Abstract
Anti-PD1 and anti-PD-L1 agents may have intrinsic and clinically relevant differences in the treatment of non-small cell lung cancer (NSCLC) patients. By reviewing currently available indirect evidence on these agents for NSCLC treatment, highlighting possible inter- and intra-class dissimilarities, anti-PD1 agents showed a higher response rate and a better outcome when combined with chemotherapy for the first-line treatment of patients with squamous and PD-L1 low advanced NSCLC, as compared to anti-PD-L1 agents. Conversely, anti-PD-L1 agents were responsible for less severe adverse events (AEs), particularly, immunerelated AEs. These differences could be explained by their different specific properties. Considering possible differences between anti-PD1 and anti-PD-L1 agents could be clinically relevant for treatment tailoring and inspiring new investigational approaches.
Key words: Immune-checkpoint inhibitor, PD1, PD-L1, lung cancer, immune-related adverse events
Introduction
Immune-checkpoint inhibitors (ICIs) have hugely contributed to the outcome improvement of patients with lung cancer as several phases III clinical trials have shown (Table 1).1-19
Emerging evidence from published studies suggests that antiprogrammed cell death 1 (PD1) and anti-programmed cell death ligand 1 (PD-L1) agents are very similar to each other from a clinical point of view.
As far as the treatment for the second and beyond-line of advanced non-small-cell lung cancer (aNSCLC) is concerned, both anti-PD1 and anti-PD-L1 demonstrated an overall survival (OS) advantage as compared to chemotherapy, despite the lack of a gain in progression-free survival (PFS).12-15
Long-lasting disease response rates were observed in approximately 14 to 20% of patients with immune-checkpoint inhibitors (ICIs) supporting an approximately 16% 5-year OS.20
Toxicity with ICIs was less severe than with chemotherapy and immune-related adverse events (irAE) did not represent a limiting or unmanageable toxicity when single-agent ICI was used.21
Several published results confirmed that the effect of ICIs is independent by the histology.12-15,20
The expression level of PD-L1 in tumor cells may matter in terms of benefits since high-PD-L1 patients show the best outcome. 14,14,20,22
In the first-line setting, PFS may be increased alongside OS provided that an ICI is given to high-PD-L1 patients, or in combination with chemotherapy, or when ICIs with different mechanisms of action are combined (i.e. anti-CTLA4 with anti-PD-L1). Furthermore, ICI combination treatments are effective on PFS and OS regardless of histology (i.e. squamous or non-squamous) and PD-L1 tumor cells expression level (i.e., negative, low or high).23
In the unresectable stage III NSCLC not-progressing following concurrent chemoradiotherapy, the PFS benefit from the use of the anti-PD-L1 durvalumab compared to placebo was even more relevant than that reported for the OS.16 Possible explanations for this effect could be: the selection of patients already responding to chemotherapy since immunotherapy tends to be more effective in those tumors with a high nonsynonymous mutational burden or DNA repair pathway mutations, which are also more sensitive to chemotherapy;24-29 the pro-inflammatory effect of radiotherapy on the tumor microenvironment that could have reversed the primary resistance of cold tumors against immunotherapy;30-32 the comparison of the anti-PD-L1 agent against placebo rather than to an active treatment.33 From this general point of view, no relevant differences stand out between anti-PD1 and anti-PD-L1 agents.
In this narrative review, we will critically examine some clinical and preclinical data suggesting that some differences actually could exist in terms of efficacy, toxicity and biological properties based on their pharmacological profile.
Efficacy: possible differences
There is some evidence from indirect clinical trial comparisons and a meta-analysis which may suggest possible inter- and intraclass differences in terms of efficacy between anti-PD1 and anti- PD-L1 agents and are following examined.
Indirect trial comparisons suggesting possible ICI inter-class ORR differences
From an indirect comparison of phase III randomized clinical trials in the second- and beyond-line treatment of aNSCLC, a numerical difference in the absolute OS benefit in favor of the anti- PD-L1 agent (atezolizumab) as compared to the anti-PD1 agents (nivolumab and pembrolizumab) came to light, with 4.2 months of OS gain versus 1.9-3.2 months, respectively (Figure 1A).12-15 However, this indirect comparison is biased by patients’ selection. Indeed, patients in the OAK trial15 with the anti-PD-L1 (atezolizumab) had more favorable characteristics than those of the Keynote 010 trial14 with the anti-PD1 (pembrolizumab): with a higher proportion of patients with good performance status (36% versus 33%), non-squamous histology (74% versus 70%), neversmokers (20% versus 18%), EGFR/ALK-positive (11% versus 9%), higher PD-L1 expression (47% versus 40%) and ≥3 treatment lines (0 versus 8%).
Yet, as above mentioned, the OS benefit from ICIs is mainly driven by long-lasting disease responses and, for the second- and beyond-line treatment, the reported ORR with the anti-PD-L1 agent (atezolizumab) was lower than reported with the anti-PD1 agents (nivolumab and pembrolizumab), of 14% versus 18-20%, respectively, and the ORR gain was of 1% versus 7-11%, respectively (Figure 1B). Such small differences could be relevant since the 16% ORR observed with the anti-PD-L1 agent could not translate into the 5 year-OS of 16% and the 3-year 23% reported in the second- and beyond-line with the anti-PD1 agents (nivolumab and pembrolizumab, respectively).20,22 Currently, data from the anti- PD-L1 atezolizumab are still limited to a 2-year OS of 31%34 and longer follow-up OS data could clarify this issue.
Meta-analysis and other trial indirect comparisons suggesting possible ICI inter-class outcome differences
Another relevant evidence suggesting possible differences between anti-PD1 and anti-PD-L1 comes from a meta-analysis of trials combining ICIs with chemotherapy for the first-line treatment of aNSCLC. The HR for trials using the anti-PD1 agent (pembrolizumab) was 0.56 (95% CI, 0.46-0.67, p<0.00001) as compared to 0.85 (95% CI, 0.76-0.94, p=0.001) of those with the anti-PD-L1 (atezolizumab).35
Furthermore, by an indirect comparison of the two trials which investigated for the first-line treatment of aNSCLC with squamous histology the addition of the anti-PD1 (pembrolizumab) and the anti-PD-L1 (atezolizumab) agents (the Keynote 407 and ImPower 131 trials, respectively),8,9 in combination with the same chemotherapy backbone (carboplatin-paclitaxel or carboplatinnab- paclitaxel), the difference in the PFS gain varied from 0.7 to 1.6 months with the anti-PD-L1 (atezolizumab) and the anti-PD1 (pembrolizumab) agent as compared to chemotherapy alone, respectively. The difference in OS gain was even more considerable (0.1 versus 4.6 months, respectively) (Figure 1C). This difference in OS in favor of the anti-PD-1 versus the anti-PD-L1 was estimated with an HR of 0.67 (95% CI, 0.47-0.94, P=0.02) and was particularly relevant in the PD-L1 low population (HR of 0.43, 95% CI, 0.24-0.76, P<0.01).36 In this regard, or for patients with aNSCLC with low-PD-L1 tumors cell expression, the above-mentioned meta-analysis has also reported a possible difference between the Keynote and Impower trials in favor of the anti-PD1 drug (pembrolizumab) as compared to the anti-PD-L1 (atezolizumab) when they were added to first-line chemotherapy.35
Indirect trial comparisons suggesting possible ICI intra-class outcome differences
Interestingly, in patients with high PD-L1 aNSCLC, either anti-PD1 (pembrolizumab, but not nivolumab)1-3,37 and anti-PDL1 agents (atezolizumab and durvalumab)4,5 have shown a significant benefit in terms of OS as compared to the first-line chemotherapy, whilst a significant benefit in PFS has only been shown by pembrolizumab (by one of the two available studies)1 and atezolizumab5 (Table 1 and Figure 1D). The reason why the anti-PD1 nivolumab failed to show OS and PFS benefit in this patient subgroup is difficult to explain by possible differences between the different platforms and related antibodies used for the selection of high-PD-L1 patients38 and might even suggest possible intrinsic intra-ICI class differences (Figure 1D).1-5 Whereas, the different cut-off (of ≥25%) used for the identification of high- PD-L1 patients for durvalumab could at least partially explain the different OS and PFS benefit observed as compared to atezolizumab (Figure 1D), although intra-class ICI differences could also be considered.
Other factors against ICI inter-class outcome differences
Against possible intrinsic differences between the ICIs discussed below, it could be argued that a different trial follow-up duration or the assessment of PD-L1 expression could have affected these results. The longer the follow-up time of a trial is the lower the OS and PFS will be since more events will be observed.39-41 The median follow-up time with the anti-PD1 agents nivolumab or pembrolizumab ranged from 7.7 to 25.2 (median, 12.0 months) as compared with 9.8 to 15.5 (median, 13.9 months) with the anti-PD-L1 atezolizumab.2,3,6,8,9,11,37,42
Table 1.
Completed phase III randomized trials with ICIs in lung cancer.
| Drug (Reference) | Histology | Trial | PDL1 expression | HR OS (95% CI) P value | HR PFS (95% CI) P value |
|---|---|---|---|---|---|
| aNSCLC - First-line single-agent | |||||
| Pembrolizumab (Reck et al., 2016) | NSCLC | KN-024 | ≥50% | 0.60 (0.41-0.89) P=0.005 | 0.50 (0.37-0.68) P<0.001 |
| Nivolumab (Carbone et al., 2017) | NSCLC | CM-026 | ≥5% | 1.02 (0.80-1.30) NR | 1.15 (0.91-1.45) P=0.25 |
| Pembrolizumab (Mok et al., 2019) | NSCLC | KN-042 | ≥1% | 0.81 (0.71-0.93) P=0.0018 | 1.07 (0.94-1.21) P=NR |
| Durvalumab (Reinmuth N et al., 2019) | NSCLC | MYSTIC | ≥25% | 0.66 (0.49-0.90) =0.002 | NR |
| Atezolizumab (Spigel DR, et al., 2019) | NSCLC | IMPower110 | 50% (TC) or IC ≥10% | 0.59 (0.40-0.89) P=0.0106 | 0.63 (0.45-0.88) P=0.007 |
| aNSCLC - First-line combination | |||||
| Pembrolizumab + Chemotherapy (Gandhi et al., 2018) | NonSq | KN-189 | any | 0.49 (0.38-0.64) P<0.001 | 0.52 (0.43-0.64) P<0.001 |
| Atezolizumab+ Bevacizumab- Chemotherapy (Socinski et al., 2018) | NonSq | IMPower150 | any | 0.78 (0.64-0.96) P=0.0164 | 0.59 (0.50-0.70) P<0.0001 |
| Atezolizumab+ Chemotherapy (Jotte et al., 2018) | Sq | IMPower131 | any | 0.96* (0.78-1.18) P=0.69 | 0.71 (0.60-0.85) P=0.0001 |
| Pembrolizumab+ Chemotherapy (Paz-Ares et al., 2018) | Sq | KN-407 | any | 0.64 (0.49-0.85) P=0.0008 | 0.56 (0.45-0.70) P<0.0001 |
| Nivolumab+/- Platinum-Pem in NonSq Platinum-Gem in Sq (Borghaei et al., 2018) NonSq (273) | Sq (90) | CM-227 | <1% | NR | 0.74 (0.58-0.94) P=NR |
| Nivolumab+ Ipilimumab° (Hellmann et al., 2019) | NSCLC | CM-227 | any | NR | 0.58° (0.41-0.81) P<0.001 |
| aNSCLC - Second- and beyond-line | |||||
| Nivolumab (Brahmer et al., 2015) | Sq | CM-017 | NA | 0.59 (0.44-0.79) P<0.001 | 0.62 (0.47-0.81) P<0.001 |
| Nivolumab (Borghaei et al., 2015) | NonSq | CM-057 | NA | 0.73 (0.59-0.89) P=0.002 | 0.92 (0.77-1.11) P=0.39 |
| Pembrolizumab (Rittmeyer et al., 2017) | NSCLC | KN-010 | ≥1% | 0.71 (0.58-0.88) P=0.0008 | 0.88 (0.74-1.05) P=0.07 |
| Atezolizumab (Herbst et al., 2016) | NSCLC | OAK | NA | 0.73 (0.62-0.87) P=0.0003 | 0.95 (0.82-1.10) NR |
| Stage III NSCLC - Maintenance | |||||
| Durvalumab (Antonia et al., 2017; Gray et al., 2019) | NSCLC | PACIFIC | NA | 0.69 (0.55-0.86) NR | 0.52 (0.42-0.65) P<0.001 |
aNSCLC, advanced NSCLC; aSCLC, advanced SCLC; CM, Checkmate; ICIs, immune-checkpoint inhibitors; CTRT, chemo-rdiotherapy; KN, Keynote; IC, tumor-infiltrating immune cells; NonSq, non-squamous; NR, not reported; NSCLC, non-small cell lung cancer; SCLC, small-cell lung cancer; Sq, squamous; TC, tumor cells.
*Results refer to first interim analysis; °Results refer only to patients with high tumor mutational burden, as defined by at least 10 mutations per megabase.
The longest follow-up with the anti-PD-L1 atezolizumab could, therefore, be considered as one of the possible explanations for the lower benefit observed in patients treated with this agent as compared with anti-PD1 ones in terms of OS or PFS but not of ORR.
Regarding the PD-L1 assessment, it is well known that possible differences between different platforms and related antibodies used, specifically the SP142-Ventana assay and the other ones, could exist.38,43 Furthermore, other differences across clinical trials regarded the definition of PD-L1 positive tumors, which in studies with the anti-PD-L1 atezolizumab included not only PD-L1 positive tumor cells but also PD-L1 positive tumor-infiltrating immune cells. For instance, in the IMpower 110 trial, high PD-L1 patients were defined by ≥50% expression of PD-L1 on tumor cells (TC3) or ≥10% expression on tumor-infiltrating immune cells (IC3) (Table 1).5 However, by a review of 400 samples of patients treated with second-line anti-PD-L1 atezolizumab, a significant OS difference between the ICI and chemotherapy was observed both in patients classified as negative or high PD-L1 by the SP142- Ventana and by the 22C3-Dako assay.44 Similar findings, by a review of 503 samples, confirmed an OS benefit independently from the classification by the SP142-Ventana or SP263-Ventana assay for negative, low and high PD-L1 patients treated with firstline anti-PD-L1 atezolizumab in combination with bevacizumab and chemotherapy as compared to bevacizumab and chemotherapy. 45 These results suggest that PD-L1 assessment should not be an issue for a possible difference in efficacy between anti-PD1 and anti-PD-L1 agents.
Summary of possible efficacy differences among anti- PD1 and anti-PD-L1 agents
Currently available evidence from indirect comparisons of phase III randomized trials and one metanalysis suggest possible ICI inter-class differences in terms of higher ORR and a better outcome for the first-line treatment in combination with the chemotherapy in favor of anti-PD1 agents, particularly for patients with squamous and low PD-L1 tumor expression. Possible ICI intra-class differences between the different anti-PD1 and anti-PDL1 agents could also be considered.
Toxicity: possible differences
ICIs are characterized by lower and different toxicity than chemotherapy.46 Particularly, irAEs, such as pneumonitis, hepatitis and colitis, could be an issue especially when ICIs are combined (i.e. anti-CTLA4 and anti-PD1 or anti-PD-L1).46 Thus, recognizing possible different toxicity profiles between anti-PD1 and anti-PD-L1 could be clinically relevant for the safest ICI delivery, and investigational purposes, or to explore potential ICIs combinations within each other and with chemotherapy or other agents (e.g., antiangiogenics or targeted treatments).
Figure 1.
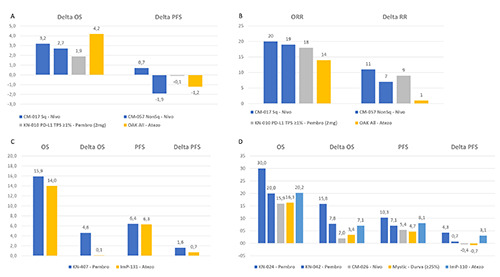
Indirect comparisons between anti-PD1 and anti-PD-L1 exploring possible clinical efficacy differences: A, indirect comparison of difference (Delta) in median months of OS and PFS between anti-PD1 and anti-PD-L1 agents and chemotherapy in the secondand beyond line treatment of aNSCLC by available phase III randomized trials; B, indirect comparison of ORR percentage between anti-PD1 and anti-PD-L1 agents and of gain in ORR (Delta) percentage between them and chemotherapy in the second- and beyond line treatment of aNSCLC by available phase III randomized trials; C, indirect comparison of median months of OS and PFS between anti-PD1 and anti-PD-L1 agents plus chemotherapy and of gain in OS and PFS (Delta) months between them and chemotherapy alone in the first-line treatment of squamous aNSCLC by available phase III randomized trials; D, indirect comparison of median months of OS and PFS between anti-PD1 and anti-PD-L1 agents and of gain in OS and PFS (Delta) months between them and chemotherapy in the first-line treatment of PD-L1 high aNSCLC by available phase III randomized trials. aNSCLC, advanced nonsmall-cell lung cancer; ADCC, antibody-dependent cell-mediated cytotoxicity (ADCC); Atezo, atezolizumab; CM, checkmate; G, grade; KN, keynote; Ig, immunoglobulin; ImP, ImPower; moAbs, monoclonal antibodies; Nivo, nivolumab; ORR, overall response rate; OS, overall survival; PD1, programmed cell death 1; PD-L1, programmed cell death ligand 1; Pembro, pembrolizumab; PFS, progressionfree survival; Sq, squamous.
Indirect trial comparisons suggesting possible ICI inter-class toxicity differences
By an indirect comparison of the second- and beyond-line treatment clinical trials where single-agent anti-PD1 or anti-PD-L1 were compared with docetaxel,12-15 the rate of overall-grade and severe (≥grade 3) toxicity seemed quite similar between the different ICIs, with the overall-grade toxicity ranging from 58 to 69% vs. 64% and the ≥ grade 3 toxicity from 7 to 13% vs. 15% for the anti- PD1 nivolumab and pembrolizumab vs. the anti-PD-L1 atezolizumab, respectively (Figure 2A). The rate of toxicity leading to treatment discontinuation was 3 to 5% vs. 8% for the anti-PD1 nivolumab and pembrolizumab vs. the anti-PD-L1 atezolizumab, respectively (Figure 2A). Yet, a possible difference in the most frequent irAEs in favour of the anti-PD-L1 atezolizumab as compared to the anti-PD1 nivolumab and pembrolizumab is detectable, with a rate of pneumonitis of 1 vs. 3-5%, hepatitis of 0.3 vs. 1-2% and colitis of 0.3 vs. 1%, respectively (Figure 2B).
Systematic- and meta-analysis suggesting possible ICI inter-class toxicity differences
By systematic analysis of 23 trials using anti-PD-1 (nivolumab and pembrolizumab) and anti-PD-L1 (atezolizumab, durvalumab, and avelumab) for 3284 patients and 2460 patients with NSCLC, respectively, investigating toxicity differences between the anti- PD-1 and anti-PD-L1 agents, their toxicity profiles appeared to be similar.47 However, patients treated with anti-PD-1 agents were found to have a slightly increased rate of irAEs (16% vs. 11%; P=0.07) and pneumonitis (4% vs 2% P=0.01) compared to patients who received anti-PD-L1 agents.47
A recent systematic review and meta-analysis including 125 clinical trials and involving 20,128 patients reported 66% of any grade AEs, 14% of ≥ grade 3 AEs and 28% of treatment-related deaths due to pneumonitis with ICIs.48 The anti-PD1 agents were associated with a higher mean incidence of ≥ grade 3 AE compared to anti-PD-L1 agents (odds ratio [OR], 1.58; 95%CI, 1.00-2.54). Furthermore, among anti-PD1 agents, nivolumab was associated with higher mean incidences of all-grade AEs compared with pembrolizumab (OR, 1.28; 95% CI, 0.97-1.79) and ≥ grade 3 AEs (OR, 1.30; 95% CI, 0.89-2.00).48
Figure 2.
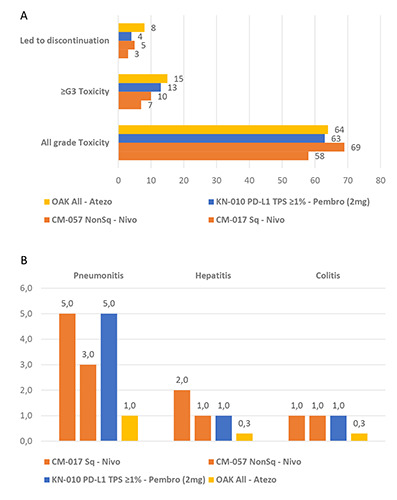
Indirect comparison between anti-PD1 and anti-PD-L1 exploring possible toxicity differences: A, indirect comparison of led to discontinuation, ≥ grade 3 and all grade toxicity percentages between anti-PD1 and anti-PD-L1 agents in the second- and beyond line treatment of aNSCLC by available phase III randomized trials; B, indirect comparison of immune-related adverse events percentages between anti-PD1 and anti-PD-L1 agents in the second- and beyond line treatment of aNSCLC by available phase III randomized trials. aNSCLC, advanced nonsmall-cell lung cancer; ADCC, antibody-dependent cell-mediated cytotoxicity (ADCC); Atezo, atezolizumab; CM, checkmate; G, grade; KN, keynote; Ig, immunoglobulin; ImP, ImPower; moAbs, monoclonal antibodies; Nivo, nivolumab; ORR, overall response rate; OS, overall survival; PD1, programmed cell death 1; PD-L1, programmed cell death ligand 1; Pembro, pembrolizumab; PFS, progressionfree survival; Sq, squamous.
Other evidence suggesting possible ICI intra-class toxicity differences
There could be different rates of infusion-related reactions (IRRs) with monoclonal antibodies (moAbs) inducing antibodydependent cell-mediated cytotoxicity (ADCC) as compared to not- ADCC mAbs (Figure 3). For instance, avelumab, which is the only anti-PD-L1 triggering the ADCC, is associated with a higher rate of IRRs as compared to not-ADCC mAbs.49
Summary of possible toxicity differences among anti- PD1 and anti-PD-L1 agents
To sum up, current evidence suggests anti-PD1 agents could be associated with more frequent ≥ grade 3 AE, particularly irAEs (e.g., pneumonitis, hepatitis and colitis), compared to anti-PD-L1 agents. The possible explanations for this difference are discussed below.
Action mechanisms: dissimilarities among anti- PD1 and anti-PD-L1
It is broadly known that immunotherapy could deliver therapeutic benefits through different mechanisms of action, firstly branching off on active and passive.
The former is designed to act by enhancing the immune response either via function empowerment of the whole immune cell spectrum, addressed by cytokines, or employing antigen– depending systems as therapeutic vaccines or antigen-independent ones as the T–cell function modulation. Proper immune-oncology (IO) stems from this latter and current drugs all funnel in the ICI subset, of which effectiveness currently lays on the blockage of immune suppression pathways as PD-1, PD-L1 and CTLA-4.50
Different target-related mechanisms
Although globally identified as ICIs, existing differences have to be focused and weighed.
For instance, anti-PD1 and anti-PD-L1 drugs prevent PD-L1 (and PD-L2) from interacting with PD-1, which would normally allow a decrease in T-cells function signaling and their activation against cells.51
Yet, the expression of these targets is not evenly spread: for a fact, PD1 belongs to T-cell membrane expression, and so anti-PD1 drugs perform a general undistinguished T-cell hyper-activation and a blockage of both PD-L1 and PD-L2. On one hand, this results in a possible higher efficacy even in low PD-L1 tumors, but this would potentially generate more severe irAEs than the anti- PD-L1 drugs.51 Up-to-date examples listed in this class are the anti-PD1 nivolumab, pembrolizumab, pidilizumab, and AMP-224.
Instead, PD-L1 distribution regards tumor cells surface and is strictly tumor-associated. Therefore, the anti-PD-L1 effect is conveyed through dysregulation of tumor cell signaling and can give milder potential irAE.51 Atezolizumab, durvalumab, avelumab and BMS-936559 are examples.
Molecular, pharmacodynamics and pharmacokinetics specifics assessment
Differences in molecular structure, in ADCC, affinity and engagement to the target, half-life, volume of distribution (Vd) and clearance may exist between different anti-PD1 and anti-PD-L1 agents and even within the same group (Table 2).52
Figure 3.
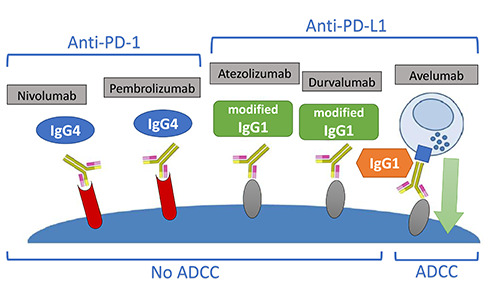
Anti-PD-1/PD-L1 isotypes. IgG1-moAbs containing an Fc region, which can bind cognate receptors on immune effector cells, could induce tumor cell lysis mediated by ADCC as compared to those with IgG4 or a mutated Fc region. aNSCLC, advanced nonsmall- cell lung cancer; ADCC, antibody-dependent cell-mediated cytotoxicity (ADCC); Atezo, atezolizumab; CM, checkmate; G, grade; KN, keynote; Ig, immunoglobulin; ImP, ImPower; moAbs, monoclonal antibodies; Nivo, nivolumab; ORR, overall response rate; OS, overall survival; PD1, programmed cell death 1; PD-L1, programmed cell death ligand 1; Pembro, pembrolizumab; PFS, progressionfree survival; Sq, squamous.
Possible inter- and intra-class differences between anti-PD1 and anti-PD-L1 in the half-maximal effective concentrations (EC50) to block the PD-1 signaling have also been demonstrated, in vitro.53
Furthermore, moAbs containing an Fc region which can bind cognate receptors on immune effector cells could induce tumour cell lysis mediated by ADCC as compared to those with IgG4 or a mutated Fc region, thus resulting in different efficacy and toxicity profiles (Figure 3).54
Anti-PD1 agents
Anti-PD-1 drugs are monoclonal antibodies (moAbs). Indeed, AMP-224 is a fusion protein, whilst nivolumab, pembrolizumab and pidilizumab belong to the Ig class, either of fully human (nivolumab, subclass IgG4) or humanized antibodies (pembrolizumab and pidilizumab, subclasses IgG4 and IgG1, respectively). Moreover, nivolumab and pembrolizumab do not boost ADCC and do not fix complement, mechanisms of action which both pidilizumab and AMP-224 are accountable for.
Affinity and engagement to their target vary as well. As to affinity, nivolumab has a low/intermediate affinity which is three times lower than pembrolizumab. Pidilizumab has a low affinity, while AMP-224 affinity is yet unknown.
Considering the engagement, a lower dose of pembrolizumab is required than of nivolumab (10 μg/mL [60%] versus 50 μg/mL [75%], respectively). Engagement in pidilizumab and AMP-224 is not known.
Half-lives, Vd and clearance are fairly comparable between nivolumab and pembrolizumab, with slight variations (half-life: 26.7 days vs. 26 days, Vd 8 L vs. 7.5 L and clearance 9.4 ml/h vs. 9.4 ml/h, respectively), while are not available for pidilizumab and AMP-224.52
Based on these properties, pembrolizumab seems to have the best affinity and engagement among the current anti-PD1 agents.
Anti-PD-L1 agents
Similar considerations can be done for anti-PD-L1 agents. They all belong to the Ig class. The only humanized one is atezolizumab (subclass IgG1 modified), while durvalumab, avelumab and BMS-936559 are all fully human (subclasses IgG1 modified, IgG1 and IgG4, respectively). Avelumab only has a role in ADCC immune processes.
As long as affinity and engagement are concerned, the former is low/intermediate, intermediate and high for atezolizumab, durvalumab and avelumab, respectively. Engagement is ≥70% after 1 cycle, ≥70% after 1 cycle and up to a 95% for atezolizumab, durvalumab and avelumab, respectively. Engagement, half-life, Vd and clearance are unknown for BMS-936559.
Atezolizumab, durvalumab and avelumab has a half-life of 27 days vs. 12 days vs. 6.1 days, Vd of 6.9 L vs. 5.6 L vs. 4.72 L and clearance of 9.4 mL/h vs. 8.24 mL/h vs. 24.6 mL/h, respectively. Remarkably, atezolizumab half-life emerges among these, being further longer than the others.52
Thus, avelumab seems to have the best affinity and engagement among the current anti-PD-L1 agents and atezolizumab the longest half-life.
Resistance mechanisms
Primary and acquired resistance mechanisms to ICIs are key clinical barriers for further improvement in outcomes and each drug may cause different resistances and may involve the following immune response phases: antigen presentation and T-cell activation, T-cell trafficking and tumor infiltration and T-cell killing activity within the tumor microenvironment (TME).55 Noteworthy, regarding the latter, the upregulation of PD-L1 is one of the possible mechanisms of primary and secondary tumors resistances.55 Particularly, PD-L1 is upregulated in squamous cell carcinoma following the depletion of phosphatase and tensin homolog (PTEN) and serine-threonine kinase11 (Stk11). Under these circumstances, anti-PD-L1 agents could be less effective than anti-PD1, although a retrospective cohort comparison data suggested lack of benefit from the addition of the anti-PD1 pembrolizumab to first-line chemotherapy in patients’ with tumors bearing Stk11 alterations.56
Conclusions
Based on this narrative review of available data on the use of anti-PD1 and anti-PD-L1 agents for NSCLC, we suggest possible differences may exist between these two groups of drugs in terms of efficacy and safety profiles. Particularly, anti-PD1 agents showed a higher response rate and better OS in the first-line treatment (in combination with chemotherapy) of squamous and PD-L1 low aNSCLC patients than anti-PD-L1 agents. Anti-PD-L1, as compared to anti-PD1 agents, could be responsible for less severe AEs and, particularly, irAEs. These differences could be explained by their different specific properties, mechanisms of actions and by tumor resistance mechanisms.
Considering possible differences between anti-PD1 and anti- PD-L1 agents could be clinically relevant for treatment tailoring based on patient’s and tumor characteristics and could also inspire future investigations on therapeutic strategies based on their combination or switching aiming at overcoming primary and acquired immune-related resistance.
Table 2.
Biological differences among anti-PD1 and anti-PD-L1.
| Anti-PD-1 | Anti-PD-L1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Nivolumab | Pembrolizumab | Pidilizumab | AMP-224 | Atezolizumab | Durvalumab | Avelumab | BMS-936559 |
| Humanized | - | ✓ | ✓ | - | ✓ | - | - | - |
| Fully human | ✓ | - | - | - | - | ✓ | ✓ | ✓ |
| Ig subclass | IgG4 | IgG4 | IgG1 | Fusion protein | IgG1 mod. | IgG1 mod. | IgG1 | IgG4 |
| ADCC/CDC | -- | -- | ✓ | ✓ | - | - | ✓ | - |
| PD-1 affinity | +/++ | +++ | + | ? | +/++ | ++ | +++ | ++ |
| PD-1 engagement | 50 μg/mL (75%) | 10 μg/mL (60%) | NA | NA | ≥70% | ≥70% after 1 cycle of 10 or 20 mg/kg Q2W | 1 μg/mL after 1 cycle | NA (95%) |
| Half-life | 26.7 days | 26 days | - | - | 27 days | 12 days | 6.1 days | - |
| Vd | 8 L | 7.5 L | - | - | 6.9 L | 5.6 L | 4.72 L | - |
| Clearance | 9.4 ml/h | 9.2 mL/h | - | - | 9.4 ml/h | 8.24 ml/h | 24.6 ml/h | - |
ADCC/CDC, antibody dependent cell-mediated cytotoxicity/complement dependent cytotoxicity; Ig, immunoglobulin; mod., modified; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; Vd, volume of distribution.
Acknowledgments
We thank the Mediterranean Cancer Support and Rehabilitation (Medicare Onlus) no-profit association for the support to communications between Authors.
Funding Statement
Funding: (36 months prior to submission) GLB had personal fees from Janssen-Cilag, Boehringer-Ingelheim, Roche, MSD; OC has no financial disclosures; MB had research funding by Roche, Pfizer, Seqirus, AstraZeneca, Novartis, Bristol-Myers Squibb and Sanofi, personal fees by Bristol-Myers Squibb, Novartis and Pfizer; MDR received personal fees by Astellas, Celgene, Ipsen, Novartis, Pfizer, Sanofi Genzyme, AstraZeneca, Janssen; Alex Friedlaender had personal fees from Roche, Pfizer, Astellas, AstraZeneca and Bristol-Myers Squibb; AC had personal fees from MSD and Astrazeneca and grant by Bristol-Myers Squibb, Roche, Novartis, Istituto Gentili and Ipsen; AA had personal fees from AstraZeneca, Pfizer, Takeda, Roche, Boehringer-Ingelheim, MSD and Bristol-Myers Squibb, grant from Boehringer-Ingelheim.
References
- 1.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [DOI] [PubMed] [Google Scholar]
- 2.Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [DOI] [PubMed] [Google Scholar]
- 4.Reinmuth N, Cho BC, Lee KH, et al. LBA4Effect of poststudy immunotherapy (IO) on overall survival (OS) outcome in patients with metastatic (m) NSCLC treated with first-line durvalumab (D) vs chemotherapy (CT) in the phase III MYSTIC study. Ann Oncol 2019;30. [Google Scholar]
- 5.Spigel D, de Marinis F, Giaccone G, et al. LBA78IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PDL1– selected NSCLC. Ann Oncol 2019;30. [Google Scholar]
- 6.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small- Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [DOI] [PubMed] [Google Scholar]
- 7.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [DOI] [PubMed] [Google Scholar]
- 8.Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36:LBA9000-LBA9000. [Google Scholar]
- 9.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [DOI] [PubMed] [Google Scholar]
- 10.Borghaei H, Hellmann MD, Paz-Ares LG, et al. Nivolumab (Nivo) + platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with <1% tumor PD-L1 expression: Results from CheckMate 227. J Clin Oncol 2018;36:9001-9001. [Google Scholar]
- 11.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [DOI] [PubMed] [Google Scholar]
- 12.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced nonsmall- cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [DOI] [PubMed] [Google Scholar]
- 16.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [DOI] [PubMed] [Google Scholar]
- 17.Gray JE, Villegas A, Daniel D, et al. Brief report: Three-year overall survival with durvalumab after chemoradiotherapy in Stage III NSCLC - Update from PACIFIC. J Thorac Oncol 2019;15:P288-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small- Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum- etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [DOI] [PubMed] [Google Scholar]
- 20.Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. [DOI] [PubMed] [Google Scholar]
- 21.Banna GL, Passiglia F, Colonese F, et al. Immune-checkpoint inhibitors in non-small cell lung cancer: A tool to improve patients’ selection. Crit Rev Oncol Hematol 2018;129:27-39. [DOI] [PubMed] [Google Scholar]
- 22.Leighl NB, Hellmann MD, Hui R, et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. The Lancet Respiratory Medicine 2019;7:347-57. [DOI] [PubMed] [Google Scholar]
- 23.Peters S, Reck M, Smit EF, et al. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann Oncol 2019;30:884-96. [DOI] [PubMed] [Google Scholar]
- 24.Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 Mutations Correlate with Cisplatin Sensitivity in Muscle- Invasive Urothelial Carcinoma. Cancer Discov 2014;4:1140-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatinbased Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol 2015;68:959-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spigel DR, Schrock AB, Fabrizio D, et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 2016;34:9017-9017. [Google Scholar]
- 28.Addeo A, Banna GL, Weiss GJ. Tumor Mutation Burden-From Hopes to Doubts. JAMA Oncol 2019;5:934-5. [DOI] [PubMed] [Google Scholar]
- 29.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly ME, Monjazeb AM, Kelly K. Clinical Trials Integrating Immunotherapy and Radiation for Non–Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1685-93. [DOI] [PubMed] [Google Scholar]
- 31.Hegde PS, Karanikas V, Evers S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin Cancer Res 2016;22:1865-74. [DOI] [PubMed] [Google Scholar]
- 32.Surace L, Guckenberger M, Broek Mvd. Radiation holidays stimulate tumor immunity. Oncotarget 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novello S, Milella M, Tiseo M, et al. Maintenance therapy in NSCLC: why? To whom? Which agent? J Exp Clin Cancer Res 2011;30:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Pawel J, Bordoni R, Satouchi M, et al. Long-term survival in patients with advanced non–small-cell lung cancer treated with atezolizumab versus docetaxel: Results from the randomised phase III OAK study. Eur J Cancer 2019;107:124-32. [DOI] [PubMed] [Google Scholar]
- 35.Addeo A, Banna GL, Metro G, Di Maio M. Chemotherapy in Combination With Immune Checkpoint Inhibitors for the First- Line Treatment of Patients With Advanced Non-small Cell Lung Cancer: A Systematic Review and Literature-Based Meta-Analysis. Front Oncol 2019;9:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Zhou H, Zhang L. Which is the optimal immunotherapy for advanced squamous non-small-cell lung cancer in combination with chemotherapy: anti-PD-1 or anti-PD-L1? J ImmunoTher Cancer 2018;6:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [DOI] [PubMed] [Google Scholar]
- 38.Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Modern Pathol 2016;29:1165-72. [DOI] [PubMed] [Google Scholar]
- 39.Landi L, Tiseo M, Chiari R, et al. Activity of the EGFR-HER2 dual inhibitor afatinib in EGFR-mutant lung cancer patients with acquired resistance to reversible EGFR tyrosine kinase inhibitors. Clin Lung Cancer 2014;15:411-7, e414. [DOI] [PubMed] [Google Scholar]
- 40.Pilotto S, Rossi A, Vavalà T, et al. Outcomes of First- Generation EGFR-TKIs Against Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Post Hoc Analysis of the BE-POSITIVE Study. Clin Lung Cancer 2018;19:93-104. [DOI] [PubMed] [Google Scholar]
- 41.Gebbia V, Bellavia M, Banna GL, et al. Treatment monitoring program for implementation of adherence to second-line erlotinib for advanced non-small-cell lung cancer. Clin Lung Cancer 2013;14:390-8. [DOI] [PubMed] [Google Scholar]
- 42.Socinski MA, Jotte RM, Cappuzzo F, et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs chemo + bev in 1L nonsquamous (NSQ) NSCLC. J Clin Oncol 2018;36:9002-9002. [Google Scholar]
- 43.Paratore S, Banna GL, D’Arrigo M, et al. CXCR4 and CXCL12 immunoreactivities differentiate primary non-smallcell lung cancer with or without brain metastases. Cancer Biomark 2011;10:79-89. [DOI] [PubMed] [Google Scholar]
- 44.Gadgeel S, Kowanetz M, Zou W, et al. 1296OClinical efficacy of atezolizumab (Atezo) in PD-L1 subgroups defined by SP142 and 22C3 IHC assays in 2L+ NSCLC: Results from the randomized OAK study. Ann Oncol 2017;28. [Google Scholar]
- 45.Kowanetz M, Socinski M, Zou W, et al. IMpower150: Efficacy of Atezolizumab Plus Bevacizumab and Chemotherapy in 1L Metastatic Nonsquamous NSCLC Across Key Subgroups. American Association for Cancer Research Annual Meeting, Chicago, IL; April 14-18, 2018. [Google Scholar]
- 46.Remon J, Mezquita L, Corral J, et al. Immune-related adverse events with immune checkpoint inhibitors in thoracic malignancies: focusing on non-small cell lung cancer patients. J Thorac Dis 2018;10:S1516-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non–small cell lung cancer: A systematic analysis of the literature. Cancer 2018;124:271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Zhou S, Yang F, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncology 2019;5:1008-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly K, Infante JR, Taylor MH, et al. Safety profile of avelumab in patients with advanced solid tumors: A pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer 2018;124:2010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galluzzi L, Vacchelli E, Bravo-San Pedro J-M, et al. Classification of current anticancer immunotherapies. Oncotarget 2014;5:12472-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muñoz-Unceta N, Burgueño I, Jiménez E, Paz-Ares L. Durvalumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 2018;10:1758835918804151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rofi E, Del Re M, Arrigoni E, et al. Clinical pharmacology of monoclonal antibodies targeting anti-PD-1 axis in urothelial cancers. Crit Rev Oncol/Hematol 2019;144:102812. [DOI] [PubMed] [Google Scholar]
- 53.De Sousa Linhares A, Battin C, Jutz S, et al. Therapeutic PDL1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci Rep 2019;9:11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Song X, Li K, Zhang T. FcgammaR-Binding Is an Important Functional Attribute for Immune Checkpoint Antibodies in Cancer Immunotherapy. Front Immunol 2019;10:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gide TN, Wilmott JS, Scolyer RA, Long GV. Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Metastatic Melanoma. Clin Cancer Res 2018;24:1260-70. [DOI] [PubMed] [Google Scholar]
- 56.Skoulidis F, Arbour KC, Hellmann MD, et al. Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non-squamous non-small cell lung cancer. J Clin Oncol 2019;37:102-102. [Google Scholar]


