:903–910 [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788. 10.1093/nar/gkg563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauden M, van Stokkum IHM, Key JM, Luehrs DC, van Grondelle R, Hagemann P, Kennis TM (2006) Hydrogen-bond switching through a radical pair mechanism in a flavin-binding photoreceptor. Proc Natl Acad Sci USA 103:10895–10900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerday C et al. (1997) Psychrophilic enzymes: a thermodynamic challenge. Biochim Biophys Acta (Protein Struct Mol Enzymol) 1342:119–131. 10.1016/s0167-4838(97)00093-9 [DOI] [PubMed] [Google Scholar]
- Gindt YM, Edani BH, Olejnikova A, Roberts AN, Munshi S, Stanley RJ (2016) The missing electrostatic interactions between DNA substrate and Sulfolobus solfataricus DNA photolyase: what is the role of charged amino acids in thermophilic DNA binding proteins? J Phys Chem B 120:10234–10242. 10.1021/acs.jpcb.6b07201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm-Alvarez S, Sancar A, Rajagopalan KV (1989) Role of enzyme-bound 5,10-methenyltetrahydropteroylpolyglutamate in catalysis by Escherichia coli DNA photolyase. J Biol Chem 264:9649–9656 [PubMed] [Google Scholar]
- Hopkins N, Stanley RJ (2003) Measurement of the electronic properties of the flavoprotein old yellow enzyme (OYE) and the OYE:p-Cl phenol charge-transfer complex using stark spectroscopy. Biochemistry 42:991–999 [DOI] [PubMed] [Google Scholar]
- Hoyoux A et al. (2004) Extreme catalysts from low-temperature environments. J Biosci Bioeng 98:317–330. 10.1263/jbb.98.317 [DOI] [PubMed] [Google Scholar]
- Huang YH, Baxter R, Smith BS, Partch CL, Colbert CL, Deisenhofer J (2006) Crystal structure of cryptochrome 3 from Arabi-dopsis thaliana and its implications for photolyase activity. Proc Natl Acad Sci USA 103:17701–17706. 10.1073/pnas.0608554103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam SDM, Penzkofer A, Hegemann P (2003a) Quantum yield of triplet formation of riboflavin in aqueous solution and of flavin mononucleotide bound to the LOV1 domain of Photl from Chlamydomonas reinhardtii. Chem Phys 291:97–114. 10.1016/s0301-0104(03)00187-3 [DOI] [Google Scholar]
- Islam SDM, Susdorf T, Penzkofer A, Hegemann P (2003b) Fluorescence quenching of flavin adenine dinucleotide in aqueous solution by pH dependent isomerisation and photo-induced electron transfer. Chem Phys 295:137–149. 10.1016/j.chemphys.2003.08.013 [DOI] [Google Scholar]
- Johnson JL, Hamm-Alvarez S, Payne G, Sancar GB, Rajagopalan KV, Sancar A (1988) Identification of the second chromophore of Escherichia coli and yeast DNA photolyases as 5,10-methenyltet-rahydrofolate. Proc Natl Acad Sci USA 85:2046–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorns MS, Wang B, Jordan SP, Chanderkar LP (1990) Chromophore function and interaction in Escherichia coli DNA photolyase: reconstitution of the apoenzyme with pterin and/or flavin derivatives. Biochemistry 29:552–561 [DOI] [PubMed] [Google Scholar]
- Kao YT, Saxena C, Wang L, Sancar A, Zhong D (2005) Direct observation of thymine dimer repair in DNA by photolyase. Proc Natl Acad Sci USA 102:16128–16132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R, Hasegawa K, Hidaka Y, Kuramitsu S, Hoshino T (1997) Characterization of a thermostable DNA photolyase from an extremely thermophilic bacterium, Thermus thermophilus HB27J. Bacteriology 179:6499–6503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay CWM, Bacher A, Fischer M, Richter G, Schleicher E, Weber S (2006) Blue light-initiated DNA repair by photolyase comprehensive series. Photochem Photobiol Sci 6:151–182 [Google Scholar]
- Kiener A, Husain I, Sancar A, Walsh C (1989) Purification and properties of Methanobacterium thermoautotrophicum DNA photolyase. J Biol Chem 264:13880–13887 [PubMed] [Google Scholar]
- Kim ST, Heelis PF, Okamura T, Hirata Y, Mataga N, Sancar A (1991) Determination of rates and yields of interchromophore (folate → flavin) energy transfer and intermolecular (fla-→ DNA)electron transfer in Escherichia coli photolyase by time-resolved fluorescence and absorption spectroscopy. Biochemistry 30:11262–11270 [DOI] [PubMed] [Google Scholar]
- Kim ST, Malhotra K, Ryo H, Sancar A, Todo T (1996) Purification and characterization of Drosophila melanogaster photolyase. Mutat Res 363:97–104 [DOI] [PubMed] [Google Scholar]
- Kim SY, Hwang KY, Kim SH, Sung HC, Han YS, Cho YJ (1999) Structural basis for cold adaptation—sequence, biochemical properties, and crystal structure of malate dehydrogenase from a psychrophile Aquaspirillium arcticum. J Biol Chem 274:11761–11767. 10.1074/jbc.274.17.11761 [DOI] [PubMed] [Google Scholar]
- Kiontke S, Geisselbrecht Y, Pokorny R, Carell T, Batschauer A, Essen LO (2011) Crystal structures of an archaeal class II DNA photolyase and its complex with UV-damaged duplex DNA. EMBO J 30:4437–4449. 10.1038/emboj.2011.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar T, Kaiser G, Hennecke U, Carell T, Batschauer A, Essen L-O (2006) Natural and non-natural antenna chromophores in the DNA photolyase from Thermus thermophilus. ChemBioChem 7:1798–1806 [DOI] [PubMed] [Google Scholar]
- Kodali G, Siddiqui SU, Stanley RJ (2009) Charge redistribution in oxidized and semiquinone E. coli DNA photolyase upon photoexcitation: stark spectroscopy reveals a rationale for the position of Trp382. J Am Chem Soc 131:4795–4807 [DOI] [PubMed] [Google Scholar]
- Komori H, Masui R, Kuramitsu S, Yokoyama S, Shibata T, Inoue Y, Miki K (2002) Crystal structure of thermostable DNA photolyase: pyrimidine-dimer recognition mechanism. Proc Natl Acad Sci USA 98:13560–13565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Nussinov R (2004) Different roles of electrostatics in heat and in cold: adaptation by citrate synthase. ChemBioChem 5:280–290. 10.1002/cbic.200300627 [DOI] [PubMed] [Google Scholar]
- Langenbacher T, Zhao X, Bieser G, Heelis PF, Sancar A, Michel-Bey-erle ME (1997) Substrate and temperature dependence of DNA photolyase repair activity examined with ultrafast spectroscopy. J Am Chem Soc 119:10532–10536 [Google Scholar]
- Leiros HKS, Pey AL, Innselset M, Moe E, Leiros I, Steen IH, Martinez A (2007) Structure of phenylalanine hydroxylase from Colwellia psychrerythraea 34H, a monomeric cold active enzyme with local flexibility around the active site and high overall stability. J Biol Chem 282:21973–21986. 10.1074/jbc.M610174200 [DOI] [PubMed] [Google Scholar]
- Light DR, Walsh C, Marletta MA (1980) Analytical and preparative high-performance liquid chromatography separation of flavin and flavin analog coenzymes. Anal Biochem 109:87–93 [DOI] [PubMed] [Google Scholar]
- Lipman RSA, Jorns MS (1992) Direct evidence for singlet-singlet energy transfer in Escherichia coli DNA photolyase. Biochemistry 31:786–791 [DOI] [PubMed] [Google Scholar]
- Liptay W (1969) Electrochromism and Solvatochromism. Angew Chem Int Ed 8:177–188 [Google Scholar]
- Liu Z et al. (2011) Dynamics and mechanism of cyclobutane pyrimidine dimer repair by DNA photolyase. Proc Natl Acad Sci. 10.1073/pnas.1110927108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi A, Gartner W (2012) The evolution of flavin-binding photoreceptors: an ancient chromophore serving trendy blue-light sensors. In: Merchant SS (ed) Annual review of plant biology, vol 63 Annual Reviews, Palo Alto, CA, USA, pp 49–72 [DOI] [PubMed] [Google Scholar]
- MacFarlane AW, Stanley RJ (2001) Evidence of powerful substrate electric fields in DNA photolyase: implications for thymidine dimer repair. Biochemistry 40:15203–15214 [DOI] [PubMed] [Google Scholar]
- MacFarlane AW, Stanley RJ (2003) Cis-syn thymidine dimer repair by DNA photolyase in real time. Biochemistry 42:8558–8568 [DOI] [PubMed] [Google Scholar]
- Mees A, Klar T, Gnau P, Hennecke U, Eker APM, Carell T, Essen L-O (2004) Crystal structure of a photolyase bound to a CPD-like DNA lesion after in situ repair. Science 306:1789–1793 [DOI] [PubMed] [Google Scholar]
- Methe BA et al. (2005) The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc Natl Acad Sci USA 102:10913–10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao LL et al. (2016) Molecular structural basis for the cold adaptedness of the psychrophilic beta-glucosidase BglU in Micrococcus antarcticus. Appl Environ Microbiol 82:2021–2030. 10.1128/aem.03158-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto E, Aso Y, Koga T, Yamashita S (2006) Thermal unfolding process of dihydrolipoamide dehydrogenase studied by fluorescence spectroscopy. J Biochem 140:349–357. 10.1093/jb/mvj156 [DOI] [PubMed] [Google Scholar]
- Ozturk N, Selby CP, Zhong D, Sancar A (2014) Mechanism of pho-tosignaling by Drosophila cryptochrome role of the redox status of the flavin chromophore. J Biol Chem 289:4634–4642. 10.1074/jbc.M113.542498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Kim ST, Sancar A, Deisenhofer J (1995) Crystal structure of DNA photolyase from Escherichia coli. Science 268:1866–1872 [DOI] [PubMed] [Google Scholar]
- parrilli E, Giuliani M, Marino G, Tutino ML (2010) Influence of production process design on inclusion bodies protein: the case of an Antarctic flavohemoglobin. Microb Cell Factories 10.1186/1475-2859-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- Sancar GB (1990) DNA photolyases: physical properties, action mechanism, and roles in dark repair. Mutat Res 236:147–160 [DOI] [PubMed] [Google Scholar]
- Sancar A (2003) Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev 103:2203–2237 [DOI] [PubMed] [Google Scholar]
- Sancar A, Sancar GB (1984) Escherichia coli DNA photolyase is a flavoprotein. J Mol Biol 172:223–227 [DOI] [PubMed] [Google Scholar]
- Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385. 10.1093/nar/gkg520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby CP, Sancar A (2006) A cryptochrome/photolyase class of enzymes with single-stranded DNA-specific photolyase activity. Proc Natl Acad Sci USA 103:17696–17700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby CP, Sancar A (2012) The second chromophore in Drosophila photolyase/cryptochrome family photoreceptors. Biochemistry 51:167–171. 10.1021/bi201536w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan PP, Panasik N, Coombs JM, Brenchley JE (2000) Approaches for deciphering the structural basis of low temperature enzyme activity. Biochim Biophys Acta (Protein Struct Mol Enzymol) 1543:417–433. 10.1016/s0167-4838(00)00237-5 [DOI] [PubMed] [Google Scholar]
- Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 75:403–433. 10.1146/annurev.biochem.75.103004.142723 [DOI] [PubMed] [Google Scholar]
- Soding J (2005) Protein homology detection by HMM–HMM comparison. Bioinformatics 21:951–960. 10.1093/bioinformatics/bti125 [DOI] [PubMed] [Google Scholar]
- Stanley RJ (2001) Advances in flavin and flavoprotein optical spectroscopy. Antioxid Redox Signal 3:847–866 [DOI] [PubMed] [Google Scholar]
- Stanley RJ, Siddiqui MS (2001) A Stark spectroscopic study of N(3)-methyl, N(10)-isobutyl-7,8-dimethylisoalloxazine in nonpolar low-temperature glasses: experiment and comparison with calculations. J Phys Chem A 105:11001–11008 [Google Scholar]
- Tamada T et al. (1997) Crystal structure of DNA photolyase from Anacystis nidulans. Nat Struct Biol 4:887–891 [DOI] [PubMed] [Google Scholar]
- Thiagarajan V, Byrdin M, Eker APM, Muller P, Brettel K (2011) Kinetics of cyclobutane thymine dimer splitting by DNA photolyase directly monitored in the UV. Proc Natl Acad Sci USA 108:9402–9407. 10.1073/pnas.1101026108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Cavicchioli R (1998) Archaeal cold-adapted proteins: structural and evolutionary analysis of the elongation factor 2 proteins from psychrophilic, mesophilic and thermophilic methanogens. FEBS Lett 439:281–286. 10.1016/s0014-5793(98)01375-1 [DOI] [PubMed] [Google Scholar]
- Tina KG, Bhadra R, Srinivasan N (2007) PIC: protein interactions calculator. Nucleic Acids Res 35:W473–W476. 10.1093/nar/gkm423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Jorns MS (1989) Reconstitution of Escherichia coli DNA photolyase with various folate derivatives. Biochemistry 28:1148–1152 [DOI] [PubMed] [Google Scholar]
- Willey JM, Sherwood L, Woolverton C, Woolverton CJ (2008) Prescott, Harley, and Klein’s microbiology McGraw-Hill, New York [Google Scholar]
- Yang K, Stanley RJ (2006) Di?erential distortion of substrate occurs when it binds to DNA photolyase: a 2-aminopurine study. Biochemistry 45:11239–11245 [DOI] [PubMed] [Google Scholar]
- Yang K, Matsika S, Stanley RJ (2007) 6MAP, a fluorescent adenine analogue, is a probe of base flipping by DNA photolyase. J Phys Chem B 111:10615–10625 [DOI] [PubMed] [Google Scholar]
- Yasui A, Eker APM, Yasuhira S, Yajima H, Kobayashi T, Takao M, Oikawa A (1994) A new class of DNA photolyases present in various organisms including aplacental mammals. EMBO J 13:6143–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.