Abstract
Helicobacter pylori is presumed to infect gastric tissue via the oral cavity in childhood, whereas risk factors for H. pylori infection in the oral cavity are unknown. In this study, we analysed the effects of Streptococcus mutans, a major cariogenic bacterial species, on H. pylori colonisation in the oral cavity, as well as gastric tissue. Rats in the weaning period were infected with S. mutans in the oral cavity, then fed a caries-inducing diet to facilitate S. mutans colonisation. One month after S. mutans infection, rats were infected with H. pylori in the oral cavity; rats were then euthanised at 1 month after H. pylori infection. H. pylori was detected in the oral cavities of rats infected with both S. mutans and H. pylori, but not in rats infected with H. pylori alone. In addition, H. pylori colonisation in the gastric tissue and typical gastrointestinal damage were observed in rats infected with both S. mutans and H. pylori. When H. pylori was co-cultured with in vitro biofilm formed by S. mutans, a large number of H. pylori bacteria invaded the biofilm formed by S. mutans. Our results suggest that S. mutans is involved in the establishment of H. pylori infection.
Subject terms: Microbiology, Bacteria, Bacterial pathogenesis
Introduction
Helicobacter pylori, a helix-shaped gram-negative microaerophilic bacterium, is a major causative agent of gastric cancer and gastric ulcers1. More than half of the world’s population is infected with H. pylori2, which is presumably acquired mainly via the oral cavity in childhood3,4. Molecular biological techniques have reportedly revealed H. pylori in oral specimens5–7. The presence of H. pylori in the oral cavity has been related to the detection of H. pylori in the gastric tissue8. However, details regarding risk factors of H. pylori infection in the oral cavity have not been clarified, which may explain the current difficulty in elimination of H. pylori infection.
Streptococcus mutans, a gram-positive facultative anaerobe, is a major causative pathogen of dental caries9. S. mutans is acquired in the oral cavity during early childhood, mainly via mother-to-child transmission10. The aetiology of dental caries caused by S. mutans was clarified in the early 1960s11; S. mutans metabolises sucrose to form a biofilm on the tooth surface, followed by demineralisation of the tooth. Nevertheless, eradication of S. mutans from the oral cavity and dental caries remains difficult12.
Some epidemiological studies have revealed that patients with dental caries or poor oral hygiene were more likely to harbour H. pylori in oral cavity or gastric tissue13,14. These findings suggest that the presence of cariogenic bacteria is involved in infection of the oral cavity with H. pylori. To the best of our knowledge, no clear evidence has been obtained regarding the effects of S. mutans on H. pylori infection in an animal model. In the present study, we hypothesised that S. mutans colonisation in the oral cavity may be involved in H. pylori colonisation in both oral cavity and gastric tissue. Therefore, we constructed a rat co-infection model with S. mutans and H. pylori. Using this model, we analysed the effects of S. mutans on H. pylori colonisation in the oral cavity and gastric tissue.
Results
Dental caries status and detection of bacteria in the rat oral cavity
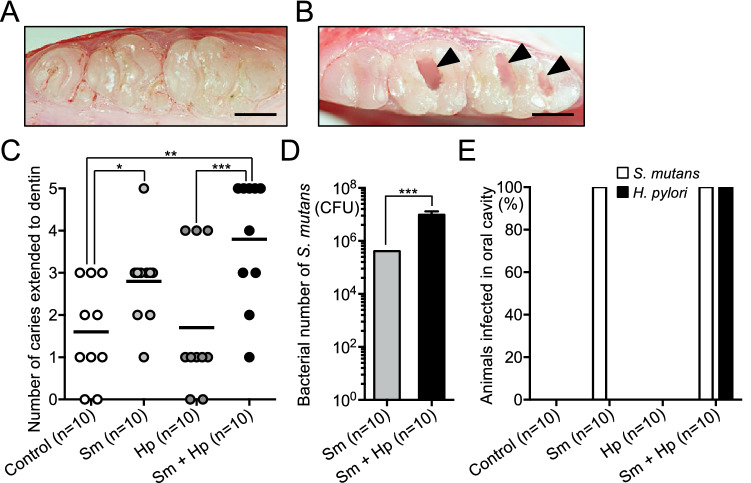
In our experimental procedure, rats were fed a caries-inducing diet containing 56% sucrose (CLEA Japan, Osaka, Japan) throughout the experiment to induce dental caries15; they were divided into four groups, depending on the presence or absence of infection with S. mutans and H. pylori (Fig. 1A,B). Rats were euthanised at 82 days of age and dental caries status was evaluated using excised maxillary and mandibular bones. Representative images of teeth from rats without and with dental caries are shown in Fig. 2A,B. Mean numbers of dental caries were significantly higher in rats that had been infected with S. mutans than in rats that had not been infected with S. mutans, regardless of H. pylori infection (P < 0.05) (Fig. 2C). The number of S. mutans isolated from the mandibular bone was significantly higher in rats infected with both S. mutans and H. pylori, compared with rats infected with S. mutans alone (P < 0.001) (Fig. 2D). Although the most severe dental caries were observed in rats that had been infected with both S. mutans and H. pylori, average number of dental caries did not significantly differ compared with rats that had been infected with S. mutans.
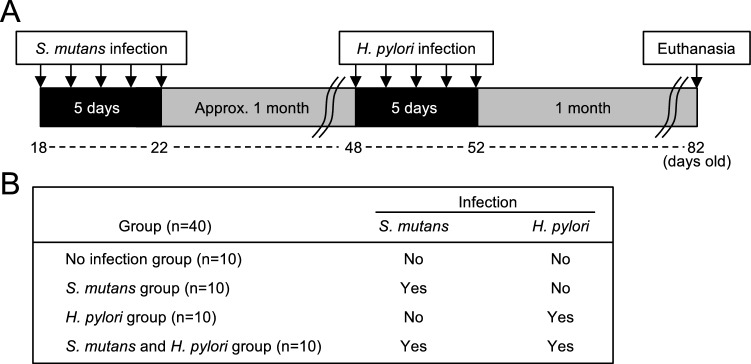
Figure 1.
Schematic of rat model experimental protocol. (A) Experimental schedule. (B) Groups of rats in this experiment.
Figure 2.
Dental caries status and detection of bacteria in the rat oral cavity. Representative images of the teeth of rats (A) without and (B) with dental caries. Arrowheads indicate dental caries. Bars = 500 μm. (C) Numbers of teeth with dental caries. Each closed circle represents the number of dental caries for a single rat. Horizontal bars indicate mean values for respective groups. (D) Numbers of S. mutans bacteria. (E) Detection rates of bacteria in the oral cavity. Significant differences were observed, using analysis of variance with Bonferroni correction (*P < 0.05, **P < 0.01 and ***P < 0.001). Sm, S. mutans; Hp, H. pylori.
Polymerase chain reaction (PCR) and nested PCR were performed to detect S. mutans and H. pylori, respectively, in dental plaque specimens collected from rats aged 55–82 days; PCR primers are shown in Table 1. S. mutans was detected in the oral cavities of all rats that had been infected with S. mutans (Fig. 2E), whereas no S. mutans was detected in rats that had not been infected with S. mutans. Notably, H. pylori was not detected in rats that had been infected with H. pylori alone; however, H. pylori was detected in all rats that had been infected with both S. mutans and H. pylori, which indicated that S. mutans was essential for H. pylori colonisation.
Table 1.
Polymerase chain reaction primers used in the present study.
| Specific primer set | Sequence (5′-3′) | Size (bp) | References |
|---|---|---|---|
| Detection of S. mutans | |||
| MKD-F | GGC ACC ACA ACA TTG GGA AGC TCA GTT | 433 | 16 |
| MKD-R | GGA ATG GCC GCT AAG TCA ACA GGA T | ||
| Detection of H. pylori | |||
| First step PCR | |||
| ureA-aF | ATG AAA CTC ACC CCA AAA GA | 488 | 17 |
| ureA-bR | CCG AAA GTT TTT TCT CTG TCA AAG TCT A | ||
| Second step PCR | |||
| ureA-bF | AAA CGC AAA GAA AAA GGC ATT AA | 383 | 17 |
| ureA-aR | TTC ACT TCA AAG AAA TGG AAG TGT GA | ||
Histopathological evaluation of rat gastric tissue
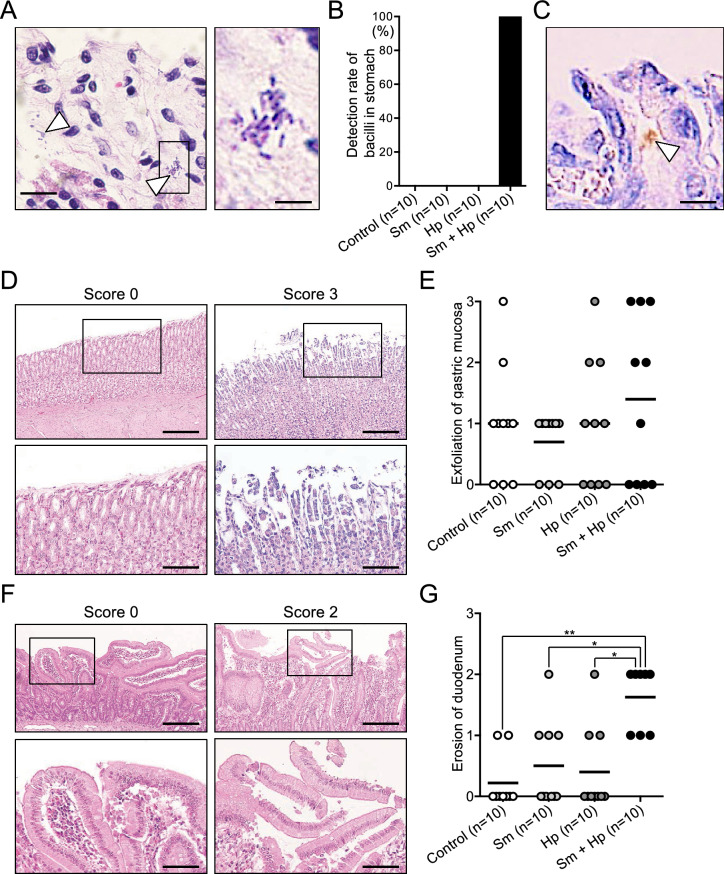
Helicobacter pylori infection in excised rat gastric tissues was analysed by histopathological evaluation. In all rats that had been infected with both S. mutans and H. pylori, invasion of bacilli into gastric tissue was confirmed by haematoxylin and eosin (HE) staining (Fig. 3A,B); immunostaining analysis confirmed that these bacilli were H. pylori (Fig. 3C). However, no bacilli were detected in other groups, including rats infected with H. pylori alone. Subsequently, qualitative analysis of HE-stained stomach and duodenum histopathological findings was performed. Representative images of gastric mucosal exfoliation are shown in Fig. 3D. The mean gastric mucosal exfoliation score was highest in rats infected with both S. mutans and H. pylori (Fig. 3E), although this score did not significantly differ from the scores of other groups. In addition, representative images of duodenal erosion are shown in Fig. 3F. The duodenal erosion score was significantly higher in rats infected with both S. mutans and H. pylori than in other groups (P < 0.05) (Fig. 3G). The scores of other histopathological findings did not significantly differ among the groups (see Supplementary Figure 1 online).
Figure 3.
Bacterial detection and histopathological evaluation in gastric tissue. (A) Representative images of histopathological features observed in HE-stained tissue. Right panel shows high-magnification image of the box on the left image. Arrowheads indicate bacilli. Bars = 50 μm (left panel) and 10 μm (right panel). (B) Detection rates of bacilli in gastric tissue. (C) Representative image of immunohistochemical staining findings, using an H. pylori-specific antibody. Arrowhead indicates positive H. pylori-specific antibody staining. Bar = 10 μm. (D) Representative images of gastric mucosal exfoliation with different histopathological scores, as determined by HE staining. Lower panels show high-magnification images of the boxes on upper images. Bars = 300 μm (upper panels) and 100 μm (lower panels). (E) Histopathological scores of gastric mucosal exfoliation. Each closed circle represents the gastric mucosal exfoliation score for a single rat. Horizontal bars indicate mean values for respective groups. (F) Representative images of duodenal erosion with different histopathological scores, as determined by HE staining. Lower panels show high-magnification images of the boxes on upper images. Bars = 300 μm (upper panels) and 100 μm (lower panels). (G) Histopathological scores of duodenal erosion. Each closed circle represents the duodenal erosion score for a single rat. Horizontal bars indicate mean values for respective groups. Significant differences were observed, using analysis of variance with Bonferroni correction (*P < 0.05 and **P < 0.01). Sm, S. mutans; Hp, H. pylori.
In vitro bacterial growth and biofilm assays with co-cultured S. mutans and H. pylori
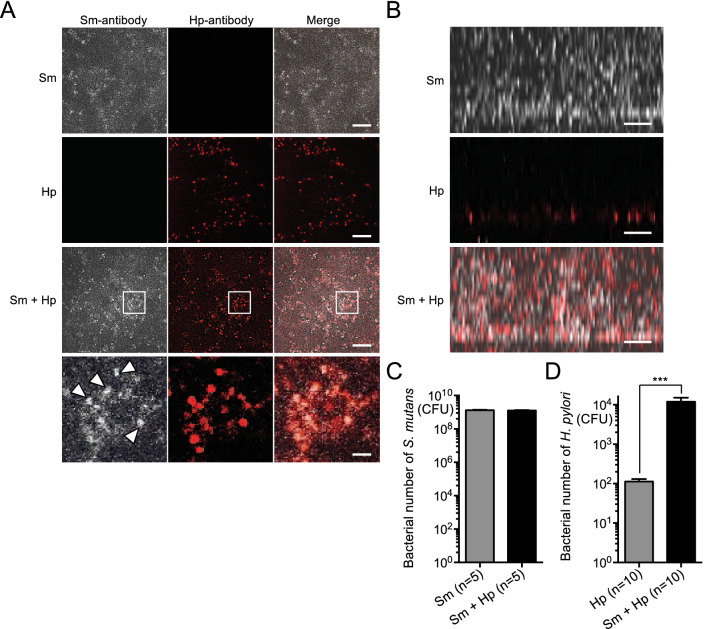
In vitro assays were performed to analyse the colonisation of H. pylori in the presence of S. mutans. Notably, the presence of the culture supernatant of S. mutans did not affect the growth of H. pylori (Supplementary Figure 2). A subsequent in vitro biofilm assay was performed using both S. mutans and H. pylori. S. mutans is known to form a biofilm with high adhesiveness in the presence of sucrose, and in vitro experimental systems for biofilm formation are widely used18–20. In our biofilm system, S. mutans is grown in a medium supplemented with sucrose on a cover glass or polystyrene plate, which are regarded as simulated tooth surfaces, and incubated at 37 °C for 18 h. To confirm that the presence of S. mutans is required for H. pylori colonisation in the oral cavity, S. mutans and H. pylori were co-cultured using the in vitro biofilm assay. H. pylori was observed to form flat layers in two-dimensional images, regardless of the presence of S. mutans (Fig. 4A). Notably, H. pylori was especially localised in dense areas of S. mutans growth. In addition, three-dimensional imaging revealed that the location of H. pylori in the biofilm was dependent upon the presence or absence of S. mutans (Fig. 4B, Supplementary Figure 3). When cultured without S. mutans, H. pylori was found adhered to the surface of the plate in a single monolayer. In contrast, when S. mutans and H. pylori were co-cultured, H. pylori was found distributed throughout the biofilm formed by S. mutans. There was no difference in the number of S. mutans between cultures of S. mutans alone and cultures containing both S. mutans and H. pylori (Fig. 4C). In contrast, the number of H. pylori was significantly higher in cultures containing both S. mutans and H. pylori (1.2 × 104 colony-forming units [CFUs]) than in cultures of H. pylori alone (1.1 × 102 CFUs) (P < 0.001) (Fig. 4D). In cultures containing both S. mutans and H. pylori, 1 CFU of H. pylori was present for approximately 1 × 105 CFUs of S. mutans.
Figure 4.
Biofilm formation by co-cultured S. mutans and H. pylori. (A) Representative two-dimensional images of biofilm, captured using confocal scanning laser microscopy. Bars = 15 μm. Lower panels of co-cultured S. mutans and H. pylori show high magnification images of square parts of upper panel. Arrowheads indicate dense growth of S. mutans. Bars = 300 nm. (B) Representative enlarged images of biofilm thickness, captured using confocal scanning laser microscopy. S. mutans and H. pylori cells are stained white and red, respectively. Bars = 10 μm. (C) Numbers of S. mutans bacteria. (D) Numbers of H. pylori bacteria. Significant differences were observed, using analysis of variance with Bonferroni correction (***P < 0.001). Sm, S. mutans; Hp, H. pylori.
Discussion
H. pylori is presumably transmitted through the oral cavity in childhood21, then resides in the human body unless eradication therapy is administered. Although several epidemiological studies have shown that H. pylori colonisation in the oral cavity is associated with its presence in gastric tissue22–24, this relationship remains controversial. In the present study, the presence of H. pylori in rat oral cavity was positively correlated with its presence in gastric tissue. In addition, the results showed that cariogenic bacteria are involved in H. pylori colonisation in these organs.
Epidemiological studies have shown that individuals with dental caries are more susceptible to H. pylori infection13,14. In contrast, a clear relationship between dental caries and H. pylori colonisation has not been demonstrated in an animal model, because only a few research groups can successfully colonise cariogenic bacteria in the oral cavity of animal models. In humans, S. mutans mainly infects infants between 19 and 31 months of age10. Similarly, it has been shown that administration of S. mutans to 18-day-old rats can establish S. mutans colonisation in the oral cavity15; moreover, a dental caries model can be induced by feeding a caries-inducing diet containing 56% sucrose for 1–2 months. Using a rat model, we orally administered H. pylori and achieved successful H. pylori colonisation.
There was no significant difference in the severity of dental caries between rats infected with S. mutans and those infected with H. pylori. In contrast, previous epidemiological studies showed that patients with H. pylori in dental plaque demonstrated a significantly higher occurrence of dental caries, compared with patients who did not exhibit H. pylori in dental plaque13,25; those results suggested that H. pylori is involved in dental caries progression. In addition, the present study evaluated the dental caries status of rats infected with S. mutans at the age of 82 days. We previously analysed the occurrence of dental caries in rats in a time-dependent manner26, which revealed that most rats infected with S. mutans had mild dental caries localised to hard tissues, despite an age greater than 90 days. This occurrence of mild dental caries may have resulted in the absence of significant differences in the numbers of dental caries between S. mutans- and H. pylori-infected groups. To more comprehensively compare the severity of dental caries between rats with S. mutans infection and rats with H. pylori infection, rats of different ages should be included in future analyses of dental caries progression. In addition, X-ray photographs of the teeth may be needed for more accurate diagnoses, whereas we evaluated the presence or absence of dental caries using a sterilised 27G needle under a stereomicroscope.
In the present study, most severe dental caries were observed in rats that had been infected with both S. mutans and H. pylori. A recent in vitro study revealed that H. pylori could change the balance of oral biofilm induced by oral streptococci27; the authors of that study suggested that H. pylori may affect signalling among oral streptococci involved in biofilm formation, and that H. pylori could create an advantageous environment for S. mutans. Indeed, higher numbers of S. mutans were isolated from excised mandibular bones of rats infected with both S. mutans and H. pylori, compared with mandibular bone from rats infected with S. mutans alone. Therefore, interactions between H. pylori and oral bacteria may affect both colonisation by H. pylori and the pathogenicity of oral bacteria. Although the main purpose of the present study was to examine the effects of S. mutans on H. pylori colonisation in oral and gastric tissue, the findings regarding effects of H. pylori on cariogenic properties of S. mutans provide important insights for future research.
In the present study, dental caries were induced in some rats which were not infected with any bacteria. This result is consistent with the findings of a previous study, in which mild dental caries occurred in some rats which were not infected with caries-causing bacteria26. In addition, oral bacteria associated with severe dental caries were detected in periapical lesions of uninfected rats which had undergone artificial exposure of dental pulp via endodontic instrumentation28. Therefore, commensal bacteria in the rat oral cavity of rats may also cause mild dental caries.
Recently, inflamed dental pulp obtained from human patients has been considered a possible source of H. pylori infection7,17. Therefore, we analysed the presence of H. pylori in pulp tissue collected by using a sterilised dental handpiece and diamond point. Our results revealed that H. pylori was not present in the dental pulp, probably because few severe dental caries extended to dental pulp in our rat model.
Gastric mucosal injury and duodenal erosion are regarded as the major symptoms of H. pylori gastritis29,30. In the present study, rats that had been infected with both S. mutans and H. pylori exhibited greater damage to the digestive tract, compared with rats that had not been infected with H. pylori. However, these rats did not exhibit severe gastrointestinal diseases, such as gastric cancer. Notably, the combination of H. pylori infection with other risk factors (e.g., excessive salt and smoking) is closely related to the occurrence of serious gastrointestinal diseases31. Thus, to induce severe gastrointestinal symptoms in a rat model, these risk factors may need to be combined with the bacterial infection approach used in the present study.
A recent study showed that H. pylori was unsuitable for growth assays involving co-cultivation with oral streptococci, due to the stringent growth requirements of H. pylori27. In that study, the H. pylori culture supernatant was added to broth cultures of oral streptococci, which allowed analysis of the effect of H. pylori on the growth of oral streptococci. In the present study, we used a modified method to analyse the growth of H. pylori in the presence of S. mutans culture supernatant. Our results showed that S. mutans did not affect the growth of H. pylori. Another previous study found that H. pylori growth inhibition depends on the H. pylori strains or the oral bacterial species used in a particular assay32. Thus, it may be necessary to further analyse the influences of specific S. mutans and H. pylori strains on the growth of H. pylori.
The S. mutans in vitro biofilm assay has been performed in prior studies19,20. When H. pylori alone was grown in the in vitro biofilm assay, only a single layer of H. pylori was observed, which suggested that a small amount of H. pylori might adhere to the tooth surface. In contrast, when both S. mutans and H. pylori were grown in biofilm, multilayer H. pylori growth was observed in biofilm formed by S. mutans. The results suggested that H. pylori can penetrate dental plaque that forms on the tooth surface; however, S. mutans is predominant in biofilm that forms in the presence of sucrose, and the amount of H. pylori is considerably smaller than the amount of S. mutans.
Bacteria generally interact with each other in oral biofilms to facilitate survival in the oral environment33. In addition, the aggregation of multiple bacterial species aids each bacteria in colonisation of the oral cavity34. In our biofilm assay, H. pylori was colocalised with S. mutans. Therefore, H. pylori presumably utilises the biofilm formed by S. mutans to survive and colonise the oral cavity.
Despite its useful findings, some questions remain unresolved in this study. For example, it is unclear how a large number of H. pylori bacteria can enter biofilm formed by S. mutans. To elucidate the mechanism of H. pylori invasion of biofilm, molecular biological analyses are necessary; these analyses should focus on pathogenic proteins or signalling systems of both H. pylori and S. mutans. In addition, it remains unknown whether S. mutans alone can support H. pylori colonisation in the oral cavity. Thus, analyses using the rat model of H. pylori co-infection should be performed with other oral bacteria. Furthermore, it remains unclear whether the presence of S. mutans alone or in combination with the occurrence of dental caries is important for H. pylori colonisation. Thus, future studies should include comparisons of rat models with S. mutans colonisation in the oral cavity, with or without severe dental caries, to determine the relationships of these specific aspects with H. pylori colonisation.
In summary, we demonstrated that the presence of S. mutans in the oral cavity was able to support H. pylori colonisation in both oral cavity and gastric tissue. In addition, we found that H. pylori was able to invade biofilm formed by S. mutans. These results suggest that prevention of S. mutans infection in childhood, as well as the establishment of preventive habits to avoid S. mutans colonisation in the oral cavity (e.g., good oral hygiene and sucrose restriction) may be effective for prevention of H. pylori infection.
Methods
Bacterial strains and growth conditions
S. mutans strain MT8148R, a streptomycin-resistant substrain of MT814835, was grown from our laboratory stock. MT8148R was cultured on Mitis Salivarius agar (Difco Laboratories, Detroit, MI, USA) plates containing bacitracin (0.2 U/ml; Sigma Chemical Co., St. Louis, MO, USA) and 15% sucrose (i.e., MSB agar) containing 1500 μg/ml streptomycin at 37 °C for 2 days under 95% N2 and 5% CO2 condition. For routine growth, S. mutans was grown in brain heart infusion broth (Difco Laboratories) containing 1500 μg/ml streptomycin at 37 °C for 18 h. H. pylori strain J99 (ATCC 700824) was purchased from Summit Pharmaceuticals International Corporation (Tokyo, Japan). H. pylori was cultured using blood agar plates (Becton Dickinson, Franklin Lakes, NJ, USA) at 37 °C for 3 days under microaerophilic conditions. Colonies were then inoculated in 10 ml brucella broth (Becton Dickinson) supplemented with 1 ml horse serum, and incubated at 37 °C for 3–5 days. Cultured S. mutans and H. pylori strains in their respective broths were harvested and washed with sterile saline, then used in the following experiments.
Rat model experimental protocol
The experimental protocol by which rats were infected with H. pylori strain J99 and/or S. mutans MT8148R, then stimulated to form dental caries, is shown in Fig. 1A. Dental caries were induced using a previously described method15. Briefly, 40 male Sprague–Dawley rats, aged 15 to 18 days, were fed a normal diet CE-2 (CLEA Japan) containing tetracycline (4 mg/g) and given water containing penicillin G (4000 U/ml), prior to the establishment of bacterial colonisation in the oral cavity. All rats were then fed a caries-inducing diet containing 56% sucrose (CLEA Japan) until the end of the experiment. At 18 days of age, oral infection of S. mutans (1 × 108 CFUs in sterile saline) was performed in 20 rats, once per day for 5 days to establish S. mutans colonisation in the oral cavity; the remaining 20 rats did not receive S. mutans. One week after infection, dental plaques were collected from the oral cavity of each rat using a sterilised cotton swab; plaque samples were then seeded in MSB agar containing 1500 μg/ml streptomycin to confirm that S. mutans colonisation had been successfully established in the oral cavity.
Twenty rats (10 S. mutans-infected rats and 10 previously uninfected rats) were infected with H. pylori at 48 days of age; the rats were divided into four groups according to the presence or absence of infection with S. mutans and H. pylori (Fig. 1B). For H. pylori infection, oral infection of H. pylori (1.5 × 106 CFUs in sterile saline) was performed for 5 consecutive days. Dental plaque was collected once per week to assess H. pylori colonisation in the oral cavity, beginning 3 days after H. pylori infection and continuing until rats were euthanised. One month after H. pylori infection, rats were euthanised; maxillary and mandibular bones were then excised and used for detection of bacteria and evaluation of dental caries. The presence or absence of dental caries was determined by a dentist, who observed the occlusal surfaces of right maxillary and mandibular molar teeth (six teeth per rat) using a sterilised 27G needle (0.4 mm diameter) (Terumo Co., Tokyo, Japan) under a stereomicroscope. In addition, stomach and duodenum were excised for use in histological evaluation.
Detection of bacteria from the oral cavity
Recovery of S. mutans strain from mandibular bone was evaluated by using a previously described method36. After rats had been euthanised, excised mandibular bones were placed in sterile saline and bacteria were separated from the bones by sonication. The resulting bacterial suspension was serially diluted with sterile saline and cultured on MSB agar plates containing 1500 μg/ml streptomycin. After the agar plates had been incubated at 37 °C for 48 h, the numbers of colonies on the agar plates were counted to determine the numbers of S. mutans present in the mandibular bones.
Bacterial DNA was extracted from dental plaque samples or excised maxillary and mandibular bones in sterile saline after sonication. S. mutans detection was performed by PCR with S. mutans-specific primers (Table 1). H. pylori detection was performed by nested PCR using previously described H. pylori-specific primers17. Briefly, first-step PCR was performed using primers ureA-aF and ureA-bR; second-step PCR was performed with the first PCR product as a template, using primers ureA-bF and ureA-aR. All PCR products were amplified using Takara Ex Taq (Takara Bio. Inc., Otsu, Japan), then visualised by electrophoresis in a 1.5% agarose gel.
Histopathological evaluation of gastric tissue
All gastric and duodenal tissues were removed from each euthanised rat. The tissues were fixed in 10% neutral buffered formalin solution (Fujifilm Wako Pure Chemical Corporation, Tokyo, Japan), then embedded in paraffin and cut into 3-μm sections. These sections were subjected to HE staining, followed by evaluation of pathological features in all sampled gastric and duodenal tissues. Histopathological features were evaluated by scoring as follows: 0 (none), 1 (mild), 2 (moderate), and 3 (severe), in accordance with a previously published method with some modification37. Scoring was performed in a double-blinded manner by a pathologist (Sept. Sapie Co., Ltd, Tokyo, Japan).
Immunohistochemical staining of sections of stomach and duodenum was performed using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA), in accordance with the manufacturer’s instructions. First, sections were blocked with 3% H2O2 and 2.5% horse serum. Then, sections were incubated with anti-H. pylori antibody (Thermo Fisher Scientific, Waltham, MA, USA; diluted 1:100 with phosphate-buffered saline [PBS]) at 4 °C for 12 h. Subsequently, sections were incubated with secondary antibody from the kit at room temperature for 30 min. In addition, counterstaining was performed with haematoxylin solution.
Bacterial growth of H. pylori
Bacterial growth of H. pylori was assessed using a previously described method27, with some modifications. H. pylori colonies were suspended in brucella broth supplemented with horse serum, then grown to an OD550 of 0.2. The culture supernatant of 1 × 107 CFUs of S. mutans filtered by a 0.45 μm filter was added to the bacterial suspension. The growth activity of H. pylori was analysed by culturing the bacterial suspension at 37 °C under microaerophilic conditions and measuring the OD550 value at 12-h intervals. Data were recorded as the average of three independent analyses.
Biofilm assay
Bacterial suspensions of S. mutans and H. pylori strains were adjusted to 1.0 × 107 CFUs/ml in BHI broth containing 1% sucrose. Then, 200 µl of the suspensions were added to a chambered cover glass system (CultureWell, Grace Bio Labs, Bend, OR, USA) and incubated at 37 °C for 18 h under microaerophilic conditions. Non-attached bacterial cells were washed with PBS, while adherent cells were fixed with 3% paraformaldehyde (Wako Pure Chemical Industries, Osaka, Japan) for 10 min. For S. mutans staining, rabbit anti-PA serum38 was used as primary antibody and Alexa Fluor 633-conjugated goat anti-rabbit immunoglobulin G (Molecular Probes, Life Technologies Co., Eugene, OR, USA) was used as secondary antibody. For H. pylori staining, rabbit anti-H. pylori antibody (Thermo Fisher Scientific) was used as primary antibody and Alexa Fluor 533-conjugated goat anti-rabbit immunoglobulin G (Molecular Probes, Life Technologies Co.) was used as secondary antibody. Each antibody was diluted 1:500 in PBS containing 0.5% bovine serum albumin, then incubated with fixed cells for 30 min at room temperature. The chambered cover glass system was washed with PBS, before and after incubation with each antibody. Biofilms were observed by confocal scanning laser microscopy using a TCS-SP5 microscope (Leica Microsystems GmbH, Wetzlar, Germany), as well as a DMI6000 B fluorescence microscope (Leica Microsystems GmbH) and a 63 × oil immersion objective. The mean volume of H. pylori contained in the biofilm was determined by analysis of 10 separate confocal images in each group, using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
To determine the number of S. mutans bacteria present in the biofilm, the formed biofilm was washed with PBS and removed by pipetting. The collected and serially diluted bacterial suspension was then cultured on MSB agar plates at 37 °C for 2 days. Subsequently, the number of S. mutans in each biofilm was calculated by counting the number of colonies on the corresponding agar plate. Data were recorded as the average of five independent analyses. The number of H. pylori was quantified by confocal scanning laser microscopy. The numbers of H. pylori colonies were initially counted in two-dimensional images. The number of H. pylori contained in the biofilm was then calculated by multiplying the number of colonies counted by the thickness of each image. Data were recorded as the average value of 10 separate images.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA). Intergroup differences were compared using analysis of variance (ANOVA). Bonferroni correction was used for post hoc analyses. Differences with P < 0.05 were considered statistically significant.
Ethical approval
All rats were treated humanely, in accordance with the guidelines of the National Institutes of Health and the AERI-BBRI Animal Care and Use Committee. All animal experiments were approved by the Institutional Animal Care and Use Committee of Osaka University Graduate School of Dentistry (Approval No. 29-031-0).
Supplementary information
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP15H05049 and JP18K17252. We thank Ryan Chastain-Gross, Ph.D., from Edanz Group (https://en-author-services.edanzgroup.com) for editing a draft of this manuscript.
Author contributions
R.N. and T.K. designed the study under the supervision of K.N. T.K. and Y.O. performed animal experiments. N.I. performed histopathological evaluation. R.N., S.M., and R.O. performed in vitro experiments. Data interpretation was conducted by R.N. and K.N. R.N. and K.N. wrote the manuscript, which all authors read and approved.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ryota Nomura and Tamami Kadota.
Supplementary information
is available for this paper at 10.1038/s41598-020-69368-2.
References
- 1.Fennerty MB. Helicobacter pylori. Arch. Intern. Med. 1994;154:721–727. doi: 10.1001/archinte.1994.00420070021003. [DOI] [PubMed] [Google Scholar]
- 2.Hooi JKY, et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Banatvala N, et al. Migration and Helicobacter pylori seroprevalence: Bangladeshi migrants in the UK. J. Infect. 1995;31:133–135. doi: 10.1016/S0163-4453(95)92135-4. [DOI] [PubMed] [Google Scholar]
- 4.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment. Pharmacol. Ther. 1995;9(Suppl 2):33–39. [PubMed] [Google Scholar]
- 5.Miyabayashi H, Furihata K, Shimizu T, Ueno I, Akamatsu T. Influence of oral Helicobacter pylori on the success of eradication therapy against gastric Helicobacter pylori. Helicobacter. 2000;5:30–37. doi: 10.1046/j.1523-5378.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, et al. Comparison of cytotoxin genotypes of Helicobacter pylori in stomach and saliva. Dig. Dis. Sci. 2002;47:1850–1856. doi: 10.1023/A:1016417200611. [DOI] [PubMed] [Google Scholar]
- 7.Ogaya Y, Nomura R, Watanabe Y, Nakano K. Detection of Helicobacter pylori DNA in inflamed dental pulp specimens from Japanese children and adolescents. J. Med. Microbiol. 2015;64:117–123. doi: 10.1099/jmm.0.079491-0. [DOI] [PubMed] [Google Scholar]
- 8.Zou QH, Li RQ. Helicobacter pylori in the oral cavity and gastric mucosa: a meta-analysis. J. Oral. Pathol. Med. 2011;40:317–324. doi: 10.1111/j.1600-0714.2011.01006.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980;44:331–384. doi: 10.1128/MMBR.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J. Dent. Res. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- 11.Keyes PH. Recent advances in dental caries research Bacteriology. Bacteriological findings and biological implications. Int. Dent. J. 1962;12:443–464. [Google Scholar]
- 12.Eckert R, et al. Targeted killing of Streptococcus mutans by a pheromone-guided "smart" antimicrobial peptide. Antimicrob. Agents. Chemother. 2006;50:1480–1488. doi: 10.1128/AAC.50.4.1480-1488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, et al. Study on the relationship between Helicobacter pylori in the dental plaque and the occurrence of dental caries or oral hygiene index. Helicobacter. 2008;13:256–260. doi: 10.1111/j.1523-5378.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 14.Aksit Bıcak D, et al. The investigation of Helicobacter pylori in the dental biofilm and saliva samples of children with dyspeptic complaints. BMC Oral. Health. 2017;17:67. doi: 10.1186/s12903-017-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooshima T, et al. Comparison of the cariostatic effects between regimens to administer oolong tea polyphenols in SPF rats. Caries Res. 1998;32:75–80. doi: 10.1159/000016433. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino T, et al. PCR detection and identification of oral streptococci in saliva samples using gtf genes. Diagn. Microbiol. Infect. Dis. 2004;48:195–199. doi: 10.1016/j.diagmicrobio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Nomura R, Ogaya Y, Matayoshi S, Morita Y, Nakano K. Molecular and clinical analyses of Helicobacter pylori colonization in inflamed dental pulp. BMC Oral Health. 2018;18:64. doi: 10.1186/s12903-018-0526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida A, Kuramitsu HK. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl. Environ. Microbiol. 2002;68:6283–6291. doi: 10.1128/AEM.68.12.6283-6291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ardin AC, et al. Identification and functional analysis of an ammonium transporter in Streptococcus mutans. PLoS ONE. 2014;9:e107569. doi: 10.1371/journal.pone.0107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura R, Morita Y, Matayoshi S, Nakano K. Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG) elute against adhesion and colonization by Streptococcus mutans. Sci. Rep. 2018;8:5056. doi: 10.1038/s41598-018-23354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasanthi CH, Prasanthi NL, Manikiran SS, Rama-Rao NN. Focus on current trends in the treatment of Helicobacter pylori infection: an update. Inter. J. Pharm. Sci. Rev. Res. 2011;1:42–51. [Google Scholar]
- 22.Morales-Espinosa R, et al. Helicobacter pylori in the oral cavity is associated with gastroesophageal disease. Oral Microbiol. Immunol. 2009;24:464–468. doi: 10.1111/j.1399-302X.2009.00541.x. [DOI] [PubMed] [Google Scholar]
- 23.Silva DG, et al. Helicobacter pylori transiently in the mouth may participate in the transmission of infection. Mem. Inst. Oswaldo Cruz. 2010;105:657–660. doi: 10.1590/S0074-02762010000500009. [DOI] [PubMed] [Google Scholar]
- 24.Yee JKC. Are the view of Helicobacter pylori colonized in the oral cavity an illusion? Exp. Mol. Med. 2017;49:e397. doi: 10.1038/emm.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P, et al. A cross-sectional survey of dental caries, oral hygiene, and Helicobacter pulori infection in adults. Asia Pac. J. Pub. Health. 2013;25:49S–56S. doi: 10.1177/1010539513495555. [DOI] [PubMed] [Google Scholar]
- 26.Nomura R, Matayoshi S, Otsugu M, Kitamura T, Teramoto N, Nakano K. Contribution of severe dental caries induced by Streptococcus mutans to the pathogenicity of infective endocarditis. Infect Immun. 2020;88:e00897–19. doi: 10.1128/IAI.00897-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Deng X, Zhou X, Hao Y, Li Y. Influence of Helicobacter pylori culture supernatant on the ecological balance of a dual-species oral biofilm. J. Appl. Oral Sci. 2018;26:e20170113. doi: 10.1590/1678-7757-2017-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuremoto K, et al. Promotion of endodontic lesions in rats by a novel extraradicular biofilm model using obturation materials. Appl. Environ. Microbiol. 2014;80:3804–3810. doi: 10.1128/AEM.00421-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smoot DT. How does Helicobacter pylori cause mucosal damage? Direct mechanisms. Gastroenterology. 1997;113:S31–S34. doi: 10.1016/S0016-5085(97)80008-X. [DOI] [PubMed] [Google Scholar]
- 30.Gisbert JP, et al. Erosive duodenitis: prevalence of Helicobacter pylori infection and response to eradication therapy with omeprazole plus two antibiotics. Eur. J. Gastroenterol. Hepatol. 1997;9:957–962. doi: 10.1097/00042737-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin. Microbiol. Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishihara K, Miura T, Kimizuka R, Ebihara Y, Mizuno Y, Okuda K. Oral bacteria inhibit Helicobacter pylori growth. FEMS Microbiol. Lett. 1997;152:355–361. doi: 10.1111/j.1574-6968.1997.tb10452.x. [DOI] [PubMed] [Google Scholar]
- 33.Yonezawa H, Osaki T, Kamiya S. Biofilm formation by Helicobacter pylori and its involvement for antibiotic resistance. Biomed. Res. Int. 2015;2015:914791. doi: 10.1155/2015/914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolenbrander PE, London J. Adhere today, here tomorrow: Oral bacterial adherence. J. Bacteriol. 1993;175:3247–3252. doi: 10.1128/JB.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura R, Nakano K, Ooshima T. Contribution of glucan-binding protein C of Streptococcus mutans to bacteremia occurrence. Arch. Oral. Biol. 2004;49:783–788. doi: 10.1016/j.archoralbio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto-Nakano M, et al. Inhibitory effect of Oenothera biennis (evening primrose) seed extract on Streptococcus mutans and S. mutans-induced dental caries in rats. Caries Res. 2011;45:56–63. doi: 10.1159/000323376. [DOI] [PubMed] [Google Scholar]
- 37.Nolte T, et al. Nonproliferative and proliferative lesions of the gastrointestinal tract, pancreas and salivary glands of the rat and mouse. J. Toxicol. Pathol. 2016;29:1S–125S. doi: 10.1293/tox.29.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano K, Tsuji M, Nishimura K, Nomura R, Ooshima T. Contribution of cell surface protein antigen PAc of Streptococcus mutans to bacteremia. Microbes. Infect. 2006;8:114–121. doi: 10.1016/j.micinf.2005.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).