Key Points
Question
Is hip fracture incidence associated with changes in time period, birth cohort, or the known risk factors for hip fracture?
Findings
This cohort study including 10 552 individuals (>105 000 person-years) in follow-up over 40 years found that those born more recently experienced a lower incidence of hip fracture for a given age. Decreases in the prevalence of smoking and heavy drinking were associated with the decrease in the incidence of hip fracture.
Meaning
In this study, the decrease in hip fracture incidence appeared to be associated with birth cohort effects and improvement in lifestyle factors, such as smoking and heavy drinking, in addition to better treatment.
Abstract
Importance
Age-adjusted hip fracture incidence is decreasing in the US. The decrease has been attributed to osteoporosis treatment, but the cause is unknown.
Objective
To examine the decrease in hip fracture incidence over the past 40 years in the US.
Design, Setting, and Participants
A population-based cohort study using participants in the Framingham Heart Study was conducted. A total of 4918 men and 5634 women were followed up prospectively for the first hip fracture between January 1, 1970, and December 31, 2010. Data were analyzed from May 1, 2019, to May 30, 2020.
Main Outcomes and Measures
Incidence of hip fracture and contemporaneous prevalence of risk factors for hip fractures analyzed with age-period-cohort models.
Results
The study contained more than 105 000 person-years in 10 552 individuals with a gradual shift toward the offspring participants in the 1980s and 1990s. Women represented more than 55% of the study sample over the years. Adjusted for age, the incidence of hip fracture decreased by 4.4% (95% CI, 6.8%-1.9%) per year from 1970 to 2010. Both period associations (P < .001) and birth cohort associations (P < .001) were statistically significant. For example, in persons aged 85 to 89 years, the incidence of hip fracture was 759 per 100 000 person-years in the offspring group compared with 2018 per 100 000 person-years in the original cohort. The decrease in hip fracture incidence was coincident with a decrease in smoking and heavy drinking. Smoking decreased from 38% in the 1970s to 15% in the late 2000s, while heavy drinking decreased from 7.0% to 4.5%. The prevalence of other risk factors for hip fracture, such as underweight (body mass index <18.5), obesity (body mass index >30), and early menopause (age <45 years) were stable over the study period. When persons who never smoked were evaluated, a change in the incidence of −3.2% (95% CI, −6.0% to −0.4%) per year was observed. The difference between the decrease of the entire population and nonsmokers of 1.5% per year was similar to the hazard ratio conferred by smoking (hazard ratio, 1.5; 95% CI, 1.14-1.96).
Conclusions and Relevance
In this study, individuals born more recently appeared to have a low risk for hip fracture. Reductions in smoking and heavy drinking were the risk factor changes coincident with the observed decrease in hip fracture. Attributing the decrease in hip fracture incidence up to 2010 solely to better treatment is not supported by these data, emphasizing the need to treat patients with osteoporosis while continuing to encourage public health interventions for smoking cessation and heavy drinking.
This cohort study examines the decrease in the incidence of hip fractures noted over 40 years in the US.
Introduction
The age-adjusted incidence of hip fractures has decreased in the US1,2 and other high-income nations3 over the past 2 decades. The cause of the reported decrease is unknown, but has been attributed to the treatment of osteoporosis.1 However other studies have noted that, while bisphosphonates are effective at reducing the risk of hip fracture, the size of the osteoporosis treatment gap and magnitude of the decrease makes effective osteoporosis treatment an unlikely explanation for the population-level decrease and postulated that birth year may play a role.4,5 Understanding the reasons behind the past decrease in hip fracture incidence is pertinent because the decrease appears to be abating while osteoporosis prescriptions are plateauing.2 Age-period-cohort modeling6 allows statistical differentiation of changes in incidence arising from birth cohort, aging, and time period, if detailed data exist. Thus, to dissect these various potential factors in hip fracture incidence rates over time, we used data from the Framingham Osteoporosis Study,7 which is uniquely positioned owing to its long duration, multiple generation structure, and carefully ascertained hip fracture outcomes. We analyzed the incidence of hip fractures in the original and offspring cohorts using age-period-cohort models and tracked changes in the prevalence of risk factors for hip fractures.
Methods
The longitudinal Framingham Heart Study enrolled the original cohort of residents of Framingham, Massachusetts, from 1948 to 1952 and the offspring cohort beginning in 1971.8 Hip fractures have been identified using self-reports followed by fracture adjudication using medical records, including discharge summaries, operative reports, emergency department records, radiographic reports, and other encounters with health care professionals. Combined data from the original and offspring cohorts were used for this study. Because of regular follow-up and detailed biennial questionnaires, the Framingham Heart Study cohorts have been used to study disease incidence over time.9 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
The study was conducted from January 1, 1970, to December 31, 2010. Data were analyzed from May 1, 2019, to May 30, 2020. Using 5-year calendar periods from 1971 onward, we tabulated the number of persons aged 60 years and older at risk of first hip fracture, stratified by age group, and then tallied the incidence of hip fracture in each 5-year calendar period until 2010. Individuals were censored after their first hip fracture or mortality. Age-period-cohort analysis tools were used to calculate the net change in age-adjusted incidence over time (a quantity known as net drift or estimated annual percentage change) and model the independent effects of birth year and time period. We analyzed fracture incidence using log-linear regression models that included linear and quadratic effects for age, period, and birth cohort.10 The parameters also describe how the fracture rate varies on the basis of an age-incidence curve, multiplied by a birth cohort–specific relative risk, multiplied by a calendar-year factor that modulates the fracture rate for all age groups simultaneously.6,10
To adjust for potential risk factors that might be associated with changing incidence rates, at the beginning of each 5-year period, we ascertained the presence or absence of risk factors for hip fracture for each participant as recorded in the Framingham Heart Study biennial physical examination and questionnaires. While there are many documented risk factors for hip fracture, we focused on the factors that are part of the FRAX (fracture risk assessment) score,11 because they are used to help clinicians judge the need for preventive medical treatment. These risk factors are smoking (yes/no), heavy drinking (≥3 drinks per day), low body mass index (<18 [calculated as weight in kilograms divided by height in meters squared]), early-onset menopause (menopause age <45 years), glucocorticoid use, and rheumatoid arthritis. Although diabetes is not part of the FRAX construct, we recorded diabetes prevalence (defined as fasting glucose level >126 mg/dL [to convert to millimoles per liter, multiply by 0.0555] or use of medication for diabetes)12 as well. Data regarding glucocorticoid use were not available before 1980, and data regarding rheumatoid arthritis status were not available before 1990. We recorded estrogen use and bisphosphonate use when available. We performed secondary analysis by repeating the age-period-cohort models on individuals who never smoked. For this analysis, individuals were classified as never smokers if they never reported smoking at any time between 1971 and 2010.
Statistical Analysis
We constructed a Cox proportional hazards model with the time-dependent covariates to estimate the hip fracture risk for each factor during the study. Because the covariates could change over the course of the study (eg, a participant might stop smoking during follow-up), time-dependent covariate models were used to ensure that the hazard ratios computed reflected the risk immediately preceding the event. We were not able to incorporate glucocorticoid use and rheumatoid arthritis status into this model owing to their low prevalence (<25 hip fracture cases with rheumatoid arthritis or glucocorticoid use). Findings were considered statistically significant at the P < .05 level with 2-tailed tests; all tests were unpaired. Statistical analyses were done using SAS, version 9.4 (SAS Institute Inc) and R, version 3.6.3 (R Project for Statistical Computing).
Results
Owing to the range of ages at enrollment, the combined original and offspring cohorts provided a stable sample size and distribution of ages over the study period (Figure 1). The study contained more than 105 000 person-years from 10 552 individuals with a gradual shift toward the offspring participants in the 1980s and 1990s. Women represented more than 55% of the study sample over the years (Table 1). The incidence of hip fracture for women from 1985 to 1995 was 789 per 100 000 person-years (95% CI, 520-1198 per 100 000 person-years) and similar to the 957 per 100 000 person-years reported from national data.1 The incidence of hip fracture for men from 1985 to 1995 was 240 per 100 000 person-years (95% CI, 93-609 per 100 000 person-years).
Figure 1. Makeup of Study Cohort Over Time.
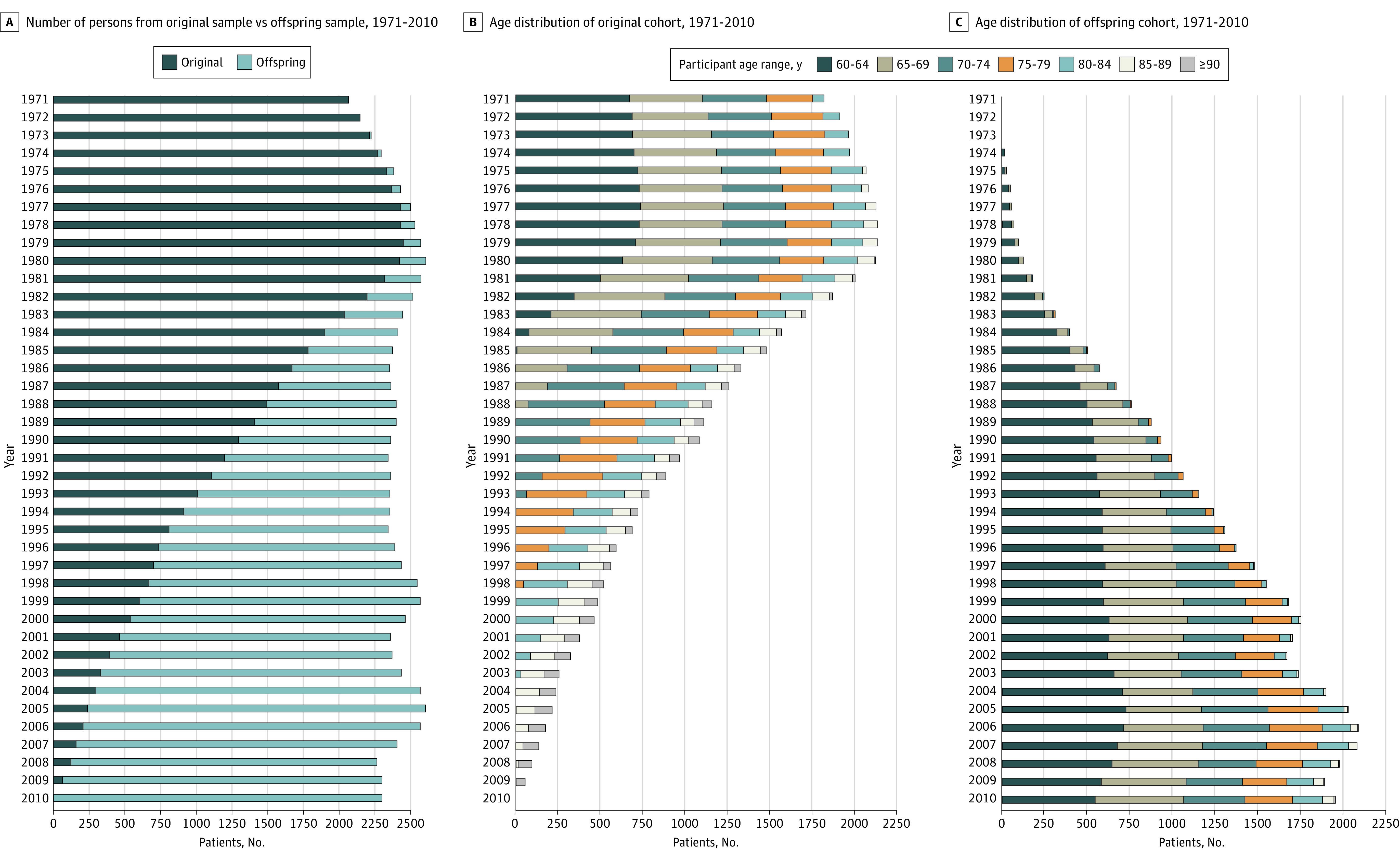
Table 1. Characteristics of Framingham Participants by Decade.
| Characteristic | 1970s | 1980s | 1990s | 2000s |
|---|---|---|---|---|
| Total No. | 3459 | 3511 | 3567 | 3646 |
| Original population, No. (%) | 3312 (95.8) | 2423 (69.0) | 1291 (36.2) | 537 (14.73) |
| Offspring, No. (%) | 147 (4.3) | 1088 (31.0) | 2276 (63.8) | 3109 (85.3) |
| Women, No. (%) | 1985 (57.4) | 1986 (56.6) | 1989 (55.8) | 2071 (56.8) |
| Age distribution (%), total person-years | ||||
| 60-64 | 2133 (32.2) | 1684 (24.0) | 1710 (24.7) | 1819 (26.2) |
| 65-69 | 1602 (24.2) | 1606 (22.9) | 1328 (19.2) | 1557 (22.4) |
| 70-74 | 1295 (19.6) | 1547 (22.0) | 1232 (17.8) | 1216 (17.5) |
| 75-79 | 959 (14.5) | 1041 (14.8) | 1128 (16.3) | 906 (13.0) |
| 80-84 | 499 (7.5) | 672 (9.6) | 887 (12.8) | 685 (9.9) |
| 85-89 | 127 (1.9) | 351 (5.0) | 445 (6.4) | 483 (7.0) |
| ≥90 | 2 (0.03) | 118 (1.7) | 184 (2.7) | 286 (4.1) |
| Risk factor prevalence (%), person-years | ||||
| Smoking | 24 992 (38.1) | 17 916 (29.3) | 8123 (17.5) | 2321 (13.5) |
| Heavy alcohol usea | 5126 (7.8) | 4361 (7.1) | 2115 (4.6) | 811 (4.4) |
| Diabetesb | 2854 (4.4) | 3055 (5.1) | 3916 (8.6) | 1804 (9.7) |
| Obesityc | 9047 (13.7) | 8107 (13.3) | 5824 (12.1) | 3143 (9.2) |
| Underweightd | 786 (1.2) | 772 (1.3) | 554 (1.2) | 391 (1.2) |
| Glucocorticoid use | NA | 65 (3.2) | 102 (2.0) | 106 (2.6) |
| Rheumatoid arthritis | NA | NA | 48 (0.9) | 41 (1.0) |
| Early menopausee | 8017 (23.4) | 7098 (24.2) | 5407 (24.3) | 3310 (23.2) |
| Hip fracture incidence (95% CI), per 100 000 person-years | 663.4 (356.2-1235.5) | 741.4 (483.7-1136.9) | 569.3 (387.9-835.6) | 246.4 (144.4-420.3) |
Abbreviation: NA, not available.
Three or more drinks per day.
Fasting glucose level above 126 mg/dL (to convert to millimoles per liter, multiply by 0.0555) or use of medication for diabetes.
Body mass index greater than 30 (calculated as weight in kilograms divided by height in meters squared).
Body mass index less than 18.5.
Percentage of women only, defined as menopause before age 45 years.
Over the 40-year study period, the age-adjusted incidence of hip fracture decreased significantly by 4.4% per year (95% CI, 6.8%-1.9%). The decrease in age-adjusted incidence (net drift) was significant for both men (5.2%; 95 CI, 9.1%-1.1%) and women (4.5%; 95% CI, 7.0%-2.0%). Because of the large net drift, the age-period-cohort modeling revealed that both period (P < .001) and birth cohort (P < .001) associations were statistically significant. Members of the offspring cohort exhibited a lower incidence of hip fracture for a given age range (Figure 2). For example, in persons aged 85 to 89 years, the incidence of hip fracture was 759 per 100 000 person-years in the offspring group compared with 2018 per 100 000 person-years in the original cohort.
Figure 2. Hip Fracture Incidence Decrease Over the Study Period.
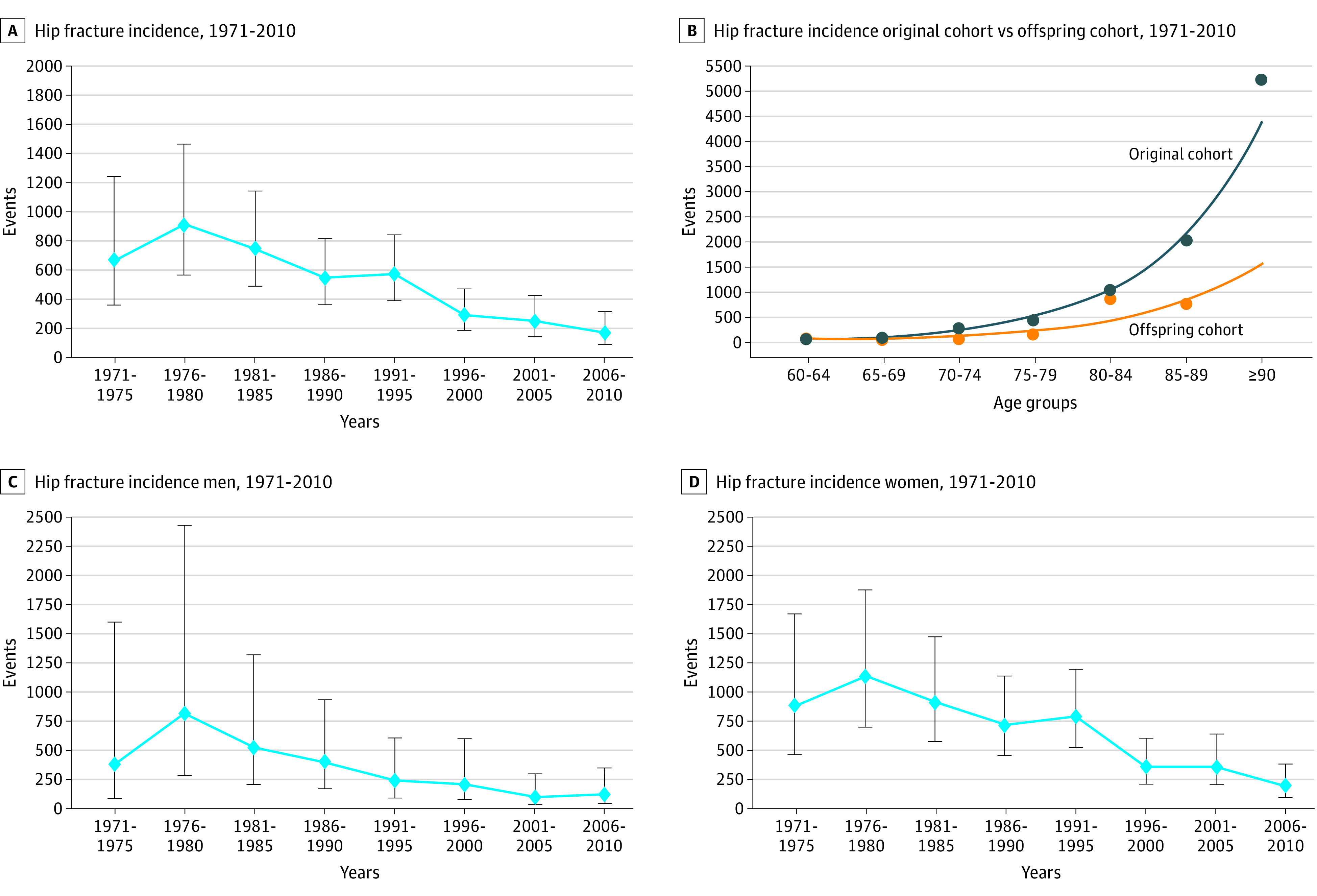
The dots represent the incidence (events per 100 000 person-years). The error bars are the 95% confidence intervals.
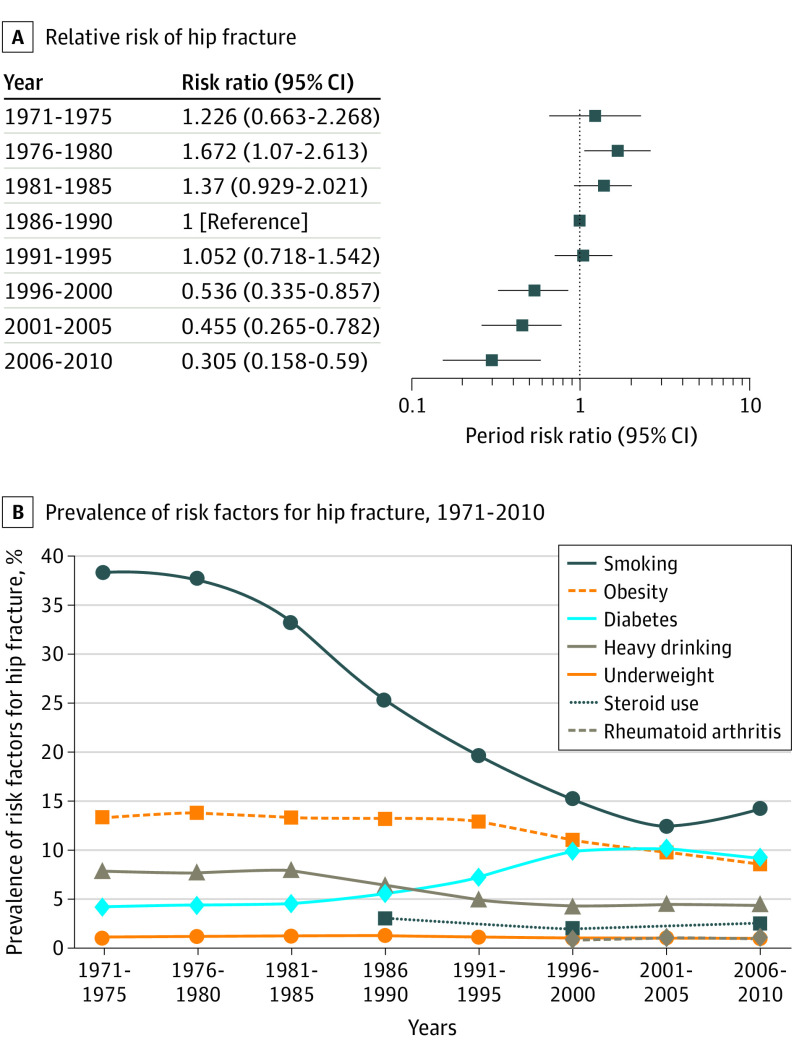
Most risk factors for hip fracture were stable over the study period (Figure 3). The prevalence of early menopause (a risk factor for secondary osteoporosis) and underweight cohort members (body mass index <18) was unchanged. The prevalence of obesity (body mass index >30), which is protective against hip fracture, decreased slightly after 1995. Diabetes increased from 4.3% to 10.2%. The prevalence of rheumatoid arthritis and glucocorticoid use were only recorded at the later visits in the Framingham Heart Study but were rare (<2%). Estrogen use among women increased from 2.8% in 1980 to a peak of 23.8% in 2000 and then decreased to 17.8%. Bisphosphonates were used by 8.5% of the population in 2000-2005. The hazard ratios for these risk factors are reported in Table 2 and the eFigure in the Supplement.
Figure 3. Relative Risk of Hip Fracture by Time Period and Prevalence of Risk Factors for Hip Fracture.
The relative risk of hip fracture (A) and prevalence of risk factors (B) in a given period compared with the reference period of 1986-1990 are shown. Risk factors included smoking (yes/no), heavy drinking (≥3 drinks per day), low body mass index (<18 [calculated as weight in kilograms divided by height in meters squared]), early-onset menopause (menopause age <45 years), glucocorticoid use, rheumatoid arthritis, and diabetes (defined as fasting glucose level >126 mg/dL [to convert to millimoles per liter, multiply by 0.0555] or use of medication for diabetes).
Table 2. Hip Fracture Risk for Factors Included in Multivariate Models.
| Risk factor | Hazard ratio (95% CI) | P value |
|---|---|---|
| Female sex | 2.26 (1.77-2.89) | <.0001 |
| Diabetesa | 0.98 (0.72-1.33) | .8958 |
| Smoking | 1.5 (1.14-1.96) | .0032 |
| Heavy alcohol intakeb | 1.94 (1.18-3.18) | .0088 |
| Obesityc | 0.54 (0.37-0.81) | .0023 |
| Underweightd | 1.83 (1.46-2.30) | <.0001 |
| Early menopausee | 0.84 (0.64-1.10) | .2103 |
| Age (per year) | 1.12 (1.10-1.13) | <.0001 |
Fasting glucose level above 126 mg/dL (to convert to millimoles per liter, multiply by 0.0555) or use of medication for diabetes.
Three or more drinks per day.
Body mass index greater than 30 (calculated as weight in kilograms divided by height in meters squared).
Body mass index less than 18.5
Women only, defined as menopause before age 45 years.
The prevalence of smoking exhibited the most precipitous decrease over the study period. From a peak of 38% in 1971-1975, smoking decreased to 15% in 2006-2010. The prevalence of heavy drinking also decreased from 7.0% to 4.5% over the study period. Heavy drinking was associated with smoking; for example, 50.9% of person-years of heavy drinking were also smoking person-years (P < .001). When only individuals who were never heavy drinkers were analyzed, the age-adjusted incidence decrease was 4.8% (95% CI, 7.1%-2.4%) per year.
When only smokers were studied, a significant decrease in hip fracture incidence was not observed (age-adjusted incidence decrease of 1.7%; 95% CI, –6.7% to 3.4%). Among individuals who never smoked, a significant age-adjusted incidence decrease of 3.2% was still present (95% CI, –6.0% to –0.4%). If the age-adjusted decrease of 3.2% in nonsmokers was multiplied by the hazard ratio conferred by smoking (1.5), the resulting 4.8% is similar to the age-adjusted decrease of the entire population.
Discussion
The decrease in the incidence of hip fractures over the past 20 years has been observed in multiple countries and in both men and women. Thus, the factors associated with the decrease must affect broad populations over a sustained period of years. The age-adjusted decrease in hip fracture incidence in our study was 4.4% per year—a substantial decrease that was observed in women and men.
The availability of the prospectively collected Framingham Heart Study data allowed us to look further back in time than previous studies.1,13 Contrary to the findings of previous studies, better diagnosis and treatment of osteoporosis appeared unlikely to be the main cause. The decrease in the incidence of hip fracture began in 1975, while bisphosphonates were first released in 1995. Estrogen use did not cross 10% prevalence until 1990 and can be considered a factor only in the decrease in the incidence of hip fracture in women. Bone mineral density testing did not become available until the 1990s. In the best-case scenario, the 8.5% use of bisphosphonates beginning immediately in 1995 would have potentially cut the rate of hip fracture by 4.8% in total,13 which is well below the observed decrease of 67%. These data do not contradict the demonstrated effectiveness of bisphosphonates to reduce hip fracture risk,14 but highlight the possibility that other factors are associated with the decreasing incidence of hip fracture.
We observed an association between the birth cohort and incidence of hip fracture in that individuals born more recently had a significantly lower risk of hip fracture. Whether this lowered risk is due to better nutrition, a more active lifestyle, or changing demographics cannot be explained.
Our data suggest that reduction in smoking may be the largest factor in the decrease in hip fractures observed in the Framingham Heart Study cohorts. The prevalence of smoking dropped from 38% to 15% in the same period as the largest decrease in hip fracture. Active smoking is an independent risk factor for hip fracture, detrimental to bone mineral density and associated with leaner body mass and a less-active lifestyle.7,11 While heavy drinking also decreased, other clinical risk factors remained stable or increased over the study period. As has been observed previously,12 we noted that body mass index remained nearly unchanged in the Framingham Heart Study cohorts while the incidence of diabetes increased. Because the hip fracture incidence decreased in individuals who never smoked, additional factors may be associated with the decrease. These data are valuable to public health officials in the other parts of the world. For example, in Asia, smoking is highly prevalent15 and the incidence of hip fracture is high.16
Limitations
A major limitation of our study is the lack of contemporaneous bone mineral density data across the study period. Bone mineral density testing was obtained for a subset of the original cohort from 1987 to 1999 and most of the offspring cohort during 1996-2008. It is possible that the prevalence of osteoporosis has decreased independently of other factors. Framingham Heart Study data on medications were limited in the 1970-2000 period. Data on bisphosphonates, which became available in 1995, were recorded only in the 2000s and thus were not analyzed. Another limitation is that the Framingham Heart Study population is exclusively White, had a lower incidence of obesity than the overall US population, and had a somewhat lower incidence of hip fracture, limiting the generalizability of the results.
Conclusions
In this study, the incidence of hip fracture appeared to decrease significantly for men and women over the Framingham Heart Study period. People born at later times during the study exhibited a lower age-adjusted incidence of hip fracture. The decrease in the hip fracture incidence that we observed over a long follow-up in large numbers of men and women may not be explained by better preventive treatment. Rather, it appears that birth cohort effects and improvement in lifestyle factors, particularly reduction in smoking, were prominent factors in the decrease in hip fracture rates over the 4 decades of our study. To reduce the hip fracture burden, treatment of osteoporosis is important as are public health interventions to reduce smoking and heavy drinking.
eFigure. Prevalence of Early Menopause and Estrogen Replacement Therapy Among Females 1971-2010
References
- 1.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579. doi: 10.1001/jama.2009.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewiecki EM, Wright NC, Curtis JR, et al. Hip fracture trends in the United States, 2002 to 2015. Osteoporos Int. 2018;29(3):717-722. doi: 10.1007/s00198-017-4345-0 [DOI] [PubMed] [Google Scholar]
- 3.Leslie WD, O’Donnell S, Jean S, et al. ; Osteoporosis Surveillance Expert Working Group . Trends in hip fracture rates in Canada. JAMA. 2009;302(8):883-889. doi: 10.1001/jama.2009.1231 [DOI] [PubMed] [Google Scholar]
- 4.Abrahamsen B, Skjødt MK, Vestergaard P. Hip fracture rates and time trends in use of anti-osteoporosis medications in Denmark for the period 2005 to 2015: missed opportunities in fracture prevention. Bone. 2019;120:476-481. doi: 10.1016/j.bone.2018.12.016 [DOI] [PubMed] [Google Scholar]
- 5.Jean S, O’Donnell S, Lagacé C, et al. ; Osteoporosis Surveillance Expert Working Group . Trends in hip fracture rates in Canada: an age-period-cohort analysis. J Bone Miner Res. 2013;28(6):1283-1289. doi: 10.1002/jbmr.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2296-2302. doi: 10.1158/1055-9965.EPI-14-0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15(4):710-720. doi: 10.1359/jbmr.2000.15.4.710 [DOI] [PubMed] [Google Scholar]
- 8.Tsao CW, Vasan RS. Cohort profile: The Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44(6):1800-1813. doi: 10.1093/ije/dyv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523-532. doi: 10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425-457. doi: 10.1146/annurev.pu.12.050191.002233 [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385-397. doi: 10.1007/s00198-007-0543-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham TM, Pencina KM, Pencina MJ, Fox CS. Trends in diabetes incidence: the Framingham Heart Study. Diabetes Care. 2015;38(3):482-487. doi: 10.2337/dc14-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrahamsen B, Vestergaard P. Declining incidence of hip fractures and the extent of use of anti-osteoporotic therapy in Denmark 1997-2006. Osteoporos Int. 2010;21(3):373-380. doi: 10.1007/s00198-009-0957-3 [DOI] [PubMed] [Google Scholar]
- 14.Ensrud KE, Crandall CJ. Bisphosphonates for postmenopausal osteoporosis. JAMA. 2019;322(20):2017-2018. doi: 10.1001/jama.2019.15781 [DOI] [PubMed] [Google Scholar]
- 15.Li S, Meng L, Chiolero A, Ma C, Xi B. Trends in smoking prevalence and attributable mortality in China, 1991-2011. Prev Med. 2016;93:82-87. doi: 10.1016/j.ypmed.2016.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung CL, Ang SB, Chadha M, et al. An updated hip fracture projection in Asia: the Asian Federation of Osteoporosis Societies study. Osteoporos Sarcopenia. 2018;4(1):16-21. doi: 10.1016/j.afos.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Prevalence of Early Menopause and Estrogen Replacement Therapy Among Females 1971-2010