Abstract
The novel coronavirus, 2019-nCoV, has quickly spread across the world and pose serious threat to public health because it can infect people very easily. The major clinical symptoms of 2019-nCoV infection include fever, dry cough, myalgia, fatigue, and diarrhea. The 2019-nCoV belongs to the betacoronavirus family, and gene sequencing results demonstrate that it is a single-stranded RNA virus, closely related to Severe Acute Respiratory Syndrome CoV (SARS-CoV) and Middle East Respiratory Syndrome CoV (MERS-CoV). It has been observed that the virus invades human body mainly through binding to angiotensin-converting enzyme 2 (ACE2) receptors similar to SARS-CoV and the main protease (Mpro) acts as a critical protease for digesting the polyprotein into functional polypeptides during the replication and transcription process of 2019-nCoV. In this review, we summarized the real-time information of 2019-nCoV treatment methods and mainly focused on the chemical drugs including lopinavir/ritonavir, chloroquine, hydroxychloroquine, arbidol, remdesivir, favipiravir and other potential innovative active molecules. Their potential targets, activity, clinical status and side effects are described. In addition, Traditional Chinese Medicine (TCM), Convalescent plasma therapy (CPT) and biological reagents available, as well as the promising vaccine candidates against 2019-nCoV are also discussed.
Keywords: Coronavirus, Target protein, Chemical drugs, Traditional Chinese medicine (TCM), Vaccines
Graphical abstract
1. Introduction
Since December 2019, the novel coronavirus (2019-nCoV, SARS-CoV-2, COVID-19) causes an epidemic of acute respiratory syndrome and has rapidly become a worldwide threat to public health. As of 15 July 2020, at least 13,691,570 cases and 586,820 deaths were identified around the world. The major clinical symptoms of 2019-nCoV infection include fever, dry cough, short of breath, chest pain, fatigue, and diarrhea [1]. Although few patients were identified without any abnormal radiological findings [2]. Many severe patients have also developed dyspnea and lymphopenia. However, until now no specific medicine is identified that could efficiently control or eliminate the virus. On 22 Feb 2020, researchers have successfully isolated the 2019-nCoV and sequenced its gene. The results showed that 2019-nCoV belongs to betacoronavirus with a single-stranded RNA [3], which is closely related to severe acute respiratory syndrome CoV (SARS-CoV) and Middle East respiratory syndrome Cov (MERS-CoV) [4]. Therefore the clinical experiences gained against SARS and MERS provide possible ways to treat 2019-nCoV infection effectively. SARS-CoV-2 encodes at least 27 proteins, including 15 non-structural proteins, 4 major structural proteins, and 8 auxiliary proteins [5]. The 4 major structural proteins are the spike (S), membrane (M), envelope (E) and the nucleocapsid (N) proteins, with the S protein mainly affecting the fusion and cell entry process of the virus, the M protein mainly defines the shape of the viral envelope, and both the E and N proteins are actively participating in the viral assembly and budding events [6]. Angiotensin-converting enzyme 2 (ACE2) is the receptor of SARS-CoV-2, and the S protein primed by the cellular serine protease TMPRSS2 might facilitate the entry process of the virus into the host cells. Therefore, the serine protease TMPRSS2 inhibitor camostat mesylate (Table 1 ) might prevent the entry of SARS-CoV-2 into cells [7]. With the imminent threat of the virus growing exponentially, it is high priority to develop specific vaccines and drugs to combat COVID-19 infection. Fortunately, experimental vaccines are promising, while, the clinical trials of small chemical molecules showed certain beneficial effects. Currently, many medications and drugs are being used in clinics worldwide to test their effectiveness including lopinavir/ritonavir, chloroquine, hydroxychloroquine, arbidol, remdesivir, favipiravir and Traditional Chinese Medicine (TCM). Besides, scientists are exploring several specific molecules and combinations of different drugs to treat the patients having SARS-CoV-2 infection. In this review, we collected and sorted out the relevant information available currently to combat SARS-CoV-2 and we mainly focused on the chemical drugs (Table 1). At the same time, we have also discussed the biological methods including convalescent plasma therapy (CPT) and the promising vaccines under development against 2019-nCoV.
Table 1.
Chemical drug in clinical against COVID-19.
| Compounds | Chemical structures | Drug Target against COVID-19 | EC50 | Severe adverse effects |
|---|---|---|---|---|
| Lopinavir/ritonavir (LPV/r) | 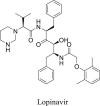 |
3CLprp | 26.63 μM | No obvious severe adverse reports |
 |
CYP3A4 | >100 μM | ||
| Chloroquine (CQ) | 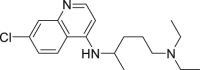 |
inhibit viral membrane fusion process | 1.13 μM | Retinopathy, cardiomyopathy, and neuromyopathy |
| Hydroxychloroquine(HCQ) | 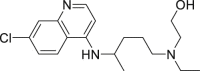 |
inhibit viral membrane fusion process | 0.72 μM | Retinopathy, cardiomyopathy, and neuromyopathy |
| Arbidol (Umifenovir) |  |
Spike glycoprotein | 4.11 μM | Hepatic insufficiency |
| Remdesivir (GS-5734) |  |
RdRp | 0.77 μM | No obvious severe adverse reports |
| Favipiravir (T-705) | 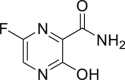 |
RdRp | 61.88 μM | Teratogenicity and embryotoxicity |
| Baricitinib | 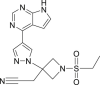 |
JAK | – | A risk of reactivation of latent infections |
| Ribavirin | 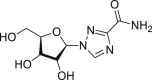 |
RdRp | >100 μM | Hemolytic anemia and teratogenic |
| Galidesivir (BCX4430) | 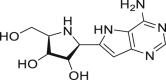 |
RdRp | >100 μM | No obvious severe adverse reports |
| Darunavir | 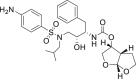 |
3CLprp | – | Hepatic insufficiency |
| Oseltamivir | 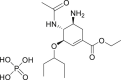 |
unknown | >100 μM | Severe rash and hemorrhagic colitis |
| Camostat mesylate | 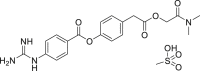 |
TMPRSS2 | – | No obvious severe adverse reports |
| 11a | 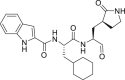 |
3CLprp | 0.53 μM | Unknown |
| 11b | 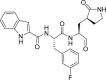 |
3CLprp | 0.72 μM | Unknown |
| 13b | 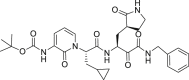 |
3CLprp | 4–5 μM | Unknown |
| N3 | 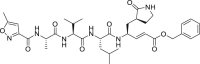 |
3CLprp | 16.77 μM | Unknown |
2. Chemical drugs
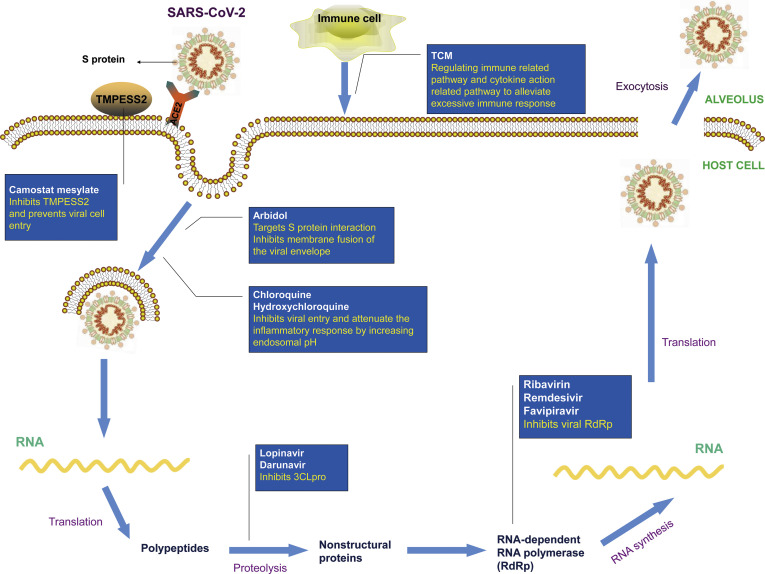
Many chemical drugs have shown promising antiviral activities against SARS-CoV-2. Here we summarized their chemical structures, effects, targets and severe adverse effects in Table 1. In addition, their possible mode of action against 2019-nCoV is depicted in Fig. 1 . Moreover, we collated the ongoing clinical trials aimed to treat COVID-19 patients from Clinicaltrials.gov in Table 2 .
Fig. 1.
The mechanism of clinical drugs against COVID-19. SARS-CoV-2 infects the host cells by binding to ACE2 receptor. S protein of SARS-CoV-2 primed by the cellular serine protease TMPRSS2 play an essential role in facilitating this entry process. Camostat mesylate inhibit TMPRSS2 to hamper virus invasion. Arbidol, chloroquine, and hydroxychloroquine mainly inhibit the membrane fusion processes, which prohibit the virial entry. 3CLpro digests the polyprotein of SARS-CoV-2 into functional polypeptides. Both Lopinavir and Darunavir hinder the viral replication processes by inhibiting 3CLpro. Ribavirin, Remdesivir and Favipiravir block the viral RNA replication by inhibiting the RdRp enzyme. Traditional Chinese Medicines dampen the immune response by decreasing the production of cytokines and regulating the immune related pathways.
Table 2.
Clinical trials identified at Clinicaltrials.gov related to drug repositioning for COVID-19 treatment.
| Compound | N° test | Clinical condition (sample size of 2019-nCoV participants) | Sponsors | Phase |
|---|---|---|---|---|
| Lopinavir/ritonavir | NCT04321174 | 1220 participants | Darrell Tan | Ⅲ |
| Lopinavir/Ritonavir, Ribavirin and IFN-beta Combination | NCT04276688 | 127 participants | University of Hong Kong, Hospital Authority | Ⅱ |
| Chloroquine Phosphate (CQ) | NCT04328493 | 250 participants | Oxford University Clinical Research Unit, Vietnam | Ⅱ |
| NCT04333628 | 210 participants (Mild) | HaEmek Medical Center, Israel | Ⅱ | |
| hydroxychloroquine (HCQ) | NCT04330144 | 2486 participants | Gangnam Severance Hospital | Ⅲ |
| Azithromycin- Hydroxychloroquine Combination or Hydroxychloroquine | NCT04336332 | 160 participants | Rutgers, The State University of New Jersey | Ⅱ |
| Remdesivir (GS-5734) | NCT04323761 | Expanded Access | Gilead Sciences | – |
| NCT04292899 | 6000 participants (Severe) | Gilead Sciences | Ⅲ | |
| Favipiravir (T-705) | NCT04336904 | 100 participants | Giuliano Rizzardini | Ⅲ |
| NCT04346628 | 120 participants (Mild) | Stanford University | Ⅱ | |
| Baricitinib | NCT04320277 | 200 participants | Hospital of Prato | Ⅱ and Ⅲ |
| NCT04340232 | 80 participants | University of Colorado, Denver | Ⅱ and Ⅲ | |
| Galidesivir (BCX4430) | NCT03891420 | 66 participants | BioCryst Pharmaceuticals | Ⅰ |
| Darunavir and Cobicistat | NCT04252274 | 30 participants | Shanghai Public Health Clinical Center | Ⅲ |
| Hydroxychloroquine, Oseltamivir, or Azithromyci | NCT04338698 | 500 participants | Shehnoor Azhar | Ⅲ |
| CamostatMesylate | NCT04321096 | 580 participants | University of Aarhus | Ⅰ |
| Arbidol (Umifenovir) | NCT04350684 | 40 participants | Shahid Beheshti University of Medical Sciences | Ⅳ |
| NCT04260594 | 380 participants | Jieming QU | Ⅳ |
2.1. Lopinavir/ritonavir (LPV/r)
Lopinavir/ritonavir (LPV/r), a protease inhibitor manufactured by AbbVie Corporation, is used for treating HIV-1 infection. The chemical structure of Lopinavir is (2S)–N-[(2S,4S,5S)-5-[[2-(2,6-dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenyl-hexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide (Table 1). It is the active component of this drug combination, which blocks the division of Gag-Pol polyproteins resulting in the production of immature virus particles incapable of infecting the patients further. However, LPV has no effect on cells having integrated viral DNA, and only prevents subsequent infections of other susceptible cells. Pharmacokinetic studies have demonstrated that lopinavir is mainly metabolized by CYP3A4 and produces low systemic concentrations when used alone. However, the metabolism of lopinavir could be inhibited by ritonavir. Hence, a combination of these two drugs could prolong the systemic exposure to lopinavir and keep the lopinavir concentration in the circulation for a longer period of time. The recommended dose for adults is 400/100 mg twice daily. The most common adverse effects in adults associated with LPV/r medication are diarrhea and other gastrointestinal disturbances, asthenia, headache and skin rashes [8]. Based on the Diagnosis and Treatment Guidelines of the 2019-nCoV in China (the seventh edition), LPV/r is included for treating the coronavirus pneumonia, however its efficacy and safety remains to be established clearly. Antiviral effects of lopinavir at EC50 value of 26.1 μM but not ritonavir against SARS-CoV-2 in vitro was reported earlier (Table 1) [9]. Later in a clinical trial, 47 patients with COVID-19 infection, admitted to Rui’an People’s Hospital, were tested along with a control group with LPV/r. This combination treatment with LPV/r had a more clearly therapeutic effect in lowering the body temperature and restoring homeostasis with no obvious toxic and side effects [10]. However, a paper published in the New England Journal of Medicine pointed out that LPV/r treatment did not significantly accelerate the clinical improvement and reduced mortality in patients with serious COVID-19 [11]. Recently, a clinical trial (NCT04321174) is recruiting participants to further evaluate the efficacy of LPV/r for treating Covid-19 patients (Table 2).
2.2. Chloroquine phosphate
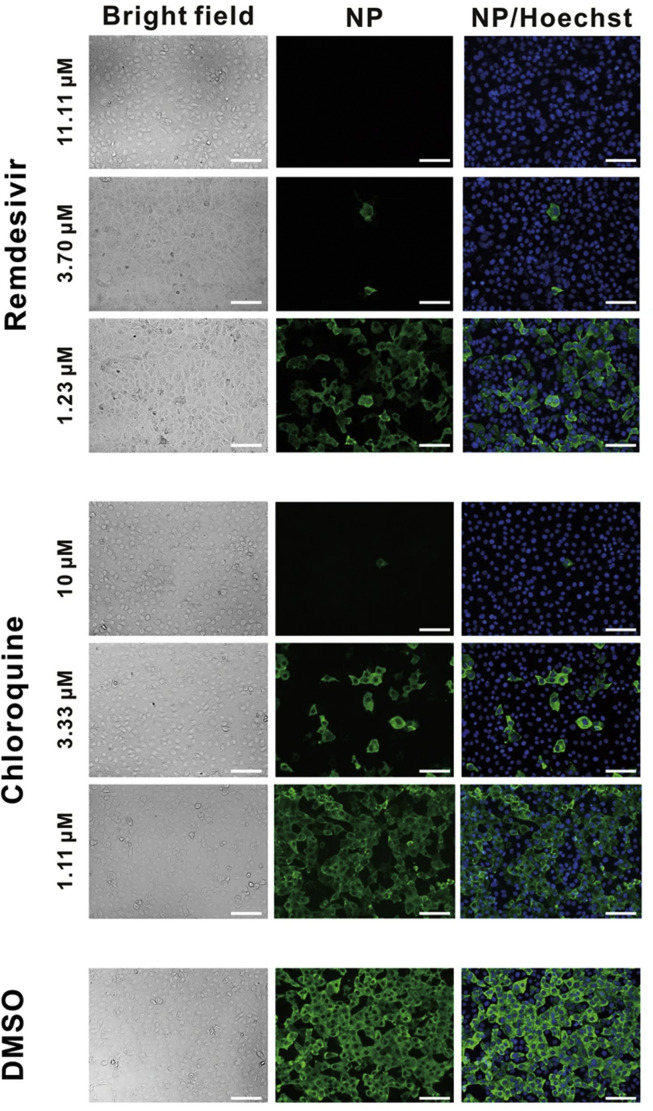
Chloroquine phosphate is a water-soluble compound with the chemical structure of 7-chloro-4-((4′-diethylamino-1-methylbutyl)amino)quinolinediphosphate (Table 1), which is widely used for treating malarial infection and autoimmune diseases, like lupus (both discoid lupus erythematosus and systemic lupus erythematosus) and arthritis. It is one of the first-line disease-modifying anti-rheumatic drugs used for treating rheumatoid arthritis (RA) and it acts by inhibiting antigen presentation capacity of dendritic cells, cytokine production in macrophages, as well as calcium and Toll-like receptor (TLR) signaling in B, T and other immune cells [12]. Later, studies also discovered the broad-spectrum virus-resistance activity of chloroquine (CQ). Chloroquine is mainly absorbed from the gastrointestinal tract, and it might block the virus infection by increasing the endosomal pH required for virus/cell fusion, as well as by interfering with the glycosylation of cellular receptors of SARS-CoV [13]. An article in Cell Research reported that CQ could prevent 2019-nCoV infection at the cellular level (EC50 = 1.13 μM, CC50 > 100 μM, SI > 88.50) (Table 1). Immunofluorescence studies on virus infection after chloroquine treatment also proved the anti-2019-nCoV activity of CQ (Fig. 2 ) [14]. Subsequently, the clinical studies confirmed its effectiveness against 2019-nCoV at a dose of 500 mg/day with no obvious serious adverse reactions [15]. Although patients showed few adverse effects in the clinics at the standard dose of chloroquine, the main severe side effects include retinopathy, cardiomyopathy and neuromyopathy, while used for longer periods, and the acute toxicity of chloroquine occurred when high dose was rapidly administered by parenteral routes [12]. Therefore clinicians should closely observe adverse reactions with chloroquine treatment, especially with elderly patients. Recently, many clinical trials with chloroquine against 2019-nCoV were initiated in many health care centers (Table 2).
Fig. 2.
Immunofluorescence images of virus infection in Vero cells with and without treatment with remdesivir and chloroquine. Virus infection and drug treatment were performed in vitro using Vero cells. At 48 h p.i., the infected cells were fixed, and probed with rabbit sera against the nucleocapsid protein (NP) of a bat SARS-related CoV2 as the primary antibody. Alexa 488-labeled goat anti-rabbit IgG (1:500; Abcam) was used as the secondary antibody. The nuclei were stained with Hoechst dye. Bars, 100 μm. (Reproduced with permission from ref.14).
2.3. Hydroxychloroquine
Hydroxychloroquine (HCQ) is an aminoquinoline compound modified by chloroquine, with an N-hydroxyethyl side chain instead of the N-diethyl group of chloroquine. An increase in hydroxyl moiety makes it highly water soluble and less toxic than chloroquine, while still retaining the antiviral activity. The immunomodulatory mechanisms are similar to chloroquine by elevating the pH through accumulation in lysosomes. Thus, HCQ interferes in the process of antigen presentation (Fig. 1) and possibly attenuating the inflammatory response by significantly decreasing the production of pro-inflammatory factors in COVID-19 patients. Similar to CQ, HCQ has beneficial effects in immunodeficient conditions and also reduces development of metabolic and cardiovascular complications apart from having anti-tumor and anti-thrombotic effects [12]. HCQ with a EC50 of 0.72 μM was reported to have anti-viral activity against COVID-19 in vitro, which suggested the potential use of HCQ in clinics [16]. Results from an open label non-randomized clinical trial of limited size in France claimed HCQ (200 mg, three times per day in 10 days) used in COVID-19 patients might be efficient in clearing nasopharyngeal carriage of SARS-CoV-2 within three to six days. A combination of HCQ and azithromycin also showed synergistic effect providing an alternate treatment strategy for SARS-CoV-2 infection [17]. However, at present there is still no clear evidence to support the effectiveness of HCQ against SARS-CoV-2 infection [18], which remains to be confirmed by clinical trials (Table 2). Although HCQ is a less toxic molecule, poisoning occurs with its prolonged use and over-dosage, particularly irreversible retinopathy possibly leading to loss of vision. Therefore, clinical monitoring and early recognition of toxic symptoms are essential for an effective management strategy. Clinicians should monitor the dose taken by the patients and pay close attention to the side effects including retinal toxicity, neuromyopathy, and cardiac disease during clinical trials.
2.4. Arbidol
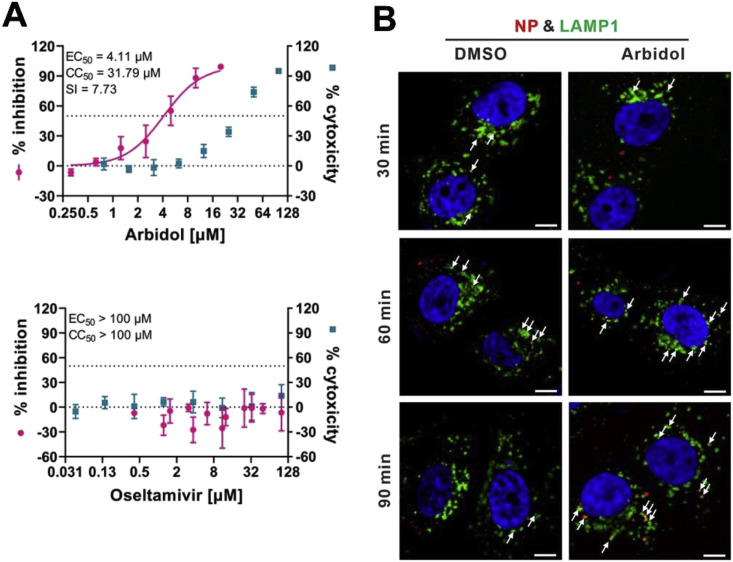
Arbidol (ARB), also known as umifenovir, is recommended as a specific anti-influenza drug to control influenza A and influenza B viral infections. It has been approved in Russia and China for human use, with no major adverse effects reported to date. It prevents contact between the virus and host cells and penetration of virus particles into the cell by inhibiting the fusion of the virus lipid shell to the cell membrane (Fig. 1) [19]. ARB is an indole analogue and, the amine in position 4 and the hydroxyl moiety in position 5 are important for the antiviral action of ARB. Insertion of a methyl group between the indole ring and 5-hydroxyl group has considerably increased its antiviral potency, whereas introduction of particular azote-based heterocyclic groups at position 4 improved anti-HBV activity. Replacement of the S-phenyl group at position 2 by a phenyl-sulfonyl group decreased its cytotoxicity, while increasing the anti-HBV activity of the compound [20]. Interestingly, arbidol has substantial antiviral activities against Zika virus (ZIKV), West Nile virus (WNV) and Tick-borne encephalitis virus (TBEV) with EC50 values ranging from 10.57 ± 0.74 to 19.16 ± 0.29 μM in HBCA and Vero cells [21]. It also has antiviral potential against herpes viruses (HHV-8), ebola virus (EBOV), arenaviruses (Tacaribe virus), poliovirus and HBV [22]. Therefore, ARB is considered as a potential compound to combat COVID-19. Later ARB (EC50 = 4.11 μM, CC50 = 31.79 μM, SI = 7.73) [23] was shown to inhibit COVID-19 infection (Fig. 3 a) more efficiently and it interferes with the release of SARS-CoV-2 from intracellular vesicles (Fig. 3b). Subsequently, a retrospective study approved by the Third People’s Hospital of Changzhou, China demonstrated the efficacy of ARB monotherapy, which might be superior to LPV/r against COVID-19 [24]. However, the existing data with clinical trials is insufficient to precisely prove the effectiveness of ARB against COVID-19 because of the limited sample size, and the unknown antiviral mechanisms. Another study confirmed that ARB combined with LPV/r might benefit the patients by delaying the progression of lung lesions and lowering the possibility of respiratory and gastrointestinal transmissions [25]. Hence, a combination therapy could be the most effective way for managing COVID-19, which needs to be validated in the near future. At present, phase IV clinical trials are being carried out to test the efficacy of ARB against COVID-19 including NCT04350684 and NCT04260594 (Table 2).
Fig. 3.
The antiviral activities of Arbidol and Oseltamivir against 2019-nCoV in vitro. (A) Antiviral activities of Arbidol and Oseltamivir in Vero E6 cells were determined by qRT-PCR analysis of virus yield at 48 h p.i. (B) Effect of Arbidol on intracellular trafficking of SARS-CoV-2. The colocalization of virions with early endosomes (EEs) or late endosomes (LEs) was analyzed by immunofluorescence. Representative confocal microscopic images of virions (red) and LAMP1+ ELs (green) in each group were shown. The nuclei (blue) were stained with Hoechst 33258 dye. White arrows: virions co-localized with ELs; bars: 10 μm. (Reproduced and arranged with permission from ref.23). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.5. Remdesivir
DNA or RNA polymerase enzymes are essential components in the process of viral replication. Nucleoside analogues are used to control the viral infections in clinics, which target the DNA or RNA polymerase. Remdesivir (GS-5734), a prodrug developed by Gilead Science, is well recognized as a potential antiviral drug against a wide array of infections with RNA viruses in cell cultures, mice and non-human primate (NHP) models. The predicted mechanism of action is by the incorporation of an active triphosphate into the viral RNA resulting in premature termination of RNA synthesis that decreased the level of viral RNA (Fig. 1) [26]. A study reported the efficacy of Remdesvir (GS-5734), which is highly active against coronaviruses (EC50 = 0.074 ± 0.023 μM in MERS-CoV-infected and EC50 = 0.069 ± 0.036 μM in SARS-CoV-infected HAE cultures) at early times post infection. Interestingly, drug resistance was not observed, which precludes development of resistant superviruses [27]. Therefore GS-5734 is a promising molecule to defeat COVID-19. Subsequently, a study from Wuhan Institute of Virology reported the EC90 value of GS-5734 against 2019-nCoV in Vero E6 cells as 1.76 μM [14], suggesting the feasibility of achieving its working concentration in NHP models. Preliminary data showed that remdesivir has also inhibited 2019-nCoV infection efficiently in a human cell line (human liver cancer Huh-7 cells). The study also proved the anti-SARS-CoV-2 activity of GS-5734 using immunofluorescence microscopy (Fig. 2) [14]. However, a report published in New England Journal of Medicine described the effect of GS-5734 was still inadequate to assess in the first COVID-19 patient recovered after remdesivir injection in the United States, because the viral load of the patient was decreased before remdesivir was used. Hence, the patient recovery was attributable to the drug or the role of self-defense mechanisms and supportive treatments was not made clear [26]. Currently, clinical trials using GS-5734 as a therapeutic against 2019-nCoV infection are in progress (NCT04323761 and NCT04292899), and the effectiveness and adverse reactions of this drug are worthy of our attention (Table 2).
2.6. Favipiravir
Favipiravir (T-705), 6-Fluoro-3-hydroxypyrazine-2-carboxamide, is a novel RNA-dependent RNA polymerase (RdRp) Inhibitor from Toyama Chemical Co., Ltd, and used to treat influenza viruses in Japan [28]. Favipiravir-RTP (favipiravir ribofuranosyl-5′-triphosphate) is the active form of T-705 converted by host enzymes, which acts as a nucleotide analogue selectively inhibiting the RdRp. At the same time, T-705 can also get incorporated into the nascent viral RNA resulting in termination of the viral replication processes (Fig. 1) [29]. Since the catalytic domain of RdRp is conserved among various types of RNA viruses, T-705 has a broad spectrum of anti-viral activities. It has inhibited the replication of ZIKV in Vero cells (EC50 = 3.5–3.8 μg/mL) and Zaire Ebola virus in Vero E6 cells (EC50 = 10.5 μg/mL) [29]. It is also used as a potential promising candidate for the treatment of COVID-19. Favipiravir (EC50 = 61.88 μM, CC50 > 400 μM, SI > 6.46) has effectively reduced the COVID-19 infection in Vero E6 cells (Table 1) [14]. Another study compared the effects of FPV and LPV/r for treating COVID-19 patients. FPV had better treatment effects on COVID-19 compared to LPV/RTV having faster viral clearance rate and a higher level of improvement from lung complications as evidenced by imaging techniques [30]. However, the production and application of T-705 are severely restricted in Japan because of the prevailing risks of teratogenicity and embryotoxicity. Currently, a Phase III clinical trial of T-705 (NCT04336904) is already underway (Table 2) [31].
2.7. Other antiviral agents
Ribavirin (Table 1) is known as an effective antiviral agent to combat hepatitis C virus (HCV) but it is highly cytotoxic [32]. Even then, it was included in the recommended combination therapy with interferon or LPV/r in the Diagnosis and Treatment Guidelines of the 2019-nCoV in China (the seventh edition). However, there was insufficient evidence available for its clinical efficacy after administration to COVID-19 patients. A janus kinase inhibitor, baricitinib (Table 1), which binds to the cyclin G-associated kinase, is a regulator of endocytosis. Researchers used AI to search for the approved drugs that could help in the treatment of COVID-19 infection and found baricitinib as a potential drug to treat 2019-nCoV acute respiratory disease [33]. Recently, the clinical trials of baricitinib against COVID-19 (NCT04320277 and NCT04340232) are ongoing and the results are eagerly awaited (Table 2). Lanjuan Li team has shown Darunavir (Table 1), a HIV-1 protease inhibitor [34], has the activity against 2019-nCoV. Darunavir could possibly be administered along with either ritonavir or cobicistat, but the effectiveness and safety profile are yet to be explored. Currently, the phase III clinical trial of Darunavir together with Cobicistat against COVID-19 is under way (Table 2). Oseltamivir (Table 1) is a drug used for preventing and treating influenza virus infection in children. It was tried for the treatment of SARS-CoV-2 patients [35], but its efficacy remains uncertain (Fig. 3a).
3. Traditional Chinese Medicine
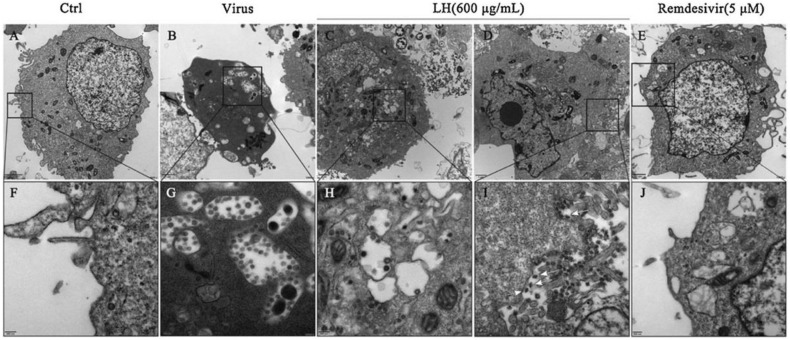
Traditional Chinese Medicine (TCM) has played a vital role in the treatment of pestilence for thousands of years in China, and also it is a vastly unexploited treasure in modern medicine. In recent years, development and application of TCM-derived herbs and formulations for evidence-based therapy is increasing exponentially [36]. TCM had notable therapeutic effects against SARS epidemic in 2003 [37]. Now, traditional Chinese medicine preparations like Lianhuaqingwen (LH) Capsule and Qingfeipaidu Decoction (QPD) have shown promising therapeutic effects against 2019-nCoV infection in clinics. According to the theory of TCM, these preparations work mainly by regulating the immune related pathways involving cytokine functions to alleviate excessive immune response and related-complications (Fig. 1) [38]. LH inhibited the replication of 2019-nCoV with an IC50 value of 411.2 μg/mL by CPE assay, and the image of viral particles in ultra-thin sections of infected cells under electron microscopy supported these observations (Fig. 4 ) [39]. Another study tested the antiviral activity of LH against 2019-nCoV by CPE and plaque reduction assay using Vero E6 cells, and the results showed that LH could inhibit the replication of 2019-nCoV and also reduce the production of pro-inflammatory cytokines (TNF-α, IL-6, CCL-2/MCP-1 and CXCL-10/IP-10) [39]. Subsequently, a retrospective clinical analysis on the treatment of SARS-CoV-2 with LH at the Ninth Hospital of Wuhan and CR & WISCO General Hospital was conducted andthe results showed that LH has significantly relieved the cough and fever symptoms associated with the infection [40]. Similarly, QPD was also widely used to treat SARS-CoV-2 in China. According to National Administration of Traditional Chinese Medicine, the disease symptoms of patients were markedly stabilized with the disappearance of cough and fever after QPD treatment. Recently, Researchers are focused to investigate and analyse the potential protective mechanisms of QPD against SARS-CoV-2, the possible side effects and new formulations. In the future, an integrated approach combining Chinese and Western drugs will be tested to cure 2019-nCoV infected patients.
Fig. 4.
Virions in the ultrathin sections of infected Vero E6 cells under electron microscope. (A, F) Uninfected and (B, G) mock-treated SARS-CoV-2 virus infected cells. (C, D, H, I) Infected cells after Lianhuaqingwen (LH) or (E, J) Remdesivir treatment. White arrows indicate the spindle shape of viral particles within the infected cells after LH treatment. (Reproduced with permission from ref.39).
4. Biological methods
4.1. Convalescent plasma therapy
Convalescent plasma therapy (CPT) is a passive immune therapy used to fight the novel coronavirus by injecting the plasma of the recovered person containing anti-virus antibodies [41]. It has been successfully used for controlling SARS infection, but the limited quantity of the plasma available impedes wider clinical applications. CPT was used to treat critically ill patients in China [42], and experts are still evaluating its efficacy and safety in clinical trials. Recently, it was observed that after CPT therapy SARS-CoV-2 RNA has disappeared completely along with a clear rise in neutralizing antibody titers in nearly all the patients, which could reduce mortality rate in severe COVID-19 patients [43].
4.2. Interferons
Interferons (IFNs) are a broad class of cytokines divided into type I, type II, and type III secreted by the host when subjected to stress or infections, and also during the development of autoinflammatory and autoimmune diseases [44]. Interferons are crucial for antiviral responses, antigen presentation, development of autoimmunity and inflammation by activating the Jak-STAT signaling pathway [45]. All IFNs share the ability to promote antiviral activities initiated by their interactions with cognate receptors [46]. It’s well known that type I IFNs could effectively inhibit virus replication, and also show critical effects on the development and activation of immune cell subsets. Given both the antiviral and immunomodulatory effects, type I IFNs are used either alone or in combination with other medicines to treat a variety of chronic and acute viral infections in clinics [47]. During the SARS-CoV and EBOV disease outbreak, Type I IFNs were used as auxiliary drugs together with other antiviral medicines to alleviate patients’ complications. However, prolonged clinical use of IFNs is limited because of their adverse side effects including neutropenia, thrombocytopenia, hyper-and hypothyroidism, pancreatitis, type I diabetes mellitus, and irreversible pulmonary hypertension [47]. Recently, IFN-α (5 million U each time for adults twice per day) was recommended to use along with Lopinavir/ritonavir or Ribavirin to combat 2019-nCoV infection [48].
4.3. Vaccines
Vaccines are effective weapons to fight against the viruses like smallpox, measles, polio and other infectious diseases that were once raged around the world. The isolation of new coronavirus makes vaccine development a possibility, but success is limited because of the strenuous development processes involved in constructing and validating a vaccine [49]. At present, researchers have developed five technical routes for the development of new COVID-19 virus vaccines, including inactivated vaccines, recombinant genetically engineered vaccines, adenovirus vector vaccines, nucleic acid vaccines, and vaccines made from attenuated influenza virus vaccine vectors [50]. It may require more than a year to start phase I clinical trials for most of the candidate vaccines. Up to May 12, 2020, 10 candidates of COVID-19 vaccines are undergoing clinical trials, including Adenovirus Type 5 Vector (CanSino Biologics Inc), ChAdOx1 nCoV-19 (Moderna), INO-4800 (Inovio Pharmaceuticals), mRNA-1273 (University of Oxford), BNT162 (BioNTech), bacTRL-Spike (Symvivo Corporation), Pathogen-specific aAPC (Shenzhen Geno-Immune Medical Institute), LV-SMENP-DC (Shenzhen Geno-Immune Medical Institute) and other 2 vaccines developed by Sinovac Biotech, and Wuhan Institute of Biological Products. Notably, on May 22, 2020, an article published in The Lancet Journal reported the first-in-human trial of a recombinant adenovirus type-5 (Ad5) vector COVID-19 vaccine (NCT04313127) [51]. This study mainly assessed the safety, tolerability, and immunogenicity of the vaccine in which 108 healthy participants were participated. The participants were divided into 3 groups on the basis of dosage [low dose (n = 36), middle dose (n = 36), or high dose (n = 36)]. This vaccine was well tolerated and the most common adverse reactions were mild or moderate in severity including fever, fatigue, headache and muscle pain. In addition, humoral responses against COVID-19 were peaked at day 28 post-vaccination and rapid specific T-cell responses were noted from day 14 post-vaccination. This data implied the potential of Ad5 vectored COVID-19 vaccine in preventing COVID-19 infection. However, this study was limited by the sample size, short follow-up period and the absence of a randomized control groups. Another ongoing phase II trial in China (NCT04341389) will provide more information on the safety and immunogenicity of the Ad5 vector COVID-19 vaccine and the results are expected soon.
5. Innovative research
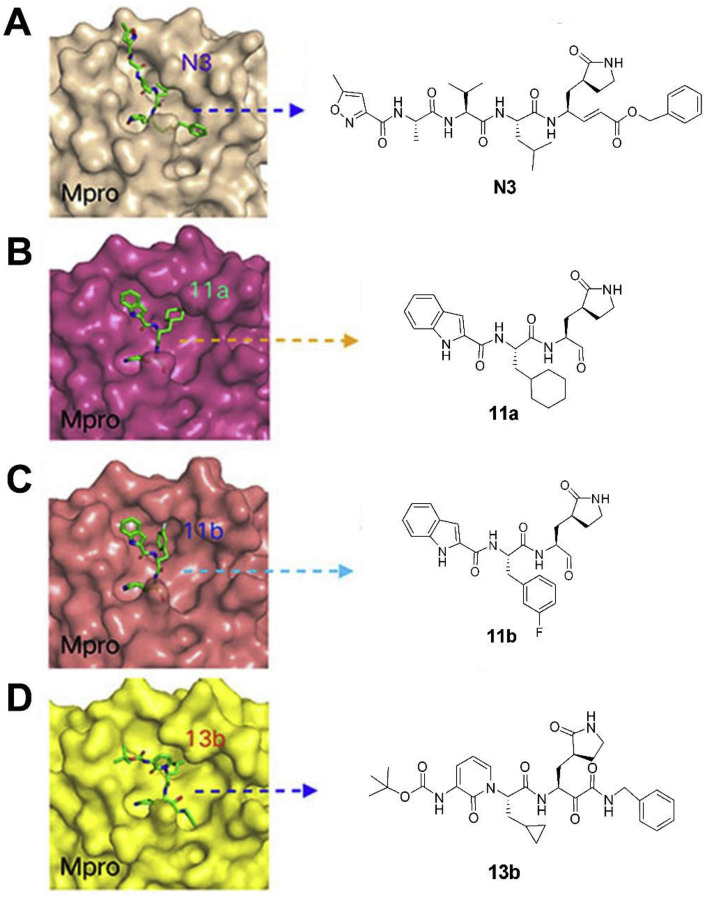
During this 2019-nCoV outbreak, scientists all over the world are focused on actively exploring different aspects of virus structure, mode of transmission, pathology and clinical complications in the infected patients and, made some outstanding contributions. The main protease (Mpro), also termed 3CLpro, acts as a critical protease for digesting the polyprotein into functional polypeptides involved in 2019-nCoV replication and transcription processes. It is also an ideal antiviral target because of no homologues present in the humans. Professor Rao and his team have identified N3 as a potent irreversible inhibitor of COVID-19 virus Mpro. They have determined the crystal structure of COVID-19 virus Mpro in complex with N3 to 2.1 Å resolution (Fig. 5 A) [52]. This structure provides a model for discovering lead inhibitors to target COVID-19 virus Mpro through computer-aided drug designs as well as virtual and high-throughput screening methods, which is fundamental for identifying potential active molecules against COVID-19. Subsequently, these authors developed a fluorescence resonance energy transfer (FRET) assay to screen a library of ∼10,000 compounds, and found 7 clinical drug candidates including Ebselen (IC50 = 0.67 μM), Disulfiram, Carmofur, Shikonin, Tideglusib, PX-12 and TDZD-8 [53]. Thus this study provided a reliable strategy for rapid drug discovery against COVID-19 in the near future. Based on the structure of the substrate-binding pocket of SARS-CoV Mpro, Hualiang Jiang and Hong Liu team have designed and synthesized novel inhibitors targeting the SARS-CoV-2 Mpro and obtained compounds 11a (Table 1) (EC50 = 0.53 ± 0.01 μM, CC50 > 100 μM, SI > 189) and 11b (Table 1) (EC50 = 0.72 ± 0.09 μM, CC50 > 100 μM, SI > 139). Both these compounds showed excellent inhibitory potency against SARS-CoV-2 Mpro enzyme [53]. The pharmacokinetic properties of 11a and 11b demonstrate their good bioavailability with no obvious toxicity. Their data indicated that these 2 compounds are good candidates for further clinical studies (Fig. 5B and C). Rolf Hilgenfeld and his team reported the x-ray structures of the un-liganded SARS-CoV-2 Mpro and its complex with an α-ketoamide inhibitor [54]. Compound 11r is a broad-spectrum inhibitor of main proteases, which could inhibit betacoronaviruses, alphacoronaviruses as well as the 3CLpro of enteroviruses. They have optimized 11r and synthesized the compounds 13a, 13b (Figs. 5D) and 14b. All of them were tolerated well in mice. More interestingly, compounds 13a and 13b tend to concentrate more in the lungs, which implied the possibility of direct administration of these compounds to the lungs. This study provided a solid framework for the development of pyridone-containing inhibitors against the virus. Shuai Xia and his team have successfully solved the X-ray crystal structure of six-helical bundle (6-HB) core of the HR1 and HR2 domains of the SARS-CoV-2 S protein S2 subunit, and generated a series of lipopeptides derived from EK1 (a pan-coronavirus fusion inhibitor). It was found that EK1C4 is the most potent fusion inhibitor available against SARS-CoV-2 S protein-mediated membrane fusion and pseudovirus infection with IC50 of 1.3 and 15.8 nM, respectively [55]. This suggests the possibility of using EK1C4 for prevention and treatment of 2019-nCoV infection.
Fig. 5.
Structure and binding pocket of different inhibitors to COVID-19 main protease, Mpro. (A) The crystal structure of COVID-19 virus Mpro-N3 complex (PDB: 6LU7) and the chemical structure of N3. (B) The crystal structure of COVID-19 virus Mpro-11a complex (PDB: 6LZE) and the chemical structure of 11a. (C) The crystal structure of COVID-19 virus Mpro-11b complex (PDB: 6MOK) and the chemical structure of 11b. (D) The crystal structure of COVID-19 virus Mpro-13b complex (PDB: 6Y2F) and the chemical structure of 13b.
6. Conclusion and perspectives
At present the 2019-nCoV infection is rapidly spreading worldwide and as of 15 July 2020, the death toll has reached 586,820. Hence, there is an urgent requirement to identify more effective antiviral agents to combat the disease and validate their clinical effects. Although the development of new drugs has shown some progress, currently, no specific treatment against this new virus is available. Certainly, vaccines are the most effective tools to fight the viruses, and many scientists all over the world are actively involved in developing specific vaccines. The phase I clinical trial data of Ad5 vectored COVID-19 vaccine is promising and there is hope to defeat the virus eventually. Considering the possible invalidity of vaccines because of the rapid gene mutations in COVID-19, the chemical drugs and active molecules offer exciting opportunities. China is one of the first-line countries in developing anti-epidemic strategies and, it has quickly and efficiently controlled the domestic epidemic situation. Living conditions and production work have gradually begun to get normalized to pre-COVID19 situation. For treating this virus infection, many antiviral drugs were used in China, and this experience gained about beneficial strategies and methods is being shared to other countries to contain the COVID-19 infection. Enormous data generated is also facilitating further scientific studies in several laboratories. According to the Diagnosis and Treatment Guidelines of 2019-nCoV in China (the seventh edition), Lopinavir/ritonavir (combined with Ribavirin), chloroquine and arbidol are recommended as antiviral drugs for mild COVID-19 patients. In addition, IFN-α can be used as an adjuvant therapy by inhalation. For severe patients, convalescent plasma therapy and blood purification treatment are advisable. Tocilizumab is suitable to use for severely 2019-nCoV infected patients having critical pulmonary lesions, especially with the increase of IL-6R expression. Chinese Traditional medicines like Lianhuaqingwen capsules, Shufengjiedu capsules, Jinhuaqinggan granules and Qingfeipaidu Decoction are also suggested to control the progression of disease. Although some chemical drugs tested in vitro have shown anti-coronavirus effects, their potential remain to be investigated in vivo. It is worth noting that more scientific research and reasonable design of clinical trials are essential to assess the effectiveness of drugs. Some existing clinical reports are lacking in their credibility because of the limitation in sample size. Researchers should design scientific and logical experiments with reasonable sample size for clinical trials to evaluate the accuracy of the results in the future.
As to the traditional therapy with chemical drugs, monotherapy may be inadequate to control the virus, hence combination therapies should be considered seriously for combating COVID-19 more efficiently. Different drug combinations and dosages as well as potential toxic effects are worth exploring further. TCM also shows huge potential for alleviating and moderating the symptoms of patients infected with 2019-nCoV. However, the composition of TCM is complex and they also act on unknown targets. Hence, development of precise TCM prescriptions and analysis of accompanied potential toxicities will be the major focus in future studies. Furthermore, the crystal structure of COVID-19 virus Mpro provides a new and efficient strategy to develop potential antiviral drugs that are based on computer-aided drug designs, virtual and high-throughput screening in the near future, which could greatly speed up the drug development processes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank National Natural Science Foundation of China (No. 81773558), Natural Science Foundation of Guangdong Province (Nos. 2020A151501518, 2018B030312010, 2019B020202002), Science and Technology Program of Guangzhou (201904010380) for the financial support to do this work.
References
- 1.Deng S.Q., Peng H.J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 2020;9(2):575. doi: 10.3390/jcm9020575. Published 2020 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu X., Zhang R., An T., et al. Impact of heat-inactivation on the detection of SARS-CoV-2 IgM and IgG antibody by ELISA [published online ahead of print, 2020 Jun 20] Clin. Chim. Acta. 2020;509:288–292. doi: 10.1016/j.cca.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S., Jiang L., Cao Z., et al. Deep learning for detecting corona virus disease 2019 (COVID-19) on high-resolution computed tomography: a pilot study. Ann. Transl. Med. 2020;8(7):450. doi: 10.21037/atm.2020.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z., Xiao X., Wei X., et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020;92(6):595–601. doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik Y.A. Properties of coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020;42(1):3–11. [PubMed] [Google Scholar]
- 7.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280–e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cvetkovic R.S., Goa K.L. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63(8):769–802. doi: 10.2165/00003495-200363080-00004. [DOI] [PubMed] [Google Scholar]
- 9.Choy K.T., Wong A.Y., Kaewpreedee P., et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro [published online ahead of print, 2020 Apr 3] Antivir. Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye X.T., Luo Y.L., Xia S.C., et al. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur. Rev. Med. Pharmacol. Sci. 2020;24(6):3390–3396. doi: 10.26355/eurrev_202003_20706. [DOI] [PubMed] [Google Scholar]
- 11.Cao B., Wang Y., Wen D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19 [published online ahead of print, 2020 mar 18] N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Bari M.A. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015;70(6):1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent M.J., Bergeron E., Benjannet S., et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. Published 2005 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahraei Z., Shabani M., Shokouhi S., Saffaei A. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine [published online ahead of print, 2020 Mar 17] Int. J. Antimicrob. Agents. 2020:105945. doi: 10.1016/j.ijantimicag.2020.105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao X., Ye F., Zhang M., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [published online ahead of print, 2020 mar 9] Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautret P., Lagier J.C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [published online ahead of print, 2020 Mar 20] Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Li X., Wang Y., Agostinis P., et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11(7):512. doi: 10.1038/s41419-020-2721-8. Published 2020 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villalaín J. Membranotropic effects of arbidol, a broad anti-viral molecule, on phospholipid model membranes. J. Phys. Chem. B. 2010;114(25):8544–8554. doi: 10.1021/jp102619w. [DOI] [PubMed] [Google Scholar]
- 20.Blaising J., Polyak S.J., Pécheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haviernik J., Štefánik M., Fojtíková M., et al. Arbidol (umifenovir): a broad-spectrum antiviral drug that inhibits medically important arthropod-borne flaviviruses. Viruses. 2018;10(4):184. doi: 10.3390/v10040184. Published 2018 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pécheur E.I., Borisevich V., Halfmann P., et al. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J. Virol. 2016;90(6):3086–3092. doi: 10.1128/JVI.02077-15. Published 2016 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Cao R., Zhang H., et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6(28) doi: 10.1038/s41421-020-0169-8. Published 2020 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Z., Lu Z., Xu T., et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19 [published online ahead of print, 2020 Apr 10] J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.060. S0163-4453(20)30188-2. [DOI] [Google Scholar]
- 25.Deng L., Li C., Zeng Q., et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study [published online ahead of print, 2020 Mar 11] J. Infect. 2020;(20):30113–30114. doi: 10.1016/j.jinf.2020.03.002. S0163-4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Y.C., Deng Q.X., Dai S.X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Trav. Med. Infect. Dis. 2020;35:101647. doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agostini M.L., Andres E.L., Sims A.C., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2) doi: 10.1128/mBio.00221-18. e00221-18. Published 2018 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oestereich L., Lüdtke A., Wurr S., Rieger T., Muñoz-Fontela C., Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir. Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Q., Yang M., Liu D., et al. Engineering (Beijing); 2020. Experimental Treatment with Favipiravir for COVID-19: an Open-Label Control Study [published Online Ahead of Print, 2020 Mar 18] 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu Y.F., Chien C.S., Yarmishyn A.A., et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657. Published 2020 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feld J.J., Jacobson I.M., Sulkowski M.S., Poordad F., Tatsch F., Pawlotsky J.M. Ribavirin revisited in the era of direct-acting antiviral therapy for hepatitis C virus infection. Liver Int. 2017;37(1):5–18. doi: 10.1111/liv.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson P., Griffin I., Tucker C., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spagnuolo V., Castagna A., Lazzarin A. Darunavir for the treatment of HIV infection. Expet Opin. Pharmacother. 2018;19(10):1149–1163. doi: 10.1080/14656566.2018.1484901. [DOI] [PubMed] [Google Scholar]
- 35.Ding Q., Lu P., Fan Y., Xia Y., Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China [published online ahead of print, 2020 Mar 20] J. Med. Virol. 2020 doi: 10.1002/jmv.25781. 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J., Wong Y.K., Liao F. What has traditional Chinese medicine delivered for modern medicine? Expet Rev. Mol. Med. 2018;20:e4. doi: 10.1017/erm.2018.3. Published 2018 May 11. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Zhang M., He L., Li Y.P., Kang Y.K. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS) Cochrane Database Syst. Rev. 2006;1:CD004882. doi: 10.1002/14651858.CD004882.pub2. Published 2006 Jan 25. [DOI] [PubMed] [Google Scholar]
- 38.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment [published correction appears in Pharmacol Res. 2020 Mar 25;:104768] Pharmacol. Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li R.F., Hou Y.L., Huang J.C., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao K.T., Liu M.Y., Li X., Huang J.H., Cai H.B. Retrospective clinical analysis on treatment of novel coronavirus-infected pneumonia with traditional Chinese medicine lianhua qingwen. Chin. J. Exp. Tradit. Med. Form. 2020 doi: 10.13422/j.cnki.syfjx.20201099. [DOI] [Google Scholar]
- 41.Marano G., Vaglio S., Pupella S., et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. J. Am. Med. Assoc. 2020;27 doi: 10.1001/jama.2020.4783. Published online March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajendran K., Krishnasamy N., Rangarajan J., Rathinam J., Natarajan M., Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: systematic review [published online ahead of print, 2020 May 1] J. Med. Virol. 2020 doi: 10.1002/jmv.25961. 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrat F.J., Crow M.K., Ivashkiv L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019;20(12):1574–1583. doi: 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negishi H., Taniguchi T., Yanai H. The interferon (IFN) class of cytokines and the IFN regulatory factor (IRF) transcription factor family. Cold Spring Harb. Perspect. Biol. 2018;10(11) doi: 10.1101/cshperspect.a028423. a028423. Published 2018 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang B.X., Fish E.N. Global virus outbreaks: interferons as 1st responders. Semin. Immunol. 2019;43:101300. doi: 10.1016/j.smim.2019.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen D., Yang H., Cao Y., et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int. J. Gynaecol. Obstet. 2020;149(2):130–136. doi: 10.1002/ijgo.13146. [published correction appears in Int J Gynaecol Obstet. 2020 Jul;150(1):136] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francis M.J. Recent advances in vaccine technologies. Vet. Clin. North Am. Small Anim. Pract. 2018;48(2):231–241. doi: 10.1016/j.cvsm.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pang J., Wang M.X., Ang I.Y.H., et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J. Clin. Med. 2020;9(3):E623. doi: 10.3390/jcm9030623. Published 2020 Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Folegatti P.M., Bittaye M., Flaxman A., et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial [published online ahead of print, 2020 Apr 20] [published correction appears in Lancet Infect Dis. 2020 May 12;:] Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30160-2. S1473-3099(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin Z., Du X., Xu Y., et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors [published online ahead of print, 2020 Apr 9] Nature. 2020 doi: 10.1038/s41586-020-2223-y. 10.1038/s41586-020-2223-y. [DOI] [Google Scholar]
- 53.Dai W., Zhang B., Su H., et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease [published online ahead of print, 2020 Apr 22] Science. 2020 doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L., Lin D., Sun X., et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved á-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia S., Liu M., Wang C., et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]