Abstract
The enhanced intracellular survival (Eis) protein of Mycobacterium tuberculosis (Mtb) is a versatile acetyltransferase that multiacetylates aminoglycoside antibiotics abolishing their binding to the bacterial ribosome. When overexpressed as a result of promoter mutations, Eis causes drug resistance. In an attempt to overcome the Eis-mediated kanamycin resistance of Mtb, we designed and optimized structurally unique thieno[2,3-d]pyrimidine Eis inhibitors toward effective kanamycin adjuvant combination therapy. We obtained 12 crystal structures of enzyme−inhibitor complexes, which guided our rational structure-based design of 72 thieno[2,3-d]pyrimidine analogues divided into three families. We evaluated the potency of these inhibitors in vitro as well as their ability to restore the activity of kanamycin in a resistant strain of Mtb, in which Eis was upregulated. Furthermore, we evaluated the metabolic stability of 11 compounds in vitro. This study showcases how structural information can guide Eis inhibitor design.
Graphical Abstract

INTRODUCTION
The advent of antibiotics, including antitubercular agents, has been one of the most powerful technological advances responsible for the reduction of mortality due to bacterial infections.1 Unfortunately, in the last few decades, due to the overuse and misuse of the same antibiotics and the paucity of new antibacterial agents, resistance to antibiotics has emerged as a global crisis. There is a pressing need for discovery of new molecules and strategies to combat antibiotic resistance. In 2018, tuberculosis (TB), caused by infections with Mycobacterium tuberculosis (Mtb), killed 1.5 million people, which is more than any other infection worldwide, and caused 10 million new cases.2 Misuse of anti-TB drugs, poor treatment compliance, and over 50 years of using many of the same antibiotics to fight this disease have led to the emergence and spread of Mtb strains resistant to one or more of these agents. Multidrug-resistant (MDR) Mtb strains that cause MDR TB are, by definition, resistant to first-line drugs isoniazid and rifampin, taken orally. A second-line antibiotic, aminoglycoside kanamycin (KAN) is used to treat MDR TB, but resistance to KAN, a hallmark of extensively drug-resistant (XDR) TB (along with resistance to a fluoroquinolone), has also emerged and spread. XDR TB has a high mortality rate and requires prolonged treatment that can last as long as 2 years, even if successful. A clinically significant mechanism of KAN resistance in TB is KAN acetylation by the acetyltransferase Eis as a result of point mutations in the eis promoter or the 5′ untranslated region of the gene encoding a transcription factor WhiB7.3–5
We previously showed that Eis transfers an acetyl group from acetyl coenzyme A (AcCoA) to one or more amino groups of all clinically relevant aminoglycosides,6–10 abolishing their antibacterial activity. In addition, Eis can acetylate the peptide antibiotic capreomycin.11 This versatility is apparently enabled by a large substrate binding site that is lined largely by acidic and hydrophobic residues.6,8,12 The acetylation reaction proceeds via a random sequential mechanism, where either AcCoA or the aminoglycoside substrate can bind first to a free enzyme.13
Two orthogonal approaches to overcoming Eis-mediated aminoglycoside resistance have been considered: (1) chemical modifications of existing aminoglycosides or development of novel aminoglycosides that would not succumb to acetylation by Eis, and (2) development of Eis inhibitors as adjuvants of the clinically used aminoglycosides. The first approach has been used successfully to design β-lactam antibiotics that are not susceptible to inactivation by β-lactamases.14 Because of the very broad substrate versatility of Eis, an analogous approach of developing aminoglycosides that would not be susceptible to acetylation by Eis does not appear to be promising. Instead, our groups have focused on development of potent small molecule Eis inhibitors for their therapeutic use as aminoglycoside adjuvants against MDR TB.15–21 We have discovered by high-throughput screening (HTS) Eis inhibitors of several structural families. Optimization of these molecules by classical medicinal chemistry has yielded several compounds that displayed potency (nanomolar in some cases) in inhibiting Eis in the test tube and in overcoming KAN resistance of a relevant resistant Mtb strain in culture.15–20 In these studies, the crystal structures of Eis in complexes with several of these inhibitors showed that these inhibitors occupied the aminoglycoside binding site, in agreement with steady-state kinetic studies. These structures also established the molecular basis behind the inhibitor specificity and explained the structure− activity relationships (SAR).
In the current study, we report the discovery of Eis inhibitors with a chemical scaffold that is distinct from the previously reported ones. Here, crystal structures of Eis− inhibitor complexes are used to guide synthetic efforts in generating derivatives for further preclinical testing.
RESULTS AND DISCUSSION
Thieno[2,3-d]pyrimidine-Containing Eis Inhibitor.
To identify potential Mtb Eis inhibitors, HTS of ∼23 000 structurally diverse compounds was carried out using our previously established aminoglycoside acetylation assay with Mtb Eis (Figure 1; step 1).15 The HTS assay had a Z′ score22 of 0.65, indicating its robustness. Among the ∼23 000 molecules tested, compound 2i containing a thieno[2,3-d]pyrimidine moiety was an HTS hit (Z = 4.9, Figure 1; step 2). Eis inhibitors with this structural core have not been characterized previously.
Figure 1.

Cone diagram showing the evaluation and triage of ∼23 000 structurally diverse compounds.
In order to validate 2i as an Eis inhibitor, we measured the dose response of the acetylation in the same assay (Figure 1, step 3) using a purchased fresh powder of 2i. We then resynthesized 2i and all analogues (Figure 1, step 4; characterization of all analogues in this study is shown in Figures S1−S222). We observed robust inhibition of Eis in this dose−response experiment, with an IC50 of 1.6 ± 0.2 μM. To test and validate the mechanism of action of 2i as an Eis inhibitor in the Mtb cell, we determined the effect of 2i on the MIC of KAN for the model strain Mtb K204, which bears a single clinically relevant mutation in the eis promoter resulting in Eis overexpression and KAN resistance3 (MICKAN in K204 is >10 μg/mL) in the background of strain H37Rv (MICKAN = 1.25 μg/mL). Mtb K204 was used extensively to observe the effect of our previously reported Eis inhibitors.16–20 We observed that when tested at 100 μM, 2i restored, within a 2-fold dilution, the sensitivity of Mtb K204 to KAN to the level of the parent strain H37Rv (MICKAN = 2.5 μg/mL against Mtb K204 in the presence of 2i).
Structural Basis for Lead Compound Binding and Analogue Design.
The HTS library contained 70 other molecules with a thieno[2,3-d]pyrimidine moiety, which were not identified as hits (Figure S223). These inactive compounds aided our analogue design by demonstrating what chemical modifications to avoid (e.g., aromatic groups in the thioether side chain; heteroatoms in ring A; or substituents on the primary amine of ring C; see Figure 2 for ring labeling). To guide our inhibitor design, we determined a crystal structure of Mtb Eis in a complex with lead inhibitor 2i (Figure 2A,B; PDB ID 6VUT; Table S1). As a reference structure, we chose the crystal structure of Eis in complex with inhibitor 39b from a previous study (Figure 2C; PDB ID 6B3T).20 Inhibitor 39b contains a 1,2,4-triazino[5,6b]indole-3-thioether core that is partially isosteric with the tricyclic moiety of 2i. Both 2i and 39b contain flexible substituents on the same side of the tricyclic moieties, each on a nitrogen-containing ring. Similar to 39b and all the other previously reported inhibitors, 2i is bound in the aminoglycoside binding pocket (Figure S224). Strikingly, despite their similar shapes, the tricyclic moieties of 2i and 39b lie in the same plane, sandwiched between Trp36 and Phe84 but in different orientations that are related by a 180° rotation around an axis lying in the plane of these moieties and passing through the three rings. This difference, nevertheless, preserves the common binding characteristic, where the entirely hydrophobic ring A that is unsubstituted (in 2i) or modified by small nonpolar or weakly polar substitutions (a fluorine in 39b) abuts the hydrophobic wall of the binding pocket (lined with Trp13, the aliphatic portion of Arg37, Val40, Leu63, and Met65), whereas the large (R1) substituent on the opposite ring C is extended along the substrate binding cleft toward the solvent, a general direction of the thioether side chain. We used these properties of rings A and C and their substituents in our analogue design (Figure 3).
Figure 2.
Crystal structures of Eis (one monomer is shown) in complex with inhibitors. (A) Crystal structure of Eis−2i complex. Eis is shown in cyan and 2i as orange sticks. (B) Zoomed-in view of the Eis−2i interface. The polder omit map for the inhibitor is contoured at 4σ and is shown by the brown mesh. The amino acid residues interacting with compound 2i are shown by dark turquoise sticks. The nitrogen atoms are in light blue, oxygen atoms in red, sulfur atoms in yellow, and fluorine atoms in cyan. The C-terminus is labeled “C-ter”. (C) Previously reported crystal structure of Eis−39b complex (PDB ID 6B3T).20 A water molecule in the active site is shown as a red sphere. The chemical structures of the bound inhibitors are shown on the bottom of panels B and C. Note: This color scheme is preserved in all the other figures depicting crystal structures.
Figure 3.

Chemical structures of all compounds synthesized and investigated in this study. The three rings of the tricyclic inhibitors in the library are labeled as A, B, and C for simplicity. The library is divided into three families (I−III) based on the size of ring A. Further subdivision of the families is based on R1, R2, and R3 substituents.
Since relatively small hydrophobic substitutions on ring A were allowed, consistent with its environment, we varied the size of this ring and the presence and the nature of small hydrophobic substituents R2 and R3 at two positions on this ring. The difference in the orientations indicates that the specific chemical structures of the rings and their substitutions specify how the inhibitors are bound to the enzyme. Indeed, ring C in the thieno[2,3-d]pyrimidine core of 2i is decorated with a primary amino group, absent in 39b, which forms a salt bridge with the C-terminal carboxyl group of Eis. The primary amine on ring C is also preserved in all analogues, as it defines the overall disposition of the core of these the thieno[2,3-d]pyrimidines in the Eis binding pocket. There is not enough room to accommodate substitutions on this amino group, which explains inactivity of library compounds with such substitutions (Figure S223). The nonpolar sulfur of the central ring B of 2i is in favorable hydrophobic contact with the side chain of Ala33 (the Cβ−S distance is 3.6 Å), whereas the orientation of 39b places its nitrogen-containing ring C approximately in the same site as the sulfur of 2i but somewhat closer to the protein surface, enabling water-mediated hydrogen bonding of this ring with the amide nitrogens of Ile28 and Gly29. The sulfur-containing ring B is preserved in the analogues to maintain the interactions of this ring with Eis. For 2i, a water mediates hydrogen bonding of a nitrogen of ring C with the side chain of Asp26. In both structures, the R1 is a thioether-linked side chain on ring C. In 2i, R1 contains an additional amide ultimately connecting to a terminal morpholine ring, whereas in 39b R1 is shorter and is terminated by a piperidine ring on an entirely hydrophobic linker. As a result of the different core orientations, the directions and interactions with the protein of R1 linkers (i.e., what links the sulfur atom and the tertiary amine of the thioether side chain) are somewhat different for 2i and 39b. While in 39b the R1 linker is directed to sterically contact the backbone of Phe27-Asp26, the R1 of 2i is directed along the other side of the binding cleft, in steric contact with the backbone and the side chain of Glu401. For both 39b and 2i, the tertiary amines of R1 formed a salt bridge with Asp26, which for 2i is enabled by its longer linker assuming a U-turn conformation. The position and the overall dimensions of the substituent on ring C, as a thin linker fitting the narrow Eis cleft, were also preserved in the analogues, whereas the nature of the substituents was different. An Eis loop spanning residues 25−30 is in two somewhat different conformations in the two structures, apparently induced by binding of the two different inhibitors. Most significantly, rotamers of Ile28 and Asp26 differ considerably in the two structures to optimally interact with the R1 linker and the terminal R1 group, respectively. The side chain of Arg37 is also in two different conformations, interacting with either the unmodified ring A of 2i or with the fluorinated ring A of 39b.
The drastic differences in core orientations, locations of bound water molecules, and consequently the inhibitor− protein interactions, which all arise as a result of seemingly minor chemical differences of the core structures, illustrate potential serious pitfalls of modeling and computational docking based on a crystal structure with a similar pharmacophore. We used the interactions observed in the crystal structure of Eis in a complex with 2i to design analogues with the thieno[2,3-d]pyrimidine-containing core.
Design and Chemical Synthesis of Eis Inhibitors.
By using the guidelines derived from the crystal structure of the Eis−2i complex and its comparison with the structure of Eis− 39b interactions, as described in the previous section, we made substitutions on the A−B−C core of 2i (Figure 3; family II) as well on the same thieno[2,3-d]pyrimidine B−C core but with a five-membered ring A (Figure 3; family I) as well as a seven-membered ring A (Figure 3; family III). To explore optimal filling in the binding pocket for ring A, we modified ring A with various hydrophobic moieties in family II. In this family, the R3 position in ring A was unsubstituted (H) or substituted with a methyl, an ethyl, a tert-butyl, or a phenyl group (Figure 3). To optimize hydrophobic interactions with residues Met65 and Leu63, we generated molecules in which the R2 position in ring A was either unsubstituted (H) or substituted with a methyl group. The analogues were divided into eight series (1−8) based on the ring A structure, with family I defining series 1, family II defining series 2−7 with different R2 and R3 substituents on ring A, and family III defining series 8. Furthermore, within all eight major series, we also installed side chains R1 on the sulfur of ring C, in which tertiary amino groups were connected to the sulfur of ring C by an aliphatic linker (with or without an amide), consistent with the restrictions of the narrow binding cleft. These tertiary amines mimic the amino groups of aminoglycoside substrates in the active site, located in the negatively charged environment of the Eis active site (Asp26, Glu401, and the C-terminal carboxyl; Figure 2). We used our previous experience in diversifying substituents at this binding site to install a pyrrolidine or a piperidine, which were observed to improve inhibitor potency in other inhibitors, whereas substituents like carbonyl, hydroxyl, ether, morpholine, or cyano group were not well tolerated.20 Here, we varied the size of the terminal group on R1 (for example, a pyrrolidine vs a piperidine ring), the position of the nitrogen, for a constant chain length, the linker length, the presence of an amide bond (imposing constraints in the R1 side chain and introducing hydrogen bonding potential). A total of nine R1 variants were generated as indicated by the letters a−i after the series number. The entire library contained 72 molecules (Figure 3).
To access the thieno[2,3-d]pyrimidine core, we initially prepared a series of 2-aminothiophenes (17−24) as building blocks via the classic Gewald aminothiophene synthesis starting from the commercially available malononitrile, sulfur, and various cyclic ketones (9−16) (Scheme 1A). The building blocks 17−24 were reacted with benzoyl isothiocyanate in 1,4-dioxane to afford the thioureas (25−32). The intermediates 25−32 were cyclized in 2 N NaOH and EtOH to afford the thieno[2,3-d]pyrimide core scaffolds (33−40). Side chains were introduced to compounds 33−40 via thiol alkylations to afford the final products 1−8(a−i). Cesium carbonate was found to be superior to potassium carbonate in terms of reaction time and yields. Please note that alkyl halides 41−44 were not commercially available and were prepared by reacting commercially available primary amines with bromoacetyl chloride (Scheme 1B). The alkyl halides 41−44 were used in alkylation reactions with intermediates 33−40 to afford final products with side chains f, g, h, and i (R1 groups in Figure 3).
Scheme 1.
Synthetic Scheme for the Preparation of (A) Compounds 1−8(a−i) and (B) Intermediates 41−44
SAR Deduced from Structural and Functional Studies.
We next sought to identify SAR for the 72 rationally designed Eis inhibitor analogues. The inhibitory potency of the analogues was determined by measuring their effect (IC50) on the acetylation of the clinically relevant KAN by Eis (Figure 1; step 5; Table 1 and Figures S225−S228). In order to interpret these data, in addition to the crystal structure of the Eis−2i complex, we determined crystal structures of Eis in complexes with 11 other inhibitors (Tables S1−S3) to avoid making potentially incorrect assumptions based on the structure of Eis in a complex with the parent compound.
Table 1.
IC50 and MIC Valuesa
| cmpd | IC50 (μM) | K204 MICKAN (μg/mL) with 100 μM cmpdb | H37Rv mc2 6230 (6206) MIC (μg/mL) | H37Rv mc2 6230 (6206) MIC (μM) | cmpd | IC50 (μM) | K204 MICKAN (μg/mL) with 100 μM cmpdb | H37Rv mc2 6230 (6206) MIC (μg/mL) | H37Rv mc2 6230 (6206)MIC (μM) |
|---|---|---|---|---|---|---|---|---|---|
| KAN | N/A | >10, >10d | <1 | <1.72 | 5e | 7.0 ± 0.7 | 10, 10 | >128 | >340 |
| 1a | 2.2 ± 0.3 | 10, 10 | 8 | 25 | 6e | 4.6 ± 0.6 | toxic | 4 (8) | 9.9 (20) |
| 2a | 0.99 ± 0.06 | 5, 5 | 4 | 12 | 7e | 0.46 ± 0.17 | toxic | 8 | 19 |
| 3a | 6.7 ± 0.7 | 10, 10 | 2−4 | 5.7−12 | 8e | 2.5 ± 0.1 | 5, toxic | 32 | 88 |
| 4a | 2.4 ± 0.2 | 5, 2.5, toxic | 4 | 12 | 1f | 11 ± 1 | 10, 10 | >128 | >363 |
| 5a | >200 | NT | >128 | >353 | 2f | 1.5 ± 0.1 | 5, 5 | >128 | >350 |
| 6a | 23 ± 5 | toxic | 4 | 10 | 3f | 52 ± 7 | >10, >10 | >128 | >337 |
| 7a | 4.0 ± 1.1 | toxic | 4 | 9.7 | 4f | 3.6 ± 0.4 | 10, 10 | 128 | 337 |
| 8a | 6.6 ± 0.6 | 10, >10 | 4 | 11 | 5f | 1.1 ± 0.1 | NT | 64 | 162 |
| 1b | 1.9 ± 0.2 | 5, 5 | 64 | 191 | 6f | ∼200 | NT | >128 | >303 |
| 2b | 1.1 ± 0.1 | 5, 5 | 32 | 92 | 7f | 3.0 ± 0.6 | toxic | 16 | 36 |
| 3b | 14 ± 1 | 10, 10 | 32 | 88 | 8f | 8.7 ± 1.2 | 10, 10 | >100 | >263 |
| 4b | 2.0 ± 0.3 | 5, 10 | 8 | 22 | 1gc | 1.9 ± 0.6 | NT | >128 | >337 |
| 5b | 5.4 ± 0.5 | toxic | 4 (8) | 11 (21) | 2gc | 0.25 ± 0.02 | 2.5, 2.5 | >128 | >325 |
| 6b | 16 ± 2 | toxic | 4 (4−8) | 9.9 (9.9−20) | 3g | ∼2.4 | NT | 128 | 314 |
| 7b | 16 ± 2 | 10, 10 | 16 | 38 | 4g | 0.20 ± 0.03 | 5, 2.5 | 64 | 157 |
| 8b | 7.6 ± 1.1 | toxic | 32 | 88 | 5g | 1.1 ± 0.2 | toxic | 16 | 38 |
| 1cc | 0.75 ± 0.06 | 2.5, 2.5 | 32−64 | 92−183 | 6g | >200 | NT | >128 | >284 |
| 2c | 0.17 ± 0.04 | 2.5, 2.5 | 64 | 176 | 7g | 0.48 ± 0.06 | toxic | 32 | 68 |
| 3c | 3.8 ± 0.5 | 5, 5, 2.5 | 16 | 42 | 8g | 3.1 ± 0.3 | 5, 5 | 8 | 20 |
| 4cc | 0.61 ± 0.08 | toxic | 16 | 42 | 1hc | 2.5 ± 0.4 | 5, 5 | >128 | >327 |
| 5c | 0.91 ± 0.13 | toxic | 16 | 41 | 2h | 0.46 ± 0.06 | 2.5, 2.5 | >128 | >315 |
| 6c | 6.6 ± 1.2 | toxic | 32 | 76 | 3h | 6.8 ± 0.6 | 10, 10 | >128 | >305 |
| 7cc | 0.23 ± 0.08 | toxic | 4 (8) | 9.1 (18) | 4h | 3.7 ± 0.6 | 2.5, 2.5 | 64 | 152 |
| 8cc | 1.2 ± 0.1 | toxic | 16 | 42 | 5hc | 0.68 ± 0.09 | toxic | 32 | 74 |
| 1d | 1.8 ± 0.1 | 5, 5 | 16 | 48 | 6h | 10 ± 4 | toxic | 16 | 35 |
| 2d | 0.45 ± 0.03 | 5, 5 | 16 | 46 | 7h | 0.93 ± 0.29 | toxic | 8 | 17 |
| 3d | 3.6 ± 0.2 | 2.5, 5 | 16 | 44 | 8hc | 0.30 ± 0.06 | NT | >128 | >305 |
| 4d | 0.81 ± 0.13 | toxic | 8 | 22 | 1i | 17 ± 1 | 5, 5 | >128 | >325 |
| 5d | >200 | NT | >128 | >340 | 2ic | 1.6 ± 0.2 | 2.5, 2.5 | >128 | >314 |
| 6d | 6.3 ± 1.5 | toxic | 4 (8−16) | 9.9 (20−40) | 3i | 56 ± 6 | 10, 10 | >128 | >303 |
| 7d | 0.53 ± 0.04 | NT | 16 | 38 | 4i | 0.47 ± 0.08 | 1.25, 2.5, 2.5 | >100 | >237 |
| 8d | 2.3 ± 0.2 | 5, 10 | 16 | 44 | 5i | 19 ± 3 | 5, 5 | 128 | 294 |
| 1e | 0.80 ± 0.10 | 10, 5 | 128 | 382 | 6i | >200 | toxic | 32 | 69 |
| 2ec | 0.15 ± 0.02 | 2.5, 2.5 | 64 | 183 | 7i | 16 ± 3 | 5, toxic, toxic | 32 | 66 |
| 3e | 2.0 ± 0.1 | 5, 2.5, 5, 5 | 64 | 176 | 8i | 8.5 ± 1.3 | 5, 5 | >128 | >303 |
| 4ec | 0.36 ± 0.05 | toxic | 8 | 21 |
N/A = not applicable; NT = not tested.
Multiple MIC values are the results of independent experiments.
X-ray structures of Eis in complex with these inhibitors are presented in this study.
Note that the MIC of KAN in the parent H37Rv from which the K204 strain is derived is 1.25 μg/mL.
Effect of the Size of Ring A.
The inhibitors with the six-membered ring A were nearly always much more potent Eis inhibitors than those with the five- or the seven-membered ring A and the same R1 side chain, regardless of the identity of R1, whereas the molecules with the seven-membered ring A were generally the least potent of the three (Table 1). This observation explains why most substitutions were made on the six-membered ring (Figure 3; family II). For example, the IC50 values for compounds 1g, 2g, and 8g were 1.9 ± 0.6 μM, 0.25 ± 0.02 μM, and 3.1 ± 0.3 μM, respectively. To interpret this effect, we determined crystal structures of Eis−1g and Eis−2g complexes (Figure 4). The six-membered ring A of 2g appears to fill the hydrophobic pocket of Eis more efficiently than the five-membered ring of 1g. A Cγ atom of Val40 is 3.8 Å away from the closest C atom of 2g, whereas it is 4.0 Å away in the case of 1g. Analogously, the Sδ of Met65 is at 4.2 and 4.3 Å from the closest C atoms in 2g and 1g, respectively. These C− C distances are likely optimal for the six-membered ring, and the seven-membered ring of 8g is not accommodated as well. While this straightforward structural interpretation of the effect of the ring A size on inhibitor potency explains the general trend, there are other consequences to ring A size changes that propagate to the rest of the inhibitor structure and that are not readily interpretable. A subtle (0.5 Å) positional shift of the tricyclic cores apparently occurred due to ring A differences, which resulted in different conformations of the R1 side chain and the side chain of Glu401, as well as differences in water-mediated inhibitor−Eis interactions involving these moieties. The differences in the R1 conformations resulting from the positional shifts of the cores with different A rings must be responsible in rare cases for the exceptions of the general rank order of potencies for A ring sizes (6 > 5 > 7). Such a rare exception was observed for inhibitor 8h with the seven-membered ring, which exhibited the highest potency among the compounds with this R1 side chain. This potency was similar to that of 2h with the six-membered ring: the IC50 values for 1h, 2h, and 8h were 2.5 ± 0.4 μM, 0.46 ± 0.06 μM, and 0.30 ± 0.06 μM, respectively.
Figure 4.
Zoomed-in views of the Eis−inhibitor interfaces from crystal structures of Eis−1g and Eis−2g complexes, and the inhibitor structures: (A) Eis−1g interface and (B) Eis−2g interface.
To investigate this effect in more detail, we determined crystal structures of Eis−1h and Eis−8h complexes (Figure 5). As anticipated, the seven-membered ring A of 8h is bent against the hydrophobic pocket of Eis, and its large size caused a 0.5 Å shift of the thieno[2,3-d]pyrimide core with respect to that of 1h, resulting in a different conformation of R1, where it makes additional hydrophobic contacts with the indole ring of Trp36 and positions the tertiary amine of R1 closer to an Oδ of Asp26 (N−O distance of 3.6 Å) than in 1h (N−O distance of 4.2 Å). Shifts in positions of bound waters in the vicinity of this interface were also observed. The significant conformational changes that propagate through the inhibitor and the surrounding solvent, ultimately accounting for the observed effects on inhibitor potency, would not have been possible to model based on the single crystal structure of Eis in a complex with the parent compound or based on molecular docking.
Figure 5.
Zoomed-in views of the Eis−inhibitor interfaces from crystal structures of Eis−1h and Eis−8h complexes and the inhibitor structures. (A) Eis−1h interface and (B) Eis−2h interface.
Effect of Substituents R2 and R3 in Family II.
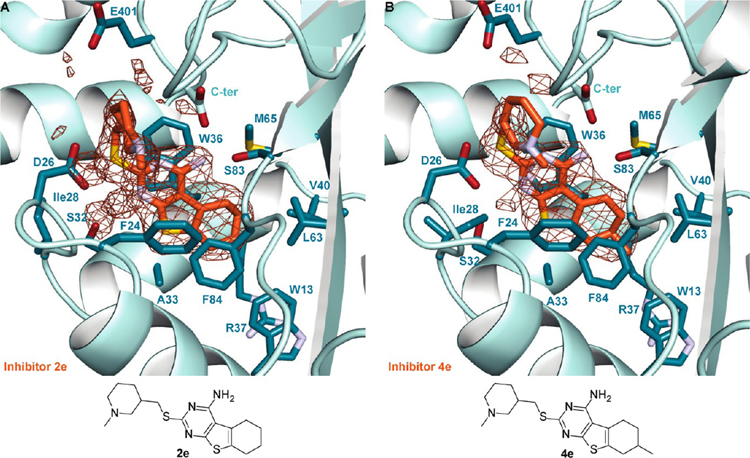
Next, we planned to verify the effect of various substitutions on ring A of the core, by considering the effect of R2 and R3 substituents (series 2−7; Figure 3 while keeping the R1 side chain (a−i) constant. We found that for each R1, regardless of its identity, the compounds with no substituents at R2 and R3 (series 2) were nearly always the most potent. Even the small methyl group at R2 (series 3) was not tolerated, generally resulting in at least a 10-fold higher IC50 value than that of its unsubstituted counterpart from series 2. Indeed, the methyl group at R2, depending on the stereoconfiguration, would sterically clash with the Sγ of Met65 or with the Cβ atom of Phe84, because the distances from the C atom of the core and the respective Sγ and Cβ are 4.2 and 4.1 Å along the potential C−C bond between the core and the R2 methyl group (Figure 4B). Nonpolar substituents, such as a methyl, an ethyl, and a phenyl group at R3, were well tolerated while a tert-butyl substituent was the least tolerated, regardless of R1 (Table 1). The relatively small methyl and ethyl substituents at R3 were predicted to be less hindered than at R2, because in either stereoconfiguration they would not clash with the protein (Figure 4B). Furthermore, the flexible side chain of Arg37 could change its conformation to enlarge the binding pocket. On the other hand, a tert-butyl group (series 6) or a phenyl ring (series 7) would not be readily accommodated. Indeed, series 6 nearly always contained the least potent compounds. Unexpectedly, some of compounds in series 7 were highly potent Eis inhibitors. To learn about the structural consequences of a methyl substitution at R3, we determined crystal structures of Eis in complexes with highly potent inhibitors 2e (IC50 = 0.15 ± 0.02 μM; PDB ID 6VUR) and 4e (IC50 = 0.36 ± 0.05 μM; PDB ID 6VUW), which differ from each other only by the presence of a methyl group at R3 in 4e (Figure 6). As anticipated, the methyl group was accommodated largely owing to a change of the conformation of the side chain of Arg37 and a minor shift by 0.4 Å of the inhibitor. The methyl group interacts favorably with Arg37 and Trp13, with the closest C−C distances of 3.8 and 3.6 Å, respectively. To investigate how a bulky phenyl group was accommodated at R3, we determined a crystal structure of Eis in a complex with the most potent inhibitor in series 7, 7c (IC50 = 0.23 ± 0.08 μM; PDB ID 6VUY; Figure 7A). As expected, large conformational changes had to occur to fit the phenyl group into the binding pocket. The indole ring of Trp13 rotated by 90° around the Cβ-Cγ bond and stacked orthogonally against the R3 phenyl group of 7c. In turn, the side chain of Arg37 changed conformation to stack orthogonally with the indole of Trp13 against its edge. Notably, computational molecular docking, which relies heavily on rigid-body assumptions, would not predict that 7c is a potent inhibitor of Eis.
Figure 6.
Zoomed-in views of the Eis−inhibitor interfaces from crystal structures of Eis−2e and Eis−4e complexes and the inhibitor structures: (A) Eis−2e interface and (B) Eis−4e interface.
Figure 7.
Zoomed-in views of the Eis−inhibitor interfaces from crystal structures of Eis−7c, Eis−1c, Eis−4c, and Eis−8c complexes and the inhibitor structures: (A) Eis−7c interface, (B) Eis−1c interface, (C) Eis−4c interface, and (D) Eis−8c interface.
Effects of R1 Substituents for Each of the Three Inhibitor Families.
The effect of different R1 substituents is complex, because the R1 side chain is extended into a large hydrophilic cleft lined with polar and charged residues. SAR have emerged, as follows.
a. Effect of the Ring Size at the Terminus of R1.
First, we investigated an effect of a terminal pyrrolidine (R1 = a) vs a piperidine ring (R1 = b) for the same 2-carbon linker. These substituents generally did not lead to significant improvement in potency from that of the parent compound 2i. In families I and III and in series 2 and 4 of family II, the R1 ring size had no effect. In the rest of the series of family II the effect was generally 2-fold or greater, favoring either the five- or the six-membered ring. In all of these last cases, the inhibitors had relatively low potencies (in the IC50 range from 4.0 ± 1.1 μM to >200 μM).
b. Effect of the Length of the Linker in R1.
To test the impact of the length of the R1 linker, we compared compounds with a 2-carbon linker to those with a 3-carbon R1 linker, both containing a terminal piperidine ring (series b vs c). The longer, 3-carbon linker (R1 = c), was strongly favored over the 2-carbon linker (R1 = b) in all three families (Table 1). In order to visualize the structural basis for this significant improvement, in addition to Eis−7c, we determined crystal structures of Eis in complexes with three additional potent inhibitors: 1c (IC50 = 0.75 ± 0.06 μM; PDB ID 6VUZ), 4c (IC50 = 0.61 ± 0.08 μM; PDB ID 6VUU), and 8c (IC50 = 1.2 ± 0.1 μM; PDB ID 6VV2) (Figure 7). These structures revealed that this longer R1 linker places the positively charged amino group of the piperidine so that it is equidistant from the carboxyl groups of Asp26, Glu401, and the C-terminal carboxyl group, all three at appropriate distances for strong salt bridge formation. The piperidine ring was found in different conformations, all related by rotations along the C−N bond connecting the ring to the R1 linker.
c. Effect of the Position of the Tertiary Amino Group in R1.
Since all R1 side chains contain a tertiary amino group in them, we examined the impact of the position of the tertiary amino group in the pyrrolidine ring on the potency of the compounds (R1 = a vs d). We observed that the ring nitrogen that is not directly attached to the 2-carbon linker (R1 = d) is preferred (Table 1). Having noted this, we then asked if we could further improve potency by keeping four bonds between the ring nitrogen and the core but enlarging the R1 ring to the piperidine (R1 = d vs e). In all but two series, 7 and 8, the inhibitors with the piperidine ring (e) were more potent than those with the pyrrolidone ring (d), and for series 7 and 8 the potency was not affected. The crystal structures of Eis−2e and Eis−4e complexes (Figure 6) showed that in series e, the tertiary amino group is placed directly between the carboxyl groups of Asp26 and the C-terminus for salt bridge formation, whereas the aliphatic side of the appropriately sized piperidine ring interacts with the aliphatic portion of the side chain of Glu401, explaining the observed trend of potencies.
d. Substituents with an Amide in R1.
We explored a potential benefit from further lengthening of an R1 linker and an introduction of an amide into it, to add polar character. In addition, we assessed the variability of the terminal R1 groups, which included a dimethyl amine (f), a diethyl amine (g), a piperidine (h), and a morpholine (i) groups. The compounds with a diethyl amino group (g) and a piperidine ring (h) were more potent that those with the other two choices of the R1 termini (Table 1). To examine the interactions of side chains g and h with Eis, in addition to the structures of Eis in complex with the potent inhibitors 2g (Figure 4B) and 8h (Figure 5B) described above, we determined the crystal structure of Eis in complex with inhibitor 5h (IC50 = 0.68 ± 0.09 μM; PDB ID 6VUX; Figure 8), the most potent inhibitor in series 5. Apparently, the side chain R1 = h overcame the unfavorable effect of the ethyl group at R3 observed with other choices of R1 in this series. Analogously, the side chain R1 = h was the most favored in family 8. In all these three structures, the R1 side chain is in the same U-turn conformation, where the thioether moiety is coplanar with the tricyclic core and the amide is solvent exposed. The tertiary amino group formed a salt bridge with Asp26. The diethyl group of 2g and the piperidine rings of 5h and 8h abutted the phenyl ring of Phe24, explaining why the morpholine ring in series i containing a polar oxygen was less preferred. The rigidity of the amide-containing linker and the restraint imposed by Phe24 likely accounted for the high potency of these molecules. Other structural factors seemed to modulate the potency. For example, the ethyl group at R3 of 5h wedged against Trp13 and Arg37, pushing the latter off and compromising its stacking against Trp13, likely caused a 2-fold weaker potency of this analogue than those of the equipotent 2g and 8h, in which ring A was unsubstituted.
Figure 8.

Zoomed-in view of the Eis−inhibitor interface from crystal structure of Eis−5h complex and the structure of 5h.
Buried Surface Area of Eis−Inhibitor Complexes.
Binding of macromolecules to each other and to druglike molecules commonly depends on geometrical and physical complementarity of the two interacting interfaces. Upon such binding, water is excluded from the interface between the nonpolar surfaces, providing a favorable entropic contribution in addition to the favorable van der Waals energy gain by the contact of the two properly spaced surfaces. Formation of the interface between the two polar or charged surfaces, in addition to the geometrical complementarity similarly needed to ensure favorable van der Waals interactions, requires that the energy stored in hydrogen bonds and salt bridges involved in hydration and solvation of such surfaces is not lost upon binding. Therefore, one can observe that in the interfaces between polar surfaces buried from bulk solvent, hydrogen bonds, and salt bridges are formed across the interface (when solvent is also excluded with a favorable gain of entropy) or with water molecules or ions trapped in the interface, without losing these energetically costly interactions. With the benefit of obtaining a set of crystal structures of Eis in complexes with 12 inhibitors, we calculated23 the total solvent accessible surface area buried in the interface between the protein and the inhibitors (ASAb,t) and examined whether there is any trend in the inhibitor potency with respect to ASAb,t. We also considered separately the buried nonpolar (ASAb,np) and polar (ASAb,p) surface areas (Figure S229). ASAb,np comprised approximately two-thirds of ASAb,t and, as expected based on the complementarity principles discussed above, was the dominant contribution to the trend in ASAb,t with respect to IC50. The larger ASAb,t and ASAb,np were associated with higher potency (smaller IC50 values), underscoring the importance of hydrophobic interactions, although the correlation was relatively weak: R = 0.040 and −0.259, respectively. To our surprise, ASAb,p had a stronger correlation with the opposite trend (R = 0.691), where the larger ASAb,p were associated with lower potency. We interpreted this to mean that in burying polar surfaces, the resulting hydrogen bonds and salt bridges in the interface were not as favorable as those with bulk solvent in the unbound state. Additionally, the loss of the conformational freedom of the polar and charged side chains upon inhibitor binding may be another contributor. These calculations are consistent with the notion that the interactions between complementary nonpolar surfaces are drivers of inhibitor potency and they advocate for frugal and rational incorporation of polar and charge groups.
Competitive Inhibition Mode.
All 12 crystal structures of Eis−inhibitor complexes presented here show that the inhibitors are bound at the Eis active site in the pocket that overlaps with the aminoglycoside substrate binding pocket (Figure S224), strongly suggesting that these molecules inhibit the acetyl transfer by competing with the aminoglycosides for binding Eis. We tested this directly by monitoring kinetics of acetylation of KAN by Eis in the steady-state, as a function of KAN in the absence of the inhibitor and at several concentrations of each of three potent inhibitors, 4c, 7c, and 4g (Figure 9). These data show that, within experimental uncertainty, these inhibitors display a competitive (with respect to KAN) inhibition mode, in agreement with the crystal structures. The global fit of the competitive mode of inhibition to these data yielded the Ki values for these three inhibitors (Table 2), all in the 0.15−0.34 μM range.
Figure 9.
Analysis of inhibition kinetics for inhibitors 4c, 7c, and 4g. Dose−response curves are shown in panels A, D, and G; Michaelis−Menten analysis is in panels B, E, and H; and the Lineweaver−Burk representation of this analysis is in panels C, F, and I. These data indicate that the molecules are competitive with KAN. These data are used in obtaining Ki by global nonlinear regression (Table 2).
Table 2.
Kinetic Parameters of Eis and Ki Values for Inhibitors 4c, 4g, and 7c
| cmpd | [inhibitor] (μM) | Km,KAN (μM) | kcat (s−1) | kcat/Km,KAN (s−1 μM−1) | Ki (μM) |
|---|---|---|---|---|---|
| 4c | 0 | 460 ± 150 | 14 ± 2 | 0.031 ± 0.01 | 0.34 ± 0.02 |
| 0.4 | 460 ± 120 | 9.8 ± 1.2 | 0.021 ± 0.006 | ||
| 0.6 | 530 ± 120 | 8.9 ± 1.0 | 0.017 ± 0.005 | ||
| 0.8 | 650 ± 150 | 8.3 ± 1.0 | 0.013 ± 0.003 | ||
| 4g | 0 | 310 ± 74 | 11 ± 1 | 0.036 ± 0.009 | 0.15 ± 0.01 |
| 0.1 | 370 ± 59 | 9.6 ± 0.6 | 0.027 ± 0.004 | ||
| 0.2 | 440 ± 82 | 8.8 ± 0.7 | 0.020 ± 0.004 | ||
| 0.4 | 390 ± 64 | 6.4 ± 0.4 | 0.016 ± 0.003 | ||
| 7c | 0 | 510 ± 160 | 15 ± 2 | 0.029 ± 0.01 | 0.31 ± 0.03 |
| 0.125 | 500 ± 140 | 14 ± 2 | 0.027 ± 0.008 | ||
| 0.25 | 490 ± 140 | 11 ± 1 | 0.022 ± 0.007 | ||
| 0.5 | 1200 ± 400 | 13 ± 3 | 0.011 ± 0.004 |
Effect of Inhibitors on the MICKAN against Mtb in Vitro.
In addition to the parent inhibitor 2i, we measured the effect of other Eis inhibitors at 100 μM on KAN resistance caused by Eis overexpression (Table 1). Inhibitors that had relatively low potencies (IC50 > 10 μM) generally had no effect on the MICKAN of the KAN-resistant Mtb strain K204, whereas compounds that were highly potent generally lowered the MICKAN as much as 4-fold, to 2.5 μg/mL. Intriguingly, while many of the compounds did not affect the growth of Mtb K204 in the absence of KAN, several compounds from families II and III were toxic (at MIC < 100 μM) to this strain even in the absence of KAN, which means that the compounds act via an additional mechanism besides Eis inhibition. This unknown mechanism merits investigation in the future. As a control, we also determined MIC values of all the inhibitors in the absence of KAN against another Mtb strain that contained the wild-type eis promoter, H37Rv mc2 6230 (Table 1). In agreement with the inhibitory effect on growth of Mtb K204, these compounds displayed MIC < 100 μM, some inhibitors as low as 9 μM. Five of the molecules were also tested against the culture of another H37Rv variant, mc2 6206. These MIC values were comparable to those for Mtb H37Rv mc2 6230 (Table 1).
Mammalian Cytotoxicity.
The cytotoxic effect of three Eis inhibitors and KAN was evaluated against three mammalian cell lines: human adenocarcinoma (A549), human embryonic kidney (HEK-293), and mouse macrophage (J774A.1) (Figure 10 and Figure S230). Inhibitors 2e and 2i were chosen as at 100 μM they restore the activity of KAN in Mtb K204 (Table 1). Compound 7c was chosen as it is toxic to Mtb strains with MIC values of 4 and 8 μM for strains H37Rv mc2 6230 and mc2 6206, respectively. We observed that 7c displayed toxicity against all three cell lines at 25 μM. Against A549 cells, 7c displayed no toxicity at 6.3 μM and approximately 20% cell survival at 12.5 μM. Similarly, against the J774A.1 cell line, no toxicity was observed up to 3.1 μM, with 30% cell survival at 6.3 μM. These values are very similar to the concentrations that are toxic to both strains of Mtb tested. In contrast, inhibitors 2e and 2i displayed no toxic effect at the highest concentration tested, 50 μM, which is highly promising.
Figure 10.
Evaluation of cytotoxicity for compounds 2e (red), 2i (yellow), 7c (purple), and KAN (gray) against three cell lines: (A) A549, (B) HEK-293, and (C) J774A.1. Controls include treatment with Triton-X (TX, 1% v/v, positive control) and 0.5% DMSO (negative control). It is important to note that testing xenobiotics at sub-IC50 concentrations can result in increase in cell growth, resulting in >100% cell survival in the treatment groups. In instances where >100% cell survival was observed, we displayed the data as 100% cell survival. Note: The raw data are presented in Figure S230.
Microsomal Stability.
In any drug discovery process, preclinical drug metabolism assessment plays a vital role in drug optimization. We tested the metabolic stability in human and mouse microsomes of several compounds in family II that were selected to represent the structural diversity of the family and predicted as stable by using StarDrop software (Optibrium) (Table 3). As expected, stabilities in human microsomes were grouped according to the nature of the R1 side chain, whereas in mouse microsomes most compounds were quite labile. Two compounds (6i and 7i) were completely metabolized by both human and mouse microsomes, while most of the other tested compounds were modestly metabolized by human microsomes. For these and several other molecules, the metabolic liability can be predicted. The instability of 7i may be due to the presence of an unsubstituted phenyl ring with oxidation at the para position of the phenyl ring or on the morpholine ring as well as hydrolysis of the amide bond. Similarly, 6i could be metabolized by oxidation at three major sites, namely, the tert-butyl group, the amide linker, and the sulfur linkage. Several compounds showed moderate stability (25−40% metabolized) with human microsomes (7a, 4c, 4d, 7e). Metabolism of 4d, 6d, and 7e is likely to be demethylation. Several compounds had improved stability with <25% metabolized in human microsomes (6a, 5c, 7c, 6d). The molecules that were most labile in the human microsomes were also highly labile in the mouse microsomes, but the converse was not true. For example, 6d and 5c were stable with human microsomes (20% and 7%, respectively), but not with mouse microsomes (62% and 52%, respectively).
Table 3.
Metabolic Stability of Eis Inhibitors
| inhibitor | human | mouse |
|---|---|---|
| 6a | 23 ± 21a | 53 ± 5 |
| 7a | 36 ± 24 | 38 ± 16 |
| 4c | 31 ± 4 | 99 ± 0 |
| 5c | 7 ± 5 | 52 ± 21 |
| 7c | 22 ± 2 | 47 ± 25 |
| 4d | 38 ± 6 | 99 ± 0 |
| 6d | 20 ± 2 | 62 ± 16 |
| 7e | 38 ± 4 | 75 ± 3 |
| 7h | 47 ± 5 | 58 ± 4 |
| 6i | 100 ± 0 | 100 ± 0 |
| 7i | 94 ± 7 | 100 ± 0 |
The values in the table are % metabolized compound after a 30 min incubation with microsomes. The data are the mean ± standard deviation for a minimum of three samples (except 4d, which was tested in duplicates).
CONCLUSION
A new class of Eis inhibitors having thieno[2,3-d]pyrimidine core was discovered, with various analogues synthesized and rationally optimized guided by structural studies, followed by their extensive characterization using chemical, biochemical, structural, and biological studies. Several analogues with potent inhibitory activity against purified Eis were able to restore the MICKAN in cellular assays, while displaying no toxicity against either Mtb or mammalian cells on their own. For compounds that displayed toxicity against Mtb, it remains to be investigated whether all these compounds are also toxic to mammalian cells (inhibitor 7c is toxic to both). In vitro metabolism assays show that several side chains were tolerated well metabolically, with R1 = c being especially promising. Pending further toxicity testing, compounds 1c and 2c, which are nontoxic to Mtb on their own, but potent in Eis inhibition and synergistic with KAN, emerged as promising compounds for further testing.
EXPERIMENTAL SECTION
Details of all experimental procedures for synthesis and characterization of all compounds and intermediates, HTS, hit validation, selectivity of inhibitors toward Eis, Mtb MIC value determination, dose-dependent Mtb MIC values determination, crystallization, and crystal structure determination of Eis−inhibitor complexes, solvent accessible surface area calculations, and metabolic stability assays are included in the Supporting Information. The Supporting Information also includes all 1H and 13C NMR spectra as well as HPLC traces for the molecules generated (Figures S1−S222).
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by a National Institutes of Health (NIH) Grant AI090048 (to S.G.-T.), a grant from the Firland Foundation (to S.G.-T.), a grant from the Center for Chemical Genomics (CCG) at the University of Michigan (to S.G.-T.), as well as by startup funds from the College of Pharmacy at the University of Kentucky (to S.G.-T. and O.V.T). We thank the UK PharmNMR Center (in the College of Pharmacy) for NMR support and S. Van Lanen for managing the LCMS system used in this study. We thank S. Vander Roest, M. Larsen, and P. Kirchhoff from the CCG at the University of Michigan for their help with HTS. We thank J. J. Johnson and R. M. Holmes for synthesizing and characterizing a few of the molecules presented in this manuscript. We thank C. Hou for assistance with the protein purification and crystallization. We also thank S. Chowdhury and B. Berube for discussion and technical assistance with metabolic stability assays. Use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, the U.S. Public Health Service, or the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
ABBREVIATIONS
- AcCoA
acetyl coenzyme A
- ASA
accessible surface area
- Eis
enhanced intracellular survival
- HPLC
high-performance liquid chromatography
- HTS
high-throughput screening
- IC50
inhibitory concentration at half-maximum inhibition
- KAN
kanamycin
- MDR
multidrug-resistant
- MIC
minimum inhibitory concentration
- Mtb
Mycobacterium tuberculosis
- PDB
Protein Data Bank
- SAR
structure−activity relationship
- TB
tuberculosis
- XDR
extensively drug-resistant
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.0c00184.
Additional details of the chemistry and the biochemical, biological, and biophysical assays (PDF)
Accession Codes
PDB accession codes: Eis−1c (6VUZ), Eis−1g (6VV0), Eis− 1h (6VV1), Eis−2e (6VUR), Eis−2g (6VUS), Eis−2i (6VUT), Eis−4c (6VUU), Eis−4e (6VUW), Eis−5h (6VUX), Eis−7c (6VUY), Eis−8c (6VV2), Eis−8h (6VV3).
The authors declare no competing financial interest.
Contributor Information
Ankita Punetha, Department of Pharmaceutical Sciences, University of Kentucky, Lexington, Kentucky 40536-0596, United States.
Huy X. Ngo, Department of Pharmaceutical Sciences, University of Kentucky, Lexington, Kentucky 40536-0596, United States.
Selina Y. L. Holbrook, Department of Pharmaceutical Sciences, University of Kentucky, Lexington, Kentucky 40536-0596, United States
Keith D. Green, Department of Pharmaceutical Sciences, University of Kentucky, Lexington, Kentucky 40536-0596, United States
Melisa J. Willby, Division of Tuberculosis Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia 30333, United States
Shilah A. Bonnett, TB Discovery Research, Infectious Disease Research Institute, Seattle, Washington 98102, United States
Kyle Krieger, TB Discovery Research, Infectious Disease Research Institute, Seattle, Washington 98102, United States; Center for Global Infectious Disease, Seattle Children’s Research Institute, Seattle Children’s Hospital, Seattle, Washington 98145, United States.
Emily K. Dennis, Department of Pharmaceutical Sciences, University of Kentucky, Lexington, Kentucky 40536-0596, United States
James E. Posey, Division of Tuberculosis Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia 30333, United States
Tanya Parish, TB Discovery Research, Infectious Disease Research Institute, Seattle, Washington 98102, United States; Center for Global Infectious Disease, Seattle Children’s Research Institute, Seattle Children’s Hospital, Seattle, Washington 98145, United States.
Oleg V. Tsodikov, Department of Pharmaceutical Sciences, University of Kentucky, Lexington, Kentucky 40536-0596, United States.
Sylvie Garneau-Tsodikova, Department of Pharmaceutical Sciences, University of Kentucky, Lexington, Kentucky 40536-0596, United States.
REFERENCES
- (1).Rice LB (2008) Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis 197, 1079–1081. [DOI] [PubMed] [Google Scholar]
- (2).Global Tuberculosis Report 2019, World Health Organization, Geneva, Switzerland, 2019, licence CCBY-NC-SA3.01GO. [Google Scholar]
- (3).Zaunbrecher MA, Sikes RD Jr., Metchock B, Shinnick TM, and Posey JE (2009) Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A 106, 20004–20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Campbell PJ, Morlock GP, Sikes RD, Dalton TL, Metchock B, Starks AM, Hooks DP, Cowan LS, Plikaytis BB, and Posey JE (2011) Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob. Agents Chemother 55, 2032–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Reeves AZ, Campbell PJ, Sultana R, Malik S, Murray M, Plikaytis BB, Shinnick TM, and Posey JE (2013) Aminoglycoside cross-resistance in Mycobacterium tuberculosis due to mutations in the 5′ untranslated region of whiB7. Antimicrob. Agents Chemother 57, 1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Chen W, Biswas T, Porter VR, Tsodikov OV, and Garneau-Tsodikova S (2011) Unusual regioversatility of acetyltransferase Eis, a cause of drug resistance in XDR-TB. Proc. Natl. Acad. Sci. U. S. A 108, 9804–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Chen W, Green KD, Tsodikov OV, and Garneau-Tsodikova S (2012) Aminoglycoside multiacetylating activity of the enhanced intracellular survival protein from Mycobacterium smegmatis and its inhibition. Biochemistry 51, 4959–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Houghton JL, Biswas T, Chen W, Tsodikov OV, and Garneau-Tsodikova S (2013) Chemical and structural insights into the regioversatility of the aminoglycoside acetyltransferase Eis. ChemBioChem 14, 2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Green KD, Biswas T, Chang C, Wu R, Chen W, Janes BK, Chalupska D, Gornicki P, Hanna PC, Tsodikov OV, Joachimiak A, and Garneau-Tsodikova S (2015) Biochemical and structural analysis of an Eis family aminoglycoside acetyltransferase from Bacillus anthracis. Biochemistry 54, 3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Green KD, Pricer RE, Stewart MN, and Garneau-Tsodikova S (2015) Comparative study of Eis-like enzymes from pathogenic and nonpathogenic bacteria. ACS Infect. Dis 1, 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Houghton JL, Green KD, Pricer RE, Mayhoub AS, and Garneau-Tsodikova S (2013) Unexpected N-acetylation of capreomycin by mycobacterial Eis enzymes. J. Antimicrob. Chemother 68, 800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Pricer RE, Houghton JL, Green KD, Mayhoub AS, and Garneau-Tsodikova S (2012) Biochemical and structural analysis of aminoglycoside acetyltransferase Eis from Anabaena variabilis. Mol. BioSyst 8, 3305–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Tsodikov OV, Green KD, and Garneau-Tsodikova S (2014) A random sequential mechanism of aminoglycoside acetylation by Mycobacterium tuberculosis Eis protein. PLoS One 9, No. e92370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Tehrani K, and Martin NI (2018) Beta-lactam/beta-lactamase inhibitor combinations: an update. MedChemComm 9, 1439–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Green KD, Chen W, and Garneau-Tsodikova S (2012) Identification and characterization of inhibitors of the aminoglycoside resistance acetyltransferase Eis from Mycobacterium tuberculosis. ChemMedChem 7, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Willby MJ, Green KD, Gajadeera CS, Hou C, Tsodikov OV, Posey JE, and Garneau-Tsodikova S (2016) Potent inhibitors of acetyltransferase Eis overcome kanamycin resistance in Mycobacterium tuberculosis. ACS Chem. Biol 11, 1639–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Garzan A, Willby MJ, Green KD, Gajadeera CS, Hou C, Tsodikov OV, Posey JE, and Garneau-Tsodikova S (2016) Sulfonamide-based inhibitors of aminoglycoside acetyltransferase Eis abolish resistance to kanamycin in Mycobacterium tuberculosis. J. Med. Chem 59, 10619–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Garzan A, Willby MJ, Green KD, Tsodikov OV, Posey JE, and Garneau-Tsodikova S (2016) Discovery and optimization of two Eis inhibitor families as kanamycin adjuvants against drug-resistant M. tuberculosis. ACS Med. Chem. Lett 7, 1219–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Garzan A, Willby MJ, Ngo HX, Gajadeera CS, Green JD, Holbrook SY, Hou C, Posey JE, Tsodikov OV, and Garneau-Tsodikova S (2017) Combating enhanced intracellular survival (Eis)-mediated kanamycin resistance of Mycobacterium tuberculosis by novel pyrrolo[1,5-a]pyrazine-based Eis inhibitors. ACS Infect. Dis 3, 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ngo HX, Green KD, Gajadeera CS, Willby MJ, Holbrook SYL, Hou C, Garzan A, Mayhoub AS, Posey JE, Tsodikov OV, and Garneau-Tsodikova S (2018) Potent 1,2,4-triazino[5,6b]indole-3-thioether inhibitors of the kanamycin resistance enzyme Eis from Mycobacterium tuberculosis. ACS Infect. Dis 4, 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Green KD, Punetha A, Hou C, Garneau-Tsodikova S, and Tsodikov OV (2019) Probing the robustness of inhibitors of tuberculosis aminoglycoside resistance enzyme Eis by mutagenesis. ACS Infect. Dis 5, 1772–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zhang JH, Chung TD, and Oldenburg KR (1999) A simple statistical parameter for use in rvaluation and validation of high throughput screening assays. J. Biomol. Screening 4, 67–73. [DOI] [PubMed] [Google Scholar]
- (23).Tsodikov OV, Record MT Jr., and Sergeev YV (2002) Novel computer program for fast exact calculation of accessible and molecular surface areas and average surface curvature. J. Comput. Chem 23, 600–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.