Abstract
A novel and simple method was described for preparation of carbonaceous adsorbent (CA) from corncob under phosphoric acid conditions. The method succeeded to introduce oxygen-containing groups onto the product surface through hydrothermal carbonization (HTC) at low temperature of 160 °C. Adsorption of methylene blue (MB) was studied systematically through the effect of pH, contact time and initial dye concentrations. The MB adsorption kinetics and isotherms experiments showed that Langmuir model and pseudo-second-order model could better describe the adsorption behavior, with a maximum adsorption capacity of MB was 140.25 mg/g. The high adsorption capacity could be ascribed to the presence of surface oxygen-containing functional groups and pore channels. In conclusion, it could be a potential adsorbent in the removal of methylene blue from wastewater.
Subject terms: Bioinspired materials, Environmental monitoring
Introduction
The presence of dyes in the wastewater can cause various detriment effects on human health and living organisms due to their not biodegradable and become a major environmental concern problem. These dyes are introduced in the freshwater from many industries, including cosmetics, leather, textile, paper, printing and dyeing industries1–8. Methylene blue (MB) is one of the most typical dye pollutants in aqueous systems9. Until now, various techniques such as photochemical oxidation, reverse osmosis, membrane filtration, adsorption and ozonation have been developed for the purification of organic contaminants from aqueous solutions10–18. Among these techniques, adsorption is regarded as a superior technique for treating wastewater considering its process simplicity, nontoxic and inexpensive. In particular, tremendous attention has been attracted to the study of different types of biomass-derived adsorbents removing organic pollutants in waterways19–25.
According to a recent China’s National Bureau of Statistics report, the Chinese annual yield of corn in 2017 was more than 21.5 million tons. The radio between corn gain and corncob may be about 100:1826, rough estimate 3.8 million tons of corncobs could be generated in China every year. Corncob is a sort of environment friendly and renewable resource, widely existing in biomass. Currently, most of the corncobs are burned as low-grade fuels and not fully utilized, which cause both environmental pollution and natural resources waste. Therefore, reasonable and harmless utilization of corncob is important to change waste into valuables.
However, to date, the most widely biomass-derived adsorbents were prepared under the most demanding conditions with high pressure and high temperature27–29. In addition, widespread application is seriously restricted because of the relatively poor capability30. Therefore, chemical modification method is widely used presently to enhance their capabilities of biochar by introduction of oxygen-containing groups31–35. These oxygen-containing groups on the surface serve as adsorption sites for organic pollutants in solution. For example, Xu et al. reported that citric acid-promoted water hyacinth (Eichornia crassipes) was an efficient method for enhancing the adsorption capability of biochar for MB36. Rhamnolipid was employed to enhance the adsorption capability of biochar for removal of MB37. However, there have been few reports that phosphorylated modification at low temperature is investigated for improving adsorption capability of biochar in wastewater treatment.
As one of most frequently used agents for chemical modifications, phosphoric acid is preferred recently for environmental and production costs. The phosphoric acid has played a vital role in the HTC process including the degradation of biomass and the formation of oxygen-containing surface functional groups. Thus, the main purpose of this research was to develop an effective modification method for preparing an alternative corncob-derived adsorbent with H3PO4 at low temperature. These works help not only to improve adsorption capability of biochar but also greatly reduce the preparation temperature. In addition, we aim to characterize the properties of as-prepared adsorbent and study their sorption behaviors for MB.
Methods
Materials
Corncob used in this study was obtained from the a farm around Fuyang City, Anhui Province of China. Before processing, the corncob was washed by water, dried at 105 °C for 24 h and smashed to pass through a 0.15 mm (100 mesh) sieve. Methylene blue (MB) was purchased from Sinopharm in Beijing, All chemicals were of analytical grades from the Shantou Xilong Chemical Factory, China.
Preparation of the corncob-derived adsorbent
The corncob powder (5 g) was added to 40 mL distilled water with a certain amount of phosphoric acid (5 mL) in a 100 mL Telon-lined reactor, then sonicated for 1 h. The resulting mixture was heated to desired temperature for 10 h in the oven. After cooling, the sample was washed with distilled water, and then obtained by drying at 85 °C over night.
Characterization techniques
The microstructure and functional groups of the samples was studied by the X-ray diffraction (XRD), Brunauer–Emmet–Teller (BET) and infrared spectroscopy (IR). Analyses of the morphology of the sample were conducted by a scanning electron microscope (SEM), Hitachi S-4800. The MB concentration was analyzed by using a UV–Vis spectrophotometer.
Adsorption experiments
All adsorption experiments were carried out in 50 mL polypropylene (PP) centrifuge tubes. With a procedure, the effect of initial pH, contact time and initial MB concentrations on the adsorption of MB were examined in a series of experiments. Analytical simples were taken from the mixture solution at predetermined time intervals during the adsorption. The content of MB in the PP centrifuge tubes was determined by means of UV spectrophotometer.
For tests on the adsorption isotherm, the adsorbent concentration of 1 g/L and the MB concentration varied from 5 to 300 mg/L under constant shaking on a rotary shaker at room temperature for 8 h.
Desorption studies
In the experiments, adding a certain amount of the MB-adsorbed CA powder to 50 mL of 0.1 M HCl solution and stirring continuously for 120 min at ambient temperature, and then the MB-desorbed CA powders were filtered from the solution. After regeneration, the regenerated adsorbents were recycled 4 times in the recycle adsorption study. The experiments were performed at the same initial conditions.
Results and discussion
Characterization of adsorbent
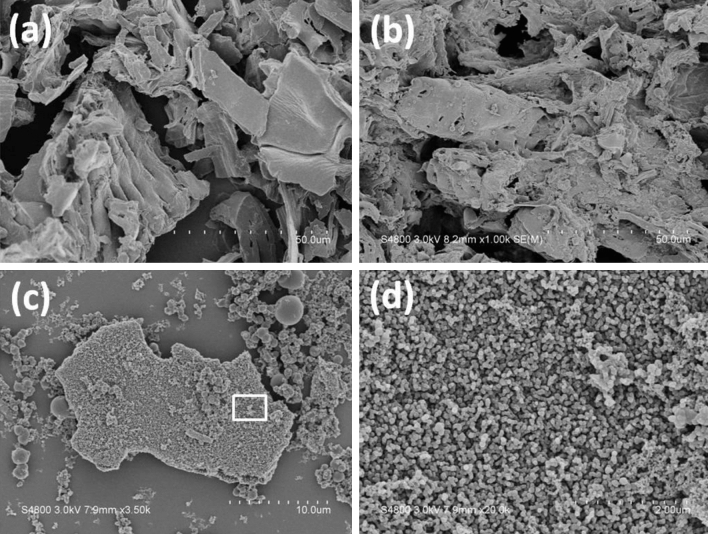
In order to highlight the effect of H3PO4 in HTC process, the carbonized corncob prepared without adding H3PO4 was employed as a reference. The SEM images showed that the surface of corncob relative smooth with some flakes (Fig. 1a,b).
Figure 1.
SEM images of corncob powder (a) before and (b) after hydrothermal carbonization at 160 °C for 10 h without adding H3PO4, (c) after hydrothermal carbonization at 160 °C for 10 h adding H3PO4 and the enlarged view of the black frame of c (d).
After hydrothermal reaction in the presence of phosphoric acid, the SEM images showed that the formation of a great deal of particles and aggregated dispersion on the surface (Fig. 1c). Detailed information on the structure was further obtained by enlarging SEM images. Figure 1d illustrated that the resulting particles are regularly distributed. These results revealed that adding H3PO4 could successfully increase the surface area, resulting in the increase of pore volume. Nitrogen adsorption/desorption isotherms in Fig. 2 were used to analyze structurally pores of the carbon samples. CA showed the surface area of 480 m2/g.
Figure 2.
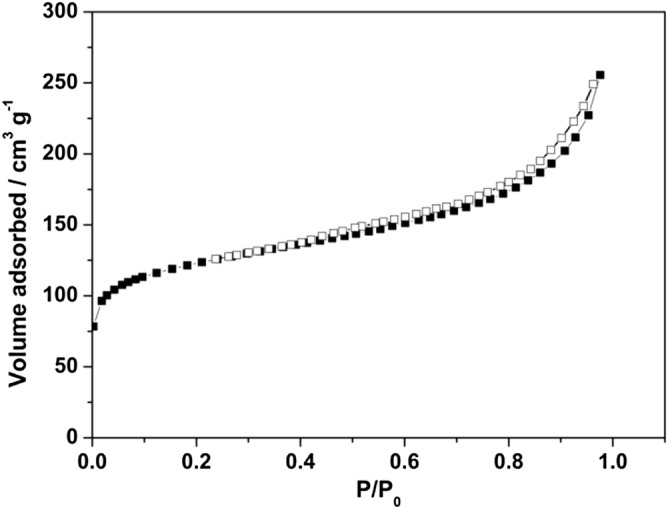
Nitrogen adsorption–desorption isotherms of carbonaceous adsorbent (CA).
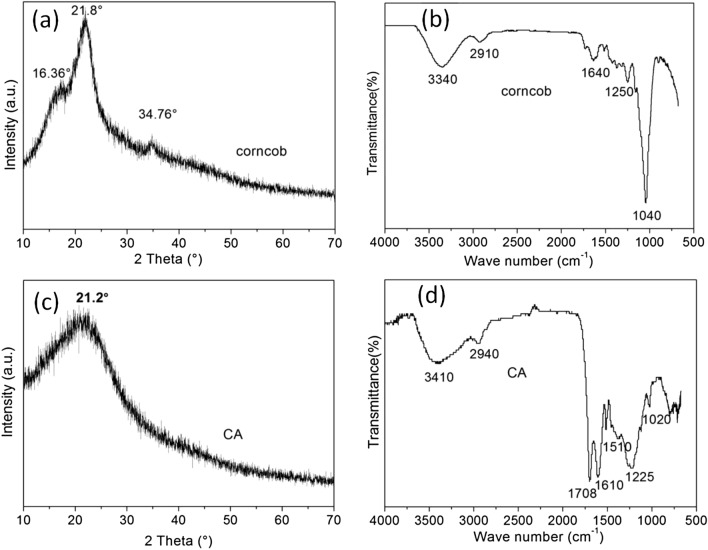
The phases of the raw material and hydrothermal carbonized samples were observed by the XRD patterns. In comparison with the raw corncob, the characteristic peaks of the prepared CA became more obvious, indicating that single HTC process only depleted impurities but without destroying the “core’’ structure of corncob. As shown in Fig. 3c, the XRD pattern of the prepared CA exhibited one broad diffraction peaks (2θ = 15–25°), indicating that the prepared CA existed in an amorphous carbon composed of aromatic carbon sheets form38. This result clearly suggests that the presence of phosphoric acid yields a carbonaceous structure composed of aromatic carbon sheets under HTC condition.
Figure 3.
XRD pattern of corncob powder (a) and CA (c), FTIR spectra of corncob powder (b) and CA (d).
The adsorption efficiency of CA depends on the number and chemical reactivity of surface functional groups39. The FT-IR technique was used to identify the characteristic functional groups. According to Fig. 3d, the FT-IR spectra of CA was obvious difference from that of the raw corncob, which imply that major spectral changes occurred in the presence of phosphoric acid after HTC process. The bands at 1,040 assigned to aromatic C–O have nearly disappeared, while C=C peak at 1,610 cm−1 and the C=O peak at 1708 cm−1 were enhanced. All of these observations were in agreement with the structural characteristics of standard HTC process40, indicating that the presence of phosphoric acid promoted the carbonization of lignocellulosic biomass through HTC process at low temperature (160 °C) and introduced carboxyl group into the structure of the raw corncob41. These oxygen-containing functional groups can play a significant role in enhancing CA adsorption efficiency by means of specific adsorption such as H-bonding and π–π interaction42.
Adsorption of MB
Effect of the initial pH
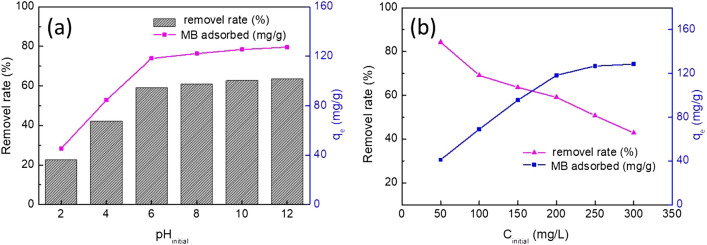
The pH is one of the most important parameter in the adsorption process, which regulates the adsorbents surface charge and influences the particles adsorption affinity in various aqueous solutions43. In order to evaluate the influence of pH on adsorption of MB, experiments were performed under the following condition: adsorbent dose 1.0 g/L, initial MB concentration 200 mg/L, pH (from 2 to 12), reaction temperature 20 ± 1 ℃ and contact time 120 min. The removal ratio of MB increased with an increase of pH from 2 to 7 and thereafter slightly increased with increasing pH range from 7 to 12, and the maximum removal was observed at pH 7 (Fig. 4a). At very low pH, lower adsorption efficiency was recorded due to the H+ competed with MB molecules for the negative active sites on the adsorbent surface. Thus cationic dye (MB) cannot move toward the positively charge surface area of adsorbent as a result of electrostatic repulsive force and hence a lower removal was observed at low pH values44. At high pH, a significant enhancement in adsorption was observed owing to electrostatic attraction increased between the adsorbent and cationic dye (MB)26,45. Therefore, all further adsorption experiments were performed at pH values of 7.
Figure 4.
Effect of (a) pH and initial concentrations (b) for MB adsorbed on CA.
Effect of the initial MB concentrations
Adsorption behavior of cationic dye onto the adsorbent at various MB concentrations of 50–300 mg/L was also investigated as shown in Fig. 4b. The figure showed that the amount of MB adsorption adsorbed increased with increasing in the initial MB concentration, but the removal efficiency decreased from 81.28 to 42.81%. The percentage of adsorption decreased may be explained that sorption sites on the surface area of adsorbent were insufficient to meet much more MB molecules available in aqueous solution18,46.
Effect of the contact time
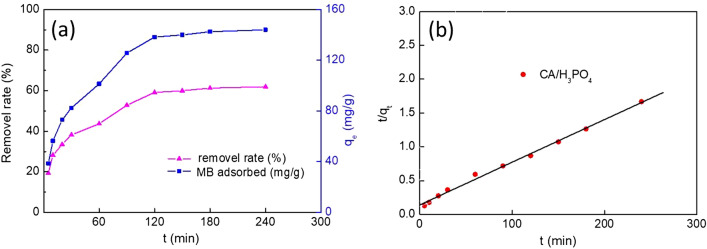
To evaluate the effect of contact time on the adsorption of MB molecules, the uptake capacity of the adsorbent with contact time was tested in Fig. 5a. It can be observed that removal efficiency was characterized by a rapid sorption phase followed by a slow phase as the contact time increases from 0 to 240 min. The results revealed that an initial very fast step where > 80% of MB molecules were adsorbed within the first 120 min and a slow phase where equilibrium was attained within the later 120 min, which might be attribute to that the availability of a large number of active sites on the surface of adsorbent at the initial stage were provided in aqueous solutions2,47.
Figure 5.
Effect of contact time on MB adsorbed on CA (a), and the pseudo second-order kinetic plots of MB adsorption on CA (b).
Adsorption isotherm
The adsorption isotherm studies are basic requirements for optimization of the adsorption systems, describing the relationship between MB molecules adsorbed onto the adsorbent and MB molecules in aqueous solution48. In current study, two types of well-known adsorption isotherms models Langmuir and Freundlich were fitted to analyze the adsorption equilibrium data16.
Langmuir isotherm model, which is valid for a monolayer adsorption with a homogeneous distribution of the adsorption sites and sorption energies, is mathematically given as follows49:
| 1 |
where the values of qe (mg/g) and ce (mg/L) represent the sorption capacity at equilibrium and the equilibrium concentration, respectively; KL (L/mg) is the Langmuir isotherm equilibrium constant; qm (mg/g) is the maximum sorption capacity.
Freundlich isotherm model is derived by assuming that the MB molecules can be applied for multilayer sorption. It is used to describe heterogeneous systems by the following equation50:
| 2 |
where the values of qe (mg/g) and ce (mg/L) represent the sorption capacity at equilibrium and the equilibrium concentration, respectively; Kf (mg/g) is the Freundlich isotherm constant; indicating the adsorption capacity related to bond strength; 1/n is also Freundlich isotherm constant that stand for adsorption intensity.
The adsorption isotherms of MB molecules onto the adsorbent were shown in Fig. 6. The Langmuir and Freundlich isotherms parameters of corncob and CA were given in Table 1. The results show that the value of R2 for Langmuir isotherm model (0.993 for CA) was higher than that of Freundlich isotherm model (0.938 for CA), indicating that Langmuir isotherm model was better fitted to describe the adsorption of MB molecules onto CA. In addition, the calculated maximum sorption capacity of MB by Langmuir model was 140.25 mg/g in Fig. 6a, which was quite close to their corresponding experimental data (128.43 mg/g). These results suggest that the monolayer adsorption occur on the surface of the CA for cationic dye31.
Figure 6.
Adsorption isotherms for MB on CA (a), and the Langmuir isotherm plots for adsorption of MB on CA (b).
Table 1.
Isotherms parameters of MB on CA.
| Isothermal models | Parameters | CA |
|---|---|---|
| Langmuir | KL (L/mg) | 0.5795 |
| qm (mg/g) | 140.25 | |
| R2 | 0.993 | |
| Freundlich | KF (mg/g (1/mg)1/n) | 87.102 |
| n | 5.1524 | |
| R2 | 0.938 |
Adsorption kinetics
Adsorption kinetic study is important to provide the vital information about the mechanism of adsorption process and the reaction pathways in the treatment of waste water. Thus, to investigate the adsorption kinetics, three types of well-known kinetic models were studied: pseudo-first-order kinetic model, pseudo-second-order kinetic model and intra-particle diffusion model. The three models were expressed as follows51:
| 3 |
| 4 |
| 5 |
where the values of qe (mg/g) and qt (mg/g) are the amounts of MB absorbed at equilibrium and at time t (min); k1 (1/min) and k2 (g/(mg min)) are the equilibrium rate constant of pseudo-first-order adsorption and the equilibrium rate constant of pseudo-second-order adsorption, respectively. ki (mg/(g min1/2)) is the intra-particle diffusion rate constant; C (mg/g) is a constant.
Three types of kinetic models for MB molecules onto adsorbent were investigated. The calculated relevant parameters of the three kinetic equations for the adsorption of MB molecules onto adsorbent were shown in Table 2. Comparison of the correlation coefficient (R2) values for three models suggested that the pseudo-second-order model was better fitted to describe the adsorption process due to its highest values. However, the value of the correlation coefficient (R2 < 0.98) of intra-particle diffusion model demonstrated that this model was not feasible to describe the adsorption process owing to a weak correlation. Moreover, Fig. 4b showed that the experimental data fitted the theoretic pseudo-second-order model simulated curves fairly well. These results indicated that chemical adsorption might be the rate-limiting step that controls the adsorption MB onto adsorbent, which were consistent with the previous studies reported in literatures52.
Table 2.
Kinetic constants of MB on CA.
| Kinetic models | Parameters | CA |
|---|---|---|
| Pseudo-first-kinetic models | qe (mg/g) | 103.94 |
| K1 (1/min) | 0.02559 | |
| R2 | 0.989 | |
| Pseudo-second-kinetic models | K2 (g/(mg min) | 0.0002206 |
| h (mg/(g min)) | 40.44 | |
| R2 | 0.996 | |
| Intra-particle diffusion equation | K3 (mg g−1 min−1/2) | 5.9978 |
| C | 24.2520 | |
| R2 | 0.966 |
Comparison with other adsorbents
Comparisons of the maximum adsorption capacity (Qmax value) of CA with other biomass-derived adsorbents are presented in Table 325,53–58. The CA shows the comparable adsorption capacity for MB compared to other biomass-derived adsorbents. And given its adsorption capacity and preparation at low temperature, CA was promising for MB removal from the waste water.
Table 3.
Comparison of adsorption capacities of other biomass-derived adsorbents for removal of MB.
| Adsorbent | Qm (mg/g) | Preparing temperature (°C) | Reference |
|---|---|---|---|
| Phosphoric acid treated corncob | 140.25 | 160 | This study |
| Acetic acid treated rice bran | 25.1 | 90 | 25 |
| Waste apricot-based activated carbon | 102.04 | 500 | 53 |
| Sulfuric acid treated Parthenium | 88.29 | 300 | 54 |
| Zinc chloride treated Stipa tenacissima | 178.44 | 600 | 55 |
| Sodium hydroxide treated corn stalk | 49.01 | 75 | 56 |
| Tartaric acid treated bagasse | 57.14 | 120 | 57 |
| Surfactant-modified pineapple leaf powder | 52.6 | 90 | 58 |
Desorption studies
The reusability of the adsorbent is also very important for its potential commercial application and economic feasibility in wastewater treatment. The tests of MB desorption were carried out with four times repeated batch adsorption–desorption cycles as shown in Fig. 7. It can be observed that CA still retained satisfactory adsorption efficiency of MB molecules even after four cycles of reuses, indicating the good reusability of CA.
Figure 7.
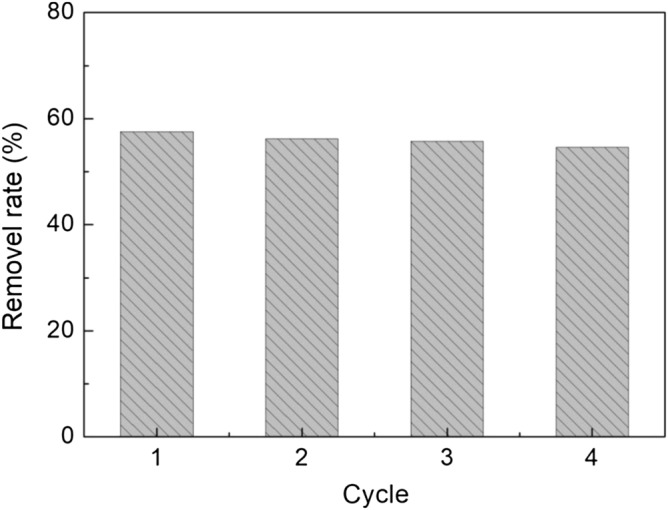
The removal rate of MB was employed to evaluate their operational stability.
Conclusions
In this work, an effective modification method based corncob with phosphoric acid through HTC at low temperature conditions is described. As we know, this is the first report of the synthesis of carbonaceous adsorbent from lignocellulosic biomass by using a low-temperature hydrothermal method. A large number of oxygen-containing groups and pore channels were introduced, resulting in a significantly enhance in the as-obtained adsorbent adsorption capability. The synthesized carbonaceous adsorbent may be used for organic pollutants removal from water treatment at large scale economically.
Acknowledgements
The authors gratefully acknowledge the financial support by the National Natural Science Foundation of China (Grant Nos. 21673002, 31371285), Provincial university student innovation project (Grant No. XJDC2019235) and National University Students Innovation Project of China (Grant No. 201510364011).
Author contributions
Y.W. and P.C. designed experiments; Y.W., Y.Z. and G.J. carried out experiments; Y.W. and P.C. analyzed experimental results. Y.W. and P.C. wrote the manuscript. P.C. and Z.C. supervised the project.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peirong Chen, Email: chenpeirong@ahau.edu.cn.
Zhen Chen, Email: zchen@ahau.edu.cn.
References
- 1.Spagnoli AA, Giannakoudakis DA, Bashkova S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017;229:465–471. [Google Scholar]
- 2.Ali I, Alothman ZA, Alharbi OML. Supra molecular mechanism of the removal of 17-β-estradiol endocrine disturbing pollutant from water on functionalized iron nano particles. J. Mol. Liq. 2017;441:123–129. [Google Scholar]
- 3.Ali I, et al. Artificial neural network modelling of amido black dye sorption on iron composite nano material: Kinetics and thermodynamics studies. J. Mol. Liq. 2018;250:1–8. [Google Scholar]
- 4.Ali I. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018;261:583–593. [Google Scholar]
- 5.Alharbi OML, et al. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 2018;263:442–453. [Google Scholar]
- 6.Burakova EA, et al. Novel and economic method of carbon nanotubes synthesis on a nickel magnesium oxide catalyst using microwave radiation. J. Mol. Liq. 2018;253:340–346. [Google Scholar]
- 7.Basheer AA, Ali I. Stereoselective uptake and degradation of (±)-o, p-DDD pesticide stereomers in water sediment system. Chirality. 2018;30:1088–1095. doi: 10.1002/chir.22989. [DOI] [PubMed] [Google Scholar]
- 8.Basheer AA. Chemical chiral pollution: Impact on the society and science and need of the regulations in the 21st century. Chirality. 2018;30:402–406. doi: 10.1002/chir.22808. [DOI] [PubMed] [Google Scholar]
- 9.Popa N, Visa M. The synthesis, activation and characterization of charcoal powder for the removal of methylene blue and cadmium from wastewater. Adv. Powder Technol. 2017;28:1866–1876. [Google Scholar]
- 10.Ali I. Microwave assisted economic synthesis of multi walled carbon nanotubes for arsenic species removal in water, batch and column operations. J. Mol. Liq. 2018;271:677–685. [Google Scholar]
- 11.Ali I, et al. Water treatment by new generation graphene materials. Hope for Bright Future. Environ. Sci. Pollut. Res. 2018;25:7315–7329. doi: 10.1007/s11356-018-1315-9. [DOI] [PubMed] [Google Scholar]
- 12.Ali I, et al. Modeling of fenuron pesticide adsorption on CNTs for mechanistic insight and removal in water. Environ. Res. 2019;170:389–397. doi: 10.1016/j.envres.2018.12.066. [DOI] [PubMed] [Google Scholar]
- 13.Ali I, et al. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019;127:160–180. doi: 10.1016/j.envint.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Ali I, et al. Water treatment by new-generation graphene materials: Hope for bright future. Environ. Sci. Pollut. Res. 2018;25:7315–7329. doi: 10.1007/s11356-018-1315-9. [DOI] [PubMed] [Google Scholar]
- 15.Ali I, et al. Facile and eco-friendly synthesis of functionalized iron nanoparticles for cyanazine removal in water. Colloids Surf. B. 2018;171:606–613. doi: 10.1016/j.colsurfb.2018.07.071. [DOI] [PubMed] [Google Scholar]
- 16.Shakoor S, Nasar A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. E. 2016;66:154–163. [Google Scholar]
- 17.Chen L, et al. Removal of methylene blue from water by cellulose/grapheme oxide fibres. J. Exp. Nanosci. 2016;11:1156–1170. [Google Scholar]
- 18.Tamilselvi S, Asaithambi M, Sivakumar V. An eco-friendly non-conventional adsorbent from silk cotton fiber for the removal of methylene blue dye, Indian. J. Chem. Technol. 2016;23:497–505. [Google Scholar]
- 19.Ali I, et al. Preparation of a carboxymethylcellulose-iron composite for uptake of atorvastatin in water. Int. J. Biol. Macromol. 2019;132:244–253. doi: 10.1016/j.ijbiomac.2019.03.211. [DOI] [PubMed] [Google Scholar]
- 20.Al-Shaalan NH, et al. High performance removal and simulation studies of diuron pesticide in water on MWCNTs. J. Mol. Liq. 2019;289:111039. [Google Scholar]
- 21.Ali I, et al. Removal of copper(II) and zinc(II) ions in water on a newly synthesized polyhydroquinone/graphene nanocomposite material: Kinetics, thermodynamics and mechanism. ChemistrySelect. 2019;4:12708–12718. [Google Scholar]
- 22.Kallel F, et al. Sorption and desorption characteristics for the removal of a toxic dye, methylene blue from aqueous solution by a low cost agricultural by-product. J. Mol. Liq. 2016;219:279–288. [Google Scholar]
- 23.Bharathi KS, Ramesh ST. Removal of dyes using agricultural waste as low-cost adsorbents: A review. Appl. Water Sci. 2013;3:773–790. [Google Scholar]
- 24.Oyelude EO, Owusu UR. Adsorption of methylene blue from aqueous solution using acid modified Calotropis procera leaf powder. J. Appl. Sci. Environ. Sanitation. 2011;6:477–484. [Google Scholar]
- 25.Yao S, Lai H, Shi Z. Adsorption of methylene blue from aqueous solution using acetic acid modified rice bran. J. Indian Chem. Soc. 2013;90:629–635. [Google Scholar]
- 26.Liu X, et al. Characterization of corncob-derived biochar and pyrolysis kinetics in comparison with corn stalk and sawdust. Bioresour. Technol. 2014;170:76–82. doi: 10.1016/j.biortech.2014.07.077. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, et al. Novel synthesis of a versatile magnetic adsorbent derived from corncob for dye removal. Bioresour. Technol. 2017;190:13–20. doi: 10.1016/j.biortech.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Huang YX, Keller AA. Magnetic nanoparticle adsorbents for emerging organic contaminants. ACS Sustain. Chem. Eng. 2013;1:731–736. [Google Scholar]
- 29.Mohan D, et al. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent-a critical review. Bioresour. Technol. 2014;160:191–202. doi: 10.1016/j.biortech.2014.01.120. [DOI] [PubMed] [Google Scholar]
- 30.Rajapaksha AU, et al. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modifica-tion. Chemosphere. 2016;148:276–291. doi: 10.1016/j.chemosphere.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 31.Cho HH, et al. Sorption of aqueous Zn[II] and Cd[II] by multiwall carbon nanotubes: The relative roles of oxygen-containing functional groups and graphenic carbon. Langmuir. 2010;26:967–981. doi: 10.1021/la902440u. [DOI] [PubMed] [Google Scholar]
- 32.Uchimiya M, Chang S, Klasson KT. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard Mater. 2011;190:432–441. doi: 10.1016/j.jhazmat.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 33.Fang Q, et al. Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ. Sci. Technol. 2014;48:279–288. doi: 10.1021/es403711y. [DOI] [PubMed] [Google Scholar]
- 34.Abdel Hakim AA, Nassar M, Emam A. Preparation and characterization of rigid polyurethane foam prepared from sugar-cane bagasse polyol. Mater. Chem. Phys. 2011;129:301–307. [Google Scholar]
- 35.Ge ML, et al. A maleic anhydride grafted sugarcane bagasse adsorbent and its performance on the removal of methylene blue from related wastewater. Mater. Chem. Phys. 2017;192:147–155. [Google Scholar]
- 36.Xu Y, et al. Enhanced adsorption of methylene blue by citric acid modification of biochar derived from water hyacinth (Eichornia crassipes) Environ. Sci. Pollut. Res. Int. 2016;23:23606–23618. doi: 10.1007/s11356-016-7572-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhen M, et al. Decontamination of methylene blue from aqueous solution by rhamnolipid-modified biochar. BioResources. 2018;13:3061–3081. [Google Scholar]
- 38.Liu WJ, et al. Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci. Rep. 2013;3:2419–2424. doi: 10.1038/srep02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren CR, et al. Highly efficient adsorption of heavy metals onto novel magnetic porous composites modified with amino groups. J. Chem. Eng. Data. 2017;62:1865–1875. [Google Scholar]
- 40.Fechler N, et al. Salt and sugar: Direct synthesis of high surface area carbon materials at low temperatures via hydrothermal carbonization of glucose under hypersaline conditions. J. Math. Chem. A. 2013;1:9418–9421. [Google Scholar]
- 41.Calucci L, Rasse DP, Forte C. Solid-state nuclear magnetic resonance characterization of chars obtained from hydrothermal carbonization of corncob and miscanthus. Energy Fuels. 2013;27:303–309. [Google Scholar]
- 42.Ma HJ. Hydrothermal preparation and characterization of novel corncob-derived solid acid catalysts. J. Agric. Food Chem. 2014;62:5345–5353. doi: 10.1021/jf500490m. [DOI] [PubMed] [Google Scholar]
- 43.Foo KY, Hameed BH. Potential of jackfruit peel as precursor for activated carbon prepared by microwave induced NaOH activation. Bioresour. Technol. 2012;112:143–150. doi: 10.1016/j.biortech.2012.01.178. [DOI] [PubMed] [Google Scholar]
- 44.Zhou XP, Xie XL. Study on PP/PMMA grafted sisal fiber composited(II) effect of surface treatment on the structure and properties of sisal fiber-reinforced polypropylene composites. J. Polym. Mater. Sci. Eng. 2004;20:138–141. [Google Scholar]
- 45.Bhatti HN, Akhtar N, Saleem N. Adsorptive removal of methylene blue by low-cost citrus Sinensis bagasse: Equilibrium, kinetic and thermodynamic characterization. Arab. J. Sci. Eng. 2012;37:9–18. [Google Scholar]
- 46.Wang YX, et al. Cellulose-based porous adsorbents with high capacity for methylene blue adsorption from aqueous solutions. Fiber Polym. 2017;18:891–899. [Google Scholar]
- 47.Sharmeen Afroze S, et al. Adsorption performance of continuous fixed bed column for the removal of methylene blue (MB) dye using Eucalyptus sheathiana bark biomass. Res. Chem. Intermed. 2016;42:2343–2364. [Google Scholar]
- 48.Kamaru AA, et al. Raw and surfactant-modified pineapple leaf as adsorbent for removal of methylene blue and methyl orange from aqueous solution. Desalin. Water Treat. 2016;57:18836–18850. [Google Scholar]
- 49.Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918;40:1361. [Google Scholar]
- 50.Freundlich HMF. Over the adsorption in solution. J. Phys. Chem. 1906;57:385. [Google Scholar]
- 51.Kumar R, Sharma RK, Singh AP. Cellulose based grafted biosorbents—Journey from lignocellulose biomass to toxic metal ions sorption applications—A review. J. Mol. Liq. 2017;232:62–93. [Google Scholar]
- 52.Hameed BH, Krishni RR, Sata SA. A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. J. Hazard Mater. 2009;162:305–311. doi: 10.1016/j.jhazmat.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 53.Basar CA. Applicability of the various adsorption models of three dyes adsorption onto activated carbon prepared waste apricot. J. Hazard Mater. 2006;135:232–241. doi: 10.1016/j.jhazmat.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 54.Lata H, Grag VK, Gupta RK. Removal of a basic dye from aqueous solution by adsorption using Parthenium hysterophorus: An agricultural waste. Dyes Pigm. 2007;74:653–658. [Google Scholar]
- 55.Bouguettouchaa AD, et al. Novel activated carbon prepared from an agricultural waste, Stipa tenacissima, based on ZnCl2 activation-characterization and application to the removal of methylene blue. Desalin. Water Treat. 2016;57:24056–24069. [Google Scholar]
- 56.Ma DZ, et al. Fabrication of the novel hydrogel based on waste corn stalk for removal of methylene blue dye from aqueous solution. Appl. Surf. Sci. 2017;422:944–952. [Google Scholar]
- 57.Low LW, et al. Studies on the adsorption omethylene blue dye from aqueous solution onto low-cost tartaric acid treated Bagasse. Apcbee Procedia. 2012;1:103–109. [Google Scholar]
- 58.Kamaru AA, et al. Raw and surfactant-modified pineapple leaf as adsorbent for removal of methylene blue and methyl orange from aqueous solution. Desalin. Water Treat. 2016;59:18836–18850. [Google Scholar]