Highlights
-
•
Dedifferentiated chondrosarcoma is composed of highly differentiated chondrosarcoma and highly malignant non-cartilaginous sarcomas with abruptly-defined.
-
•
The question of whether the two components originated from the same archaeocyte has not yet been clarified.
-
•
Clonality analysis showed that the two components were same X chromosome inactivation.
-
•
The mutation states of IDH1 and IDH2 gene were consistent in the two components of a DDCS.
-
•
We conclude that the two components of a DDCS originate from the same primitive cell.
Keywords: Dedifferentiated chondrosarcoma, Clonality analysis, Origin, IDH1 and IDH2, Mutation
Abstract
Dedifferentiated chondrosarcoma (DDCS) is a highly malignant tumor that belongs to an uncommon subtype of chondrosarcoma with a poor prognosis. Microscopically, it is composed of highly differentiated chondrosarcoma and highly malignant noncartilaginous sarcomas with an abrupt interface. The question of whether the two components originated from the same archaeocyte has not yet been clarified. To further investigate this issue, DNA was separately extracted from the two components of the same patient. In total, 18 DDCS patients were analyzed. A portion of DNA samples from 9 female patients was used for clonality analysis. Another portion of DNA from 9 female and DNA from 9 male patients was used for isocitrate dehydrogenase 1(IDH1) and IDH2 gene mutation detection. The results of clonality analysis showed that the same X chromosome inactivation and consistent mutation states of the IDH1 and IDH2 genes in the two DDCS components. We conclude that the two DDCS components originate from the same primitive cell and that DDCS is monoclonal in origin.
1. Introduction
Dedifferentiated chondrosarcoma (DDCS) is an uncommon histologic variant of chondrosarcoma and comprises approximately 10% of all chondrosarcomas. It is described high-grade sarcoma juxtaposed with low-grade chondrosarcoma with a sharp interface [1]. The tumor almost always develops between 40 and 60 years of age and affects both men and women almost equally. There is no effective treatment except for surgical resection [2], [3]. Its prognosis is very poor, with a 5-year survival rate of <20% [4], [5]. The typical radiological features are biphasic, indicating the coexistence of chondral matrix mineralization and unmineralized soft tissue [6]. Microscopically, the low-grade cartilaginous and high-grade noncartilaginous sarcomas have clear boundaries and tend to change suddenly. The dedifferentiated components in DDCS may show features similar to those osteosarcoma, undifferentiated pleomorphic sarcoma (UPS), fibrosarcoma, rhabdomyosarcoma, angiosarcoma, benign giant cell tumor, etc. [7], [8]. Due to the pleomorphic nature of their histologic appearances, we are interested in the histological origin of these two components. However, the derivation of the cartilaginous and noncartilaginous components of DDCS remains unclear: some researchers have stated that the dedifferentiated component represents a separate genotypic lineage (collision tumor) [9], [10]; others have reported that DDCS is of monoclonal origin based on molecular research [9], [11], [12], [13], [14]; Furthermore, some scholars believe that the dedifferentiated component is produced by the malignant transformation of the fibrous matrix in the necrotic region of the chondrosarcoma component [15]; but there is still no conclusive evidence to prove these theories.
Based on the Lyon hypothesis, tumors are formed through the unlimited division of an original cell, which can be confirmed by clonality analysis [16], [17]. One of the two X chromosomes in each cell of a female is inactivated by random methylation during the process of embryogenesis, and this inactivation is permanent and conserved in the process of mitosis. A recent study of the derivation of ovarian intestinal-type mucinous tumors using clonality analysis revealed a shared clonal relationship between the two different tumor components in mucinous and Brenner tumors [18]. An adenomatoid tumor is a true monoclonal neoplasm rather than reactive mesothelial hyperplasia because it shows an uniform, nonrandom pattern of X chromosome inactivation consistent with monoclonality [19]. Fumi Karino used clonality analysis in a rare case of undifferentiated thymic carcinoma coexisting with type AB thymoma revealed the same clonal pattern in both tumors [20]. However, there is no report on the origin of the low malignant chondrosarcoma and noncartilaginous sarcoma components of DDCS using clonality analysis. There are two different histological manifestations of DDCS, the clonality analysis can be used to detect whether the two tumor components of DDCS are derived from the same primary cell.
Similarly, if two components of DDCS have the same gene mutation, we can speculate the monoclonal hypothesis. In recent years, studies have shown that isocitrate dehydrogenase 1 (IDH1) and IDH2 mutations in cartilaginous tumors are relatively specific: the mutation rate in chondrosarcoma (50–70%) is second to that in glioma (70–80%) [21], [22], but this does not occur in other mesenchymal tumors, such as osteosarcoma and UPS [23], [24]. Additionally, IDH1 and IDH2 mutations are early events in tumorigenesis [22], [25], [26]. If the same IDH1 or IDH2 mutation is detected in both components in the same DDCS, we can speculate that the two components share a common origin. In addition, it is difficult and highly subjective to distinguish DDCS from other sarcomas of the bone with morphologic overlap, especially in a small biopsy specimen. However, there are only two reports on the differential diagnosis of DDCS using IDH1 and IDH2 mutations [23], [24]. Some researchers believe that the IDH1 and IDH2 mutations can distinguish DDCS from osteosarcoma and UPS because the chondrosarcoma harbors IDH1 and IDH2 mutations, which are not detected in other types of noncartilaginous sarcoma. However, there is no report on IDH1 and IDH2 mutations in fibrosarcoma of the bone and other common types of tumors that, like DDCS, have similar dedifferentiated components. It is necessary to detect IDH1 and IDH2 mutations in DDCS, fibrosarcoma, osteosarcoma and UPS of the bone. We speculate that IDH1 and IDH2 mutation detection may be used for the differential diagnosis of DDCS and other sarcomas, including osteosarcoma, UPS, and fibrosarcoma in the bone.
In this study, by using both clonality analysis and IDH1 and IDH2 mutation detection, we aimed to identify the derivation of the cartilaginous and noncartilaginous components of DDCS. IDH1 and IDH2 mutations may also be detected in fibrosarcoma, UPS and osteosarcoma in the bone to distinguish DDCS from these sarcomas, which have dedifferentiated components that are morphologically similar to those of DDCS.
2. Materials and methods
2.1. Collection of tumor specimens
Procedures involving human subjects were performed in accordance with the Helsinki Declaration of 1975, as revised in 1983, and ethical committee approval was given for this study (Approval No: 2017-026, Date:2017-03-30 Shanghai Jiaotong University Affiliated Sixth People’s Hospital). All DDCSs were confirmed by pathologists. Patient information was collected from Shanghai Jiao Tong University Affiliated Sixth People's Hospital and included clinical, imaging, pathologic and follow-up data. The hematoxylin and eosin (H&E) stained slides were reviewed by pathologists with more than 30 years of experience in the diagnosis of bone and soft tissue tumors, and then paraffin-embedded tissue blocks were selected and used for DNA extraction. The slides were reviewed, diagnosed and graded according to the WHO Classification of Soft Tissue and Bone (2013 editions). Twenty-nine patients with DDCS were diagnosed based on samples collected from 2011 to 2017. Follow-up information was available for all of the patients. Normal female blood samples were used as control tissue. Last, 41 sarcomas with a similar histologic appearances that of the high-grade component of DDCSs, including 11 conventional chondrosarcomas, 10 chondroblastic osteosarcomas, 10 UPSs, and 10 fibrosarcomas, were examined in this study to detect IDH1 and IDH2 mutations.
2.2. DNA extraction
DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissue without decalcification from samples that contained >85% tumor cells. Both the chondrosarcoma component and the dedifferentiated component of DDCS were separately embedded in paraffin tissue blocks. The procedure was performed using a Spin Column DNA FFPE Tissue Kit (AmoyDx, Fujian, China) according to the instructions provided by the manufacturer. Approximately 10 slices of 5 μm thick sections from paraffin-embedded blocks were collected in a 1.5 mL EP tube. These FFPE tissue sections were first deparaffinized with xylene and ethanol and then incubated in buffer DTL and proteinase K solution at 56 °C for 1 hour to release DNA from the sections. A short incubation of approximately 1 hour in buffer DES at a high temperature (90 °C) partially reversed the formalin crosslinking of the released nucleic acids, and last, the DNA was eluted in buffer DTE.
2.3. Restriction endonuclease HpaⅡ treatment and PCR amplification
DNA was extracted from female patients with DDCS according to the above procedure, and normal female blood sample DNA was used as a control. The digestion reaction system (final volume = 20 μl) included 10 μl DNA, 1 μl HpaII (Takara, Dalian, China), 2 μl 10× loading buffer, and 7 μl dH2O. Distilled water instead of enzyme was used in the nondigested system as a control. The reaction conditions were 37 °C digestion for 3 hours and then incubation at 85 °C for 15 minutes to stop the reaction. This was followed by PCR using a 50 μl reaction system including 10 μl 5× PrimeSTAR GXL Buffer, 4 μl dNTP mix, 1 μl PrimeSTAR GXL DNA polymerase (Takara, Dalian, China), 1 μl each primer (AR) (Sangon Biotech, Shanghai, China), 28 µl sterile water, and 5 μl digested or nondigested DNA. The sequences of the primers were 5′-CTACCGAGGAGCTTTCCAGAAT-3′ (forward primer, labeled with FAM green fluorescence on the 5′ end) and 5′-CGATGGGCTTGGGGAGAACCAT-3′ (reverse primer). The mixture underwent predenaturation at 95 °C for 5 minutes, followed by 30 cycles at 98 °C for 10 s and 68 °C for 30 seconds. The PCR product was used for capillary electrophoresis on an ABI 3730 sequencer (Applied Biosystems), and the data were analyzed by GeneMapper 4.0 Software (Applied Biosystems).
2.4. Data interpretation
Theoretically, in female patients, AR gene PCR amplification in the nonenzymatic group will produce two main peaks; however, in heterozygotes, it will produce only one peak, which is uninformative. However, after enzyme treatment, the normal tissue will also produce two main peaks, and the tumor tissue will produce only one main peak due to its monoclonal characteristics. There is a slipping of the Taq enzyme during the amplification process, which leads to a slight deviation in the amplified fragment size. To accurately express the enzyme cutting effect, we used the following formula to calculate the cleavage ratio (CR): (area under peak 1 in the nonenzymatic group ÷ area under peak 2 in the nonenzymatic group)/(area under peak 1 in the enzymatic group ÷ area under peak 2 in the enzymatic group). When the ratio is greater than 2 or < 0.5, it signifies that the activated X chromosome is cut by the enzyme, proving that the sample is monoclonal [18], [19], [27].
2.5. IDH1 and IDH2 gene fluorescence PCR capillary electrophoresis sequencing analysis
This process, including fluorescence PCR and capillary electrophoresis sequencing analysis, was performed with the IDH1 Gene Mutation Detection Kit and the IDH2 Gene Mutation Detection Kit (SinoMDgene, Beijing, China), which include negative and positive controls, according to the manufacturer’s instructions. First, fluorescence PCR was performed on an ABI 7500 machine (Applied Biosystems) using 45 cycles of denaturation at 94 °C for 15 seconds and annealing and extension of fluorescence signal acquisition at 60 °C for 45 seconds. The mixture included buffer, dNTPs, primer, Taq DNA polymerase and a probe. The target gene channel showed an amplification curve with an S shape, and the Ct value was <30, which indicated that the amplification was successful. Then, PCR products were enzymatically isolated with a mixture of SAP at 37 °C for 60 min, followed by 80 °C for 15 minutes. Then, sequencing PCR was performed using a mixture of the product from the above step, genotypic reagent and sequencing primers as follows: 45 cycles of denaturation at 96 °C for 10 s, annealing and extended fluorescence signal acquisition at 50 °C for 5 seconds, and elongation at 60 °C for 2 minutes. The products were purified using mixtures of sodium acetate and ethanol before being transferred to the bottom of a 96-well sequencing plate.
The analysis was performed on an ABI 3500 Dx gene analyzer according to the instructions provided in the operating manual. The sequencing results were analyzed using the Chromas 2.6 software programs. The relationship between IDH1 and IDH2 gene mutations and the prognosis of the 18 DDCS patients was statistically analyzed. All survival analyses were performed using Kaplan-Meier single-factor survival function analysis methods. All probabilities were two-tailed. P < 0.05 was considered statistically significant.
3. Results
3.1. Clinicopathological features
Eleven of 29 patients were excluded due to decalcification or because the two DDCS components could not be separately obtained. Therefore, 18 patients (9 men and 9 women) were included in this study. The average age of the series was 50.6 years, ranging from 32 to 72 years. The most common site of disease was the pelvis (n = 8), followed by the femur (n = 4), humerus (n = 2), tibia (n = 1), sternum (n = 1), phalanx (n = 1) and T8-T12 (n = 1). The follow-up period ranged between 2 and 74 months; 13 patients died of disease, with an average survival period of 13 months; 5 patients are currently alive, and the longest survival period was 74 months (see Table 1). Typical radiographic manifestations were “biphasic features“ with mineralized and noncalcified areas. Histologically, chondrosarcoma components juxtapose a highly malignant dedifferentiation component, which may appear as follows: osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, or spindle cell sarcoma that cannot be clearly classified.
Table 1.
Clonality analysis and IDH1/2 Mutation analysis of dedifferentiated chondrosarcoma specimens.
| Case | Age (y) | Sex | Site | Follow-up (months) | Clonality | IDH1 mutation | IDH2 mutation | ||
|---|---|---|---|---|---|---|---|---|---|
| C-DDCS | D-DDCS | C-DDCS | D-DDCS | ||||||
| 1 | 44 | F | Femur | DOD8m | Monoclonal | R132C | R132C | WT | WT |
| 2 | 62 | F | Femur | DOD7m | Failed | WT | WT | WT | WT |
| 3 | 39 | F | Pelvis | DOD10m | Monoclonal | R132S | R132S | R159H | R159H |
| 4 | 42 | F | Pelvis | DOD32m | Failed | R132C | R132C | WT | WT |
| 5 | 48 | F | Pelvis | DOD5m | Uninformative | WT | WT | WT | WT |
| 6 | 49 | F | tibia | AWD74m | Monoclonal | WT | WT | WT | WT |
| 7 | 46 | F | Humerus | DOD72m | Failed | WT | WT | WT | WT |
| 8 | 62 | F | Pelvis | AWD6m | Monoclonal | R132S | R132S | WT | WT |
| 9 | 45 | F | Humerus | AWD36m | Failed | WT | WT | WT | WT |
| 10 | 51 | M | T8-12 | DOD7m | Not tested | WT | WT | WT | WT |
| 11 | 44 | M | sternum | DOD27m | Not tested | R132F | R132F | WT | WT |
| 12 | 50 | M | Pelvis | DOD5m | Not tested | R132H | R132H | WT | WT |
| 13 | 72 | M | Femur | DOD17m | Not tested | R132G | R132G | WT | WT |
| 14 | 53 | M | Pelvis | DOD5m | Not tested | R132G | R132G | WT | WT |
| 15 | 45 | M | Pelvis | DOD9m | Not tested | R132G | R132G | WT | WT |
| 16 | 58 | M | Pelvis | DOD2m | Not tested | WT | WT | WT | WT |
| 17 | 33 | M | finger | AWD19m | Not tested | R132C | R132C | WT | WT |
| 18 | 69 | M | Femur | AWD11m | Not tested | WT | WT | R172S | R172S |
Abbreviations: F, female; M, male; DOD, died of disease; AWD, alive with disease; m, months; C-DDCS, chondrosarcoma component of DDCS; D-DDCS, dedifferentiated component of DDCS; WT, wild type.
All patients were subjected to IDH gene mutation detection. However, only 9 female patients were subjected to clonality analysis.
3.2. Clonality assessment
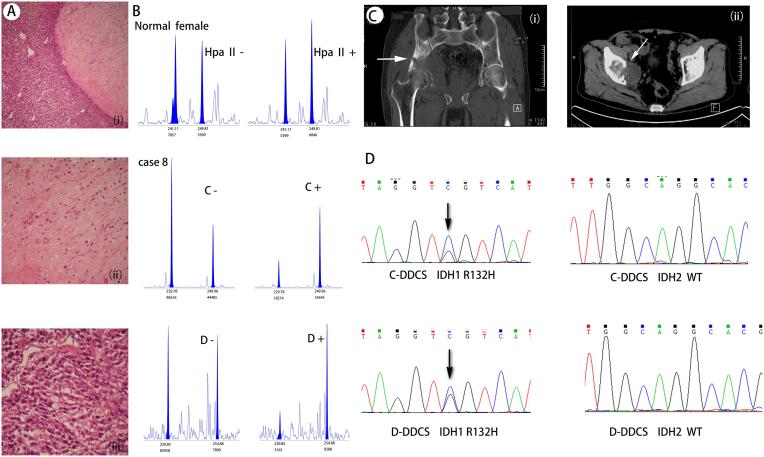
Capillary electrophoresis was successfully completed in five cases of 9 DDCS patients, in which one showed a single peak both with and without digestion, indicating that it was homozygous and could not be analyzed. CR values of the other 4 DDCS patients (patients 1, 3, 6, and 8; shown in Table 1 and supplement Fig. 1), regardless of whether the chondrosarcoma component or the dedifferentiated component was analyzed., were greater than 2 or <0.5, indicating that all were monoclonal. Two main peaks in the nonenzymatic group were observed (Fig. 1). However, a sharp decrease in or disappearance of one of the main peaks was observed in the enzyme digestion group but not in the nondigestion group, indicating that the chromosome that was not inactivated by methylation was cleaved by the endonuclease. However, the normal female blood sample showed two main peaks in both the digested and nondigested groups, suggesting that inactivation of the X chromosome in normal female somatic cells is random. The sizes of the mean peak allele in the two DDCS components were highly similar, suggesting that they have a concordant X chromosome inactivation pattern, indicative of a shared clonal origin. Four samples failed the analysis because they could not be amplified.
Fig. 1.
Histological and imaging appearances of case8 and it’s results of cloning analysis and IDH gene mutation detection. A The morphology of HE under the microscope, (Ai) the junction of two components of DDCS; (Aii) the chondrosarcoma component; (Aiii) the dedifferentiated component. B Results of clonality analysis. (C, chondrosarcoma component; D, dedifferentiated components; +, treated with HpaII enzyme; −, not digested by HpaII enzyme). Two main peaks were found in both the enzymatic and non enzymatic groups of normal female, There was a significant decrease in short size peak in the enzyme-treat group compared with the non enzyme treat group in both component of DDCS. C Imaging data, the arrow is marked with a double manifestation of calcification and non calcification. (Ci) Coronal CT, (Cii) cross section CT. D shows the result of IDH gene mutation detection, and the arrow marks the mutation site. IDH1 R132S mutation (C > G) was detected in both components; IDH2 mutation was not detected in the two components.
3.3. Mutational analysis
Mutational analysis revealed that 11 of 18 DDCS patients (61.1%) harbored IDH1 or IDH2 mutations. IDH1 mutations were observed in 10 patients, and IDH2 mutation was found in 2 patients (Table 1). One patient had an IDH1 (R132S) mutation together with a relatively rare IDH2 (R159H) mutation. All of the IDH1 mutations were found to be point mutations of the arginine residue in codon 132, yielding 5 different amino acids: glycine (N = 3), cysteine (N = 3), serine (N = 2), histidine (N = 1), and isoleucine (N = 1). The mutations in the two DDCS components were consistent. Moreover, five of 11 (45%) patients with conventional chondrosarcoma harbored IDH1 or IDH2 mutations, including 4 mutations in IDH1 R132 and one mutation in IDH2 (Table 2). Neither IDH1 nor IDH2 mutations were detected in control samples, which included 10 fibrosarcomas, 10 UPSs and 10 osteosarcomas.
Table 2.
IDH1 and IDH2 mutation analysis of 11 conventional chondrosarcoma specimens.
| Case | Age | Sex | Site | IDH1 | IDH2 |
|---|---|---|---|---|---|
| 1 | 52 | F | Femur | WT | WT |
| 2 | 34 | F | Femur | WT | WT |
| 3 | 33 | M | Femur | WT | R172T |
| 4 | 23 | F | Femur | R132S | WT |
| 5 | 60 | F | finger | WT | WT |
| 6 | 16 | M | finger | R132H | WT |
| 7 | 24 | F | Femur | WT | WT |
| 8 | 66 | F | Femur | R132C | WT |
| 9 | 23 | M | tibia | WT | WT |
| 10 | 61 | M | Pelvis | R132G | WT |
| 11 | 25 | F | temporal bone | WT | WT |
4. Discussion
The question of whether the two DDCS components, the cartilaginous and the noncartilaginous anaplastic components, are derived from the same archaeocyte has not been clearly confirmed. Many attempts have been made to address this problem; some studies on genetic and epigenetic alterations, such as chromosomal changes, loss of heterozygosity (LOH) analysis, array-CGH analysis, DNA analysis, flow cytometry, and CpG island methylation analysis of tumor suppressor genes, have suggested that the two DDCS components are derived from a single precursor [11], [12], [13], [14]. However, some studies based on phenotype, (e.g., the composition of the extracellular tumor matrix and S-100 protein expression) have shown that the dedifferentiated component lacks the characteristics of the cartilaginous component, supporting the ‘collision tumor’ theory [13], [28]. Here, we investigated this issue with two methods: clonality analysis and IDH1 and IDH2 mutation detection.
We proved the monoclonal origin of two DDCS components for the first time by using clonality analysis and validated that these two components of the same DDCS seem to arise from a single common precursor cell. The tumor DNA group showed one main peak after digestion, indicating a monoclonal origin. The sizes of the peak allele on capillary electrophoresis were the same in the two components, indicating that they were derived from the same individual, and the same single peak remained after enzyme digestion, illustrating that they had inactivated the same X chromosome. These data indicate that the two components originated from the same primitive cells and support a monoclonal origin. Notably, our results provided stronger evidence than do previous findings.
In this study, the two DDCS components harbored identical IDH1 and IDH2 mutations in all samples, supporting the above conclusion. Furthermore, the results showing that IDH1 and IDH2 mutations were detected in chondrosarcoma samples but not in other 10 osteosarcoma samples are consistent with the results showing IDH1 and IDH2 gene mutations detection in 220 cartilaginous tumors, 222 osteosarcoma tissues and 19 osteosarcoma cell lines [24]. The results obtained from the 10 UPSs were the same as those obtained from 14 UPSs of the bone by Shaoxiong Chen [23], in that no mutations were detected. The above results show that mutations were detected in chondrogenic tumors but not in noncartilaginous tumors. Additionally, IDH1 and IDH2 gene mutation results in the accumulation of the novel oncometabolite D-2-hydroxyglutarate (D-2-HG), which results in the inhibition of various cellular dioxygenases and then affects cell metabolism, epigenetic regulation, the redox status and DNA repair, resulting in carcinogenic effects. Therefore, IDH1 and IDH2 gene mutations are an early event in tumorigenesis [29], [30]. Based on the results described above, we speculate that the precursor cell have the potential to differentiate not only into a cartilaginous tumor but also into another type of noncartilaginous sarcomas. We would like to believe that this original cell may be a chondroid progenitor cell with multidifferentiation potential.
Neither IDH1 nor IDH2 mutations were detected in 10 fibrosarcoma samples providing a new characteristic for the clinical differential diagnosis of DDCS from sarcomas, which shares a similar morphology and histology to those of the dedifferentiated component of DDCS. Interestingly, if wild-type IDH1 and IDH2 mutations occur in DDCS, then the differential diagnosis will be meaningless.
IDH1R132 and IDH2R172 mutations are the most common mutations in the IDH1 and IDH2 genes in cartilaginous tumors [22], [25], [31], [32]. It is striking that the IDH2 R159H mutation was observed in one of the 18 DDCS patients. There are no data on the IDH2R159H mutation in DDCS, or in other tumors such as conventional chondrosarcoma, osteosarcoma, and glioma. Therefore, the role of the IDH2R159H mutation in the development of DDCS remains to be further studied.
It is undeniable that combining clonality analysis and gene mutation detection has great advantages in studying the origin of tumor cells in DDCS. Clonality analysis is a credible method of studying the origin of cells in the embryonic period [16], [17], [18], [20]. However, its drawback is that it can be used only in females. In addition, it has high requirements for DNA quality (fragment length ≥ 300 bp). Four of the 9 patients subjected to clonality analysis in this experiment failed due to low DNA quality, and 3 failed due to the long preservation time (DNA degradation and fragmentation in paraffin-embedded samples that have been stored for too long). Another patient failed because the needle biopsy specimen, while the large specimens of the tumor segment underwent decalcification and DNA was unable to be extracted. Fortunately, IDH gene mutation detection compensates for the defects of in clonality analysis. This method has no sex limitation and compensates the defect that clonality analysis cannot be used in males. On the other hand, gene mutation detection is more sensitive than the former method and has certain significance samples that failed clonality analysis. However, in some cases in which the IDH mutation is negative and the clonality analysis is successful, the advantages of the combination are prominent. In short, the combination of the two methods to study this problem has great advantages and innovation.
In conclusion, by combining clonality analysis with IDH1 and IDH2 mutation detection, we confirmed that the anaplastic and the cartilaginous components of DDCS originate from the same primitive cells, which must have the potential to differentiate into cartilage. When preoperative biopsy shows only the high-grade sarcomatous component and the imaging findings are atypical, IDH1 and IDH2 mutations analysis may be used as molecular diagnostic markers to distinguish DDCS from the histologically similar sarcomas, such as fibrosarcoma, osteosarcoma and UPS.
Declaration of Interests
No conflict of interest exits in the submission of this manuscript, which is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed. And this work was supported by Shanghai Science and technology development Fund (19MC1911000), the Natural Science Foundation of China project (81602099), Medicine and Engineering Combination Project of Shanghai Jiaotong University (YG2015QN12) and Shanghai Municipal Health Bureau Program (20134052).
Acknowledgments
Acknowledgement
The authors would like to thanks the patients and their families for agreeing to participate in this work. And this work was supported by Shanghai Science and technology development fund(19MC1911000), the Natural Science Foundation of China project(81602099), Medicine and Engineering Combination Project of Shanghai Jiaotong University (YG2015QN12) and Shanghai Municipal Health Bureau Program (20134052). The authors thank Junhui Ge and Lingyan Sun for technical help with the clonality analysis, Dingjun Hu for help in the collection of imaging data, and all the staff of the Department of pathology of Shanghai Jiao Tong University Affiliated Sixth People's Hospital for the help and support.
Author contributions
Review of the pathology was undertaken by histopathologists Huizhen Zhang, Yueqing Bai and Wentao Huang. Preparation of the DNA was by Tingting Yang, Keyang Sun, Jie Chen, Yueqing Bai and the experimental procedure of cloning analysis and IDH1 and IDH2 gene fluorescence PCR Capillary Electrophoresis Sequencing Analysis were completed by Tingting Yang. Yanli Luo provided many ideas of guiding the experiments. Statistical analysis was performed by Tingting Yang. The MS was written by Tingting Yang and reviewed by Huizhen Zhang, Yanli Luo and Wentao Huang.
Contributor Information
Yanli Luo, Email: luoyanli2000@163.com.
Wentao Huang, Email: hrc_2002@163.com.
Huizhen Zhang, Email: huizhenzhang2015@163.com.
References
- 1.Dahlin D.C., Beabout J.W. Dedifferentiation of low-grade chondrosarcomas. Cancer. 1971;28:461–466. doi: 10.1002/1097-0142(197108)28:2<461::aid-cncr2820280227>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Mavrogenis A.F., Gambarotti M., Angelini A. Chondrosarcomas revisited. Orthopedics. 2012;35:e379–390. doi: 10.3928/01477447-20120222-30. [DOI] [PubMed] [Google Scholar]
- 3.Staals E.L., Bacchini P., Bertoni F. Dedifferentiated central chondrosarcoma. Cancer. 2006;106:2682–2691. doi: 10.1002/cncr.21936. [DOI] [PubMed] [Google Scholar]
- 4.Mercuri M., Picci P., Campanacci L. Dedifferentiated chondrosarcoma. Skelet. Radiol. 1995;24:409–416. doi: 10.1007/BF00941235. [DOI] [PubMed] [Google Scholar]
- 5.Grimer R.J., Gosheger G., Taminiau A. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur. J. Cancer (Oxford, Engl.) 1990;2007(43):2060–2065. doi: 10.1016/j.ejca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Henderson E.R., Pala E., Angelini A. Dedifferentiated peripheral chondrosarcoma: a review of radiologic characteristics. Sarcoma. 2013;2013 doi: 10.1155/2013/505321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J., Jiang Z., Yang Q. Benign looking giant cell component in dedifferentiated chondrosarcoma: benign or malignant? A case report. Int. J. Surg. Pathol. 2013;21:48–53. doi: 10.1177/1066896912451322. [DOI] [PubMed] [Google Scholar]
- 8.Knosel T., Werner M., Jung A. Dedifferentiated chondrosarcoma mimicking a giant cell tumor. Is this low grade dedifferentiated chondrosarcoma? Pathol. Res. Pract. 2014;210:194–197. doi: 10.1016/j.prp.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto A. The molecular pathogenesis of dedifferentiated chondrosarcoma. Ind. J. Orthopaed. 2014;48:262–265. doi: 10.4103/0019-5413.132506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aigner T., Unni K.K. Is dedifferentiated chondrosarcoma a ‘de-differentiated’ chondrosarcoma? J. Pathol. 1999;189:445–447. doi: 10.1002/(SICI)1096-9896(199912)189:4<445::AID-PATH468>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Meijer D., de Jong D., Pansuriya T.C. Genetic characterization of mesenchymal, clear cell, and dedifferentiated chondrosarcoma. Genes Chromosomes Cancer. 2012;51:899–909. doi: 10.1002/gcc.21974. [DOI] [PubMed] [Google Scholar]
- 12.Bovee J.V., Cleton-Jansen A.M., Rosenberg C. Molecular genetic characterization of both components of a dedifferentiated chondrosarcoma, with implications for its histogenesis. J. Pathol. 1999;189:454–462. doi: 10.1002/(SICI)1096-9896(199912)189:4<454::AID-PATH467>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Ropke M., Boltze C., Neumann H.W. Genetic and epigenetic alterations in tumor progression in a dedifferentiated chondrosarcoma. Pathol. Res. Pract. 2003;199:437–444. doi: 10.1078/0344-0338-00443. [DOI] [PubMed] [Google Scholar]
- 14.Yang L., Chen Q., Zhang S. A novel mutated cell line with characteristics of dedifferentiated chondrosarcoma. Int. J. Mol. Med. 2009;24:427–435. doi: 10.3892/ijmm_00000249. [DOI] [PubMed] [Google Scholar]
- 15.Sanerkin N.G., Woods C.G. Fibrosarcomata and malignant fibrous histiocytomata arising in relation to enchondromata. J. Bone Joint Surg. Br. Volume. 1979;61-b:366–372. doi: 10.1302/0301-620X.61B3.225333. [DOI] [PubMed] [Google Scholar]
- 16.Lyon M.F. Sex chromatin and gene action in the mammalian X-chromosome. Am. J. Human Genet. 1962;14:135–148. [PMC free article] [PubMed] [Google Scholar]
- 17.Lyon M.F. X-chromosome inactivation: a repeat hypothesis. Cytogenet. Cell Genet. 1998;80:133–137. doi: 10.1159/000014969. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Wu R.C., Shwartz L.E. Clonality analysis of combined Brenner and mucinous tumours of the ovary reveals their monoclonal origin. J. Pathol. 2015;237:146–151. doi: 10.1002/path.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W., Zhu H., Wang J. Clonality assessment of adenomatoid tumor supports its neoplastic nature. Human Pathol. 2016;48:88–94. doi: 10.1016/j.humpath.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Karino F., Yokose T., Matsukuma S. Clonality analysis performed using human androgen receptor assay in a rare case of undifferentiated thymic carcinoma coexisting with type AB thymoma. Pathol. Int. 2016;66:398–403. doi: 10.1111/pin.12426. [DOI] [PubMed] [Google Scholar]
- 21.Turkalp Z., Karamchandani J., Das S. IDH mutation in glioma: new insights and promises for the future. JAMA Neurol. 2014;71:1319–1325. doi: 10.1001/jamaneurol.2014.1205. [DOI] [PubMed] [Google Scholar]
- 22.Schaap F.G., French P.J., Bovee J.V. Mutations in the isocitrate dehydrogenase genes IDH1 and IDH2 in tumors. Adv. Anatom. Pathol. 2013;20:32–38. doi: 10.1097/PAP.0b013e31827b654d. [DOI] [PubMed] [Google Scholar]
- 23.Chen S., Fritchie K., Wei S. Diagnostic utility of IDH1/2 mutations to distinguish dedifferentiated chondrosarcoma from undifferentiated pleomorphic sarcoma of bone. Human Pathol. 2017;65:239–246. doi: 10.1016/j.humpath.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Amary M.F., Bacsi K., Maggiani F. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 25.Dang L., Yen K., Attar E.C. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann. Oncol. J. Eur. Soc. Med. Oncol. 2016;27:599–608. doi: 10.1093/annonc/mdw013. [DOI] [PubMed] [Google Scholar]
- 26.Polychronidou G., Karavasilis V., Pollack S.M. Novel therapeutic approaches in chondrosarcoma. Fut. Oncol. (London, Engl.) 2017;13:637–648. doi: 10.2217/fon-2016-0226. [DOI] [PubMed] [Google Scholar]
- 27.Moniz S., Catarino A.L., Marques A.R. Clonal origin of non-medullary thyroid tumours assessed by non-random X-chromosome inactivation. Eur. J. Endocrinol. 2002;146:27–33. doi: 10.1530/eje.0.1460027. [DOI] [PubMed] [Google Scholar]
- 28.Dornauer K., Soder S., Inwards C.Y. Matrix biochemistry and cell biology of dedifferentiated chondrosarcomas. Pathol. Int. 2010;60:365–372. doi: 10.1111/j.1440-1827.2010.02530.x. [DOI] [PubMed] [Google Scholar]
- 29.Molenaar R.J., Maciejewski J.P., Wilmink J.W. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene. 2018;37:1949–1960. doi: 10.1038/s41388-017-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molenaar R.J., Radivoyevitch T., Maciejewski J.P. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim. Biophys. Acta. 2014;1846:326–341. doi: 10.1016/j.bbcan.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Krell D., Mulholland P., Frampton A.E. IDH mutations in tumorigenesis and their potential role as novel therapeutic targets. Fut. Oncol. (London, Engl.) 2013;9:1923–1935. doi: 10.2217/fon.13.143. [DOI] [PubMed] [Google Scholar]
- 32.Jin Y., Elalaf H., Watanabe M. Mutant IDH1 dysregulates the differentiation of mesenchymal stem cells in association with gene-specific histone modifications to cartilage- and bone-related genes. PloS one. 2015;10 doi: 10.1371/journal.pone.0131998. [DOI] [PMC free article] [PubMed] [Google Scholar]