Abstract
Objective
The compositions of the gut microbiota and its metabolites were altered in individuals with Autism Spectrum Disorder (ASD). The aim of this study was to assess whether plasma levels of gut-derived metabolite trimethylamine N-oxide (TMAO) were associated with ASD and the degree of symptom severity.
Methods
From September 2017 to January 2019, a total of three hundred and twenty-eight Chinese children (164 with ASD and 164 their age-sex matched control subjects) aged 3–8 years were included. TMAO levels in plasma were determined using high-performance liquid chromatography tandem mass spectrometry (LC/MS/ MS). Logistic regression analysis was used to examine the TMAO-ASD association.
Results
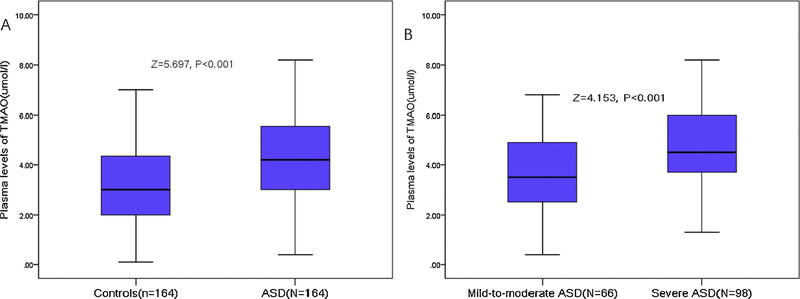
In the study, the median age of the ASD group was 5 years (interquartile range [IQR], 4–6 years) and 129 (78.7%) were boys. The median plasma levels of TMAO in children with ASD and typically-developing (TD) children at admission were 4.2 (IQR, 3.0–5.6) μmol/l and 3.0 (2.0–4.4) μmol/l, respectively (P < 0.001). For each 1 μmol/l increase of plasma TMAO, the unadjusted and adjusted risk of ASD would be increased by 54% (with the odds ratios [OR] of 1.54; 95% confidence intervals [CI]: 1.32–1.78; P < 0.001) and 27% (1.27 [1.10–1.45], P < 0.001), respectively. Symptom severity was classified as mild-to-moderate (CARS < 37) for 66 children with ASD (40.2%). In these children, the plasma levels of TMAO were lower than in the 98 children with ASD (59.8%) whose symptoms were classified as severe (CARS > 36) (3.5[2.5–4.9] μmol/l vs. 4.5(3.7–6.0) μmol/l; P < 0.001). For each 1 μmol/l increase of plasma TMAO, the unadjusted and adjusted risk of severe autism would be increased by 61% (with the OR of 1.61 [95% CI 1.28–2.01], P < 0.001) and 31% (1.31 [1.08–1.49], P < 0.001), respectively.
Conclusions
Elevated plasma levels of TMAO were associated with ASD and symptom severity.
Keywords: Gut microbiota, Trimethylamine N-oxide, Autism spectrum disorder, Chinese
1. Introduction
Autism Spectrum Disorder (ASD) is a term used to describe a constellation of early-appearing social communication deficits and restricted or repetitive interests and behaviors (Lord et al., 2018). It is prevalent in children and is increasing at a steady rate in recent years. A previous study reported that in the Autism and Developmental Disabilities Monitoring (ADDM) Network sites in 2014, the overall estimated ASD prevalence was 16.8 per 1000 children aged 8 years (one in 59) (Christensen et al., 2018). In 3-year-old Chinese children, the prevalence was suggested to be 1.11% (95% CI, 0.99%–1.23%) (Wu et al., 2018).
The etiology of autism is still poorly understood, but it is accepted that both genetic and environmental factors contribute to the etiology of ASD (Colvert et al., 2015; Tu et al., 2013a). A high prevalence of gastrointestinal (GI) symptoms in autistic individuals had been proposed in previous studies (Li et al., 2017a; Chaidez et al., 2014). Liu et al. (2019) found that the compositions of the gut microbiota and its metabolites (short-chain fatty acids, SCFAs) were altered in individuals with ASD. Another study showed that children with more severe ASD were likely to have more severe gastrointestinal symptoms, and the authors concluded that ASD symptoms may have been mediated or partially-moderated by these underlying gastrointestinal problems (Adams et al., 2011).
Trimethylamine N-oxide (TMAO) is generated from the oxidation of trimethylamine (TMA) that occurs in the gut microbiota (Janeiro et al., 2018). TMA is generated in the gut from betaine, L-carnitine and its metabolite-butyrobetaine (GBB), choline, and other choline-containing compounds, which are present in the typical diet (Zeisel and Warrier, 2017). TMAO was thought to be a waste product of choline metabolism without action in our organism, but recent evidence suggests an association between TMAO and inflammation (Chen et al., 2017; Rohrmann et al., 2016). Zhu et al. (2016) suggested that TMAO was produced by gut microbial metabolism of dietary quaternary amines, and TMAO has been linked to several chronic diseases. The plasma level of TMAO was determined by several factors including diet, gut microbial flora, drug administration, and liver flavin monooxygenase activity (Janeiro et al., 2018). Recent clinical studies reported that dysregulated levels of TMAO have been linked with renal diseases, cardiovascular disorders, and neurological disorders (Tang et al., 2015; Qi et al., 2018; Meng et al., 2019). The relationship between gut microbiota (its metabolites) and ASD has been studied previously (Li et al., 2017a; Chaidez et al., 2014; Liu et al., 2019). However, it remains unclear whether TMAO, a gut microbiota-dependent metabolite, is associated with ASD. The present study is the first to directly examine the relationship between TMAO, ASD, and symptom severity.
2. Material and methods
2.1. Children
From September 2017 to January 2019, a cross-sectional study was conducted in first Affiliated Hospital of Nanchang University, Nanchang, China. A total of three hundred and twenty-eight 3- to 8year old Chinese children (164 with ASD and 164 age- and sex-matched control subjects) were included. For participants in the ASD group, diagnosis was confirmed by two pediatricians according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) (First, 2013). Children in the ASD group were newly diagnosed, drug-naïve, and exhibited the core diagnostic symptoms of ASD (social communication impairments and restricted, repetitive patterns of interests or behavior).
Sex- and age-matched TD children were recruited to the control group from a kindergarten near the hospital. In order to exclude the possibility that the controls could have any sub-clinical features (e.g., broader autism phenotype), all control subjects were also evaluated against the DSM-5 criteria for ASD by the study pediatricians. ASD and TD children with (1) congenital and/or genetic disease; (2) an acute or chronic infectious disease during the previous three months; (3) any food or drug allergy, and (4) use of any medication to address behavior/ focus/attention during the previous three months were excluded. The protocol and informed consent for this study were reviewed and approved by the Institutional Review Board at the First Affiliated Hospital of Nanchang University. Written informed consent was obtained from parents before their children participated.
2.2. Clinical data collected
At admission, we collected demographic data (age, sex, and ethnicity), body mass index (BMI, kg/m2), family history of ASD, and time from ASD onset to admission. ASD symptom severity was assessed using the Chinese version of the Childhood Autism Rating Scale (CARS) score, which was used to classify the ASD group into two categories of symptom severity: mild-to-moderate (CARS total score = 30–36) and severe (CARS total score >36) (Ning et al., 2019).
2.3. Laboratory testing
Fasting plasma blood samples of all participants were collected using EDTA tubes on the morning of the first day after admission, and immediately processed and frozen at −80 °C until analysis. TMAO, choline, and betaine levels in plasma were determined using stable isotope dilution high-performance liquid chromatography with online electrospray ionization tandem mass spectrometry (LC/MS/MS) on an AB SCIEX 5500 triple quadrupole mass spectrometer using d9-(trimethyl)-labelled internal standards as described previously (Tang et al., 2015). A volume of 50 μl of either the serum sample or standards were combined with 100 μl of acetonitrile containing 10 μM of internal standards (d9-choline, d9-carnitine, and d9-TMAO) and then centrifuged at 14,000 g for 15 min. After an additional centrifugation step, the supernatant was analyzed after injection into a normal-phase silica column (2.1 × 50 mm, 2.7 μm) and equilibrated with 19% solution A (10 mmol/l ammonium formate and 0.1% formate acid in water) and 81% solution B (acetonitrile) under isocratic elution with the flow rate of 0.3 mL/min. The assay described shows good inter- and intra-day reproducibility (all CVs <6%) and accuracy (> 99.0% across low, mid, and high values).
2.4. Statistical analysis
All statistical analysis was performed with SPSS for Windows, version 22.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as percentages or frequencies; continuous variables were expressed as medians (interquartile range; IQR). Between-group comparisons were performed with Chi-Square tests or Mann–Whitney U tests (Tu et al., 2013b). Correlations among continuous variables were assessed using the Spearman rank-correlation coefficient.
The relationship between TMAO, ASD risk, and symptom severity was assessed using univariate and multivariate logistic regression analysis. In the multivariate logistic regression analysis, confounding factors included age, sex, ethnicity, BMI, the time from symptom onset to admission, and plasma levels of choline and betaine. The results were presented as adjusted odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). The effects of TMAO levels on ASD was further estimated by TMAO quartiles (lowest TAMO quartile as the reference). Receiver operating characteristic (ROC) curves were utilized to evaluate the accuracy of TMAO to predict ASD risk and severity. Area under the curve (AUC) was calculated as measurements of the accuracy of the test Tu et al. (2019). Statistical significance was defined as p < 0.05.
3. Results
3.1. Basic information
In this study, 164 children with ASD and 164 age- and sex-matched TD children were included. The characteristics of those children are presented in Table 1; the median age of the ASD group was 5 years (IQR, 4–6) and 129 (78.7%) were boys. In addition, 158 out of the 164 children with ASD were from the Chinese Han population, and 15 (9.1%) children had a family history of ASD. The median length of hospital stay was 86 (IQR, 56–109) days.
Table 1.
Characteristics of the ASD and control cases.
| Variable | ASD | Control cases | p-value † |
|---|---|---|---|
| N | 164 | 164 | _ |
| Age, years | 5(4-6) | 5(4-6) | 1.00 |
| Sex-male | 129(78.7) | 129(78.7) | 1.00 |
| Ethnic-Han | 158(96.3) | 155(94.5) | 0.43 |
| BMI, kg/m2 | 16.6(16.2- | 17.0(16.1- | 0.18 |
| 17.4) | 17.6) | ||
| The time from symptom onset to | 105(78-141) | — | — |
| admission, days | |||
| Family history of ASD | 15(9.1) | 2(1.2) | 0.001 |
| CARS | 40(36-47) | 22(18-25) | < 0.001 |
| Mild to moderate autism‡ | 66(40.2) | — | — |
| Laboratory findings | |||
| TMAO (umol/1) | 4.2(3.0-5.6) | 3.0(2.0-4.4) | < 0.001 |
| Choline (^imol/1) | 9.0(6.4-13.6) | 7.8(5.2-12.3) | 0.012 |
| Betaine (^imol/1) | 21.4(16.5- | 19.8(14.8- | 0.019 |
| 26.4) | 25.3) | ||
The discrete variable was expressed in the percentage or frequency, continuous variable was expressed in the medians (interquartile range, IQR).
ASD: Autism spectrum disorders; BMI, body mass index; CARS, Childhood Autism Rating Scale; TMAO, Trimethylamine N-oxide.
P value was tested by chi-squared test or Mann-Whitney U test.
A total score of between 30 and 36 indicates mild-to-moderate autism, whereas the interval between 37 and 60 denotes severe autism.
3.2. Plasma levels of TMAO and risk of ASD
The median plasma levels of TMAO in the ASD and TD groups at admission were 4.2 (IQR, 3.0–5.6) μmol/l and 3.0 (2.0–4.4) μmol/l, respectively (Fig. 1A; P < 0.001). As shown in Table 1, plasma levels of choline and betaine were also higher in the ASD group than the TD group (P < 0.05). Levels of TMAO increased with increasing severity of ASD as defined by CARS total score. A positive correlation between the plasma levels of TMAO and CARS score was found (r =0.428; P < 0.001). In addition, positive correlation was also found between TMAO and choline (r=0.248; P=0.001) and betaine (r=0.277; P < 0.001).
Fig. 1.
Distribution of plasma levels of TMAO in diffident groups. (A) TMAO in children with ASD and typical development. (B) TMAO in mild-to-moderate ASD and severe ASD. All data are median and IQR. P values refer to Mann–Whitney U test for differences between groups. A total CRAS score of between 30 and 36 indicates mild-to-moderate autism, whereas the interval between 37 and 60 denotes severe autism. ASD =Autism spectrum disorder; CARS =Childhood Autism Rating Scale; TMAO =Trimethylamine N-oxide.
Based on the ROC curve, the optimal cutoff value of TMAO as biomarker discriminating between ASD and TD was projected to be 3.7 μmol/l, which yielded a sensitivity of 65.9% and a specificity of 64.6% (Fig. 2A), with the area under the curve at 0.68 (95%CI: 0.620.0.74; P < 0.001). With the AUC of 0.68, TAMO had greater discriminatory value than choline (AUC: 0.57; 95%: 0.51–0.63; P < 0.001) and betaine (0.58; 0.52–0.64; P < 0.001).
Fig. 2.
Receiver operator characteristic curve demonstrating sensitivity as a function of 1-specificity for predicting the ASD risk or severity based on TMAO. (A) Receiver operator characteristic curve demonstrating sensitivity as a function of 1-specificity for predicting the risk of ASD based on TMAO. (B) Receiver operator characteristic curve demonstrating sensitivity as a function of 1-specificity for predicting the risk of severe ASD based on TMAO. A total CRAS score of between 37 and 60 denotes severe autism. ASD =Autism spectrum disorder; CARS = Childhood Autism Rating Scale; TMAO =Trimethylamine N-oxide.
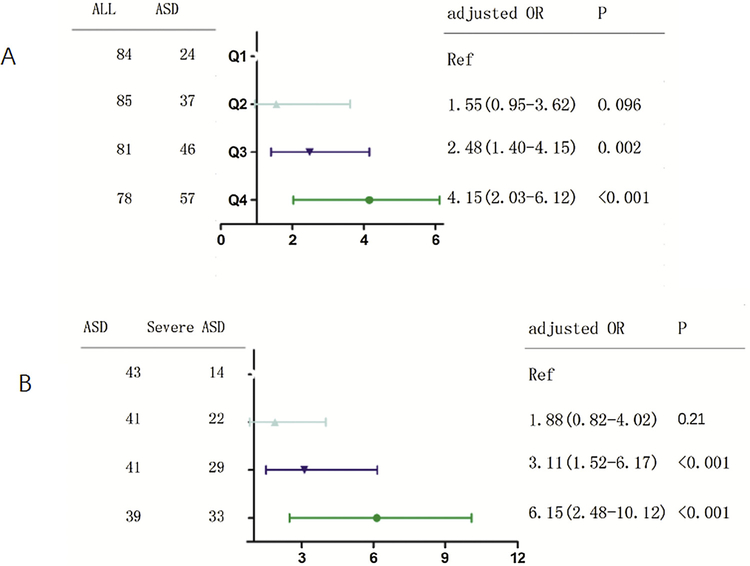
A logistic regression analysis was performed to characterize the prediction of ASD by TMAO levels with the OR (95%CI). For each 1 μmol/l increase of plasma concentration of TMAO, the unadjusted and adjusted (factors including for, age, sex, ethnicity, BMI, family history of ASD, the time from symptom onset to admission, plasma levels of choline and betaine) risk of ASD would be increased by 54% (with the OR of 1.54 [95% CI 1.32–1.78], P < 0.001) and 27% (1.27 [1.10–1.45], P < 0.001), respectively. In addition, multivariate analysis models were also used to assess ASD risk according to TMAO quartiles (the lowest quartile [Q1] as the reference), with the adjusted OR (95% CIs) were recorded. As shown in the Fig. 3A, the Q3 and Q4 quartile of TMAO were compared against the Q1, and the risks were increased by 148% (OR = 2.48; 95%CI: 1.40–4.15; P =0.002) and 315% (4.15; 2.03–6.12; P < 0.001), respectively.
Fig. 3.
Multivariate logistic regression analysis for the ASD risk or severity based on TMAO quartiles. (A) Multivariate logistic regression analysis for the risk of ASD based on TMAO quartiles. (B) Multivariate logistic regression analysis for the risk of severe ASD based on TMAO quartiles. A total CRAS score of between 37 and 60 denotes severe autism. The adjusted factors included age, sex, ethnic, BMI, family history of ASD, the time from symptom onset to admission, plasma levels of choline and betaine. ASD = Autism spectrum disorder; CARS = Childhood Autism Rating Scale; TMAO =Trimethylamine N-oxide.
Furthermore, for each 1 μmol/l increase of plasma concentration of choline and betaine, the unadjusted risk of ASD would be increased by 31% (with the OR of 1.31 [95% CI 1.09–1.63], P = 0.012) and 7% (1.07 [1.01–1.19], P =0.019), respectively. In the multivariate logistic regression analysis adjusted for TAMO and other factors, plasma concentration of choline and betaine were not associated with ASD (with the OR of 1.16[95% CI 0.98–1.79; P =0.083] and 1.03 [0.93–1.18; P = 0.102], respectively).
3.3. Plasma levels of TMAO and severity of autism
For analysis of the relationship between TMAO and symptom severity as a categorical variable, 66 children with ASD (40.2%) were classified as having mild-to-moderate symptoms (CARS < 37) and 98 children with ASD (59.8%) were classified as having severe symptoms (CARS > 36). Plasma levels of TMAO were lower in children with mild-to-moderate symptoms than in children with severe symptoms (3.5[2.5–4.9] μmol/l vs. 4.5(3.7–6.0) μmol/l; P < 0.001; Fig. 1B). As shown in Table 2, plasma levels of choline and betaine were also higher than in children with severe symptoms than in children with mild-tomoderate symptoms (P < 0.05).
Table 2.
Characteristics of the Mild to moderate and severe ASD‡.
| Variable | ASD | Control cases | p-value † |
|---|---|---|---|
| N | 66 | 98 | - |
| Age, years | 4(3-6) | 5(3-7) | 0.42 |
| Sex-male | 54(81.8) | 75(76.5) | 0.059 |
| Ethnic-Han | 63(95.5) | 95(96.9) | 0.62 |
| BMI, kg/m2 | 16.7(16.3-17.6) | 16.3(16.0-17.2) | 0.49 |
| The time from symptom onset to admission, days | 104(66-158) | 106(82-125) | 0.94 |
| Family history of ASD | 5(7.6) | 10(10.2) | 0.57 |
| CARS | 43(39-48) | 33(31-35) | < 0.001 |
| Laboratory findings | |||
| TMAO (umol/l) | 3.5(2.5-4.9) | 4.5(3.7-6.0) | < 0.001 |
| Choline (μmol/l) | 7.7(6.2-10.5) | 10.2(6.8-15.0) | 0.001 |
| Betaine (μmol/l) | 20.3(15.9-22.8) | 23.4(18.5-30.6) | 0.001 |
The discrete variable was expressed in the percentage or frequency, continuous variable was expressed in the medians (interquartile range, IQR).
ASD: Autism spectrum disorders; BMI, body mass index; CARS, Childhood Autism Rating Scale; TMAO, Trimethylamine N-oxide.
P value was tested by chi-squared test or Mann-Whitney U test.
A total score of between 30 and 36 indicates mild-to-moderate autism, whereas the interval between 37 and 60 denotes severe autism.
Based on the ROC curve, the optimal cutoff value of TMAO as biomarker discriminating between mild-to-moderate and severe symptoms in children with ASD was projected to be 3.6 μmol/l, which yielded a sensitivity of 79.6% and a specificity of 53.3% (Fig. 2B), with the area under the curve at 0.69 (95%CI: 0.61–0.77; p < 0.001). With the AUC of 0.69, TAMO had greater discriminatory value than choline (AUC: 0.65; 95%: 0.57–0.74; P=0.039) and betaine (0.65; 0.57–0.73; P=0.036). Interestingly, the combined model including TMAO, choline, and betaine improved the value of TMAO to discriminate between mild-to-moderate and severe symptoms (AUC of the combined model, 0.76; 95% CI: 0.68–0.83; P=0.009). This improvement was stable in an internal 5-fold cross-validation that resulted in an average AUC of 0.69 (standard error [SE]=0.042) for the TMAO and 0.76 (SE=0.037) for the combined model(TMAO/choline/betaine), corresponding to a difference of 0.07 (SE=0.005, P =0.009).
A logistic regression analysis was performed to characterize the prediction of ASD symptom severity by TMAO levels with the OR (95%CI). For each 1 μmol/l increase of plasma concentration of TMAO, the unadjusted and adjusted (factors including for, age, sex, ethnicity, BMI, family history of ASD, the time from symptom onset to admission, plasma levels of choline and betaine) risk of severe symptoms would be increased by 61% (with the OR of 1.61 [95% CI 1.28–2.01], P < 0.001) and 31% (1.31 [1.08–1.49], P < 0.001), respectively. In addition, multivariate analysis models were also used to assess symptom severity according to TMAO quartiles (the lowest quartile [Q1] as the reference), with the adjusted OR (95% CIs) were recorded. As shown in the Fig. 3B, the Q3 and Q4 quartile of TMAO were compared against the Q1, and the risks were increased by 211% (OR =3.11; 95%CI: 1.52–6.17; P < 0.001) and 515% (6.15; 2.48–10.12; P < 0.001), respectively.
4. Discussion
Intestinal disturbances are reported clinically in ASD, and compositional changes in gut microbiota are described in the literature (Li et al., 2017a; Chaidez et al., 2014; Liu et al., 2019; Adams et al., 2011). However, the role of microbiota in brain disorders is poorlydocumented (De Theije et al., 2014). Gut-derived metabolite TMAO is a circulating metabolite that has been implicated in the development of atherosclerosis and cardiovascular disease (CVD) (Manor et al., 2018). To the best of our knowledge, this is the first study to measure plasma levels of TMAO in ASD and assess its association with symptom severity. Our results demonstrated that: (1) elevated levels of TMAO were associated with ASD, suggesting that TMAO might be useful as a biomarker of ASD that moderate response to intervention and early developmental trajectories for early prevention strategies; (2) elevated levels of TMAO were associated with severe autism defined by CARS score; (3) TMAO showed significantly greater discriminatory value than choline or betaine alone, especially when considered in a combined model including all three.
Environmental factors have been proposed as possible influences on the etiology of ASD (Bölte et al., 2019), and specifically, prior work demonstrated associations between TMAO blood levels and intestinal environment and flora (Romano et al., 2015). Current evidence suggests that the impairment of gut microbiota plays a key role in the development of ASD and mood disorders (Mangiola et al., 2016). Li et al. (2017b) reviewed the bidirectional interactions between the central nervous system and the gastrointestinal tract (brain-gut axis) and the role of the gut microbiota in the central nervous system (CNS) and ASD. An altered intestinal microbial community associated with ASD was proposed in a previous study (Strati et al., 2017). One study showed that autism-like behavior and its intestinal phenotype was associated with altered microbial colonization and activity in a murine model for ASD (De Theije et al., 2014). Wang et al. (2019a) reported that changes in the gut microenvironment may influence the pathogenesis of autism spectrum disorders. Wang et al. (2019b) found that thirty-four gut microbiota-associated epitopes (MEs) identified were potential biomarker of ASD, and alterations in MEs may contribute to abnormalities in gut immunity and/or homeostasis in ASD children. Consistent with above findings, we found that TMAO was associated with ASD risk and symptom severity.
Accumulating evidence has shown a link between alterations in the composition of the gut microbiota and both gastrointestinal and neurobehavioral symptoms in children with ASD (Fattorusso et al., 2019). However, our data did not imply any causal relationship between TMAO and development of ASD due to the cross-sectional design. We also could not determine from these data whether the higher levels of TMAO observed in our ASD group were a direct result of underlying ASD pathology or other originating factors in isolation or combination (e.g., diet, genetic predisposition, other metabolic differences). Further research is needed to determine whether reduction of plasma levels of TMAO to normal levels can improve the symptoms of children with ASD, in alignment with others’ assertions that altering the gut microbiome and virome (Kang et al., 2017), for example through microbiota transfer therapy (MTT) (Kang et al., 2019), can improve GI and behavioral symptoms of ASD.
Interestingly, in this study, we found that increased levels of betaine and choline in the children with autism while previous literatures reported that, at least in Italian and American children with autism (Lussu et al., 2017; Hamlin et al., 2013), these were deficient and another research showed that supplementation betaine could ameliorate autism-like features and play a beneficial role in a mouse autism model induced by prenatal valproic acid (VPA) exposure (Huang et al., 2019).
This discrepancy among those studies might be due to their ethnic/ racial differences, diet and nutrition status and activity level. Further studies are advocated to explain this difference, which may provide new proposal for the treatment of ASD.
Common mechanisms of action for gut microbiota and inflammation on the neural basis of ASD have been proposed (Doenyas, 2018). In this study, we found that levels of TMAO increased with increasing symptom severity in children with ASD. We speculate that TMAO plays an important role in the pathogenesis of ASD through the following pathways: (1) TMAO induces inflammation and endothelial dysfunction via activating ROS-TXNIP-NLRP3 inflammasome (Chen et al., 2017; Sun et al., 2016). Neuro-inflammation and neuro-immune abnormalities have now been established in ASD as key factors in its development and maintenance (Siniscalco et al., 2018). One study showed that ongoing inflammatory responses may be linked to disturbances in behavior in ASD (Ashwood et al., 2011). (2) Immune dysfunction and autoimmunity were pathological mechanisms in ASD (Hughes et al., 2018), and TMAO can initiate several innate immune responses lead to immune dysfunction (Ketelhuth and Hansson, 2011). (3) Circulating TMAO levels can impair eNOS-derived NO bioavailability by increasing vascular inflammation and oxidative stress (Li et al., 2017c). Oxidative stress has been suggested as an etiological factor for ASD, given its role in central nervous system function (Smaga et al., 2015).
Some limitations should be considered. First, this was a small sample (N =164) obtained from a single center. Second, we did not have any information on gastrointestinal symptoms or dietary conditions before blood sampling. As a result, the potential for dietary intake of TMAO (e.g., via large consumption of some fish species) within 24 h before blood sampling could not be excluded. Third, plasma level of TMAO was only measured one time at admission, and therefore are unable to draw conclusions about within-subjects variability in this biomarker over time. Fourth, the specific microbiome bacterium was not assessed in this study. However, previous studies demonstrated that alterations in the gut microbiota were observed in ASD individuals compared with neurotypical control subjects (Parracho et al., 2005; Tomova et al., 2015; Kang et al., 2013; Williams et al., 2011; Wang et al., 2013; Vuong and Hsiao, 2017). (Parracho et al., 2005) found that fecal bacterial profiling revealed a higher abundance of bacteria in the genus Clostridium among individuals with ASD. Another study showed that individuals with ASD also exhibited decreased Bacteroidetes/Firmicutes ratio, increased Lactobacillus and Desulfovibrio species, which correlated with symptom severity (Tomova et al., 2015). Furthermore, bacterial genera important for carbohydrate degradation and fermentation, including Prevotella, Coprococcus, and Veilonellaceae, were decreased in individuals with ASD (Kang et al., 2013; Williams et al., 2011). In addition, elevated abundance of Sutterella, which regulates mucosal metabolism and intestinal epithelial integrity had been found in individuals with ASD (Wang et al., 2013). Thus, these studies suggested that ASD was associated with altered composition and function of the gut microbiota (Vuong and Hsiao, 2017). Finally, our observational study did not allow us to identify specific cause-and-effect relationships.
5. Conclusion
In summary, elevated plasma levels of TMAO were associated with ASD and symptom severity, suggesting it as a potential causal factor for ASD. Further studies should be carried out to demonstrate the causal relationship between TMAO and ASD.
Acknowledgement
We express our gratitude to all the children, the parents and physicians who participated in this study, and thereby made this work possible. We especially want to express our gratitude to those doctors who participated in the clinical data collection.
Footnotes
Declaration of Competing Interest
None.
Ethics approval and consent to participate
The protocol and informed consent for this study were reviewed and approved by the Institutional Review Board at the first Affiliated Hospital of Nanchang University. The written informed consents were obtained from the parents before the children included.
Consent for publication
None.
Availability of data and material
Please contact the correspondence author for the data request.
References
- Adams JB, Johansen LJ, Powell LD, et al. , 2011. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 11 (1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, et al. , 2011. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun 25 (1), 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölte S, Girdler S, Marschik PB, 2019. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci 76 (7), 1275–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaidez V, Hansen RL, Hertz-Picciotto I, 2014. Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord 44 (5), 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhu X, Ran L, et al. , 2017. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J. Am. Heart Assoc 6 (9), e006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Braun KVN, Baio J, et al. , 2018. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2012. Mmwr Surveill. Summ 65 (13), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvert E, Tick B, McEwen F, et al. , 2015. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry 72 (5), 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Theije CGM, Wopereis H, Ramadan M, et al. , 2014. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav. Immun 37, 197–206. [DOI] [PubMed] [Google Scholar]
- Doenyas C, 2018. Gut microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience 374, 271–286. [DOI] [PubMed] [Google Scholar]
- Fattorusso A, Di Genova L, Dell’Isola GB, et al. , 2019. Autism spectrum disorders and the gut microbiota. Nutrients 11 (3), 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, 2013. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J. Nerv. Ment. Dis 201, 727–729. [DOI] [PubMed] [Google Scholar]
- Hamlin JC, Pauly M, Melnyk S, et al. , 2013. Dietary intake and plasma levels of choline and betaine in children with autism spectrum disorders. Autism Res. Treat, 578429 10.1155/2013/578429.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Chen X, Jiang X, et al. , 2019. Betaine ameliorates prenatal valproic-acid- induced autism-like behavioral abnormalities in mice by promoting homocysteine metabolism. Psychiatry Clin. Neurosci 73 (6), 317–322. [DOI] [PubMed] [Google Scholar]
- Hughes H, Mills Ko E, Rose D, et al. , 2018. Immune dysfunction and autoimmunity as pathological mechanisms in autism spectrum disorders. Front. Cell. Neurosci 12, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeiro M, Ramírez M, Milagro F, et al. , 2018. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients 10 (10), 1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Adams JB, Gregory AC, et al. , 2017. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Adams JB, Coleman DM, et al. , 2019. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep 9 (1), 5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. , 2013. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8, e68322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelhuth DFJ, Hansson GK, 2011. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb. Haemost 106 (11), 779–786. [DOI] [PubMed] [Google Scholar]
- Li Q, Han Y, Dy ABC, et al. , 2017a. The gut microbiota and autism spectrum disorders. Front. Cell. Neurosci 11, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Han Y, Dy ABC, et al. , 2017b. The gut microbiota and autism spectrum disorders. Front. Cell. Neurosci 11, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chen Y, Gua C, et al. , 2017c. Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front. Physiol 8, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li E, Sun Z, et al. , 2019. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep 9 (1), 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Elsabbagh M, Baird G, et al. , 2018. Autism spectrum disorder. Lancet 392, 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussu M, Noto A, Masili A, et al. , 2017. The urinary 1H-NMR metabolomics profile of an Italian autistic children population and their unaffected siblings. Autism Res. 10 (6), 1058–1066. [DOI] [PubMed] [Google Scholar]
- Mangiola F, Ianiro G, Franceschi F, et al. , 2016. Gut microbiota in autism and mood disorders. World J. Gastroenterol 22 (1), 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor O, Zubair N, Conomos MP, et al. , 2018. A multi-omic association study of trimethylamine N-oxide. Cell Rep. 24 (4), 935–946. [DOI] [PubMed] [Google Scholar]
- Meng G, Zhou X, Wang M, et al. , 2019. Gut microbe-derived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 10.1016/j.ebiom.2019.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning J, Xu L, Shen CQ, et al. , 2019. Increased serum levels of macrophage migration inhibitory factor in autism spectrum disorders. NeuroToxicology 71, 1–5. [DOI] [PubMed] [Google Scholar]
- Parracho HM, Bingham MO, Gibson GR, McCartney AL, 2005. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol 54, 987–991. [DOI] [PubMed] [Google Scholar]
- Qi J, You T, Li J, et al. , 2018. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J. Cell. Mol. Med 22 (1), 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann S, Linseisen J, Allenspach M, von Eckardstein A, Müller D, 2016. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J. Nutr 146, 283–289. [DOI] [PubMed] [Google Scholar]
- Romano KA, Vivas EI, Amador-Noguez D, et al. , 2015. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 6 (2), e02481–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalco D, Schultz S, Brigida A, et al. , 2018. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals 11 (2), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaga I, Niedzielska E, Gawlik M, et al. , 2015. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep 67 (3), 569–580. [DOI] [PubMed] [Google Scholar]
- Strati F, Cavalieri D, Albanese D, et al. , 2017. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5 (1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Jiao X, Ma Y, et al. , 2016. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROSTXNIP- NLRP3 inflammasome. Biochem. Biophys. Res. Commun 481 (1–2), 63–70. [DOI] [PubMed] [Google Scholar]
- Tang WHW, Wang Z, Kennedy DJ, et al. , 2015. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res 116 (3), 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. , 2015. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav 138, 179–187. [DOI] [PubMed] [Google Scholar]
- Tu WJ, Dong X, Zhao SJ, Yang DG, Chen H, 2013b. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischemic stroke. J. Neuroendocrinol 25 (9), 771–778. [DOI] [PubMed] [Google Scholar]
- Tu WJ, Qiu HC, Cao JL, et al. , 2019. Circulating serum retinoic acid for prediction of mortality in ischemic stroke. Neurology 92 (15), e1678–e1687. [DOI] [PubMed] [Google Scholar]
- Tu W, Yin C, Guo Y, et al. , 2013a. Serum homocysteine concentrations in Chinese children with autism. Clin. Chem. Lab. Med 51 (2), e19–e22. [DOI] [PubMed] [Google Scholar]
- Vuong HE, Hsiao EY, 2017. Emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry 81 (5), 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA, 2013. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wan J, Rong H, et al. , 2019a. Alterations in gut glutamate metabolism associated with changes in gut microbiota composition in children with autism spectrum disorder. MSystems 4 (1), e00321–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhou J, He F, et al. , 2019b. Alteration of gut microbiota-associated epitopes in children with autism spectrum disorders. Brain Behav. Immun 75, 192–199. [DOI] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, et al. , 2011. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 6, e24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DM, Wen X, Han XR, et al. , 2018. Relationship between neonatal vitamin D at birth and risk of autism spectrum disorders: the NBSIB study. J. Bone Miner. Res 33 (3), 458–466. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Warrier M, 2017. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr 37, 157–181. [DOI] [PubMed] [Google Scholar]
- Zhu W, Gregory JC, Org E, et al. , 2016. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165 (1), 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]