https://doi.org/10.5217/ir.2018.00078
Intest Res 2019;17(1):87-93
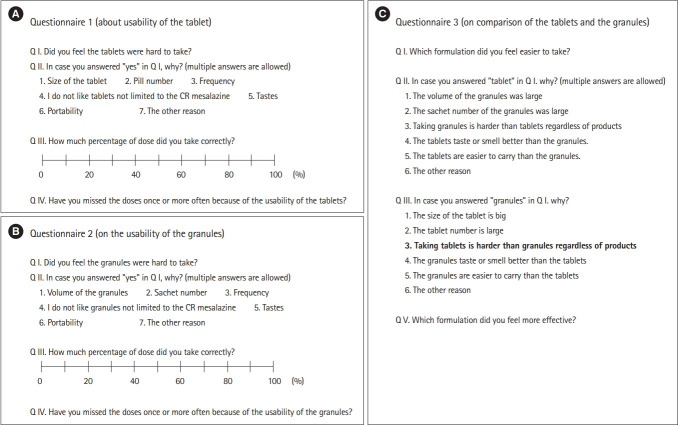
In the version of this article initially published, Figure 2 contains errors in the content of the questionnaire.
On page 89, the figure should be corrected as following.
From:
Fig. 2.
(A) Questionnaire 1: acceptability of tablets. (B) Questionnaire 2: acceptability of granules. (C) Questionnaire 3: comparison of tablets and granules. All the original questionnaires were written in Japanese. CR, controlled-release.
To:
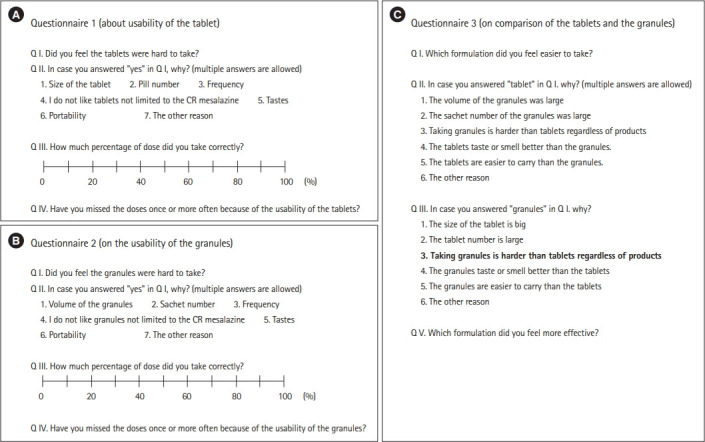
Fig. 2.
(A) Questionnaire 1: acceptability of tablets. (B) Questionnaire 2: acceptability of granules. (C) Questionnaire 3: comparison of tablets and granules. All the original questionnaires were written in Japanese. CR, controlled-release.