Abstract
The novel coronavirus disease 2019 (COVID-19) pandemic is a global crisis, challenging healthcare systems worldwide. Many patients present with a remarkable disconnect in rest between profound hypoxemia yet without proportional signs of respiratory distress (i.e. happy hypoxemia) and rapid deterioration can occur. This particular clinical presentation in COVID-19 patients contrasts with the experience of physicians usually treating critically ill patients in respiratory failure and ensuring timely referral to the intensive care unit can, therefore, be challenging. A thorough understanding of the pathophysiological determinants of respiratory drive and hypoxemia may promote a more complete comprehension of a patient’s clinical presentation and management. Preserved oxygen saturation despite low partial pressure of oxygen in arterial blood samples occur, due to leftward shift of the oxyhemoglobin dissociation curve induced by hypoxemia-driven hyperventilation as well as possible direct viral interactions with hemoglobin. Ventilation-perfusion mismatch, ranging from shunts to alveolar dead space ventilation, is the central hallmark and offers various therapeutic targets.
Keywords: COVID-19, SARS-CoV-2, Respiratory failure, Hypoxemia, Dyspnea, Gas exchange
Take home message
This review describes the pathophysiological abnormalities in COVID-19 that might explain the disconnect between the severity of hypoxemia and the relatively mild respiratory discomfort reported by the patients.
Background
In early December 2019, the first cases of a pneumonia of unknown origin were identified in Wuhan, the capital of Hubei province in China. The pathogen responsible for coronavirus disease 2019 (COVID-19) has been identified as a novel member of the enveloped RNA betacoronavirus family and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), due to similarities with SARS-CoV and Middle East Respiratory Syndrome (MERS) viruses. Although much is known about the epidemiology and the clinical characteristics of COVID-19, little is known about its impact on lung pathophysiology. COVID-19 has a wide spectrum of clinical severity, data classifies cases as mild (81%), severe (14%), or critical (5%) [1–3]. Many patients present with pronounced arterial hypoxemia yet without proportional signs of respiratory distress, they not even verbalize a sense of dyspnea [4–8]. This phenomenon is referred as silent or ‘happy’ hypoxemia. Tobin et al. recently presented three cases of happy hypoxemia with PaO2 ranging between 36 and 45 mmHg in the absence of increased alveolar ventilation (PaCO2 ranging between 34 and 41 mmHg) [5]. In patients with COVID-19, the severity of hypoxemia is independently associated with in-hospital mortality and can be an important predictor that the patient is at risk of requiring admission to the intensive care unit (ICU) [9, 10]. Since correct recognition of hypoxemia has such an impact on prognosis and timely treatment decisions, we here offer an overview of the pathophysiological abnormalities in COVID-19 that might explain the disconnect between hypoxemia and patient sensation of dyspnea.
Dyspnea as a sensation
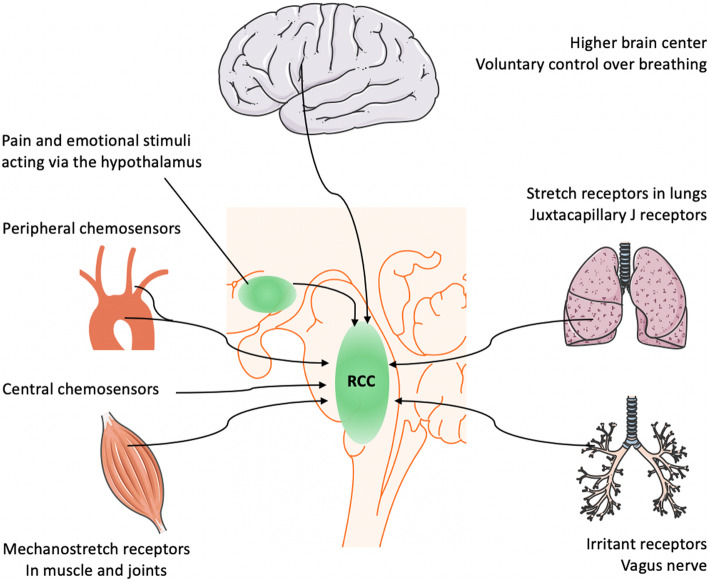
Breathing is centrally controlled by the respiratory center in the medulla oblongata and pons regions of the brainstem (see Fig. 1) that control the ‘respiratory drive’ to match respiration to the metabolic demands of the body [11, 12]. The main input affecting the respiratory drive is derived from chemical feedback among peripheral and central chemoreceptors. The center is, however, also influenced by higher brain cortex, hypothalamic integrative nociception, feedback from mechanostretch receptors in muscle and lung, and metabolic rate. The output of the respiratory center can be divided into rhythm- (e.g. respiratory rate) and pattern generating (e.g. depth of breathing effort) signals, and these outputs may be controlled independently [11, 13, 14]. Dyspnea is generally defined as a sensation of ‘uncomfortable, difficult, or labored’ breathing and occurs, in general, when the demand for ventilation is out of proportion to the patient’s ability to respond. It should be distinguished from tachypnea (rapid breathing) or hyperpnea (increased ventilation). Dyspnea grading relates to whether this feeling occurs in rest or upon exercise. This semi-quantitative approach of scoring is best exemplified by the frequently used modified Medical Research Council (MRC) dyspnea scale, which categorizes dyspnea from grade 0 (dyspnea only with strenuous exercise) to grade 4 (too dyspneic to leave house or breathless when dressing) in relation to subjects of the same age [15, 16].
Fig. 1.
Main inputs affecting respiratory center (RCC)
Various sensory, pain and emotional stimuli affect the sensation of breathing via the cerebral cortex and hypothalamus [17, 18]. The abnormal sense of muscle effort is another contributor to dyspnea. Conscious awareness of the activation of respiratory muscles is absent in healthy breathing. However, when the respiratory muscles are fatigued or weakened due to altered lung mechanics (e.g. decreased thoracic compliance), breathing may be perceived as a substantial effort [12]. Dyspnea can also be caused by input from the mechanoreceptors in the respiratory tract and the chest wall. Stimulation of vagal irritant receptors (e.g. bronchoconstriction, breathing through an external resistance) appears to intensify dyspnea [12, 19, 20]. The contribution of metabolic rate in modulating sense of dyspnea in critically ill patients remains unclear, despite its well-established role during exercise [11, 21]. The best-known determinants of the respiratory drive are the central and peripheral chemoreceptors. Changes in partial gas pressure of dissolved carbon dioxide in the blood (PaCO2) seem the most important component, causing shifts in pH at the level of both the peripheral and central chemoreceptors [11, 12, 22]. At steady state, the arterial PaCO2 is determined by the following equation:
| K | constant (863 mmHg) |
| VCO2 | Rate of CO2 production |
| VE | minute ventilation |
| VD | dead space |
| Vt | tidal volume |
The normal response to hypercapnia (caused by increased VD, hypoventilation or increased VCO2) is an increase in respiratory drive and minute volume ventilation [23]. Hypoxemia itself rather plays a limited role in the sensation of breathlessness experienced by patients with cardiopulmonary disease on the opposite of hypercapnia that creates per se dyspnea [12, 24, 25]. In healthy subjects, the respiratory drive shifts minimally in mild hypoxemia (PaO2 60–65 mmHg), such as resulting from stays at high altitude or experimental hypoxic chambers [11, 26]. Many patients with dyspnea are not hypoxemic, while those who are, usually experience only a slight improvement in symptoms after hypoxemia is corrected with supplemental oxygen therapy [12]. When arterial PaO2 drops below 40 mmHg, dyspnea often occurs [12]. Of note, the normal response to hypoxemia is a rise in minute ventilation, primarily by increasing tidal volume and respiratory rate. Increased respiratory rate (tachypnea) and tidal volume (hyperpnea) - and not dyspnea - are therefore the most important clinical signs of impending hypoxemic respiratory failure [11, 27]. Furthermore, PaCO2 serves as one of the fundamental regulators of cerebral blood flow. Hyperventilation causes decreased PaCO2 which subsequently leads to arterial vasoconstriction thus lowering cerebral blood flow and intracranial pressure. In contrary, increase in PaCO2 leads to increased intracranial pressure ultimately leading to deteriorating level of consciousness, altered brainstem reflexes, and altered postural and motor responses [28, 29]. At the bedside, a profound understanding of the pathophysiological determinants of respiratory drive and hypoxemia may promote a more complete comprehension of a COVID-19 of a patient’s clinical presentation and timely management [11].
Happy hypoxemia in COVID-19
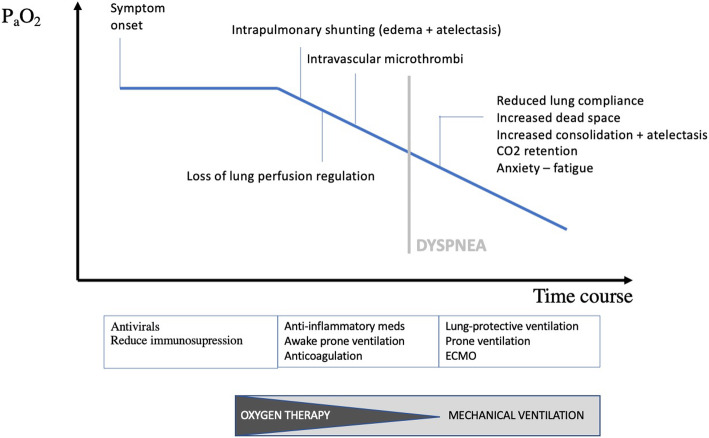
The disconnect between the severity of hypoxemia and the relatively mild respiratory discomfort reported by the COVID-19 patients contrasts with the experience of physicians usually treating critically ill patients in respiratory failure [30]. Guan reported dyspnea in only 18.7% of 1099 hospitalized COVID-19 patients, despite low PaO2/FiO2 ratios, abnormal CT scans (86%) and common requirement for supplemental oxygen (41%) [31]. Happy or silent hypoxemia is not exclusively seen in COVID-19, but may also occur in patients with atelectasis, intrapulmonary shunt (i.e. arterio-venous malformations) or right-to-left intracardiac shunt. The adequacy of gas exchange is primarily determined by the balance between pulmonary ventilation and capillary blood flow, referred as ventilation/perfusion (V/Q) matching [32]. In the initial phase of COVID-19, several mechanisms contribute to the development of arterial hypoxemia (see Fig. 2), without a concomitant increase in work of breathing. Rapid clinical deterioration may occur.
Fig. 2.
Mechanisms of hypoxemia in COVID-19
Changes in oxyhemoglobin dissociation curve
Oxygen saturation measured by pulse oximetry (SpO2) is often used to detect hypoxemia. However, SpO2 should be interpreted with caution in COVID-19. The sigmoid shaped oxyhemoglobin dissociation curve seems to shift to the left, due to induced respiratory alkalosis (drop in PaCO2) because of hypoxemia-driven tachypnea and hyperpnea. During hypocapnic periods, the affinity of hemoglobin for oxygen and thus oxygen saturation increases for a given degree of PaO2, explaining why SpO2 can be well-preserved in the face of a profoundly low PaO2 [33–35]. This finding is also seen in high altitude hypoxemia, in which hypocapnia significantly shifts the oxygen-hemoglobin dissociation curve and improves blood oxygen saturation [36]. The alveolar gas equation also predicts that hyperventilation and the resulting drop in the alveolar partial pressure of carbon dioxide leads to an increase in the alveolar partial pressure of oxygen and ultimately lead to an increase in SpO2 [22].
There might also be a biological explanation for the leftward shift of the curve in COVID-19. Liu et al. put forward hypotheses about direct viral interaction with the heme group of hemoglobin. According to this theory, heme serum levels are increasing in COVID-19 along with harmful iron ions (Fe3+) causing inflammation and cell death (ferroptosis). This leads to the production of large amounts of serum ferritin to bind these free irons in order to reduce tissue damage [37]. In conclusion, SpO2 should be interpreted in the light of the presence of hyperventilation (tachypnea, low PaCO2) and, if possible, PaO2 via arterial puncture. Measuring the alveolar to arterial oxygen (P(A-a)O2 gradient (150 mmHg - PaCO2/0.8 - PaO2 at sea level) and relating this value to age and supplemental oxygen (age/4 + 4 + 50 (FiO2–0.21) in mmHg) can be insightful. This can be performed rapidly on a smartphone app [38]. The P(A-a)O2 gradient is increased either by V/Q mismatch or by intrapulmonary shunting. Hypoxemia due to V/Q mismatch can be easily corrected by supplemental oxygen therapy whereas pulmonary shunts have a poor response to oxygen therapy [39].
Causes of hypoxemia in COVID-19
Intrapulmonary shunting
Arterial hypoxemia early in SARS-CoV-2 infection is primarily caused by V/Q mismatch and thus persistence of pulmonary arterial blood flow to non-ventilated alveoli, reflected by a marked increase in P(A-a)O2 gradient. The infection leads to a modest local interstitial edema, particularly located at the interface between lung structures with different elastic properties, where stress and strain are concentrated [27]. Due to increased lung edema (leading to ground-glass opacities and consolidation on chest imaging), loss of surfactant and superimposed pressure, alveolar collapse ensues and a substantial fraction of the cardiac output is perfusing non-aerated lung tissue, resulting in intrapulmonary shunting [27]. As previously discussed, tidal volume increases during the disease course leading to rising negative inspiratory intrathoracic pressure. The latter, in combination with increased lung permeability due to inflammation, will eventually result in progressive edema, alveolar flooding, and patient self-inflicted lung injury (P-SILI), as first described by Barach in 1938 [11, 40, 41]. Over time, the increased edema will further enhance lung weight, alveolar collapse, and dependent atelectasis, resulting in progressively increasing shunt fraction and further decline of oxygenation which cannot completely be corrected by increasing FiO2.
Loss of lung perfusion regulation
The persistence of high pulmonary blood flow to non-aerated lung alveoli appears to be caused by the relative failure of the hypoxic pulmonary vasoconstriction mechanism (constriction of small intrapulmonary arteries in response to alveolar hypoxia) during SARS-CoV-2 infection, as recently illustrated by Lang et al. using dual-energy CT [42, 43]. Whether the latter mechanism is only triggered by the release of endogenous vasodilator prostaglandins, bradykinin, and cytokines associated with the inflammatory process or also by other yet undefined mechanisms remains to be investigated [33, 44, 45]. Vasoplegia also seems to be influential in the loss of lung perfusion regulation, possibly induced by shear stress on the interfaces between lung structures, as part of the P-SILI spectrum [45–47]. Further, dysregulation of the renin-angiotensin system (RAS) contribute to the pathophysiology of COVID-19 [48–52]. Angiotensin-converting enzyme 2 (ACE2) is the principal functional receptor used by SARS-CoV-2 for cell entry, implying ACE2 internalization [52–54]. ACE2 converts angiotensin II (Ang II) to angiotensin 1–7 (Ang 1–7) and is also important for degrading bradykinin. Hence, diminished levels of ACE2 lead to an increase in Ang II, mediating pulmonary vasoconstriction through agonism at Ang II-receptor, while Ang 1–7 opposes the actions of Ang II [50, 51, 54]. Recently, Liu et al. revealed that serum Ang II levels were linearly associated with viral load and lung injury in COVID-19 [55].
Intravascular microthrombi
Endothelial injury is emerging as a central hallmark of COVID-19 pathogenesis, and the cytopathic virus can directly infect lung capillary endothelial cells that express ACE2 [54, 56]. Intravascular microthrombi are the net result of an imbalance between procoagulant and fibrinolytic activity in the presence of acute inflammation and endothelial injury [45, 57–59]. The pro-coagulant activity might result from complement system-mediated activation of clotting, similar to some forms of thrombotic microangiopathy (TMA), or could be due to inhibition of plasminogen activation and fibrinolysis via increased activity of plasminogen activator inhibitor (PAI-1 and -2) which are induced as acute-phase proteins under the influence of IL-6. Diffuse intravascular coagulation (DIC) is also seen in patients with severe COVID-19, mediated via endothelial release of tissue factor and activation of clotting factor VII and XI. Many patients with COVID-19 develop elevated D-dimers suggesting the formation of blood clots. D-dimer levels on admission are used to predict in-hospital mortality in COVID-19, and DIC present much more frequently (71%) in COVID-19 patients with a dismal prognosis, versus only 0,6% of survivors [60–63]. Autopsy of the lungs after severe disease showed fibrin deposition, diffuse alveolar damage, vascular wall thickening, and frequently occurring complement-rich microthrombi occluding lung capillaries and larger thrombi causing pulmonary artery thrombosis and embolism [63–65]. Hypercoagulable state leads to further deterioration in V/Q mismatch and lung tissue damage. Moreover, coagulation is also modulated by activating C-reactive protein and ensuing complement activation and hepatic synthesis of fibrinogen as an acute phase protein in COVID-19 [66].
Impaired diffusion capacity
Lung diffusion capacity (DLCO) can be impaired, although pure diffusion defects are rarely a cause for increased P(A-a)O2 gradient at rest [67, 68]. SARS-CoV-2 propagates within alveolar type II cells, where a large number of viral particles will be produced and released, followed by immune response mediated destruction of infected cells (virus-linked pyroptosis) [54]. Loss of alveolar epithelial cells and a pro-coagulant state cause the denuded basement membrane to be covered with debris, consisting of fibrin, dead cells, and complement activation products, collectively referred to as hyaline membranes [54, 69]. With incremental exercise and in the face of absent hypoxic vasoconstriction in COVID-19, a hyperdynamic pulmonary circulation might not allow sufficient time for red blood cells to equilibrate their oxygen uptake. A diffusion limitation may, therefore, occur in COVID-19 leading to a raised P(A-a)O2 gradient and exercise-induced arterial hypoxemia (EIAH) [68]. Recently, Xiaoneng Mo et al. confirmed a decrease in DLCO in COVID-19 patients at the time of discharge. The prevalence of impaired diffusing-capacity was linked to the severity of disease, respectively 30.4% in mild illness, 42.4% in pneumonia and 84.2% in severe pneumonia [70]. Long-term studies are needed to address whether these deficits are persistent as seen in MERS where 37% of MERS survivors still presented with an impairment of DLCO [39].
Preservation of lung mechanics
The outline presented in the previous paragraphs largely clarifies the dissociation between the severity of hypoxemia in COVID-19 and relatively well-preserved lung mechanics. Gas exchange abnormalities in some patients with COVID-19 occur earlier than increases in mechanical loads [41]. During the first days of infection, there is no increased airway resistance, and there is presumably no increased anatomical or physiological dead space ventilation. The breathing effort also remains rather low because lung compliance is normal in many patients without pre-existing lung disease. As recently shown by Gattinoni et al. in a cohort of 16 critically ill patients, relatively normal values for respiratory system compliance (50.2 ± 14.3 ml/cmH2O) went hand in hand with a dramatically increased shunt fraction of 0.50 ± 0.11 [47]. Such a wide discrepancy is highly unusual for most forms of disorders that lead to acute lung injury and ARDS [47, 71]. Relatively high compliance indicates a well-preserved lung gas volume and explains in part the absence of dyspnea early in the course of illness [42, 47, 61, 72, 73]. In contrast, Ziehr et al. described a low compliance and a uniform presentation consistent with the Berlin definition for ARDS in a cohort of COVID-19 patients [30, 70]. Of note, patients on mechanical ventilation have the highest COVID-19 severity and thus probably the lowest respiratory system compliance. Dyspnea itself may have precipitated mechanical ventilation, and the latter may be a surrogate marker for low compliance in COVID-19 [41]. Understanding of the respiratory mechanics found in COVID-19 will continue to evolve as further research is reported.
Rapid deterioration
Hypoxemia-driven tachypnoea, hyperpnea and altered oxygenation predict clinical deterioration induced by either disease severity and/or host response and/or suboptimal management [35, 61]. As the disease progresses, the more consolidated air spaces do not inflate as easily at higher transpulmonary pressures. The volume loss is proportionally greater at higher lung volumes. This loss of volume reduces total lung compliance and increases the work of breathing [45]. There is also evidence that the dynamic compliance of the remaining ventilated lung is reduced in SARS-CoV-2 pneumonia (as seen in pneumococcal pneumonia) most possibly by a reduction in surfactant activity, further increasing the work of breathing [45]. Physiological dead space is also increasing due to reduced blood flow caused by intravascular thrombi. Importantly, the anxiety experienced by COVID-19 patients also affects the cortical feedback to the respiratory centers. Consequently, as the disease progresses, dyspnea becomes increasingly apparent.
Thoughts on management
At the stage that COVID-19 patients are admitted to the hospital with hypoxemia, viral replication is well underway and in addition to giving antiviral medication, optimization of the V/Q mismatch and reduction of cytokine storm remain the major therapeutic goals. Regarding perfusion, avoiding microthrombi and ongoing fibrin deposition is one of the therapeutic strategies. It seems prudent to use thromboprophylaxis in all COVID-19 patients, particularly in those with high D-dimers on admission [59, 66, 74]. Moore et al. recently suggested the use of tissue plasminogen activator (tPA) to treat ARDS in COVID-19 [75]. In addition, tackling the systemic prothrombotic complication using anti-inflammatory medications (such as anti-IL6R tocilizumab or sarilumab, or the anti-IL6 antibody siltuximab or complement inhibiting strategies) to prevent macro- and microthrombi represents another potential approach and several trials are currently verifying this hypothesis [54]. Effective hypoxic pulmonary vasoconstriction may be another target to improve the matching of regional perfusion and ventilation in the lung. There is an excessive release of inflammatory mediators that disturbs the balance between nitric oxide (NO), endothelin, and prostanoids in the pulmonary capillaries [76], although inhaled NO has consistently failed to show an improvement in mortality in ARDS [53–55, 84]. RAS modulation (e.g. angiotensin receptor blockers, recombinant soluble ACE2, and inhibition of the bradykinin system) may have a potential role in restoring lung perfusion regulation, and trials are ongoing [50–52].
Regarding ventilation, supplemental oxygen is the first step in facilitating oxygenation. In patients with refractory hypoxemic respiratory failure (increasing shunt fraction), timely but not premature intubation and invasive ventilation support may be superior to non-invasive ventilation in boosting transpulmonary pressure, opening collapsed alveoli, improving oxygenation, decreasing oxygen debt, avoiding P-SILI and offering a better chance for the lungs to heal [61, 76, 77]. Considering the critical cases, most patients fulfil the Berlin criteria of ARDS, where lung-protective ventilation, prone ventilation, effective sedation and analgesia, and high positive end-expiratory pressure (PEEP) are key [54, 61, 70, 77]. COVID-19 patients are exquisitely PEEP sensitive [78–80]. Tolerance for modest permissive hypercapnia minimizes ventilator-induced lung injury (VILI) [61]. Since prone position recruits the dorsal lung regions and diverts blood flow to these caudal regions, it may have particular importance in COVID-19 when used early and in relatively long sessions [42]. Although further trials are needed to evaluate the impact on disease severity and mortality, several authors confirmed that awake proning can improve oxygenation in COVID-19 [81–83].
Conclusion
The remarkable dissociation between profound hypoxemic respiratory failure and a clinically ‘happy’ patient is frequently seen and should prompt physicians and health care workers not only to rely on the patient’s apparent wellbeing but closely monitor respiratory rate, signs of hyperventilation, oxygen saturation and invasive measurements of hypoxemia/hypocapnia at regular time intervals. Pulse oximetry should be interpreted with caution, because left-sided shifting of the oxyhemoglobin dissociation curve. The arterial hypoxemia is induced by intrapulmonary shunting, dysregulated hypoxic pulmonary vasoconstriction, impaired lung diffusion, and formation of intravascular microthrombi. As in the first days of the disease, the lung mechanics are well-preserved and there is no increased airway resistance or dead space ventilation. The respiratory center thus does not sense an uncomfortable sensation of breathing. However, sudden and rapid respiratory decompensation may occur, and tachypnea and hyperpnea might be the most important clinical warning signs of impending respiratory failure in COVID-19 patients.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- MERS
Middle East Respiratory Syndrome
- ICU
Intensive Care Unit
- PaCO2
Partial gas pressure of dissolved carbon dioxide in the blood
- PaO2
Partial gas pressure of dissolved oxygen in the blood
- SpO2
Oxygen saturation measured by pulse oximetry
- P(A-a)O2 gradient
the alveolar to arterial oxygen gradient
- VCO2
Rate of CO2 production
- VE
Minute ventilation
- VD
Dead space
- Vt
Tidal volume
- V/Q
Ventilation/Perfusion
- FiO2
Fraction of inspired oxygen
- P-SILI
Patient self-inflicted lung injury
- VILI
Ventilator-induced lung injury
- RAS
Renin-angiotensin system
- ACE2
Angiotensin-converting enzyme 2
- Ang
Angiotensin
- TMA
Thrombotic microangiopathy
- PAI
Plasminogen activator inhibitor
- DIC
Diffuse intravascular coagulation
- DLCO
Lung diffusion capacity
- NO
Nitric oxide
- PEEP
Positive end-expiratory pressure
Authors’ contributions
SD, ED and BNL wrote the manuscript. BNL, EVB, PD and ED read and corrected were needed. All authors took part in the discussion leading up to the manuscript. The authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Omer SB, Malani P, Del Rio C. The COVID-19 pandemic in the US: a clinical update. JAMA. 2020. Epub ahead of print. [DOI] [PubMed]
- 3.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Articles estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020; [cited 2020 Apr 30]; Available from: www.thelancet.com/infection. [DOI] [PMC free article] [PubMed]
- 4.Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46:837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020; [cited 2020 Jun 23]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32539537. [DOI] [PMC free article] [PubMed]
- 6.Couzin-Frankel J. The mystery of the pandemic’s ‘happy hypoxia’ [internet]. Science (80- ). 2020:455–6 [cited 2020 Jun 23]. American Association for the Advancement of Science; Available from: https://science.sciencemag.org/content/368/6490/455. [DOI] [PubMed]
- 7.Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020; W.B. Saunders; [cited 2020 Jun 23]; Available from: https://pubmed.ncbi.nlm.nih.gov/32471783/. [DOI] [PMC free article] [PubMed]
- 8.Allali G, Marti C, Grosgurin O, Morélot-Panzini C, Similowski T, Adler D. Dyspnea: the vanished warning symptom of COVID-19 pneumonia. J Med Virol. 2020;jmv.26172 Wiley; [cited 2020 Jul 4]. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.26172. [DOI] [PMC free article] [PubMed]
- 9.Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020; Elsevier; [cited 2020 Apr 19];0. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0025619620303670. [DOI] [PMC free article] [PubMed]
- 10.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. Elsevier BV. 2020;0. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 11.Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically ill patients pathophysiology and clinical implications. Am J Respir Crit Care Med. 2020;201:20–32. doi: 10.1164/rccm.201903-0596SO. [DOI] [PubMed] [Google Scholar]
- 12.Epstein FH, Manning HL, Schwartzstein RM. Pathophysiology of dyspnea [internet]. Epstein FH, editor. N Engl J Med. 1995:1547–53 Massachusetts Medical Society ; [cited 2020 Apr 19]. Available from: http://www.nejm.org/doi/10.1056/NEJM199512073332307. [DOI] [PubMed]
- 13.Costa R, Navalesi P, Cammarota G, Longhini F, Spinazzola G, Cipriani F, et al. Remifentanil effects on respiratory drive and timing during pressure support ventilation and neurally adjusted ventilatory assist. Respir Physiol Neurobiol. 2017;244:10–16. doi: 10.1016/j.resp.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Corne S, Webster K, C Ginn GM, St-john W, Younes M. Medullary metastasis causing impairment of respiratory pressure output with intact respiratory rhythm. Am J Respir Crit Care Med. 1999; Available from: www.atsjournals.org. [DOI] [PubMed]
- 15.Richards JB. Calculated decisions: mMRC (modified Medical Research Council) dyspnea scale. Emerg Med Pract. 2017;19:1–2. [PubMed] [Google Scholar]
- 16.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2:257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raux M, Ray P, Prella M, Duguet A, Demoule A, Similowski T. Cerebral cortex activation during experimentally induced ventilator fighting in normal humans receiving noninvasive mechanical ventilation. Anesthesiology. 2007;107:746–755. doi: 10.1097/01.anes.0000287005.58761.e8. [DOI] [PubMed] [Google Scholar]
- 18.Esnault P, Roubin J, Cardinale M, D’Aranda E, Montcriol A, Cungi PJ, et al. Spontaneous hyperventilation in severe traumatic brain injury: incidence and association with poor neurological outcome. Neurocrit Care. 2019;30:405–413. doi: 10.1007/s12028-018-0639-0. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton RD, Winning AJ, Perry A, Guz A. Aerosol anesthesia increases hypercapnic ventilation and breathlessness in laryngectomized humans. J Appl Physiol. 1987;63:2286–2292. doi: 10.1152/jappl.1987.63.6.2286. [DOI] [PubMed] [Google Scholar]
- 20.Manning HL, Shea SA, Schwartzstein RM, Lansing RW, Brown R, Banzett RB. Reduced tidal volume increases “air hunger” at fixed PCO2 in ventilated quadriplegics. Respir Physiol. 1992;90:19–30. doi: 10.1016/0034-5687(92)90131-F. [DOI] [PubMed] [Google Scholar]
- 21.Dempsey JA, Smith CA. Pathophysiology of human ventilatory control: number 6 in the series “physiology in respiratory medicine”. Eur Respir JEuropean Respiratory Society. 2014:495–512. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 22.Leusen IR. Chemosensitivity of the respiratory center; influence of CO2 in the cerebral ventricles on respiration. Am J Physiol. 1954;176:39–44. doi: 10.1152/ajplegacy.1953.176.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. NeuronCell Press. 2015:946–61. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 24.Kvale PA, Conway WA, Coates EO. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. A clinical trial. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 25.Gandevia SC, Killian K, McKenzie DK, Crawford M, Allen GM, Gorman RB, et al. Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J Physiol. 1993;470:85–107. doi: 10.1113/jphysiol.1993.sp019849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Easton PA, Slykerman LJ, Anthonisen NR. Ventilatory response to sustained hypoxia in normal adults. J Appl Physiol. 1986;61:906–911. doi: 10.1152/jappl.1986.61.3.906. [DOI] [PubMed] [Google Scholar]
- 27.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia : different respiratory treatment for different phenotypes ? Intensive Care Med. 2020:1–6 Springer; [cited 2020 Apr 19]. Available from: https://www.esicm.org/wp-content/uploads/2020/04/684_author-proof.pdf. [DOI] [PMC free article] [PubMed]
- 28.Mchenry LC, Slocum HC, Bivens HE, Mayes HA, Hayes GJ. Hyperventilation in awake and anesthetized man: effects on cerebral blood flow and cerebral metabolism. Arch Neurol. 1965;12:270–277. doi: 10.1001/archneur.1965.00460270046006. [DOI] [PubMed] [Google Scholar]
- 29.Meng L, Gelb AW. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology. 2015:196–205 Lippincott Williams and Wilkins; [cited 2020 Jun 23]. Available from: https://pubmed.ncbi.nlm.nih.gov/25401418/. [DOI] [PubMed]
- 30.Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020; W.B. Saunders; [cited 2020 May 31]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735675720303909. [DOI] [PMC free article] [PubMed]
- 31.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J MedMassachusetts Medical Society. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 32.D’Alonzo GE, Dantzker DR. Respiratory failure, mechanisms of abnormal gas exchange, and oxygen delivery. Med Clin North Am. 1983;67:557–571. doi: 10.1016/S0025-7125(16)31189-0. [DOI] [PubMed] [Google Scholar]
- 33.Ottestad W, Søvik S. COVID-19 patients with respiratory failure: what can we learn from aviation medicine? Br J AnaesthElsevier BV. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 34.Hamilton C, Steinlechner B, Gruber E, Simon P, Wollenek G. The oxygen dissociation curve: quantifying the shift. Perfusion. 2004;19:141–144. doi: 10.1191/0267659104pf734oa. [DOI] [PubMed] [Google Scholar]
- 35.Woyke S, Rauch S, Ströhle M, Gatterer H. Modulation of Hb-O2 affinity to improve hypoxemia in COVID-19 patients. Clin NutrChurchill Livingstone. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 36.Sainburg RL, Clark AL, Billman GE, Schlader ZJ, Mündel T, Milne K, et al. Hypoxia, focus hypobaric hypoxia. Encycl Exerc Med Heal DisSpringer Berlin Heidelberg. 2012:428–31. Epub ahead of print.
- 37.Liu W, Hualan L. COVID-19:attacks the 1-Beta chain of hemoglobin and captures the porphyrin to inhibit human Heme metabolism. ChemRxiv. 2020. Epub ahead of print.
- 38.A-a O2 Gradient - MDCalc [Internet]. [cited 2020 Apr 30]. Available from: https://www.mdcalc.com/a-a-o2-gradient.
- 39.Park WB, Il JK, Kim G, Choi JP, Rhee JY, Cheon S, et al. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J Korean Med SciKorean Academy of Medical Science. 2018:33. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 40.Barach AL, Martin J, Eckman M. Positive pressure respiration and its application to the treatment of acute pulmonary edema. Ann Intern Med. 1938;12:754. doi: 10.7326/0003-4819-12-6-754. [DOI] [Google Scholar]
- 41.Komorowski M, Aberegg SK. Using applied lung physiology to understand COVID-19 patterns. Br J Anaesth. 2020;0 Elsevier; [cited 2020 May 31]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0007091220303755. [DOI] [PMC free article] [PubMed]
- 42.Archer SL, Sharp WW, Weir EK. Differentiating COVID-19 pneumonia from acute respiratory distress syndrome (ARDS) and high altitude pulmonary edema (HAPE): therapeutic implications. CirculationOvid Technologies (Wolters Kluwer Health). 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 43.Lang M, Som A, Mendoza DP, Flores EJ, Reid N, Carey D, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect DisLancet Publishing Group. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 44.Nagaraj C, Tabeling C, Nagy BM, Jain PP, Marsh LM, Papp R, et al. Hypoxic vascular response and ventilation/perfusion matching in end-stage COPD may depend on p22phox. Eur Respir JEuropean Respiratory Society. 2017;50. Epub ahead of print. [DOI] [PubMed]
- 45.Light RB. Pulmonary pathophysiology of pneumococcal pneumonia. Semin Respir Infect. 1999:218–26. Epub ahead of print. [PubMed]
- 46.van Heerden PV, Cameron PD, Karanovic A, Goodman MA. Orthodeoxia - an uncommon presentation following bilateral thoracic sympathectomy. Anaesth intensive care. SAGE PublicationsSage UK: London. England. 2003;31:581–583. doi: 10.1177/0310057X0303100516. [DOI] [PubMed] [Google Scholar]
- 47.Gattinoni L, Coppola S, Cressoni M, Busana M, Chiumello D. Covid-19 does not Lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care MedAmerican Thoracic Society. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 48.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med VirolJohn Wiley and Sons Inc. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 49.South AM, Diz D, Chappell MC. COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol Heart Circ PhysiolNLM (Medline). 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 50.Kickbusch I, Leung G. Response to the emerging novel coronavirus outbreak. BMJBMJ Publishing Group. 2020. Epub ahead of print. [DOI] [PubMed]
- 51.Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care. 2017:305 BioMed Central Ltd. [cited 2020 Apr 28]. Available from: https://ccforum.biomedcentral.com/articles/10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed]
- 52.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J MedMassachusetts Medical Society. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 53.Wang S, Guo F, Liu K, Wang H, Rao S, Yang P, et al. Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res. 2008;136:8–15. doi: 10.1016/j.virusres.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev ImmunolNature Research. 2020:363–74. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 55.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020; Journal of the American College of Cardiology; [cited 2020 Apr 20]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109720350087. [DOI] [PMC free article] [PubMed]
- 58.Guérin C, Matthay MA. Acute cor pulmonale and the acute respiratory distress syndrome. Intensive Care MedSpringer Verlag. 2016:934–6. Epub ahead of print. [DOI] [PubMed]
- 59.Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID-19 related systemic thrombosis? [cited 2020 Apr 20]; Available from: http://ahajournals.org. [DOI] [PubMed]
- 60.Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020; [cited 2020 Apr 25]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32306492. [DOI] [PMC free article] [PubMed]
- 61.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020; [cited 2020 Apr 26]; Available from: https://jamanetwork.com/journals/jama/fullarticle/2765302. [DOI] [PubMed]
- 62.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb HaemostNLM (Medline). 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 63.Monsalve-Naharro JÁ, Domingo-Chiva E, Castillo SG, Cuesta-Montero P, Jiménez-Vizuete JM. Inhaled nitric oxide in adult patients with acute respiratory distress syndrome. Farm HospSociedad Espanola de Farmacia Hospitalaria. 2017:292–312. Epub ahead of print. [DOI] [PubMed]
- 64.Luo W, Yu H, Gou J, Li X, Sun Y, Li J, et al. Title: clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19) list of authors. 2020. [Google Scholar]
- 65.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao S-Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost. 2020;jth.14872 [cited 2020 Apr 25]. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jth.14872. [DOI] [PMC free article] [PubMed]
- 67.Di Marco F, Devaquet J, Lyazidi A, Galia F, Da Costa NP, Fumagalli R, et al. Positive end-expiratory pressure-induced functional recruitment in patients with acute respiratory distress syndrome. Crit Care Med. 2010;38:127–132. doi: 10.1097/CCM.0b013e3181b4a7e7. [DOI] [PubMed] [Google Scholar]
- 68.Hopkins SR. Exercise induced arterial hypoxemia: the role of ventilation-perfusion inequality and pulmonary diffusion limitation. Adv Exp Med BiolSpringer New York. 2006:17–30. Epub ahead of print. [DOI] [PubMed]
- 69.Mason RJ. Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respir J. 2020; European Respiratory Society; [cited 2020 Apr 20]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32269085. [DOI] [PMC free article] [PubMed]
- 70.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care MedNLM (Medline). 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 71.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ottestad W, Seim M, Mæhlen JO. Covid-19 med stille hypoksemi. Tidsskr Nor LaegeforenNLM (Medline). 2020;140. Epub ahead of print. [DOI] [PubMed]
- 73.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA - J Am Med AssocAmerican Medical Association. 2020. Epub ahead of print. [DOI] [PubMed]
- 74.Spyropoulos AC, Ageno W, Barnathan ES. Hospital-based use of thromboprophylaxis in patients with COVID-19. Lancet. 2020;0 Elsevier; [cited 2020 Apr 25]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620309260. [DOI] [PMC free article] [PubMed]
- 75.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020; [cited 2020 Apr 25]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32267998. [DOI] [PMC free article] [PubMed]
- 76.Tarry D, Powell M. Hypoxic pulmonary vasoconstriction. BJA Educ. 2017;17:208–213. doi: 10.1093/bjaed/mkw076. [DOI] [Google Scholar]
- 77.Meng L, Qiu H, Wan L, Ai Y, Xue Z, Guo Q, et al. Intubation and ventilation amid the COVID-19 outbreak. AnesthesiologyOvid Technologies (Wolters Kluwer Health). 2020;1. Epub ahead of print.
- 78.Dondorp AM, Hayat M, Aryal D, Beane A, Schultz MJ. Respiratory support in novel coronavirus disease (COVID-19) patients, with a focus on resource-limited settings. Am J Trop Med Hyg. 2020; [cited 2020 Apr 25]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32319424. [DOI] [PMC free article] [PubMed]
- 79.Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 80.Elharrar X, Trigui Y, Dols A-M, Touchon F, Martinez S, Prud’homme E, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMAAmerican Medical Association (AMA). 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 81.Caputo ND, Strayer RJ, Levitan R. Early self-Proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Kline J, editor. Acad Emerg Med. 2020;27:375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit CareBioMed Central Ltd. 2020;24. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 83.Slessarev M, Cheng J, Ondrejicka M, Arntfield R. Patient self-proning with high-flow nasal cannula improves oxygenation in COVID-19 pneumonia. Can J AnesthSpringer. 2020:1. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 84.Monsalve-Naharro JÁ, Domingo-Chiva E, Castillo SG, Cuesta-Montero P, Jiménez-Vizuete JM. Inhaled nitric oxide in adult patients with acute respiratory distress syndrome. Farm Hosp. 2017:292–312 Sociedad Espanola de Farmacia Hospitalaria; [cited 2020 Apr 25]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28236803. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.